Abstract
Context:
Diabetes mellitus is a chronic disease, and its incidence is tremendously increasing globally. Decreasing postprandial hyperglycemia by retarding glucose absorption through inhibiting carbohydrates digesting enzymes (α-amylase and α-glucosidase) is one of many approaches used for the management of this disease.
Objectives:
The leaf and root aqueous and ethanol extracts of Albizia antunesiana were investigated for α-amylase and α-glucosidase inhibitory and cytotoxic activity in vitro.
Materials and Methods:
The α-amylase and α-glucosidase activities were measured in the presence of aqueous and ethanol extracts of the plant parts using starch and p-nitrophenyl-D-glucopyranoside as substrates respectively. Furthermore, cytotoxic effects of the extracts were investigated on HEK (human embryonic kidney) 293 cell lines using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (MTT) assay.
Results:
The results showed that ethanolic root extract of A. antunesiana had mild α-amylase and strong α-glucosidase inhibitory activity with half-maximal inhibitory concentration values of 30.68 and 4.35 µg/mL, respectively. The aqueous root extract showed mild α-glucosidase but no α-amylase inhibitory activity. Cytotoxicity studies on the extracts using the MTT assay revealed that the ethanolic (leaf and root) extracts were relatively nontoxic at tested concentrations on the HEK 293 cell lines. However, the aqueous extracts (leaf and root) were cytotoxic at concentrations above 50 µg/mL.
Conclusion:
Data from this study suggest that the ethanolic root extract of A. antunesiana possess in vitro α-amylase and α-glucosidase inhibitory activities and are not cytotoxic at least in an in vitro condition.
Keywords: Albizia antunesiana, cytotoxicity, diabetes, α-amylase, α-glucosidase
INTRODUCTION
The prevalence of type 2 diabetes mellitus (T2DM) is increasing at an alarming rate such that it is currently being estimated to be responsible for 90–95% of all diabetes cases worldwide.[1] This is a result of lifestyle and socioeconomic changes mainly characterized by lower physical activity and higher intake of high fat and saturated carbohydrate containing diets among many other factors.[2]
Hyperglycemia is a hallmark of T2DM and plays vital roles in most of the pathogenic features of the disease. This condition prevails when there is decreased insulin sensitivity or decreased insulin secretion from pancreatic β-cells, which can further inhibit insulin secretion from the pancreas and reduce insulin-mediated glucose uptake in peripheral tissues.[3,4] All diabetic complications (nephropathy, neuropathy, microangiopathy, macroangiopathy, retinopathy, and cataract) are strongly linked to hyperglycemia. This calls for the improved treatment of hyperglycemia, T2DM-related risk factors and the long-term degenerative disorders so as to dramatically lower the risk of both micro-and macro-vascular complications.
An important strategy to control hyperglycemia is through the inhibition of key carbohydrates digesting enzymes such as α-amylase and α-glucosidase, which also play a vital role in preventing diabetic complications. The inhibitors of these enzymes delay the digestion of carbohydrates, therefore, reduce the rate of glucose absorption from the small intestinal tract, as well as reduce postprandial blood glucose level. Thus, inhibition of α-amylase and α-glucosidase is a key in the management and treatment of hyperglycemia and T2DM.[3]
Currently, several conventional prescribed α-glucosidase inhibitors are available in the market, which include acarbose, voglibose, and miglitol; however, they have been shown to have some undesirable side effects such as flatulence, diarrhea, and abdominal pain, which were hostile to patients.[3] This indicates the urgent need for the development of newer alternatives. A better clinical outcome could be derived from specific agents with inhibitory activity against the α-amylase and α-glucosidase and not cytotoxic to target cells, but still effective to reduce the postprandial hyperglycemia. Furthermore, an ideal compound or drug will be the one that will mildly inhibit α-amylase and strongly inhibit α-glucosidase,[5] and it can be identified from any natural source, e.g. medicinal plants.
The use of medicinal plants has been adopted since ancient times to treat a number of ailments, and some of these plants and plant-derived products have shown impressive potentials including the discovery of some conventional medicines. Some medicinal plants have been shown to possess α-amylase and α-glucosidase inhibitory activities.[6,7] However, the presence of some potential toxic and carcinogenic[8] agents in some of these plants made them unsuitable for therapeutic applications. It is, therefore, utmost important to intensively investigate the potential cytotoxic activity not only to validate the safety but also to continue the use of these medicinal plants. It has been documented that some plants extracts do have bioactivity however, they are cancelled by their cytotoxicity; hence such scenarios need to be evaluated so as to assess the overall efficacy of the plant extracts.[8]
Albizia antunesiana Harms (Fabaceae), commonly called purple-leaved albizia, is indigenous to Zimbabwe. Locally, the Shona and Ndebele-speaking Zimbabweans call it Muriranyenze and Umnonjwana, respectively. The leaves of this plant are used by traditional healers to cure several metabolic and nonmetabolic disorders such as sore eyes, cuts, ulcers, sore throat, tonsillitis, and tuberculosis while the root part is used for the treatment of gonorrhea and cardiac problem.[9] More relevant to this article, both the leaf and root parts of the plant are used in the traditional treatment of diabetes in Mrewa District of Zimbabwe. However, despite the extensive use of these plant parts in traditional medicine, scientific reports on the biological and pharmacological actions of this plant are limited. In a recent study, we reported the anti-oxidative activities of the aqueous and ethanol extracts of the leaf and root of this plant, as well as their possible bioactive compounds.[10]
The present study was undertaken to intensively probe the in vitro α-amylase and α-glucosidase inhibitory effects and cytotoxic activities of the leaf; and root ethanolic and aqueous extracts of this plant as potential sources of therapeutically nontoxic anti-diabetic agents, which can be used for the treatment of hyperglycemia, as well as diabetes.
MATERIALS AND METHODS
Chemicals, reagents, apparatus and equipment
Gallic acid, acarbose, α-amylase, α-glucosidase, dinitrosalicylic acid (DNS), monosodium and disodium phosphate, ascorbic acid, iron chloride, sodium carbonate, trichloroacetic acid, ethylenediaminetetraacetic acid, 4-nitrophenyl-D-glucopyranoside (pNPG), and potassium ferricyanide were procured from Sigma-Aldrich through Capital Lab Supplies, New Germany, South Africa. Griess reagent, sodium hydroxide, hydrogen peroxide, dimethyl sulfoxide (DMSO), sodium nitroprusside, and thiobarbituric acid were purchased from Merck Chemical Company, Durban, South Africa. Starch was purchased from Associated Chemicals Enterprises via Polychem Supplies, Durban, South Africa.
Preliminary ethno botanical survey
We conducted a mini preliminary ethnobotanical survey based on personal communications with local traditional healers and herbalists. Plants were selected based on the number of times they were mentioned for the folkloric treatment of diabetes mellitus. Based on the above mention criteria, A. antunesiana was selected as one of the most mentioned plant.
Plant samples
Plant parts (leaf and root samples) of A. antunesiana were collected during the period of March 2012–February 2013 from Mrewa, Mashonaland East Province, Zimbabwe. The plant samples were identified and authenticated by the herbarium unit of the Harare Botanical Garden and Herbarium, Harare, Zimbabwe and a voucher specimen was deposited for A. antunesiana, with a voucher number AA31509.
These plant samples of A. antunesiana were immediately washed with distilled water, cut into small pieces and shade-dried until constant weight was attained. The dried samples were ground to a fine powder using a kitchen blender, and stored individually in airtight Ziploc bags and transported to the University of KwaZulu-Natal, Westville Campus, Durban, South Africa for further analysis.
Preparation of plant extract
The powdered plant parts were extracted with three solvents in the order of increasing polarity (hexane, ethanol, and water). In this regard, 40 g of the finely powdered sample was separately defatted with hexane. The defatted materials were sequentially extracted with ethanol and water by soaking for 48 h in 200 mL of the respective solvent. For ethanol extract, after filtration through Whatman filter paper (No. 1), the ethanol was evaporated under reduced pressure using a rotary evaporator (Buchi Rotavapor II, Buchi, Germany) at 40°C and the remaining ethanol was allowed to evaporate freely at room temperature. The aqueous extract was dried using a freeze dryer. The solvent extracts in each case were weighed, transferred to micro tubes, and stored in a refrigerator at 4°C until required.
α-amylase inhibitory activity of the plant extracts
The α-amylase inhibitory activity of plant extracts was determined according to a previously described method[11] with slight modifications. A volume of 250 µL of each extract or acarbose at different concentrations (50–250 µg/mL) was incubated with 500 µL of porcine pancreatic amylase (2 U/mL) in 100 mM phosphate buffer (pH 6.8) at 37°C for 20 min. A 250 µL of 1% starch dissolved in 100 mM phosphate buffer (pH 6.8) was then added to the reaction mixture and incubated at 37°C for 1 h. Then, 1 mL of DNS color reagent was added and boiled for 10 min. The absorbance of the resulting mixture was measured at 540 nm, and the inhibitory activity was expressed as a percentage of control without the inhibitors.
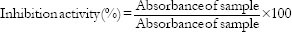
α-glucosidase inhibitory activity of the plant extracts
The α-glucosidase inhibitory activity was determined according to a previously described method[12] with slight modifications. Briefly, 250 µL of the extract or acarbose at different concentrations (50–250 µg/mL) was incubated with 500 µL of 1.0 U/mL α-glucosidase solution in 100 mM phosphate buffer (pH 6.8) at 37°C for 15 min. Thereafter, 250 µL of pNPG solution (5 mM) in 100 mM phosphate buffer (pH 6.8) was added, and the mixture was further incubated at 37°C for 20 min. The absorbance of the released p-nitrophenol was measured at 405 nm, and the inhibitory activity was expressed as a percentage of control without the inhibitors.
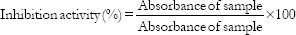
Cell line maintenance for cytotoxicity assay
Routine cell culture maintenance of the human embryonic kidney (HEK), 293 cells was done by incubating the cells at 37°C in a humidified atmosphere supplemented with 5% CO2. Cells were replenished with fresh growth medium every 2–3 days, consisting of media (minimum essential medium + glutmax + antibiotics + 10% fetal bovine serum).
Cytotoxicity activity of the plant extracts on human embryonic kidney 293 kidney cells using 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide assay
The aqueous and ethanol extracts used for this assay were reconstituted in 10% DMSO, vortexed, filtered through Whatman filter paper (No. 1) and left for 15 min, before further dilution with the respective growth medium. Subsequently, the prepared extracts were tested for in vitro cytotoxicity, using HEK293 kidney cells by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) (MTT) assay.[13,14] Confluent monolayer culture suspensions of the cells were trypsinized and plated into 96 well plates at a seeding density of 2.5 × 103 cells per well and incubated for 24 h at 37°C in a 5% CO2 incubator in a culture medium containing 10% fetal bovine serum. Following 24 h incubation and attachment, the cell culture medium was replaced with fresh medium. Thereafter, varied concentrations of plant extracts (50–200 µg/mL) were added to the cells in triplicate and the plate incubated for 48 h as previously described. Two controls, one containing only cells and one containing DMSO were also set up. After 48 h, all culture media were removed from the plates, the cells were washed with phosphate-buffered saline (PBS), and 100 µL of the cell media and 100 µL of MTT solution (5 mg/mL in PBS) was added to each well. The plates were then incubated for 4 h at 37°C. Thereafter, 100 µL DMSO solution was added to each well to stop the reaction and to dissolve the insoluble formazan crystals. Absorbance was measured at 570 nm using a Mindray MR-96A microplate reader. The assessment of cytotoxicity was based on a comparison with untreated cells and expressed as half-maximal inhibitory concentration (IC50) (the concentration of the sample required to inhibit 50% of cell proliferation), calculated from the dose-response curve (curve fit-nonlinear regression, four parameter). The values are presented as means of triplicate analyses.
Statistical analysis
All data are presented as the mean ± standard deviation of triplicates determination. Data were analyzed by SPSS statistical software (version 19, Windows IBM Inc., USA) using Tukey's -HSD multiple ranges post-hoc tests. Values were considered significantly different at P < 0.05.
RESULTS
Figure 1 shows inhibitory activity of A. antunesiana aqueous and ethanol extracts on α-amylase [Figure 1a] and α-glucosidase [Figure 1b]. The result indicates that A. antunesiana root ethanol extract is a better α-amylase inhibitor than the standard acarbose. However, aqueous root and both leaf extracts do possess inhibition potential that is significantly lower (P < 0.05) than that of the standard. Figure 1b also indicated the ability of root extracts to have better inhibitory activity on α-glucosidase than acarbose, however, the inhibitory activity was much pronounced in the ethanol root extract because it had significantly higher (P < 0.05) α-glucosidase inhibition at all concentrations tested.
Figure 1.
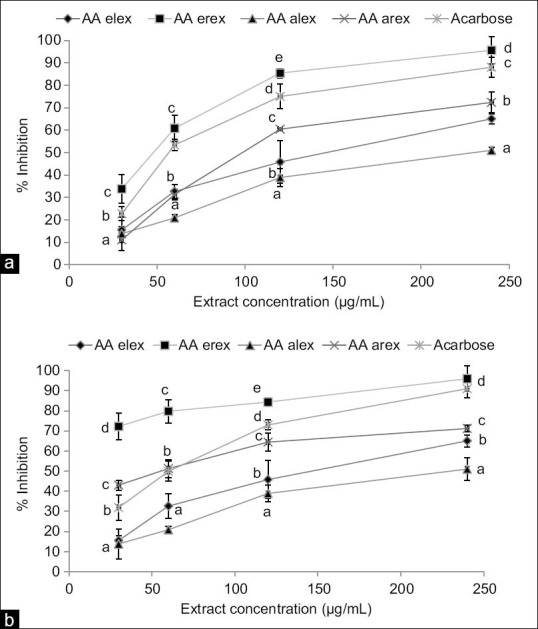
α-amylase (a) and α-glucosidase (b) inhibitory activity of Albizia antunesiana aqueous and ethanol extracts. Arex = aqueous root extract and Erex = ethanol root extract; Alex = aqueous leaf extract and Elex = ethanol leaf extract. Data are presented as mean ± standard deviation values of triplicate determinations. (a-e) Different superscripts letters for a given value within a column are significantly different from each other (Tukey's-honest significant difference multiple range post-hoc test, P < 0.05)
The IC50 values for inhibiting α-glucosidase (4.35 ± 0.56 μg/mL) and α-amylase (30.68 ± 1.09 μg/mL) of the ethanol root extract indicates that the extract is a moderate inhibitor of α-amylase and a potent inhibitor of α-glucosidase [Table 1].
Table 1.
IC50 values for α-amylase and α-glucosidase inhibition activity of Albizia antunesiana extracts
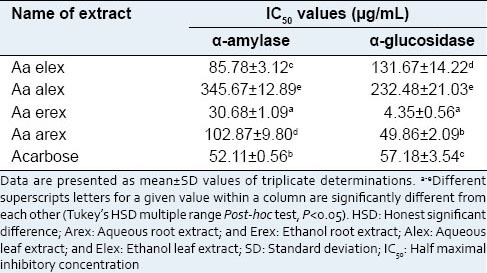
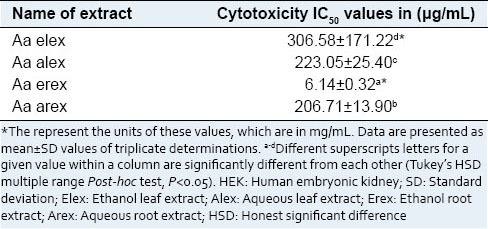
Figure 2 displays the cytotoxic activity of the A. antunesiana extracts on HEK 293 cell lines as confirmed by MTT assay. As indicated in the Figure 2, A. antunesiana ethanol root extracts did not decrease cell viability to a significant extent. However, the aqueous (leaf and root) extracts significantly (P < 0.05) decreased the cell viability across all tested concentrations (50–200 µg/mL) in a concentration-dependent pattern. This is also consistent with the high IC50 [Table 2] value obtained for A. antunesiana ethanol root extract (6.14 ± 0.32 µg/mL). Despite the lowest IC50 value of 206.71 ± 13.90 µg/mL [Table 2] aqueous root extract was found most toxic during MTT assay [Figure 2].
Figure 2.
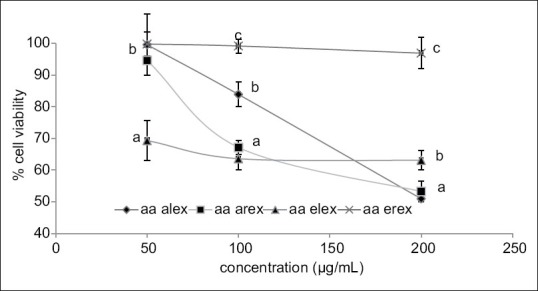
Cytotoxicity activity Albizia antunesiana extracts on human embryonic kidney 293 kidney cells lines as confirmed by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide cell proliferation assay. Arex = aqueous root extract and Erex = ethanol root extract; Alex = aqueous leaf extract and Elex = ethanol leaf extract. Data are presented as mean ± standard deviation values of triplicate determinations. (a-c) Different superscripts letters for a given value within a column are significantly different from each other (Tukey's-honest significant difference multiple ranges post-hoc test, P < 0.05)
Table 2.
IC50 values for cytotoxicity activity of Albizia antunesiana extracts on HEK 293 kidney cells
DISCUSSION
The inhibition of carbohydrate digesting enzymes (α-amylase and α-glucosidase) activity, as well as delaying the digestion, and absorption of carbohydrates from the small intestinal tract is considered as one of the major treatment options for T2DM. By inhibiting these key enzymes, minimal amounts of glucose would be absorbed into the blood stream, hence the plasma glucose will not spike after a meal.[15]
The result of the present study showed that A. antunesiana root extracts moderately inhibited α-amylase and significantly inhibited α-glucosidase activity as evidently shown by the lower IC50 values. This mimics an effective T2DM drug model, which has to be a strong inhibitor of intestinal α-glucosidases and mild inhibitor of pancreatic α-amylase.[5] This is because strong inhibition of the α-amylase is associated with stomach discomfort due to fermentation of undigested carbohydrates by the colonic bacteria.[5] In a previous study, we found that the A. antunesiana ethanol root extract contains mainly phenolic compounds (catechol, pyrogallol, and hydroxyquinol and resorcinol), coumarin, and triterpenoids (α-and β-amyrin) as the major phytochemical components.[10] Besides having the highest antioxidant activity, the ethanol root extract was found to be effective α-glucosidase and α-amylase inhibitor in this study, which could be linked to the above mentioned phenolic compounds. This is because phenolic compounds were reported to be effective α-glucosidase and α-amylase inhibitors.[16,17,18] Based on these observations, the observed inhibitory activity of A. antunesiana ethanol root extract could be linked to one or more of the above-mentioned phenolic compounds present in the extracts. On the other hand, some of the compounds found in this extract such as coumarin and triterpenoids were also reported to be effective inhibitors of α-amylase and α-glucosidase.[18] Hence, our data suggest that the A. antunesiana ethanol root extract possess bioactive compounds with potential anti-diabetic activity via inhibiting two major carbohydrate-digesting enzymes.
In order to ascertain the safety of the extracts, the cytotoxic activities of the A. antunesiana extracts were investigated and we found that aqueous leaf and root extracts showed a significant cytotoxic activity [Figure 2] by the reduction in cell viability to 51% and 53%, respectively at 200 µg/mL concentration. However, the ethanol root extract did not significantly decrease the viability of cells. Therefore, it necessitates the further investigation of the ethanolic extracts to identify the responsible compounds for the observed α-glucosidase and α-amylase inhibitory activity.
CONCLUSION
Based on the findings of this study, it can be concluded that ethanolic root extract of A. antunesiana possesses effective α-glucosidase and α-amylase inhibitory effects, and it is nontoxic in nature. The results displayed by the ethanol root extract are interesting enough to stimulate the isolation of pure bioactive compounds and further in vivo study using an animal model of diabetes.
ACKNOWLEDGMENTS
This study was supported by a competitive research grant from the research office, University of KwaZulu-Natal (UKZN), Durban; an incentive grant for rated researchers and a grant support for women and young researchers from the National Research Foundation, Pretoria, South Africa. The first author was awarded a Masters Research grant from the College of Agriculture, Engineering and Science, University of KwaZulu-Natal, Durban, South Africa.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Diabetes Atlas. 5th ed. Brussels, Belgium: International Diabetes Federation; 2011. International Diabetes Federation (IDF) [Google Scholar]
- 3.Ali MS, Jahangir M, Hussan SS, Choudhary MI. Inhibition of alpha-glucosidase by oleanolic acid and its synthetic derivatives. Phytochemistry. 2002;60:295–9. doi: 10.1016/s0031-9422(02)00104-8. [DOI] [PubMed] [Google Scholar]
- 4.Cai H, McNeilly AS, Luttrell LM, Martin B. Endocrine function in aging. Int J Endocrinol 2012. 2012:872478. doi: 10.1155/2012/872478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krentz AJ, Bailey CJ. Oral antidiabetic agents: Current role in type 2 diabetes mellitus. Drugs. 2005;65:385–411. doi: 10.2165/00003495-200565030-00005. [DOI] [PubMed] [Google Scholar]
- 6.Ortiz-Andrade RR, García-Jiménez S, Castillo-España P, Ramírez-Avila G, Villalobos-Molina R, Estrada-Soto S. Alpha-glucosidase inhibitory activity of the methanolic extract from Tournefortia hartwegiana: An anti-hyperglycemic agent. J Ethnopharmacol. 2007;109:48–53. doi: 10.1016/j.jep.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Shirwaikar A, Rajendran K, Punitha IS. Antidiabetic activity of alcoholic stem extract of Coscinium fenestratum in streptozotocin-nicotinamide induced type 2 diabetic rats. J Ethnopharmacol. 2005;97:369–74. doi: 10.1016/j.jep.2004.11.034. [DOI] [PubMed] [Google Scholar]
- 8.Fennell CW, Lindsey KL, McGaw LJ, Sparg SG, Stafford GI, Elgorashi EE, et al. Assessing African medicinal plants for efficacy and safety: Pharmacological screening and toxicology. J Ethnopharmacol. 2004;94:205–17. doi: 10.1016/j.jep.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Ndemera B, Gelfand M, Mavi S, Drummond RB. Zimbabwe: Mambo Press; 1985. The Traditional Medical Practitioner in Zimbabwe. [Google Scholar]
- 10.Chipiti T, Ibrahim MA, Koorbanally NA, Islam MS. In vitro antioxidant activities of leaf and root extracts of Albizia antunesiana harms. Acta Pol Pharm. 2013;70:1035–43. [PubMed] [Google Scholar]
- 11.Tirosh A, Shai I, Bitzur R, Kochba I, Tekes-Manova D, Israeli E, et al. Changes in triglyceride levels over time and risk of type 2 diabetes in young men. Diabetes Care. 2008;31:2032–7. doi: 10.2337/dc08-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ademiluyi AO, Oboh G. Health benefits of traditional corn, beans, and pumpkin: In vitro studies for hyperglycemia and hypertension management. J Med Food. 2013;16:88–93. doi: 10.1089/jmf.2006.234. [DOI] [PubMed] [Google Scholar]
- 13.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–33. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 15.Ferrannini E, Nannipieri M, Williams K, Gonzales C, Haffner SM, Stern MP. Mode of onset of type 2 diabetes from normal or impaired glucose tolerance. Diabetes. 2004;53:160–5. doi: 10.2337/diabetes.53.1.160. [DOI] [PubMed] [Google Scholar]
- 16.de Sousa E, Zanatta L, Seifriz I, Creczynski-Pasa TB, Pizzolatti MG, Szpoganicz B, et al. Hypoglycemic effect and antioxidant potential of kaempferol-3,7-O-(alpha)- dirhamnoside from Bauhinia forficata leaves. J Nat Prod. 2004;67:829–32. doi: 10.1021/np030513u. [DOI] [PubMed] [Google Scholar]
- 17.Hanamura T, Hagiwara T, Kawagishi H. Structural and functional characterization of polyphenols isolated from acerola (Malpighia emarginata DC.) fruit. Biosci Biotechnol Biochem. 2005;69:280–6. doi: 10.1271/bbb.69.280. [DOI] [PubMed] [Google Scholar]
- 18.Thilagam E, Parimaladevi B, Kumarappan C, Mandal SC. α-Glucosidase and α-amylase inhibitory activity of Senna surattensis. J Acupunct Meridian Stud. 2013;6:24–30. doi: 10.1016/j.jams.2012.10.005. [DOI] [PubMed] [Google Scholar]


