Abstract
Background:
Poncirus trifoliata Rafin. is a traditional medicine with known anti-inflammatory and anti-cancer properties. Traditionally, it is used to control chronic inflammation, allergy and gastrointestinal diseases such as digestive ulcers gastritis in China, Japan, and Korea.
Objectives:
To evaluate the apoptosis-inducing activity of a P. trifoliata methanol extract (MEPT) and elucidate the molecular mechanisms.
Materials and Methods:
The anti-cancer effect of MEPT and its underlying mechanisms were investigated in breast cancer cells using 3,4,5-dimethyl N-methylthiazol-2-yl-2, 5-d-phenyl tetrazolium bromide assay, cell cycle analysis, and western blotting.
Results:
MEPT suppressed the proliferation of MDA-MB-231 cells with inhibition dose 50% value of 119.44 μg/mL at 24 h, which have features typical of triple-negative breast cancer cells. MEPT also altered the characteristic features of the MDA-MB-231 cells and increased the proportion of cells undergoing sub-G1 arrest. In addition, MEPT increased levels of caspase 8 and 3 in MDA-MB-231 cells, whereas caspase 9 was not detected. In addition, MEPT-induced tumor necrosis factor receptor (TNFR) and TNFR type 1-associated death domain (TRADD) protein and the activations of c-Jun NH(2)-terminal kinase (JNK) and extracellular signal-regulated kinases (ERK).
Conclusion:
Our results indicate that MEPT has chemotherapeutic potential in triple-negative breast cancer and that at the molecular level its effects are derived from the activations of TNFR and of the mitogen-activated protein kinase pathway.
Keywords: Apoptosis, cancer therapy, Poncirus trifoliata, tumor necrosis factor receptor
INTRODUCTION
The immature fruit of Poncirus trifoliata Rafin. is traditionally used to treat inflammation, gastrointestinal diseases, and allergies.[1,2] Recently, researchers have reported that extracts of P. trifoliata have various biological activities that is, the suppression of Helicobacter pylori, the inhibition of anaphylaxis and the attenuation of the anti-cancer activities of tumor cells.[1] In addition, Yi et al. suggested that P. trifoliata extracts are cytotoxic to promyelocytic leukemia cells.[2] However, the cytotoxic effects of P. trifoliata extracts on breast cancer cells and the molecular mechanisms underlying their anti-cancer activities are not well understood.
Two classical pathways are involved in apoptosis, namely, the intrinsic pathway and the extrinsic pathway.[3] The intrinsic pathway is activated by the release of cytochrome c into the cytoplasm by the opening of the bax/bak channel, whereas the extrinsic pathway initiates cellular apoptosis by activating death receptors such as tumor necrosis factor receptor (TNFR) 1/2. In turn, the death receptor such as Fas/Fas ligand (FasL) interactions activate downstream signals such as TNFR type 1-associated death domain (TRADD), which are followed by the induction of Fas-associated protein with death domain (FADD).[3] These events are followed by the activation of caspase 8 (the initiator caspase of extrinsic pathway) then of caspase 3 (effector caspase) and Poly Adenosine diphosphate ribose (ADP-ribose) polymerase poly ADP ribose polymerase (PARP) cleavage, and eventually result in cell death.
Many chemotherapeutic agents modulate the mitogen-activated protein kinase (MAPK) pathway, and the activation of this pathway is closely associated with the apoptosis cascade.[4,5] The MAPK signaling pathway mainly involves of three kinase families: Extracellular signal-regulated kinase (ERK), c-Jun NH (2)-terminal kinase (JNK) and p38 MAPKs.[4,5] MAPK pathway members have been implicated in essential cellular processes such as differentiation, cellular reproduction, and inflammation.[4] Importantly, while JNKs and p38 kinases are considered to be pro-apoptotic in various tumor cells, the ERKs are associated with cell survival and possess anti-apoptotic activity.[5,6,7] In general, ERKs activation suppresses the cleavage of caspase 8 and inhibits chemotherapeutic agent-induced apoptosis in tumor cells. However, recent studies have suggested that ERK may also have a pro-apoptotic role.[7,8]
In the present study, we evaluated the anti-cancer effects of P. trifoliata methanol extract (MEPT) on human breast cancer cells. MEPT-induced apoptosis and its induction mechanisms related in TNFR and TRADD activation, and up-regulation of ERK and JNK were investigated using MDA-MB-231 cells.
MATERIALS AND METHODS
Reagents and antibodies
Paclitaxel, 3,4,5-dimethyl N-methylthiazol-2-yl-2, 5-d-phenyl tetrazolium bromide (MTT) and propidium iodide (PI) solution were obtained from Sigma-Aldrich (St. Louis, MO, USA). Anti-mouse IgG antibody and anti-rabbit IgG antibody were purchased from Enzo Life Sciences (Farmingdale, NY, USA). Primary antibodies for cleaved caspase 3, cleaved caspase 8, cleaved caspase 9, TNFR, TRADD, Fas, FADD, p38, phospho-p38, JNK, and phospho-JNK were purchased from Cell Signaling Technology (Beverly, MA, USA). Primary antibodies for beta actin, ERK, and phospho-ERK were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Preparation of P. trifoliata methanol extract
The immature fruit of P. trifoliata Rafin. was purchased from Hwalim Medicinal Herbs (Pusan, Korea). Extraction was performed using our standard extraction process.[9] Briefly, 50 g of P. trifoliata (dried fruit) were immersed in 1 L of methanol, sonicated for 30 min, and then extracted for 48 h. The extract obtained was filtered through No. 20 Whatman filter paper, evaporated under reduced pressure using a vacuum evaporator (Eyela, Tokyo, Japan), and lyophilized using a freeze dryer (Labconco, Kansas City, MO, USA). Finally, 8.66 g of lyophilized powder was obtained (yield, 17.32%). A sample of the lyophilized powder (MEPT, Voucher No. MH2013-007) was deposited at the Division of Pharmacology, School of Korean Medicine, Pusan National University.
Cell culture
MDA-MB-231 cells (a triple-negative breast cancer (TNBC) cell line) were purchased from the Korean Cell Bank (Seoul, Korea), and maintained in Dulbecco's Modified Eagle's Medium (DMEM, Hyclone, UT, USA) containing 10% fetal bovine serum (FBS, Hyclone) with 1% penicillin/streptomycin (Invitrogen, NY, USA), at 37°C in a humidified 5% CO2/95% air atmosphere.
Determination of cell viability
Cell viability was determined using a MTT proliferation assay. Briefly, MDA-MB-231 cells were seeded in 24-well plates at a density of 5 × 104 cells per well and cultured overnight at 37°C for attachment. Cells were treated with MEPT at the concentration of 0, 12.5, 25, 50 or 100 μg/mL for 24 or 48 h. The same volume of absolute ethanol (the solvent of MEPT) was used as a vehicle control. MTT solution was diluted in DMEM (1:9) and added 500 µL to each well, and cells were then incubated at 37°C for 4 h in a 5% CO2 atmosphere. Absorbance was measured at 570 nm using a microplate reader (Bio-Rad, California, USA).
Observation of cell morphology
The morphologies of untreated and MEPT-treated MDA-MB-231 cells were observed using photographs taken with a phase contrast microscope (Olympus, Tokyo, Japan).
Cell cycle analysis by flow cytometry
Cell cycle distributions were determined using a flow cytometric method. Briefly, cells were seeded in 24-well plates at a density of 5 × 104 cells per well and treated with MEPT at the concentration of 0, 25, 50 or 100 μg/mL for 24 h. Following MEPT treatment, cells were collected, fixed, and stained with PI solution (10 μg/mL) at 4°C for 30 min. Fluorescence intensities were measured using a fluorescence-activated cell sorting (FACS) Scan flow cytometer (BD bioscience, Heidelberg, Germany).
Western blot analysis
MEPT-treated MDA-MB-231 cells were harvested with radio-immunoprecipitation assay (RIPA) buffer (Cell Signaling Technology), according to the manufacturer's instructions. The protein concentrations of cell lysate were determined and 50 μg amount of protein was separated in Sodium dodecyl sulfate (SDS)-polyacrylamide gels and transferred to polyvinylidene fluoride membranes, which were then incubated at 4°C overnight with primary antibodies against cleaved caspase 3, cleaved caspase 8, cleaved caspase 9, TNFR, TRADD, p38, phospho-p38, JNK, phospho-JNK, ERK, phospho-ERK and beta-actin (the internal control). Horseradish peroxidase (HRP)-conjugated secondary antibody was then applied at room temperature. Bound antibodies were detected using SuperSignal West-Femto reagent (Pierce, Rockford, IL, USA).
Statistical analysis
Student's t-test in Window Predictive Analytics SoftWare (PASW) version 21.0 (SPSS Inc., NY, USA) was used to determine the significance of differences between the control and experimental groups. Values are presented as means ± standard deviation, and P < 0.05 was considered as statistically significant.
RESULTS
Effects of MEPT on proliferation rates and morphologic changes
Treatment with MEPT lowered proliferation rates of MDA-MB-231 cells in a dose-dependent manner [Figure 1a] with inhibition dose 50% of 119.44 and 62.52 μg/mL at 24 and 48 h, respectively. As shown in Figure 1b, morphological changes such as shrinkage and cell volume reduction were observed in the 50 and 100 μg/mL MEPT treated groups.
Figure 1.
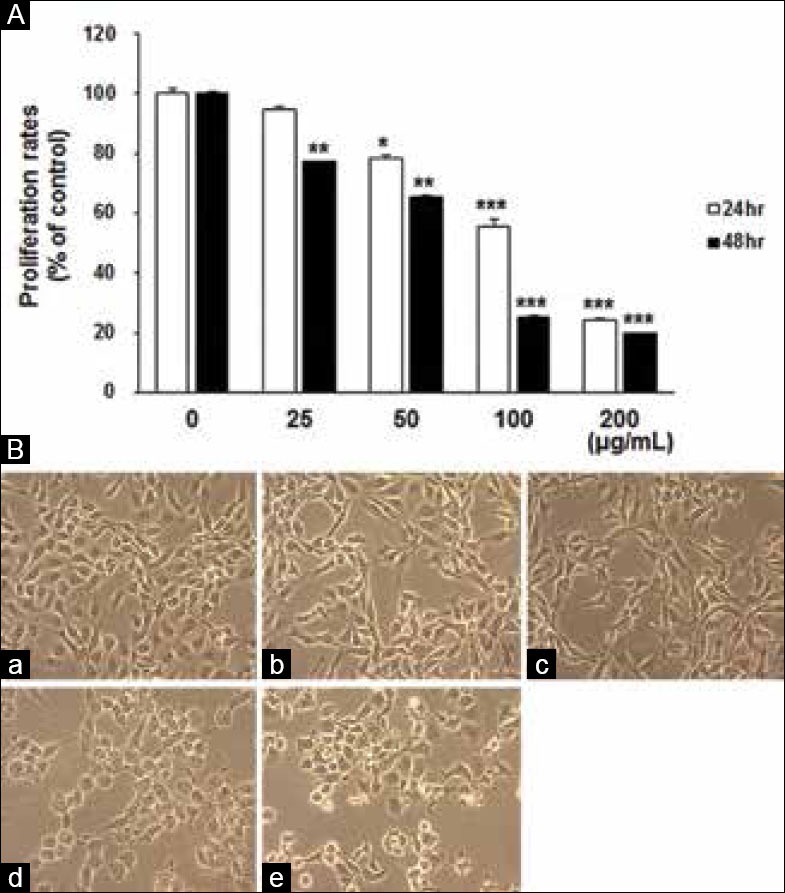
MEPT-induced inhibition of proliferation and changes in cell morphology. MDA-MB-231 cells were treated with 0, 12.5, 25, 50, or 100 μg/mL of MEPT for 24 h or 48 h and proliferation rates were analyzed using an 3,4,5-dimethyl N-methylthiazol-2-yl-2, 5-d-phenyl tetrazolium bromide assay. Results are presented as the means ± standards deviation of three independent experiments (*P < 0.05; **P < 0.01; ***P < 0.001) (A). MEPT caused a change in cell morphology consistent with apoptosis in MDA-MB-231 cells (original magnification, ×200). Cells were treated with (a) 0 μg/mL, (b) 25 μg/mL, (c) 50 μg/mL, or (d) 100 μg/mL of MEPT for 24 h or (e) 30 nM of paclitaxel for 24 h (B)
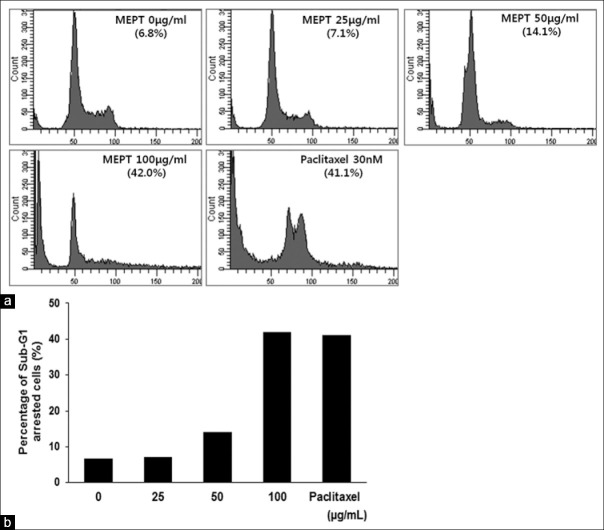
Effects of MEPT on cell cycle distribution
In the normal group, 6.8% of cells were at the sub-G1 phase. Cells treated with 100 μg/mL of MEPT (24 h) showed a marked increase in the number of cells undergoing sub-G1 arrest. The proportions of cells in sub-G1 arrest in the 100 μg/mL of MEPT treated group, and 30 nM of paclitaxel-treated groups were almost same [Figure 2].
Figure 2.
Cell cycle analysis of P. trifoliata methanol extract (MEPT)-treated MDA-MB-231 cells. Cells were treated with various concentrations of MEPT for 24 h or with 30 nM of paclitaxel for 24 h. Cell cycle distributions were analyzed by flow cytometry (a). Percentages of cells in sub-G1 arrest after treatments with MEPT at different concentrations or 30 nM of paclitaxel (b)
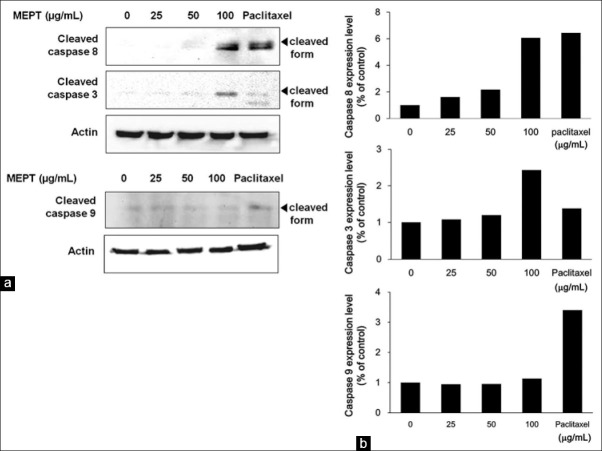
Activation of the extrinsic apoptotic pathway following MEPT treatment
As shown in Figure 3a, MEPT treatment activated extrinsic pathway-related proteins, such as caspase 8 and caspase 3. On the other hand, the level of cleaved caspase 9 was not affected by MEPT [Figure 3b].
Figure 3.
Effects of P. trifoliata methanol extract (MEPT) on the expression of apoptosis-related caspases. MDA-MB-231 cells were treated with 0, 25, 50, or 100 μg/mL of MEPT for 24 h or 30 nM paclitaxel for 24 h. Protein expressions of cleaved caspases 3 and 8 (a) and of cleaved caspase 9 (b) were analyzed by western blot analysis
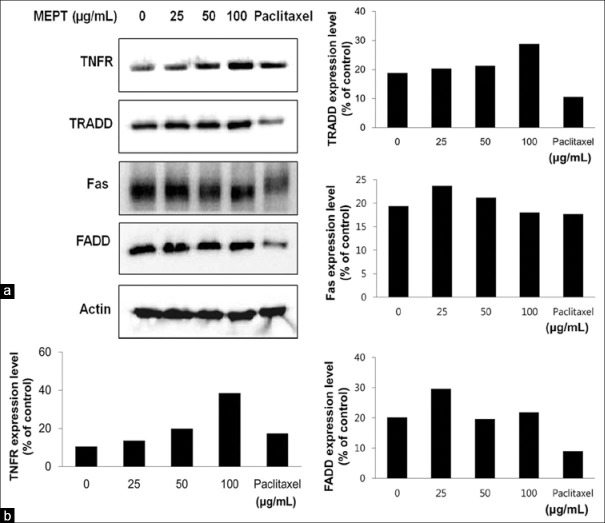
Induction of death receptor protein expression by MEPT
Treatment with MEPT induced the expressions of TNFR and TRADD [Figure 4a]. In contrast, levels of FADD or FasL proteins were not affected by MEPT treatment [Figure 4].
Figure 4.
The expressions and activations of death receptor proteins in P. trifoliata methanol extract (MEPT)-treated MDA-MB-231 cells. Western blotting was performed for tumor necrosis factor receptor, TNFR type 1-associated death domain, Fas, and FADD after administering MEPT at 0, 25, 50, or 100 μg/mL for 24 h, or 30 nM paclitaxel for 24 h (a), and expression levels were normalized using loading control (b)
Triggering of JNK and ERK pathway but not of the p38 pathway by MEPT treatment
MEPT induced the phosphorylations of JNK and ERK in a dose-dependent manner. On the other hand, phospho-p38 levels were not changed by MEPT [Figure 5].
Figure 5.
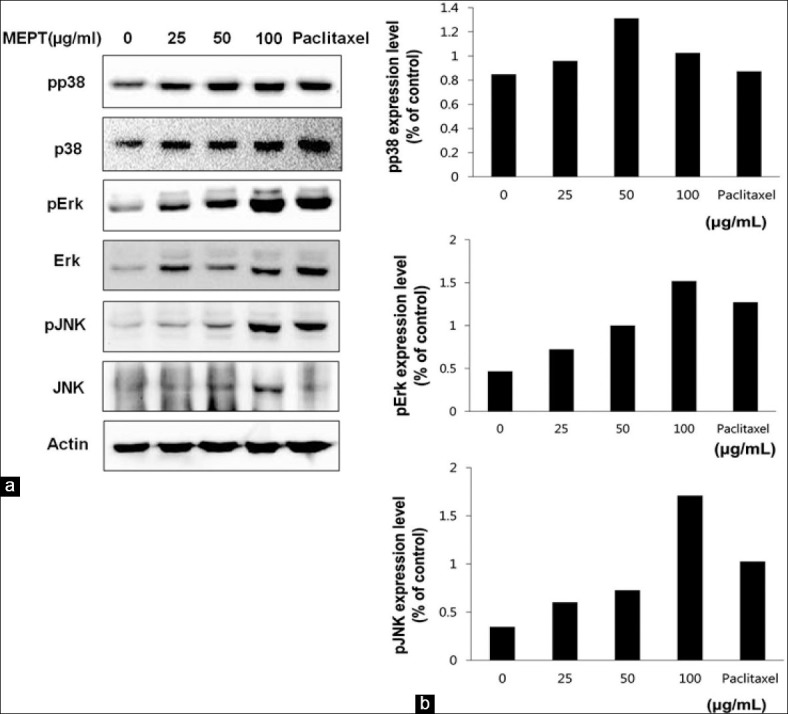
Expression and activations of MAPK proteins in P. trifoliata methanol extract (MEPT)-treated MDA-MB-231 cells. Western blotting was performed for the total and phosphorylated forms of p38, c-Jun NH (2)-terminal kinase (JNK), and extracellular signal-regulated kinases (ERK) in MDA-MB-231 cells treated with 0, 25, 50, or 100 μg/mL of MEPT for 24 h, or 30 nM paclitaxel for 24 h (a). The expression levels of phospho-p38, phospho-JNK, and phospho-ERK were normalized using loading control (b)
DISCUSSION
Tumor necrosis factor receptor (TNFR) plays key roles in cell death and cell regulation,[10,11] and activates downstream molecules such as TRADD, TRAF, FADD and RIP.[12] In addition, the activation of a death domain in the cytoplasmic tail of TNFR is crucial for the induction of apoptosis, particularly for the activation of caspase 8 and 10.[13] Fas is a member of the TNFR superfamily and can trigger apoptosis, and functional loss of Fas in breast cancer cells may be associated with tumor development and drug resistance.[14] In the present study, MEPT treatment enhanced the expression of TNFR and TRADD versus the control group but did not affect the expressions of Fas and FADD. These results imply that MEPT activated TNFR and then TRADD and caspase 8, the latter of which is the major initiator caspase of the extrinsic apoptotic pathway. These results suggest that TNFR-TRADD activation, and not Fas activation, plays a key role in the MEPT-induced apoptosis of MDA-MB-231 cells.
c-Jun NH (2)-terminal kinase protein (JNK) is regarded to be a pro-apoptotic signal that activates apoptotic pathways,[15] and our results suggest that TNFR-mediated JNK activation is essential for apoptosis induction in MEPT-treated MDA-MB-231 cells. On the other hand, ERK pathway is associated with cell survival and proliferation, although some experimental models suggested that under some circumstances ERK protein may have a pro-apoptotic role.[7,16,17] Wang et al.[7] and Frese et al.[8] demonstrated that the ERK pathway is required for cisplastin-induced apoptosis and Apo2 L/TRAIL-induced apoptosis, respectively.[7,8] When ERK is involved in the pro-apoptotic pathway, ERK activation alone seems to be insufficient to induce cell death.[18] In addition, Hu et al. indicated that there exist extensive interactions and cross-talk between JNK and ERK pathways.[5] Furthermore, activated TRADD recruits and suppresses cellular inhibitor of apoptotic protein 1 (cIAP1) to activate JNK.[19] Considering these previous results, our results suggest that MEPT-induced apoptosis, due to the activation of the extrinsic pathway via the activation of TNFR and TRADD, is accompanied by the co-activations of the JNK and ERK pathways.
Poncirin (a flavonoid glycoside) is abundant in P. trifoliata, and is used routinely to identify P. trifoliata. In our opinion, the anti-cancer properties of MEPT observed in the present study are due to influential, perhaps novel components other than poncirin, because poncirin did not exhibit cytotoxicity at concentrations up to 200 ug/mL (data not shown). Several compounds isolated from immature fruit of P. trifoliata such as 25-Methoxyhispidol A, neohesperidin, and β-sitosterol have been reported to have anti-cancer effects,[1,20,21] and in one of these reports, 25-Methoxyhispidol A was found to induce MDA-MB-23l cell death by modulating the EGFR/c-Src signaling pathway and not by inducing apoptosis.[20] Jayaprakasha et al.[22] and Yi et al.[2] reported apoptosis induction by β-sitosterol in HT-29 cells and by extract of P. trifoliata in HL-60 cells respectively,[2,22] and Xu et al. found that neohesperidin induced apoptosis in MDA-MB-231 cells via the intrinsic apoptotic pathway.[21] Therefore, our findings regarding the mode of action of MEPT and activation of the extrinsic apoptotic pathway provide new insights of the anti-cancer effects of MEPT.
Paclitaxel, an effective chemotherapeutic agent derived from Taxus brevifolia, induces the apoptosis of a number of human cancer cell lines. In the present study, paclitaxel was employed as a positive control for apoptosis induction and the results were compared to the effects of MEPT on cells. In paclitaxel-treated cells, TNFR activation was not observed and the decreased expression of TRADD and FADD differed from that of MEPT-treated cells. These observations suggest that the mechanism of apoptosis induction by MEPT and paclitaxel and the proteins involved are quite different.
MDA-MB-231 cells are classified as TNBC cells, as they do not express estrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 (Her2/neu).[21] Because TNBCs are highly malignant and associated with poorer prognosis,[23] a novel therapeutic agent that can act against them is urgently required. The present study shows TNFR, which is constitutively expressed on the cell surface of MDA-MB-231 cells, effectively induces apoptosis in response to MEPT treatment, and thus, our findings suggest that MEPT has potential as a therapeutic agent for the treatment of TNBCs.
CONCLUSIONS
Our results suggest that MEPT induces apoptosis of breast cancer cells via the extrinsic pathway. In addition, our results also suggest that the activations of TNFR, TRADD, and the JNK and ERK pathways play central role in the MEPT-induced apoptosis. MEPT may be a novel, promising chemotherapeutic agent against triple-negative breast cancer.
Footnotes
Source of Support: Supported by a grant of the Korean Health Technology R and D Project, Ministry of Health and Welfare, Republic of Korea (A101420)
Conflict of Interest: None declared.
REFERENCES
- 1.Hong J, Min HY, Xu GH, Lee JG, Lee SH, Kim YS, et al. Growth inhibition and G1 cell cycle arrest mediated by 25-methoxyhispidol A, a novel triterpenoid, isolated from the fruit of Poncirus trifoliata in human hepatocellular carcinoma cells. Planta Med. 2008;74:151–5. doi: 10.1055/s-2008-1034286. [DOI] [PubMed] [Google Scholar]
- 2.Yi JM, Kim MS, Koo HN, Song BK, Yoo YH, Kim HM. Poncirus trifoliata fruit induces apoptosis in human promyelocytic leukemia cells. Clin Chim Acta. 2004;340:179–85. doi: 10.1016/j.cccn.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 3.Elmore S. Apoptosis: A review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–90. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 5.Hu R, Kong AN. Activation of MAP kinases, apoptosis and nutrigenomics of gene expression elicited by dietary cancer-prevention compounds. Nutrition. 2004;20:83–8. doi: 10.1016/j.nut.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 6.Shin DY, Kim GY, Hwang HJ, Kim WJ, Choi YH. Diallyl trisulfide-induced apoptosis of bladder cancer cells is caspase-dependent and regulated by PI3K/Akt and JNK pathways. Environ Toxicol Pharmacol. 2014;37:74–83. doi: 10.1016/j.etap.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Martindale JL, Holbrook NJ. Requirement for ERK activation in cisplatin-induced apoptosis. J Biol Chem. 2000;275:39435–43. doi: 10.1074/jbc.M004583200. [DOI] [PubMed] [Google Scholar]
- 8.Frese S, Pirnia F, Miescher D, Krajewski S, Borner MM, Reed JC, et al. PG490-mediated sensitization of lung cancer cells to Apo2L/TRAIL-induced apoptosis requires activation of ERK2. Oncogene. 2003;22:5427–35. doi: 10.1038/sj.onc.1206842. [DOI] [PubMed] [Google Scholar]
- 9.Kim H, Kim M, Kim H, Lee GS, An WG, Cho SI. Anti-inflammatory activities of Dictamnus dasycarpus Turcz.root bark on allergic contact dermatitis induced by dinitrofluorobenzene in mice. J Ethnopharmacol. 2013;149:471–7. doi: 10.1016/j.jep.2013.06.055. [DOI] [PubMed] [Google Scholar]
- 10.Ihnatko R, Kubes M. TNF signaling: Early events and phosphorylation. Gen Physiol Biophys. 2007;26:159–67. [PubMed] [Google Scholar]
- 11.Jupp OJ, McFarlane SM, Anderson HM, Littlejohn AF, Mohamed AA, MacKay RH, et al. Type II tumour necrosis factor-alpha receptor (TNFR2) activates c-Jun N-terminal kinase (JNK) but not mitogen-activated protein kinase (MAPK) or p38 MAPK pathways. Biochem J. 2001;359:525–35. doi: 10.1042/0264-6021:3590525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dempsey PW, Doyle SE, He JQ, Cheng G. The signaling adaptors and pathways activated by TNF superfamily. Cytokine Growth Factor Rev. 2003;14:193–209. doi: 10.1016/s1359-6101(03)00021-2. [DOI] [PubMed] [Google Scholar]
- 13.Depuydt B, van Loo G, Vandenabeele P, Declercq W. Induction of apoptosis by TNF receptor 2 in a T-cell hybridoma is FADD dependent and blocked by caspase-8 inhibitors. J Cell Sci. 2005;118:497–504. doi: 10.1242/jcs.01640. [DOI] [PubMed] [Google Scholar]
- 14.Shiu LY, Chang LC, Liang CH, Huang YS, Sheu HM, Kuo KW. Solamargine induces apoptosis and sensitizes breast cancer cells to cisplatin. Food Chem Toxicol. 2007;45:2155–64. doi: 10.1016/j.fct.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Baker SJ, Reddy EP. Modulation of life and death by the TNF receptor superfamily. Oncogene. 1998;17:3261–70. doi: 10.1038/sj.onc.1202568. [DOI] [PubMed] [Google Scholar]
- 16.Alessandrini A, Namura S, Moskowitz MA, Bonventre JV. MEK1 protein kinase inhibition protects against damage resulting from focal cerebral ischemia. Proc Natl Acad Sci U S A. 1999;96:12866–9. doi: 10.1073/pnas.96.22.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sutherland CL, Heath AW, Pelech SL, Young PR, Gold MR. Differential activation of the ERK, JNK, and p38 mitogen-activated protein kinases by CD40 and the B cell antigen receptor. J Immunol. 1996;157:3381–90. [PubMed] [Google Scholar]
- 18.Xiao D, Choi S, Johnson DE, Vogel VG, Johnson CS, Trump DL, et al. Diallyl trisulfide-induced apoptosis in human prostate cancer cells involves c-Jun N-terminal kinase and extracellular-signal regulated kinase-mediated phosphorylation of Bcl-2. Oncogene. 2004;23:5594–606. doi: 10.1038/sj.onc.1207747. [DOI] [PubMed] [Google Scholar]
- 19.Walczak H. TNF and ubiquitin at the crossroads of gene activation, cell death, inflammation, and cancer. Immunol Rev. 2011;244:9–28. doi: 10.1111/j.1600-065X.2011.01066.x. [DOI] [PubMed] [Google Scholar]
- 20.Chung HJ, Park EJ, Pyee Y, Hua Xu G, Lee SH, Kim YS, et al. 25-Methoxyhispidol A, a novel triterpenoid of Poncirus trifoliata, inhibits cell growth via the modulation of EGFR/c-Src signaling pathway in MDA-MB-231 human breast cancer cells. Food Chem Toxicol. 2011;49:2942–6. doi: 10.1016/j.fct.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Xu F, Zang J, Chen D, Zhang T, Zhan H, Lu M, et al. Neohesperidin induces cellular apoptosis in human breast adenocarcinoma MDA-MB-231 cells via activating the Bcl-2/Bax-mediated signaling pathway. Nat Prod Commun. 2012;7:1475–8. [PubMed] [Google Scholar]
- 22.Jayaprakasha GK, Mandadi KK, Poulose SM, Jadegoud Y, Nagana Gowda GA, Patil BS. Inhibition of colon cancer cell growth and antioxidant activity of bioactive compounds from Poncirus trifoliata (L.) Raf. Bioorg Med Chem. 2007;15:4923–32. doi: 10.1016/j.bmc.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 23.Holliday DL, Speirs V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011;13:215. doi: 10.1186/bcr2889. [DOI] [PMC free article] [PubMed] [Google Scholar]