Abstract
Background:
Diabetes mellitus is a chronic physiological glucose metabolic disorder. It has affected millions of people all over the world thereby having a significant impact on quality of life. The management of diabetes includes both nonpharmacological and conventional interventions. Drawbacks in conventional therapy have led to seeking alternative therapy in herbal medicine. Therefore, the need to review, elucidate and classify their mode of action in therapy for diabetes disease arises.
Materials and Methods:
Comprehensive literature reports were used to review all conventional agents and herbal therapy used in the management of diabetes. An online database search was conducted for medicinal plants of African origin that have been investigated for their antidiabetic therapeutic potentials.
Results:
The results showed that of the documented sixty five plants used, fourteen inhibit intestinal absorption of glucose, three exhibit insulin-mimetic properties, seventeen stimulate insulin secretion from pancreatic beta cells, twelve enhance peripheral glucose uptake, one promotes regeneration of beta-cell of islets of Langerhans, thirteen ameliorate oxidative stress and twenty induces hypoglycemic effect (mode of action is still obscure). Thirteen of these plants have a duplicate mode of actions while one of them has three modes of actions. These agents have a similar mechanism of action as the conventional drugs.
Conclusion:
In conclusion, antidiabetic activities of these plants are well established; however, the molecular modulation remains unknown. It is envisaged that the use of herbal therapy will promote good health and improve the status of diabetic patients.
Keywords: Antidiabetic, diabetes, herbal therapy, in vitro, in vivo
INTRODUCTION
Diabetes mellitus is a chronic metabolic disorder caused by abnormal metabolism of carbohydrate, promoted by factors such as insulin deficiency and/or insulin resistance.[1] The prevalence of diabetes globally was estimated to be 4.0% in 1995 and is projected to rise to 5.4% (300 million) by the year 2025. In 2010, 12.1 million people were estimated to be living with diabetes in Africa, and this is projected to increase to 23.9 million by 2030.[2] Diabetes mellitus has affected several millions of people all over the world, thereby significantly impacting on the economy, health, quality of life and life expectancy of patients, as well as on the health care systems.[3]
There are two major forms of diabetes mellitus; insulin-dependent diabetes mellitus (IDDM) also known as type I diabetes and non-insulin-dependent diabetes mellitus (NIDDM) also known as type II diabetes.[4] IDDM is caused by failure to release insulin from the β-cells of the islets of Langerhans in the pancreas. NIDDM is caused by insulin resistance probably due to too few insulin receptors.[5]
The major causes of IDDM include genetic predisposition, environmental factors such as nutrition, exposure to viruses and allergens and autoimmunity leading to destruction of insulin-producing pancreatic β-cells.[6] The major causes of NIDDM include genetic and environmental factors. IDDM requires insulin injection to prevent ketosis and other complications as well as maintenance of life.[6] The complications of IDDM and NIDDM include; retinopathy, neuropathy, angiopathy, nephropathy, infection and diabetic ketoacidosis.[4] Diabetic foot disease which is due to changes in blood vessels and nerves, often leads to ulceration and subsequent limb amputation. Skin disorders are also more common in diabetics. Bacterial (mycobacterium and anaerobic) and fungal infections are common in diabetics.[4]
Various approaches have been used in the management of diabetes mellitus. These are nonpharmacological interventions including; diet therapy, physical activity, acupuncture and hydrotherapy and mineral supplementation.[5,6,7] The conventional management of diabetes mellitus includes; insulin therapy, oral glucose-lowering agents such as sulfonylurea, biguanides, alpha-glucosidase inhibitors, thiazolidinediones, and meglitinides.[8,9,10,11,12,13] Another approach which has been tried is the prevention of autoimmune attack using immunosuppressive compounds.[14,15,16,17,18] Transplantation of either the pancreas or preparations of islet tissues has also been tried.[17,18] Regardless of the efficiency of the above-mentioned methods used in the management of the disease, they have several drawbacks. These include; adverse side effects, cost (expensive) and inaccessible to many communities. This has led to the use of herbal therapy as an alternative method in the management of the disease.
Therefore, this review has chronicled the nonpharmacological and pharmacological interventions used in the management of diabetes mellitus and their drawbacks leading to sourcing for herbal therapy.
Nonpharmacological interventions in the management of diabetes mellitus
Diet therapy
Given the heterogeneous nature of type 2 diabetes, no single dietary approach is appropriate for all patients. Meal plans and diet modifications are generally individualized by a registered dietician to meet patient needs and lifestyle. A typical conventional approach would recommend a diet composed of 60–65% carbohydrate, 25–35% fat, and 10–20% protein with limited or no alcohol consumption.[5]
Vegetables
Vegetables are among the numerous plant adjuncts tried on the treatment of diabetes mellitus. Bitter gourd (Momordica charantia) and Ivy gourd (Coccinia indica) are hypoglycemic when administered orally. Other vegetables such as cabbage (Brassicia oleracia) green leafy vegetables, beans, and tubers are hypoglycemic in both experimental animals and humans.[19]
Mineral supplementation
The treatment of diabetes requires nutritional supplementation, as these patients have a greatly increased need for many nutrients. These improve blood sugar control and prevent many major complications of diabetes. The mineral supplements include:
Chromium (Cr) is an essential element required for normal lipid and carbohydrate metabolism. Brewers yeast appears to be the richest source of GTF-chromium, followed closely by black pepper, wheat germ, rye bread, mushrooms, prunes, wine, and beer. Most meats, fresh fruits, and cheeses are fair sources of chromium. Cereals are poorer sources, their chromium decreasing with refining and processing.[20]
Vanadium is known to play a role in the regulation of intracellular signaling and as a cofactor of enzymes essential in energy metabolism hence reduces gluconeogenesis and increases glycogen deposition.[21] A reasonable amount of supplemental vanadium is 20 µg/day. Vanadyl sulfate at a dose of 100 mg/day is effective in improving insulin sensitivity. Good sources of vanadium include seafood, mushrooms, olives, whole grain bread, carrots and vegetable oils.[22]
Magnesium (Mg) is one of the major mineral constituents of the human body. Its functions include strengthening cell membrane structure, cofactor to several enzymes like kinase, which participate in energy production processes and participation in deoxyribonucleic acid (DNA) replication.[23] A reasonable amount of supplemental magnesium is 450 mg/day.[23]
Zinc is an important trace element in diabetes. It is a cofactor for insulin. Although its real mechanisms in carbohydrate metabolism is not clear, zinc has influence in carbonic anhydrase, alkaline phosphatase, alcohol dehydrogenase, pancreatic carboxypeptidases A and B, lactate dehydrogenase, glutamate dehydrogenase, glyceraldehyde-3-phosphate dehydrogenase and maltose dehydrogenase. Zinc plays a vital role in the biosynthesis of nucleic acids, RNA polymerases, and DNA polymerases; hence its involvement in the healing processes of body tissues. Other physiological processes that require zinc include hormone metabolism, immune responses and stabilization of ribosomes and membranes.[24]
Manganese (Mn) is a trace metal in the body. It is both an activator and a constituent of several enzymes. It is necessary for the normal activity of hydrolyases, kinases, decarboxylases, transferases, leucine aminopeptidase, alkaline phosphatase and of the enzymes of oxidative phosphorylation. Manganese metalloenzymes include pyruvate decarboxylase, arginase, glutamate synthetase, and manganese superoxide dismutase.[25]
Molybdenum (Mo) affects glucose metabolism. In the hepatocytes, molybdenum stimulates glycolysis and accelerates glycogen degradation.[26] Mo also increases insulin receptor autophosphorylation and phosphorylation of its substrate and augments glucose transport, oxidation and lipogenesis in adipocytes.[26]
Molybdate is an effective antihyperglycemic agent in diabetics with severe insulin resistance. It is associated with a substantial reduction of hyperinsulinemia and an increase in pancreatic insulin stores. The glucose-lowering effect of Mo may be partly related to attenuation of hepatic glucose production. Hence, Mo proves to be an effective blood glucose-lowering agent in severely diabetic patients.[27]
Iron is an important element, it is found in the portion of the cell involved in energy production and as a cofactor for several enzymes such as succinic dehydrogenase, catalase, and cytochromes.[28] Insulin is known to cause a rapid and marked stimulation of iron uptake by fat cells, redistributing transferrin receptors from an intracellular membrane compartment to the cell surface. Insulin is also responsible for the increased ferritin synthesis.[29] Reciprocally, iron influences insulin action. Iron interferes with insulin inhibition of glucose production by the liver. Hepatic excretion and metabolism of insulin is reduced with increasing iron stores, leading to peripheral hyperinsulinemia.[30] In fact, the initial and most common abnormality seen in iron overload conditions is liver insulin resistance. Iron overload also affects skeletal muscle, the main effector of insulin action.[31]
Physical activity
In well-controlled diabetes, physical activity improves the body's ability to use glucose and lowers the insulin requirement. Exercise should start at a low level and gradually increase to avoid adverse effects such as injury, hypoglycemia, or cardiac problems.[32]
Acupuncture and hydrotherapy
Acupuncture is the best-known alternative therapy in the United States of America for chronic pain and is used in the treatment of diabetes. Acupuncture is effective in treating not only diabetes, but also in preventing and managing complications of the diseases.[7] Acupuncture activates glucose-6-phosphatase an important enzyme in carbohydrate metabolism and affects the hypothalamus. Acupuncture acts on the pancrease to enhance insulin synthesis, increase the number of receptors on target cells, and accelerate the utilization of glucose, resulting in lowering of blood sugar. Acupuncture also has an antiobesity effect, which is the most modifiable risk factor for type 2 diabetes. The therapeutic effect of acupuncture on diabetes is not the result of its action on one single organ but on multiple systems.[7] Acupuncture can be effective in treating complications of diabetes and is promising in patients with dietary control, physical exercise, breathing exercises and massage. Although acupuncture shows some effectiveness in treating diabetes, its mechanisms are still obscure.[7]
Since hot-tub therapy can increase blood flow to skeletal muscles, it has been recommended for patients with type 2 diabetes who are unable to exercise. Hot-tub therapy decreases weight, mean plasma glucose level and mean glycosylated hemoglobin.[6,7,33] However, caution should be taken that the water is not too hot as neuropathy may prevent the patient from noticing that they are burning themselves; proper water sanitation and appropriate guidance should be considered.[6]
Conventional management of diabetes mellitus
Insulin therapy (exogenous insulin)
Insulin therapy restores normoglycemia, suppressing ketogenesis, delaying or arresting diabetic complications. Insulin also stimulates the synthesis of glucokinase and moderates the degree of gluconeogenesis.[9] Weight gain, hypoglycemia, skin reactions, insulin resistance due to antibody reaction, insulin lipidystrophy, visual disturbance and allergy are common side-effects of insulin therapy. Insulin therapy is also unavailable to many communities in developing countries due to inaccessible health facilities and socioeconomic factors.[2]
Oral glucose – lowering agents
Sulfonylurea
These include sulfonylurea such as tolbutamide and glyburide. The mode of action of sulfonylureas could be chiefly explained by inhibition of KATP channels initiating insulin secretion from the pancreatic β-cells. This enhances the glycolytic flux and inhibits glucose output from the liver inhibiting gluconeogenesis.[34] Thus, these drugs could be used only in patients with type 2 diabetes having functional beta cells for endogenous insulin production. A significant side effect is hypoglycemia and weight gain due to hyperinsulinemia. The weight gain is implicated as a cause of secondary drug failure.[13]
Biguanides
These reduce hepatic glucose output, fasting glucose output and fasting glucose levels by increasing hepatic insulin sensitivity. They reduce intestinal absorption of glucose. These include the drug metformin derived from a medicinal plant, Galega officinalis.[11] Metformin is a biguanide agent that lowers blood glucose primarily by decreasing hepatic glucose production and increases muscle glucose uptake. It also reduces plasma triglyceride and low-density lipoprotein (LDL)-cholesterol levels and reducing insulin resistance. Metformin is used as monotherapy or in combination with sulfonylureas for the management of type 2 diabetes. The side-effects include weakness, fatigue, shortness of breath, nausea, dizziness, lactic acidosis, and kidney toxicity.[11]
Alpha-glucosidase inhibitors
Alpha-glucosidase inhibitors, such as acarbose (Precose) and miglitol (Glyset), are indicated as monotherapy or in combination with sulfonylureas for management of type 2 diabetes. These are inhibitors of intestinal α-glycosidase.[10] These agents inhibit the breakdown of complex carbohydrates and delay the absorption of monosaccharide from the gastrointestinal tract. The major side-effects are gas, bloating and diarrhoea.[10]
Thiazolidinediones
These are represented by troglitazone, rosiglitazone and pioglitazone. The thiazolidinediones are a unique drug class of “insulin sensitizers” that promote skeletal muscle glucose uptake and to a much lesser extent, in the liver.[35] Troglitazone is the first agent of this drug class to be introduced in the United States of America market and like metformin, it reduces insulin resistance. Troglitazone is beneficial in patients requiring large daily amounts of insulin (more than 30 units/day) whose diabetes is still uncontrolled. Troglitazone is also effective when used in combination with other oral agents thereby potentially delaying the need to start insulin therapy.[36]
Molecular mechanisms of action of these agents are through binding avidly to peroxisome proliferator-activated receptor gamma (PPARγ). Thiazolidinediones are selective agonists of PPARγ. When activated by a ligand, such as a thiazolidinedione, PPARγ binds to the 9-cis retinoic acid receptor retinoid X receptor to form a heterodimer. This binds to DNA to regulate the genetic transcription and translation of a variety of proteins involved in cellular differentiation and glucose and lipid metabolism.[37]
The potential role of the thiazolidinediones in reducing hepatic lipid content in non-alcoholic steatohepatitis is still under investigation. The thiazolidinediones do not increase insulin secretion. On the contrary, thiazolidinediones reduce insulin levels acutely, which may be a consequence of improved insulin sensitivity and/or reduced circulating fatty acids (as fatty acids stimulate insulin secretion). In the longer term, thiazolidinediones arrest the decline in β-cell function that occurs in type 2 diabetes, perhaps by protecting the β-cell from lipotoxicity. The thiazolidinediones are of no use in type 1 diabetes or in the occasional lean insulin-deficient (but insulin-sensitive) patient with type 2 diabetes.[37]
In addition to promoting adipogenesis and fatty acid uptake, thiazolidinediones improve insulin sensitivity by altering hormone production by adipocytes. Adipocytes secrete a number of important hormones, referred to as “adipokines,” including leptin, adiponectin, resistin and tumor necrosis factor-α.[38,39] The disadvantage of these drugs is that they are expensive oral agents. These drugs decrease plasma triglyceride levels, but such decrease may be associated with weight gain and an increase in LDL – cholesterol levels.[40] Hepatotoxicity is a concern requiring monthly monitoring of liver function every month for the first 8 months of treatment and every other month for 4 months thereafter.[35]
Meglitinides
One of the meglitinides is repaglinide. Repaglinide is an insulin secretagogue, the first of the meglitinide class. It is a member of the carbamoyl methyl benzoic acid family (glinides) introduced in early 1998. It is structurally different from the traditional sulfonylureas, but shows chemical resemblance to the nonsulfonylurea moiety of the glibenclamide molecule.[12] Nateglinide, the newest member of the class has recently become available. The meglitinides stimulate the release of insulin from the pancreatic β-cells. However, this action is mediated through a different binding site on the “sulfonylurea receptor” of the β-cells and the drugs have somewhat different characteristics when compared with sulfonylureas. In contrast to glibenclamide, meglitinides do not stimulate calcium-dependent exocytosis.[12] Unlike commonly used sulfonylureas, the meglitinides have a very quick onset of action and a short half-life. Repaglinide is a suitable option for patients with severe sulfa allergy who are not candidates for sulfonylurea therapy. The drug is used as monotherapy or in combination with metformin. The major side effects are weight gain, gastrointestinal disturbances and hypoglycaemia.[41]
Advancements in diabetes management
Prevention of autoimmune attack
There are several attempts made to control autoimmune attack on the β-cells and there are several on-going diabetes prevention trials worldwide. In general, it is preferable to start a specific immuno-modulatory treatment while substantial β-cells mass remains; that's during the prediabetic phase.[14,42] The vitamin B-complex nicotinamide is currently undergoing a multicentre trial in Europe. Nicotinamide is thought to protect against damage acting as an antioxidant and thus inhibits the deleterious effects of free radicals. It also inhibits the enzyme Poly (ADP-ribose) polymerase, thereby saving the cellular stores of nicotinamide adenosine diphosphate. Furthermore, it stimulates islet cell proliferation.[16] Another interesting immunosuppressive compound, which has shown encouraging results in newly diagnosed patients, is cyclosporine A, which acts by inhibiting T-helper lymphocyte function.[43] Unfortunately cyclosporin A must be given early and it has potentially serious side effects, including a toxic action on the β-cell itself.[16] Newer immunosuppressive drugs, such as FK-506Transpl, are under investigation, and some of these side effects may be avoided. Moreover, Bacillus Calmette-Guerin, a nonspecific immunostimulant, has been shown to induce extended remission in newly diagnosed patients by unknown mechanism.[15]
Transplantation
Transplanting technology of either the pancreas or preparations of islet tissues is limited by the problem of obtaining donor tissue and preventing immune rejection of the graft.[17] Nevertheless, transplanting is as yet the only available treatment that can lead to insulin independence. Human allograft transplantation cannot be used on a large scale in clinical practice. After whole pancreas transplantation the graft survival after 1-year is 85–90%. Islet transplants are much more vulnerable. Many of them fail within few weeks or months after engraftment and most islet transplants.[17] The reasons for these functional failures are largely unknown, although insufficient numbers of islets, engraftment difficulties, chronic rejection and recurrence of autoimmune disease have been suggested to be contributing factors. Moreover, hyperglycemia in the recipient after transplantation deteriorates islet graft survival and function.[18]
One of the major obstacles for clinical islet transplantation is a lack of donors. Therefore, it is important to optimize the number of β-cells harvested from each donor, stimulate the growth and/or differentiation of β-cells or to genetically manipulate insulin-producing cell lines for transplantation.[17] The differentiated β-cells have the ability to proliferate at a low pace. The proliferation rate can be affected in many ways, for example, by growth stimulating hormones like growth hormone and prolactin. Also, the size and composition of the graft and the blood glucose level in the recipient are of crucial importance for β-cell replication.[17]
Herbal management
The drawbacks of conventional therapy though effective, have led to seeking alternative therapy in herbal medicine. Many pharmaceutical drugs are derived from plants that were first used in traditional systems of medicine, and according to WHO, about 25% of medicines are plant-derived.[44] Traditional knowledge has proven a useful tool in the search for new plant-based medicines. Less than a quarter of the estimated 250,000 medicinal plant species have been investigated for hypoglycemic activity.[45] Moreover, only a small number of these have received a scientific medicinal evaluation to establish their efficacy. Examples of plants that have been documented for diabetic therapy in Africa have been reviewed and discussed subsequently.
METHODOLOGY
This review was carried out using comprehensive and systematic literature reports on the use of traditional and conventional therapy in the management of diabetes and emergence of herbal therapy. Empirical searches were conducted using Google Scholar (http://www.scholar. google.com), and Science Direct (http://www.science direct.com), PubMed and Medline for medicinal plants of African origin that have been studied and investigated for their antidiabetic therapeutic potentials both in vivo and in vitro. In addition to these databases, the University of Fort Hare's online database was also used. Some articles were found through tracking citations from other publications or by directly accessing the journals' website. The keyword combinations for the search were antidiabetic, antihyperglycemia, hypoglycemia, mode of action, medicinal plant, and Africa. Following the search, the antidiabetic plants were categorized and presented based on their mode of actions and parts of Africa they are used including East, West, North and Southern Africa.
Plants used in the management of diabetes mellitus in Africa
Green tea (Camellia sinensis)
Green tea (leaves of Camellia sinensis, Theaceae) is a popular beverage in Kenya and East Asia, and also used as a herbal remedy in Europe and North America. Green tea is considered to be antiinflammatory, antioxidative, antimutagenic, and anticarcinogenic and can prevent cardiac disorders. Epidemiologically, it has been suggested that green tea consumption prevents type 2 diabetes.[46] Green tea extract contains polyphenols like catechin, epicatechin, epigallocatechin, and their gallates, tannin, and caffeine. Furthermore, the polyphenols in green tea extract have epigllo-catechin-3-gallate as the main constituent with anti-diabetic activity.[47] The extract also has pyrroloquinoline quinone, a newly discovered vitamin.[48] Some constituent components enhance the basal and insulin-stimulated glucose uptake, inhibit intestinal glucose uptake by inhibiting the sodium-dependent glucose transporter in the intestinal epithelial cells, and reduce serum glucose level in alloxan-diabetic rats.[49] Controversially, caffeine acutely lowers insulin sensitivity in humans.[50]
Onion (Allium cepa) and garlic (Allium sativum)
Onion (A. cepa) and garlic (A. sativum) contains active hypoglycemic constituents. Garlic (A. sativum) also contains hypoglycemic organic sulfur compounds.[51] Volatile oils in raw onion and garlic cloves lower fasting glucose concentration in both diabetic animals and human subjects. The active components are believed to be sulfur-containing compounds such as allyl propyl disulfide in onions and diallyl disulfide (allicin) in garlic. These active ingredients lower glucose levels by competing with insulin (which is also a disulfide) for insulin-inactivating sites in the liver, resulting in an increase of free insulin. Onion extracts reduce blood sugar levels in a dose-dependent manner. A typical dosage of A. cepa is one 400 mg capsule daily while the general dosage of garlic is 4 g fresh garlic or 8 mg of the essential oil.[52]
Panax ginseng (Panax quinquefolius)
Panax ginseng (P. quinquefolius) is widely used in Chinese medicine for over 2000 years. The root of ginseng has been used for over 2000 years in the Far East for its health promoting properties. It is also used in Northern Africa, especially in Egypt.[53] Of the several species of ginseng, P. ginseng (Asian ginseng) and P. quinquefolius (American ginseng) are commonly used. Constituents of all ginseng species include ginsenosides, polysaccharides, peptides, polyacetylenic alcohol, and fatty acids. Most pharmacological actions of ginseng are attributed to ginsenosides, a family of steroids named steroidal saponins.[54] The chemical composition of ginseng products and potency may vary with the plant extract derivative, the age of the root, the location where grown, the season when harvested, and the methods of drying. Both Asian and American ginseng has significant hypoglycemic action. The blood lowering effect appears to be attributed to ginsenoside Rb-2 and more specifically to panaxans I, J, K and L But whether these constituents have a similar effect on type 2 diabetes is yet unknown.[55] The ginseng's mechanisms of action are thought to be: Slowing the digestion of food, decreasing the rate of carbohydrate absorption into portal hepatic circulation; ginseng may affect glucose transport, which is mediated by nitric oxide (NO); and lastly, ginseng may modulate NO-mediated insulin secretion and NO stimulates glucose-dependent secretion of insulin. However, the side-effects of ginseng are nervousness and excitation. The recommended daily ginseng dosage is 1–3 g of the crude root, or 200–600 mg of a standardized extract.[56]
Bitter Gourd (Momordica charantia)
Bitter Gourd (M. charantia), also known as balsam pear is a tropical vegetable widely cultivated in parts of Asia, Africa, and South America, which has been extensively used in folk medicine as a remedy for diabetes.[57] The active, hypoglycemic constituents include charantin, obtained from an alcohol extract of the fruit, and a polypeptide called p-insulin (plant insulin or polypeptide-p) isolated from the fruit and seeds of the plant. The p-insulin consists of 166 residues containing 17 amino acids and has a molecular weight of 11,000. It is structurally and pharmacologically comparable to bovine insulin, and is composed of two polypeptide chains with disulfide bonds. p-insulin has an onset of action similar to bovine insulin (30–60 min) and a peak hypoglycemic effect after 4 h in type I diabetics, compared with 2–3 h for regular insulin. Although the precise mechanism of action remains to be fully elucidated, M. charantia stimulates insulin release or possibly glycogen synthesis in the liver.[57] In addition, the plant is believed to contain several anti-diabetic principles. The hypoglycemic effects of this plant appears to be due to extra-pancreatic activity, including increased glucose utilization by the liver;[58] decreased glucose synthesis by depression of key gluconeogenic enzymes like glucose-6-phosphatase and fructose-1,6-biphosphatase; and enhancement of glucose oxidation through the shunt pathway via activation of glucose-6-phosphate dehydrogenase.[59] Interestingly, these herbs on an individual basis are reported to possess a variety of healthful properties, including blood glucose regulating, immunomodulation, liver detoxifying, and anti-inflammatory properties. These properties are significant to the diabetic as autoimmune processes are believed to play a role in the destruction of β-cells, and inflammation mediated by free radicals is also characteristic of the diabetic condition.[60]
The recommended dose of bitter melon depends on the form it is being consumed. Dosage for tincture ranges from 5 mL 2–3 times daily to as high as 50 ml/day. However, bitter melon juice is very difficult to make palatable since, as the name implies, it is quite bitter. To avoid the bitter taste, the Indians and Chinese crush the herbs and form tablets. In Central America, it is prepared as an extract or decoction.[60] Dosage of capsulized dried powder range from 3 to 15 g daily. That is quite a large dose so to avoid the necessity of taking so many capsules; a standardized extract may be used at dosages of 100–200 mg 3 times daily.[60]
Ackee fruit (Blighia sapida)
Ackee is the National fruit of Jamaica and was imported from West Africa in the 18th century. It is a tall, leafy tree (up to 12 m) that produces clusters of fruits widely used for human consumption and for industrial purposes. The fruit is yellow in color and shaped like an oblong capsule that contains three cream-colored arils. The arils may be consumed safely when the fruit becomes red and opens under the light of the sun. It is then commonly boiled in water or milk and eaten alone or in meat or fish dishes. It is also consumed raw in some African countries. Ackee fruit contains hypoglycin, a natural toxin. It exists as a cyclic amino acid, hypoglycin A (HG-A), and its gamma-glutamyl derivative, HG-B. When the fruit is consumed unripe, it produces an acute toxic effect within 2–3 h with symptoms including nausea, vomiting, headache, and drowsiness. Coma and death may occur within 12 h in severe cases. The most toxic is HG-A, which is found in the unripe arils. HG-A is a water-soluble liver toxin that produces hypoglycemia through the inhibition of gluconeogenesis, secondary to the limitation of cofactors (CoA and carnitine) that are essential for the oxidation of long-chain fatty acids.[61] The concentration of HG-A in the unripe ackee is 20 times greater than in the mature fruit. However the level of concentration of the toxin lowers rapidly after its exposure to the sun. The seeds contain HG-B and are always poisonous. An important factor seems to be the nutritional status of the person consuming ackee since diagnosed patients often present chronic malnutrition and vitamin deficiencies. When ingested unripe, ackee produces vomiting and fatal cases of poisoning.[61]
Khat (Catha edulis)
Catha edulis popularly called khat is an evergreen shrub of the tropics. The fresh leaf is traditionally chewed by some people in East Africa and the Arabian Peninsula to attain a state of euphoria and stimulation. Since the leaf rapidly loses its effect upon wilting, the chewing habit has remained endemic to the areas where the plant is cultivated.[62]
In South Africa, the plant has found its way to the country due to influence of availability of this plant through improved road networks, and the availability of air transport, and the habit has spread considerably in those regions and countries where the plant does not.[62] Although it has been reported that there is moderate in vitro antidiabetic property of C. edulis,[63] there is no published scientific article substantiating this claim in animal models.
Fenugreek (Trigonella foenum graecum)
Trigonella foenum graecum has been used as a remedy for diabetes, particularly in India and Africa. The active principal is in the defatted portion of the seed, which contains the alkaloid trigonelline, nicotinic acid and coumarin. Administration of the defatted seed (1.5–2.0 g/kg daily) reduces fasting and postprandial blood levels of glucose, glycagon, somatostatin, insulin, total cholesterol, and triglycerides and increased high-density lipoprotein-cholesterol levels.[60] Human studies have confirmed the glucose and lipid-lowering effects. The fiber constitutes potential mechanisms of fenugreek's beneficial effect in diabetic patients. Dosages of the fiber range from 10 to 100 g daily in divided dosages. The major side-effect is that the urine may have a maple syrup smell after fenugreek consumption.[60]
Gurmar (Gymnema sylvestre)
Gymnema sylvestre, a plant native in the tropical forests of Africa and India, has long been used as a treatment for diabetes. It is postulated that G. sylvestre enhances the production of endogenous insulin. A typical dosage of G. sylvestre extract is 400–600 mg/day. One of its side-effects may be a reduction or loss of the taste sensation of sweetness and bitterness although this occurs only if the plant is directly exposed to the tongue.[60]
Bitter leaf (Vernonia amygdalina)
Vernonia amygdalina commonly known as bitter leaf is a small tree growing up to 3 m high. It occurs wild in most countries of tropical Africa. In South Africa, the plant is found in KwaZulu Natal, Mpumalanga, Eastern and Northern Cape Provinces. It is probably the most used plant in the genus Vernonia. The common and documented medicinal uses include treatment of malaria, venereal diseases, wounds, hepatitis, and diabetes. The leaves may be consumed either as a vegetable or aqueous extracts as tonics for the treatment of various illnesses. It has been reported that chloroform extract of the plant has hypoglycemic activity in both normoglycemic and alloxan-induced hyperglycemic rats. Ebong et al.,[64] also reported the anti-diabetic efficacy of combined ethanolic extracts of Azadirachta indica (neem) and V. amygdalina in rats. Also V. amygdalina extract alone shows hypoglycemic activity in diabetic rats.[65]
Aloe vera
The dried sap (fluid) of A. vera is a traditional remedy used for diabetes in the Arabian peninsula and Africa. A. vera juice is prepared from A. vera gel, a mucilaginous preparation obtained from the leaves of the plant. Oral administration of the juice reduces fasting blood glucose and triglyceride levels in type 2 diabetic patients with or without combination of a conventional antidiabetic agent. The amount used is one tablespoon of A. vera juice with no significant adverse effects reported.[60]
Marula (Sclerocarya birrea)
Sclerocarya birrea commonly known as marula is one of the most highly valued indigenous trees in southern Africa. It grows up to 15 m high with gray fissured bark, stout branchlets, and pale foliage. The leaves are compound, pinnate and the flowers greenish-white or reddish. The fruits are yellow and closely resemble the mango fruits. The pulp of the fruit is delicious, and the large nut is also edible. In Africa, the tree is commonly found in savannah regions, and its geographical distribution stretches from Gambia in the west across to Nigeria and Cameroon, in Central Africa, and to Ethiopia and Sudan in the east. In South Africa, the plant is commonly found in the Northern Province.[62] The Zulu people use the bark decoction to treat diarrhea, dysentery, fevers, stomach ailments, ulcers and bacterial-related diseases. Traditional Zulu healers wash in bark decoctions before treating patients with gangrenous rectitis and also administer the decoction to the patient.[62] Dimo et al.[66] has shown that a methanol/methylene chloride (1:1) extract of the plant reduces blood glucose and increases plasma insulin levels in diabetic rats. The extract also prevents body weight loss and reduces plasma cholesterol, while the triglyceride and urea levels normalized with controls. It has also been reported that an aqueous stem-bark extract of the plant has hypoglycemic effect in normal and streptozotocin (STZ) treated diabetic rats.[67] These observations thus lend confidence to the folkloric use of the plant in the management and/or control of adult-onset diabetes in some African communities.
Wild cucumber (Momordica foetida)
Momordica foetida is a perennial climbing herb with tendrils and popularly known as Wild cucumber. It is commonly found in Gabon, Malawi, Ghana, Sudan, and Tanzania. The flowers are cream, often with a reddish or orange center, having the male and female flowers on the same plant. The characteristic fruit is bright orange with prickles, and the plant has a strong unpleasant smell. The herb is used to treat a number of ailments including hypertension, diabetes mellitus, fever and symptoms of malaria.[62] In diabetes management, the information is still obscure. An isolate from the plant, fetidin, has shown the exhibit hypoglycaemic effect in normal and not in diabetic rats.[68] Moreover, the mechanism of action of the plant has not been elucidated. These are classical examples of plants used in traditional medicine in the management of diabetes in Africa with different cultural background. Other plants used in East, West, North or Southern Africa are outlined in Tables 1–4.
Table 1.
Some of the plants that are documented to be used in Southern Africa to manage diabetes mellitus

Table 4.
Some of the plants that are documented to be used in North Africa to manage diabetes mellitus
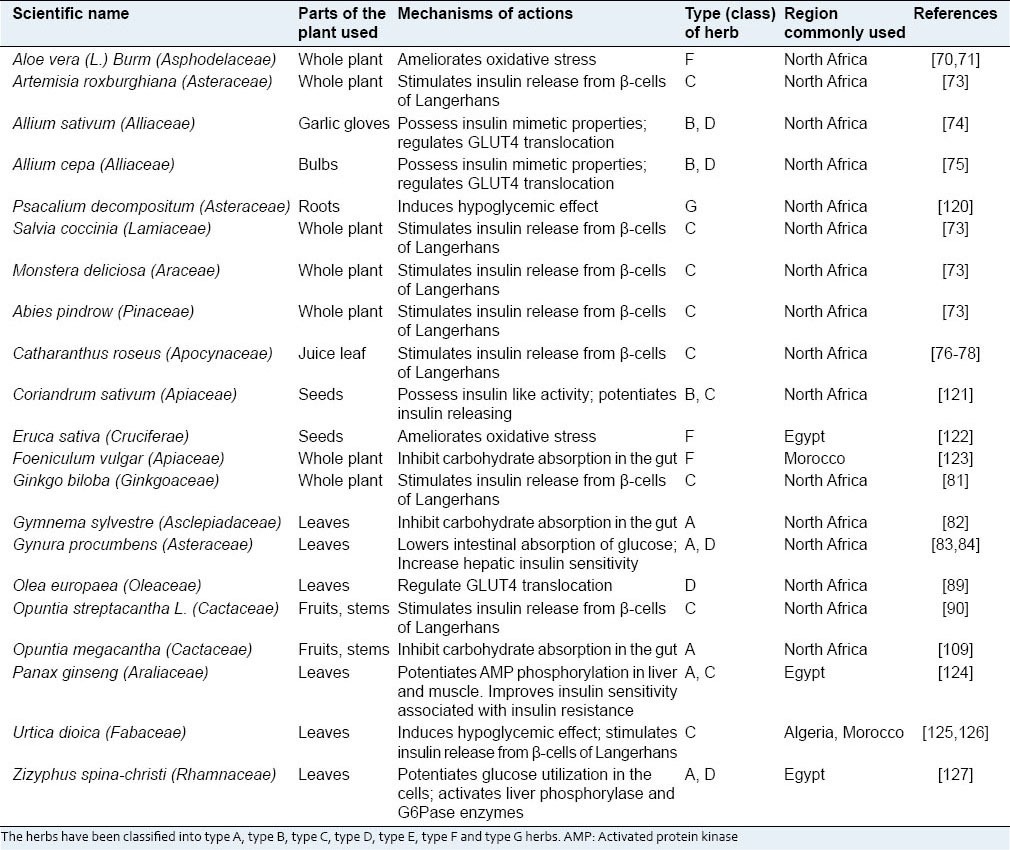
Table 2.
Some of the plants that are documented to be used in West Africa to manage diabetes mellitus
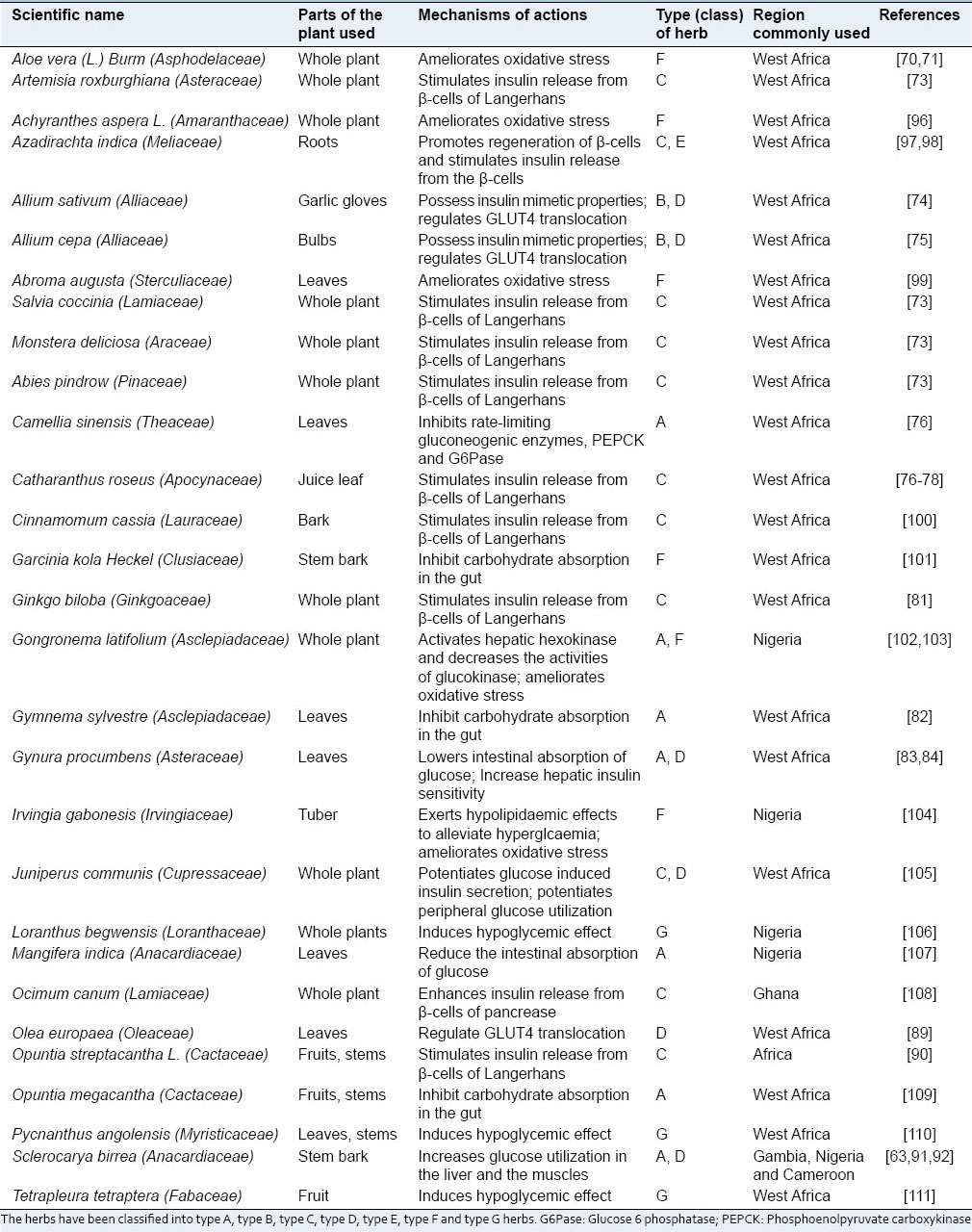
Table 3.
Some of the plants that are documented to be used in East Africa to manage diabetes mellitus
These antidiabetic plants have been reported in different parts of the Africa for the treatment of diabetes with different therapeutic targets.[128] Some have been investigated in STZ and alloxan induced diabetic rats at different dosages to evaluate their antidiabetic potentials. The majority of these plants displayed hypoglycemic effect. Some of the mechanisms of action reported are related to inhibition of mitochondrial function, stimulation of glycolysis, activation of adenosine mono-phosphate kinase (AMPK) pathway, suppression of adipogenesis, uptake of glucose and induction of LDL. Also, some plants with anti-diabetic properties have also been reported to inhibit carbohydrate digestive enzymes such as α-glucosidase and α-amylase.[129] Antioxidant properties and modification of insulin structure or insulin receptor sensitivity, as well as up-regulation of glucose transporter of some plants, have been reported in several studies.[130]
In this review, it has been noted that there are several possible mechanisms through which these herbs can act to control the blood glucose level.[130] The mechanisms of action can be related, generally, to the ability of the plant in question (or its active principle) to lower plasma glucose level by interfering with one or more of the processes involved in glucose homeostasis.[8,130,131]
In a nutshell, the reported mechanisms whereby medicinal plants act as anti-diabetic therapies can be summarized as follows:
Stimulation of insulin synthesis and/or secretion from pancreatic beta-cells
Regeneration/revitalization of damaged pancreatic beta cells
Improvement of insulin sensitivity (enhancement of glucose uptake by fat and muscle cells)
Mimicking the action of insulin (acting like insulin)
Slowing down the absorption of carbohydrates from the gut and altering glucose metabolizing enzymes
Ameliorating oxidative stress.
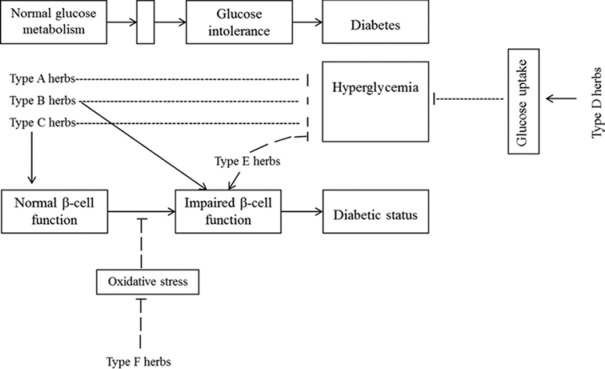
Therefore, in this review, different medicinal plants used in Africa for management of diabetes have been classified based on their modes of action [Figure 1] including; inhibiting intestinal absorption of glucose and altering glucose metabolizing enzymes (type A herbs); having insulin mimetic properties (type B herbs); potentiating glucose-induced insulin release (type C herbs); enhance peripheral glucose uptake (type D herbs); promote regeneration of β-cell of islets of Langerhans (type E herbs); and ameliorating oxidative stress (type F herbs). Some of the plants have been shown to induce hypoglycemic effect, but the mode of action still remains obscure, hence, they have been classified as type G herbs [Tables 1–4].
Figure 1.
Mechanisms underlying plants used in traditional medicine in the management of diabetes mellitus. Some inhibit intestinal absorption of glucose and alter glucose metabolizing enzymes (type A herbs); have insulin mimetic properties (type B herbs); potentiates glucose-induced insulin release (type C herbs); enhances peripheral glucose uptake (type D herbs); promotes regeneration of β-cell of islets of Langerhans (type E herbs); and ameliorates oxidative stress (type F herbs)
It is noted that, of the documented 65 plants used in treatment of diabetes in Africa as listed in Tables 1–4, 14 inhibit intestinal absorption of glucose and altering glucose metabolizing enzymes, three exhibit insulin-mimetic properties, seventeen stimulate insulin secretion from pancreatic beta cells, twelve enhance peripheral glucose uptake, one promotes regeneration of beta-cell of islets of Langerhans, 13 ameliorate oxidative stress and twenty induce hypoglycemic effect (mode of action is still obscure). Of these plants, thirteen of them have been identified to have duplicate mode of actions while one of them has three modes of actions.
CONCLUSION
It can be concluded on the basis of the above mentioned reviews that the majority of anti-diabetic medicinal plants exert their blood glucose lowering effect through stimulation of insulin release from pancreatic beta-cells or through alteration of some hepatic enzymes involved in glucose metabolism and decreasing intestinal glucose absorption. Another point of note in the above-mentioned reviews is that a given plant and/or its product may exert its blood glucose lowering effect through a combination of more than one mechanism.[131,132,133] These plants also have a similar mode of action as the conventional drugs used in the management of diabetes mellitus hence due to their advantages and accessibility; herbal therapy has surpassed conventional therapy. Moreover, traditional knowledge has proven a useful tool in the search for new plant-based medicines.
A review of literature suggests that most researchers utilize strategies that are more or less similar to one another to study medicinal plants with alleged anti-diabetic potential. That is, candidate plants are collected, extracted and screened for hypoglycemic activity using either in vitro or in vivo bioassay techniques. Then, active compounds are isolated and identified from plants through fractionation guided bioassays. Then blood glucose lowering mechanism of action of the crude plant extract and/or active ingredients is investigated. Therefore, these studies have led to findings which are still obscure. Therefore, while the metabolic activities of these plants are well established, the molecular mechanism underlying their biological activities remains unknown.
There is a need to use molecular tools to determine genes that act as molecular signatures to be involved in the diagnosis of the disease, monitor the herbal therapeutic progress and determine the effectiveness of the bioactive compounds in efficient manner. It is vital to embrace computational biology tools to study the identified compounds in relation to existing anti-diabetic drugs in order to improve potency and efficacy of bioactive compounds, hence develop novel drugs for diabetes management. Considering the rich cultural traditions of plant use and the high prevalence of diabetes mellitus in Africa, more investigations should be encouraged in order to validate the anti-diabetic activity of the identified plants as claimed by the traditional healers.
AUTHOR'S CONTRIBUTIONS
CMK carried out the study and wrote the manuscript; AJA contributed to conception of the review and supervised the manuscript writing. All authors have read and approved the final manuscript.
ACKNOWLEDGMENT
This research was supported by grants from Govan Mbeki Research and Development Centre, University of Fort Hare, South Africa.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Ozkol H, Tuluce Y, Dilsiz N, Koyuncu I. Therapeutic potential of some plant extracts used in Turkish traditional medicine on streptozocin-induced type 1 diabetes mellitus in rats. J Membr Biol. 2013;246:47–55. doi: 10.1007/s00232-012-9503-x. [DOI] [PubMed] [Google Scholar]
- 2.International Diabetes Federation: Diabetes Atlas. 2013. pp. 51–68. Available from: http://www.idf.org/diabetesatlas . [PubMed]
- 3.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 4.Holt RI. Diagnosis, epidemiology and pathogenesis of diabetes mellitus: An update for psychiatrists. Br J Psychiatry. 2004;184:S55–63. doi: 10.1192/bjp.184.47.s55. [DOI] [PubMed] [Google Scholar]
- 5.Schilichtmann J, Graber MA. Hematologic, electrolyte and metabolic disorders. In: Graber MA, Herting RL, editors. The Family Practice Handbook. 3rd ed. St. Toth, Missouri: Mosby, Year Book Inc; 1997. pp. 192–251. [Google Scholar]
- 6.Hooper PL. Hot-tube therapy for type 2 diabetes mellitus. Reply to discussion. N Engl J Med. 2000;342:218–9. [Google Scholar]
- 7.Hu H. A review of treatment of diabetes by acupuncture during the past forty years. J Tradit Chin Med. 1995;15:145–54. [PubMed] [Google Scholar]
- 8.Bastaki S. Diabetes mellitus and its treatment. Int J Diabetes Metab. 2005;13:111–34. [Google Scholar]
- 9.Garg MK. Current perspective in insulin therapy in the management of diabetes mellitus. J Indian Med Assoc. 2002;100:194–5. 202. [PubMed] [Google Scholar]
- 10.Raing HP, Dale MM, Ritter JM. Pharmacology. 4th ed. Edinburgh, London, New York, Philadelphia, Sydney, Toronto: Churchill Livingstone; 2000. The endocrine pancreas and the control of blood glucose; pp. 389–98. [Google Scholar]
- 11.DeFronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med. 1999;131:281–303. doi: 10.7326/0003-4819-131-4-199908170-00008. [DOI] [PubMed] [Google Scholar]
- 12.Fuhlendorff J, Rorsman P, Kofod H, Brand CL, Rolin B, MacKay P, et al. Stimulation of insulin release by repaglinide and glibenclamide involves both common and distinct processes. Diabetes. 1998;47:345–51. doi: 10.2337/diabetes.47.3.345. [DOI] [PubMed] [Google Scholar]
- 13.Kelly DE. Effects of weight loss on glucose homeostasis in NIDDM. Diabetes Rev. 1995;3:366–77. [Google Scholar]
- 14.Knip M, Akerblom HK. Environmental factors in the development of type 1 diabetes mellitus. Exp Clin Endocrinol Diabetes. 1999;107:93–100. doi: 10.1055/s-0029-1212160. [DOI] [PubMed] [Google Scholar]
- 15.Wright DC, Deol HS, Tuch BE. A comparison of the sensitivity of pig and human peripheral blood mononuclear cells to the antiproliferative effects of traditional and newer immunosuppressive agents. Transpl Immunol. 1999;7:141–7. doi: 10.1016/s0966-3274(99)80033-1. [DOI] [PubMed] [Google Scholar]
- 16.Sandler S, Andersson A. Stimulation of cell replication in transplanted pancreatic islets by nicotinamide treatment. Transplantation. 1988;46:30–1. doi: 10.1097/00007890-198807000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Sutherland DE, Goetz FC, Sibley RK. Recurrence of disease in pancreas transplants. Diabetes. 1989;38(Suppl 1):85–7. doi: 10.2337/diab.38.1.s85. [DOI] [PubMed] [Google Scholar]
- 18.Landgraf R. Impact of pancreas transplantation on diabetic secondary complications and quality of life. Diabetologia. 1996;39:1415–24. doi: 10.1007/s001250050593. [DOI] [PubMed] [Google Scholar]
- 19.Platel K, Srinivasan K. Plant foods in the management of diabetes mellitus: Vegetables as potential hypoglycaemic agents. Nahrung. 1997;41:68–74. doi: 10.1002/food.19970410203. [DOI] [PubMed] [Google Scholar]
- 20.Castro VR. Chromium in a series of Portuguese plants used in the herbal treatment of diabetes. Biol Trace Elem Res. 1998;62:101–6. doi: 10.1007/BF02820025. [DOI] [PubMed] [Google Scholar]
- 21.Orvig C, Thompson KH, Battell M, McNeill JH. Vanadium compounds as insulin mimics. Mol Cell Biol. 2000;208:167–8. [PubMed] [Google Scholar]
- 22.Cohen N, Halberstam M, Shlimovich P, Chang CJ, Shamoon H, Rossetti L. Oral vanadyl sulfate improves hepatic and peripheral insulin sensitivity in patients with non-insulin-dependent diabetes mellitus. J Clin Invest. 1995;95:2501–9. doi: 10.1172/JCI117951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McNair P, Christiansen C, Madsbad S, Lauritzen E, Faber O, Binder C, et al. Hypomagnesemia, a risk factor in diabetic retinopathy. Diabetes. 1978;27:1075–7. doi: 10.2337/diab.27.11.1075. [DOI] [PubMed] [Google Scholar]
- 24.Goto Y, Kida K, Ikeuchi M, Kaino Y, Matsuda H. Synergism in insulin-like effects of molybdate plus H2O2 or tungstate plus H2O2 on glucose transport by isolated rat adipocytes. Biochem Pharmacol. 1992;44:174–7. doi: 10.1016/0006-2952(92)90052-k. [DOI] [PubMed] [Google Scholar]
- 25.Friedman BJ, Freeland-Graves JH, Bales CW, Behmardi F, Shorey-Kutschke RL, Willis RA, et al. Manganese balance and clinical observations in young men fed a manganese-deficient diet. J Nutr. 1987;117:133–43. doi: 10.1093/jn/117.1.133. [DOI] [PubMed] [Google Scholar]
- 26.Fillat C, Rodríguez-Gil JE, Guinovart JJ. Molybdate and tungstate act like vanadate on glucose metabolism in isolated hepatocytes. Biochem J. 1992;282(Pt 3):659–63. doi: 10.1042/bj2820659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reul BA, Becker DJ, Ongemba LN, Bailey CJ, Henquin JC, Brichard SM. Improvement of glucose homeostasis and hepatic insulin resistance in ob/ob mice given oral molybdate. J Endocrinol. 1997;155:55–64. doi: 10.1677/joe.0.1550055. [DOI] [PubMed] [Google Scholar]
- 28.Fernández-Real JM, López-Bermejo A, Ricart W. Cross-talk between iron metabolism and diabetes. Diabetes. 2002;51:2348–54. doi: 10.2337/diabetes.51.8.2348. [DOI] [PubMed] [Google Scholar]
- 29.Yokomori N, Iwasa Y, Aida K, Inoue M, Tawata M, Onaya T. Transcriptional regulation of ferritin messenger ribonucleic acid levels by insulin in cultured rat glioma cells. Endocrinology. 1991;128:1474–80. doi: 10.1210/endo-128-3-1474. [DOI] [PubMed] [Google Scholar]
- 30.Niederau C, Berger M, Stremmel W, Starke A, Strohmeyer G, Ebert R, et al. Hyperinsulinaemia in non-cirrhotic haemochromatosis: Impaired hepatic insulin degradation? Diabetologia. 1984;26:441–4. doi: 10.1007/BF00262217. [DOI] [PubMed] [Google Scholar]
- 31.Bertelsen M, Anggård EE, Carrier MJ. Oxidative stress impairs insulin internalization in endothelial cells in vitro. Diabetologia. 2001;44:605–13. doi: 10.1007/s001250051667. [DOI] [PubMed] [Google Scholar]
- 32.American Diabetes Association. Clinical practice recommendations. Position statement: Diabetes mellitus and exercise. Diabetes Care. 1997:18–28. [Google Scholar]
- 33.Hooper PL. Hot-tub therapy for type 2 diabetes mellitus. N Engl J Med. 1999;341:924–5. doi: 10.1056/NEJM199909163411216. [DOI] [PubMed] [Google Scholar]
- 34.Parving HH, Gall MA, Skøtt P, Jørgensen HE, Løkkegaard H, Jørgensen F, et al. Prevalence and causes of albuminuria in non-insulin-dependent diabetic patients. Kidney Int. 1992;41:758–62. doi: 10.1038/ki.1992.118. [DOI] [PubMed] [Google Scholar]
- 35.Sparano N, Seaton TL. Troglitazone in type II diabetes mellitus. Pharmacotherapy. 1998;18:539–48. [PubMed] [Google Scholar]
- 36.Inzucchi SE, Maggs DG, Spollett GR, Page SL, Rife FS, Walton V, et al. Efficacy and metabolic effects of metformin and troglitazone in type II diabetes mellitus. N Engl J Med. 1998;338:867–72. doi: 10.1056/NEJM199803263381303. [DOI] [PubMed] [Google Scholar]
- 37.Greenfield JR, Chisholm DJ. Thiazolidinediones-mechanisms of action. Clin Exp Pharmacol. 2004;27:67–70. [Google Scholar]
- 38.Fürnsinn C, Waldhäusl W. Thiazolidinediones: Metabolic actions in vitro. Diabetologia. 2002;45:1211–23. doi: 10.1007/s00125-002-0899-1. [DOI] [PubMed] [Google Scholar]
- 39.Gurnell M, Savage DB, Chatterjee VK, O'Rahilly S. The metabolic syndrome: Peroxisome proliferator-activated receptor gamma and its therapeutic modulation. J Clin Endocrinol Metab. 2003;88:2412–21. doi: 10.1210/jc.2003-030435. [DOI] [PubMed] [Google Scholar]
- 40.Lee CH, Olson P, Evans RM. Minireview: Lipid metabolism, metabolic diseases, and peroxisome proliferator-activated receptors. Endocrinology. 2003;144:2201–7. doi: 10.1210/en.2003-0288. [DOI] [PubMed] [Google Scholar]
- 41.Scheen AJ. Drug treatment of non-insulin-dependent diabetes mellitus in the 1990s. Achievements and future developments. Drugs. 1997;54:355–68. doi: 10.2165/00003495-199754030-00001. [DOI] [PubMed] [Google Scholar]
- 42.Herold KC, Rubenstein AH. Immunosuppression for insulin-dependent diabetes. N Engl J Med. 1988;318:701–3. doi: 10.1056/NEJM198803173181110. [DOI] [PubMed] [Google Scholar]
- 43.Skyler JS. Immune intervention studies in insulin-dependent diabetes mellitus. Diabetes Metab Rev. 1987;3:1017–35. doi: 10.1002/dmr.5610030410. [DOI] [PubMed] [Google Scholar]
- 44.Saslis-Lagoudakis CH, Savolainen V, Williamson EM, Forest F, Wagstaff SJ, Baral SR, et al. Phylogenies reveal predictive power of traditional medicine in bioprospecting. Proc Natl Acad Sci U S A. 2012;109:15835–40. doi: 10.1073/pnas.1202242109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu SZ, Deng YX, Chen B, Zhang XJ, Shi QZ, Qiu XM. Antihyperglycemic effect of the traditional Chinese scutellaria-coptis herb couple and its main components in streptozotocin-induced diabetic rats. J Ethnopharmacol. 2013;145:490–8. doi: 10.1016/j.jep.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 46.Tsuneki H, Ishizuka M, Terasawa M, Wu JB, Sasaoka T, Kimura I. Effect of green tea on blood glucose levels and serum proteomic patterns in diabetic (db/db) mice and on glucose metabolism in healthy humans. BMC Pharmacol. 2004;4:18. doi: 10.1186/1471-2210-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Broadhurst CL, Polansky MM, Anderson RA. Insulin-like biological activity of culinary and medicinal plant aqueous extracts in vitro. J Agric Food Chem. 2000;48:849–52. doi: 10.1021/jf9904517. [DOI] [PubMed] [Google Scholar]
- 48.Kasahara T, Kato T. A new redox cofactor vitamin for mammals. Nature. 1993;4:822–32. doi: 10.1038/422832a. [DOI] [PubMed] [Google Scholar]
- 49.Sabu MC, Smitha K, Kuttan R. Anti-diabetic activity of green tea polyphenols and their role in reducing oxidative stress in experimental diabetes. J Ethnopharmacol. 2002;83:109–16. doi: 10.1016/s0378-8741(02)00217-9. [DOI] [PubMed] [Google Scholar]
- 50.Keijzers GB, De Galan BE, Tack CJ, Smits P. Caffeine can decrease insulin sensitivity in humans. Diabetes Care. 2002;25:364–9. doi: 10.2337/diacare.25.2.364. [DOI] [PubMed] [Google Scholar]
- 51.Rawi SM, Abdelmoneim A, Ahmed OM. Studies on the effect of garlic oil and glibenclamide on alloxan diabetic rats. Egypt J Zool. 1998;30:211–28. [Google Scholar]
- 52.Sharma KK, Gupta RK, Gupta S, Samuel KC. Antihyperglycemic effect of onion: Effect on fasting blood sugar and induced hyperglycemia in man. Indian J Med Res. 1977;65:422–9. [PubMed] [Google Scholar]
- 53.El-Khayat Z, Hussein J, Ramzy T, Ashour M. Anti-diabetic, anti-oxidant effect of panax ginseng. J Med Plants Res. 2011;5:4616–20. [Google Scholar]
- 54.Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: Multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–93. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 55.Liu CX, Xiao PG. Recent advances on ginseng research in China. J Ethnopharmacol. 1992;36:27–38. doi: 10.1016/0378-8741(92)90057-x. [DOI] [PubMed] [Google Scholar]
- 56.Gillis CN. Panax ginseng pharmacology: A nitric oxide link? Biochem Pharmacol. 1997;54:1–8. doi: 10.1016/s0006-2952(97)00193-7. [DOI] [PubMed] [Google Scholar]
- 57.Welihinda J, Karunanayake EH, Sheriff MH, Jayasinghe KS. Effect of Momordica charantia on the glucose tolerance in maturity onset diabetes. J Ethnopharmacol. 1986;17:277–82. doi: 10.1016/0378-8741(86)90116-9. [DOI] [PubMed] [Google Scholar]
- 58.Sarkar S, Pranava M, Marita R. Demonstration of the hypoglycemic action of Momordica charantia in a validated animal model of diabetes. Pharmacol Res. 1996;33:1–4. doi: 10.1006/phrs.1996.0001. [DOI] [PubMed] [Google Scholar]
- 59.Shibib BA, Khan LA, Rahman R. Hypoglycaemic activity of Coccinia indica and Momordica charantia in diabetic rats: Depression of the hepatic gluconeogenic enzymes glucose-6-phosphatase and fructose-1,6-bisphosphatase and elevation of both liver and red-cell shunt enzyme glucose-6-phosphate dehydrogenase. Biochem J. 1993;292(Pt 1):267–70. doi: 10.1042/bj2920267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dey L, Attele AS, Yuan CS. Alternative therapies for type 2 diabetes. Altern Med Rev. 2002;7:45–58. [PubMed] [Google Scholar]
- 61.Odutuga AA, Asemota HN, Musac I, Gloden KD, Kean EA. Fatty acid composition of Arilli from ackee fruit (Blighia sapida L) Jamaica J Sci Technol. 1992;3:30–2. [Google Scholar]
- 62.Afolayan AJ, Sunmonu TO. In vivo studies on antidiabetic plants used in South African herbal medicine. J Clin Biochem Nutr. 2010;47:98–106. doi: 10.3164/jcbn.09-126R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van de Venter M, Roux S, Bungu LC, Louw J, Crouch NR, Grace OM, et al. Antidiabetic screening and scoring of 11 plants traditionally used in South Africa. J Ethnopharmacol. 2008;119:81–6. doi: 10.1016/j.jep.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 64.Ebong PE, Atangwho IJ, Eyong EU, Egbung GE. The antidiabetic efficacy of combined extracts from two continental plants: Azadirachta indica (A. Juss) (Neem) and Vernonia amygdalina (Del) (African Bitter Leaf) Am J Biochem Biotechnol. 2008;4:239–44. [Google Scholar]
- 65.Atangwho IJ, Ebong PE, Egbung GE, Eteng MU, Eyong EU. Effect of Vernonia amygdalina Del. on liver function in alloxan-induced hyperglycaemic rats. J Pharm Bioresour. 2007;4:27–31. [Google Scholar]
- 66.Dimo T, Rakotonirina SV, Tan PV, Azay J, Dongo E, Kamtchouing P, et al. Effect of Sclerocarya birrea (Anacardiaceae) stem bark methylene chloride/methanol extract on streptozotocin-diabetic rats. J Ethnopharmacol. 2007;110:434–8. doi: 10.1016/j.jep.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 67.Ojewole JA. Hypoglycemic effect of Sclerocarya birrea [(A. Rich.) Hochst.] [Anacardiaceae] stem-bark aqueous extract in rats. Phytomedicine. 2003;10:675–81. doi: 10.1078/0944-7113-00295. [DOI] [PubMed] [Google Scholar]
- 68.Marquis VO, Adanlawo TA, Olaniyi AA. The effect of foetidin from Momordica foetida on blood glucose level of albino rats. Planta Med. 1977;31:367–74. doi: 10.1055/s-0028-1097545. [DOI] [PubMed] [Google Scholar]
- 69.Sunmonu TO, Afolayan AJ. Evaluation of antidiabetic activity and associated toxicity of artemisia afra aqueous extract in wistar rats. Evid Based Complement Alternat Med 2013. 2013:929074. doi: 10.1155/2013/929074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jain N, Vijayaraghavan R, Pant SC, Lomash V, Ali M. Aloe vera gel alleviates cardiotoxicity in streptozocin-induced diabetes in rats. J Pharm Pharmacol. 2010;62:115–23. doi: 10.1211/jpp.62.01.0013. [DOI] [PubMed] [Google Scholar]
- 71.Okyar A, Can A, Akev N, Baktir G, Sütlüpinar N. Effect of Aloe vera leaves on blood glucose level in type I and type II diabetic rat models. Phytother Res. 2001;15:157–61. doi: 10.1002/ptr.719. [DOI] [PubMed] [Google Scholar]
- 72.Mogale MA, Lebelo SL, Shai LJ, Eloff JN. Aloe arborescens aqueous gel extract alters the activities of key hepatic enzymes and blood concentration of triglycerides, glucose and insulin in alloxan-induced diabetic rats. Afr J Biotechnol. 2011;10:4242–8. [Google Scholar]
- 73.Hussain Z, Waheed A, Qureshi RA, Burdi DK, Verspohl EJ, Khan N, et al. The effect of medicinal plants of Islamabad and Murree region of Pakistan on insulin secretion from INS-1 cells. Phytother Res. 2004;18:73–7. doi: 10.1002/ptr.1372. [DOI] [PubMed] [Google Scholar]
- 74.Eidi A, Eidi M, Esmaeili E. Antidiabetic effect of garlic (Allium sativum L.) in normal and streptozotocin-induced diabetic rats. Phytomedicine. 2006;13:624–9. doi: 10.1016/j.phymed.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 75.Mathew PT, Augusti KT. Hypoglycaemic effects of onion, Allium cepa Linn. on diabetes mellitus – A preliminary report. Indian J Physiol Pharmacol. 1975;19:213–7. [PubMed] [Google Scholar]
- 76.Koyama Y, Abe K, Sano Y, Ishizaki Y, Njelekela M, Shoji Y, et al. Effects of green tea on gene expression of hepatic gluconeogenic enzymes in vivo. Planta Med. 2004;70:1100–2. doi: 10.1055/s-2004-832659. [DOI] [PubMed] [Google Scholar]
- 77.Nammi S, Boini MK, Lodagala SD, Behara RB. The juice of fresh leaves of Catharanthus roseus Linn. reduces blood glucose in normal and alloxan diabetic rabbits. BMC Complement Altern Med. 2003;3:4. doi: 10.1186/1472-6882-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Satyanarayana S, Sarma GS, Ramesh A, Sushruta K, Srinivas N. Evaluation of herbal preparations for hypoglycemic activity in normal and diabetic rabbits. Int J Pharm. 2003;41:466–72. [Google Scholar]
- 79.Ojewole JA. Hypoglycaemic effect of Clausena anisata (Willd) hook methanolic root extract in rats. J Ethnopharmacol. 2002;81:231–7. doi: 10.1016/s0378-8741(02)00085-5. [DOI] [PubMed] [Google Scholar]
- 80.Njagi JM, Piero MN, Ngeranwa JJ, Njagi EN, Kibiti CM, Njue WM, et al. Assessment of anti-diabetic potential of Ficus sycomorus on alloxan-induced diabetic mice. Int J Diabetes Res. 2012;1:47–51. [Google Scholar]
- 81.Kudolo GB. The effect of 3-month ingestion of Ginkgo biloba extract on pancreatic beta-cell function in response to glucose loading in normal glucose tolerant individuals. J Clin Pharmacol. 2000;40:647–54. [PubMed] [Google Scholar]
- 82.Mutalik S, Chetana M, Sulochana B, Devi PU, Udupa N. Effect of dianex, a herbal formulation on experimentally induced diabetes mellitus. Phytother Res. 2005;19:409–15. doi: 10.1002/ptr.1570. [DOI] [PubMed] [Google Scholar]
- 83.Zhang XF, Tan BK. Anti-diabetic property of ethanolic extract of Andrographis paniculata in streptozotocin-diabetic rats. Acta Pharmacol Sin. 2000;21:1157–64. [PubMed] [Google Scholar]
- 84.Akowuah GA, Amirin S, Mariam A, Aminah I. Blood sugar lowering activity of Gynura procumbens leaf extracts. J Trop Med Plants. 2001;2:5–10. [Google Scholar]
- 85.Mahomed IM, Ojewole JA. Analgesic, antiinflammatory and antidiabetic properties of Harpagophytum procumbens DC (Pedaliaceae) secondary root aqueous extract. Phytother Res. 2004;18:982–9. doi: 10.1002/ptr.1593. [DOI] [PubMed] [Google Scholar]
- 86.Mahomed IM, Ojewole JA. Hypoglycemic effect of Hypoxis hemerocallidea corm (African potato) aqueous extract in rats. Methods Find Exp Clin Pharmacol. 2003;25:617–23. doi: 10.1358/mf.2003.25.8.778082. [DOI] [PubMed] [Google Scholar]
- 87.Ojewole JA. Antinociceptive, antiinflammatory and antidiabetic effects of Leonotis leonurus (L.) R. BR. [Lamiaceae] leaf aqueous extract in mice and rats. Methods Find Exp Clin Pharmacol. 2005;27:257–64. doi: 10.1358/mf.2005.27.4.893583. [DOI] [PubMed] [Google Scholar]
- 88.Mukherjee PK, Saha K, Pal M, Saha BP. Effect of Nelumbo nucifera rhizome extract on blood sugar level in rats. J Ethnopharmacol. 1997;58:207–13. doi: 10.1016/s0378-8741(97)00107-4. [DOI] [PubMed] [Google Scholar]
- 89.Gonzalez M, Zarzuelo A, Gamez MJ, Utrilla MP, Jimenez J, Osuna I. Hypoglycemic activity of olive leaf. Planta Med. 1992;58:513–5. doi: 10.1055/s-2006-961538. [DOI] [PubMed] [Google Scholar]
- 90.Frati-Munari AC, Del Valle-Martínez LM, Ariza-Andraca CR, Islas-Andrade S, Chávez-Negrete A. Hypoglycemic action of different doses of nopal (Opuntia streptacantha Lemaire) in patients with type II diabetes mellitus. Arch Invest Med (Mex) 1989;20:197–201. [PubMed] [Google Scholar]
- 91.Ojewole JA. Evaluation of the analgesic, anti-inflammatory and anti-diabetic properties of Sclerocarya birrea (A. Rich.) Hochst. Stem-bark aqueous extract in mice and rats. Phytother Res. 2004;18:601–8. doi: 10.1002/ptr.1503. [DOI] [PubMed] [Google Scholar]
- 92.Kar DM, Maharana L, Pattnaik S, Dash GK. Studies on hypoglycaemic activity of Solanum xanthocarpum Schrad. and Wendl. fruit extract in rats. J Ethnopharmacol. 2006;108:251–6. doi: 10.1016/j.jep.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 93.Oyedemi SO, Bradley G, Afolayan AJ. In-vitro and in-vivo antioxidant activities of aqueous extract of Strychnos henningsii Gilg. Afr J Pharm Pharmacol. 2010;4:70–8. [Google Scholar]
- 94.Ojewole JA. Analgesic, antiinflammatory and hypoglycemic effects of Sutherlandia frutescens R. BR. (variety Incana E. MEY.) [Fabaceae] shoot aqueous extract. Methods Find Exp Clin Pharmacol. 2004;26:409–16. [PubMed] [Google Scholar]
- 95.Musabayane CT, Mahlalela N, Shode FO, Ojewole JA. Effects of Syzygium cordatum (Hochst.) [Myrtaceae] leaf extract on plasma glucose and hepatic glycogen in streptozotocin-induced diabetic rats. J Ethnopharmacol. 2005;97:485–90. doi: 10.1016/j.jep.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 96.Akhtar MS, Iqbal J. Evaluation of the hypoglycaemic effect of Achyranthes aspera in normal and alloxan-diabetic rabbits. J Ethnopharmacol. 1991;31:49–57. doi: 10.1016/0378-8741(91)90143-2. [DOI] [PubMed] [Google Scholar]
- 97.Akinola OB, Caxton-Martins EA, Dini L. Chronic treatment with ethanolic extract of the leaves of Azadirachta indica ameliorates lesions of pancreatic islets in streptozotocin diabetes. Int J Morphol. 2010;28:291–302. [Google Scholar]
- 98.Bhat M, Kothiwale SK, Tirmale AR, Bhargava SY, Joshi BN. Antidiabetic properties of Azardiracta indica and Bougainvillea spectabilis: In vivo studies in murine diabetes model. Evid Based Complement Alternat Med 2011. 2011:561625. doi: 10.1093/ecam/nep033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nahar L, Ripa FA, Zulfiker AH, Rokonuzzaman M, Haque M, Islam KM. Comparative study of anti-diabetic effect of Abroma augusta L and Syzygium cumuni on alloxan-induced diabetic rat. Agric Biol J North Am. 2010;1:1268–72. [Google Scholar]
- 100.Verspohl EJ, Bauer K, Neddermann E. Antidiabetic effect of Cinnamomum cassia and Cinnamomum zeylanicum in vivo and in vitro. Phytother Res. 2005;19:203–6. doi: 10.1002/ptr.1643. [DOI] [PubMed] [Google Scholar]
- 101.Adaramoye OA, Adeyemi EO. Hypoglycaemic and hypolipidaemic effects of fractions from kolaviron, a biflavonoid complex from Garcinia kola in streptozotocin-induced diabetes mellitus rats. J Pharm Pharmacol. 2006;58:121–8. doi: 10.1211/jpp.58.1.0015. [DOI] [PubMed] [Google Scholar]
- 102.Ugochukwu NH, Babady NE. Antihyperglycemic effect of aqueous and ethanolic extracts of Gongronema latifolium leaves on glucose and glycogen metabolism in livers of normal and streptozotocin-induced diabetic rats. Life Sci. 2003;73:1925–38. doi: 10.1016/s0024-3205(03)00543-5. [DOI] [PubMed] [Google Scholar]
- 103.Ugochukwu NH, Cobourne MK. Modification of renal oxidative stress and lipid peroxidation in streptozotocin-induced diabetic rats treated with extracts from Gongronema latifolium leaves. Clin Chim Acta. 2003;336:73–81. doi: 10.1016/s0009-8981(03)00325-5. [DOI] [PubMed] [Google Scholar]
- 104.Omonkhua AA, Onoagbe IO, Fajimeye IA, Adekola MB, Imoru ZA. Long-term anti-diabetic and anti-hyperlipidaemic effects of aqueous stem bark extract of Irvingia gabonensis in streptozotocin-induced diabetic rats. Int J Niger Soc Exp Biol. 2014;26:1–8. [Google Scholar]
- 105.Sánchez de Medina F, Gámez MJ, Jiménez I, Jiménez J, Osuna JI, Zarzuelo A. Hypoglycemic activity of juniper “berries”. Planta Med. 1994;60:197–200. doi: 10.1055/s-2006-959457. [DOI] [PubMed] [Google Scholar]
- 106.Obatomi DK, Bikomo EO, Temple VJ. Anti-diabetic properties of the African mistletoe in streptozotocin-induced diabetic rats. J Ethnopharmacol. 1994;43:13–7. doi: 10.1016/0378-8741(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 107.Aderibigbe AO, Emudianughe TS, Lawal BA. Evaluation of the antidiabetic action of Mangifera indica in mice. Phytother Res. 2001;15:456–8. doi: 10.1002/ptr.859. [DOI] [PubMed] [Google Scholar]
- 108.Nyarko AK, Asare-Anane H, Ofosuhene M, Addy ME. Extract of Ocimum canum lowers blood glucose and facilitates insulin release by isolated pancreatic beta-islet cells. Phytomedicine. 2002;9:346–51. doi: 10.1078/0944-7113-00124. [DOI] [PubMed] [Google Scholar]
- 109.Bwititi P, Musabayane CT, Nhachi CF. Effects of Opuntia megacantha on blood glucose and kidney function in streptozotocin diabetic rats. J Ethnopharmacol. 2000;69:247–52. doi: 10.1016/s0378-8741(99)00123-3. [DOI] [PubMed] [Google Scholar]
- 110.Fort DM, Ubillas RP, Mendez CD, Jolad SD, Inman WD, Carney JR, et al. Novel antihyperglycemic terpenoid-quinones from Pycnanthus angolensis. J Org Chem. 2000;65:6534–9. doi: 10.1021/jo000568q. [DOI] [PubMed] [Google Scholar]
- 111.Ojewole JA, Adewunmi CO. Anti-inflammatory and hypoglycaemic effects of Tetrapleura tetraptera (Taub) [Fabaceae] fruit aqueous extract in rats. J Ethnopharmacol. 2004;95:177–82. doi: 10.1016/j.jep.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 112.Ngugi MP, Njagi MJ, Kibiti MC, Maina DM, Ngeranwa JN, Njagi NM, et al. Trace elements content of selected Kenyan anti-diabetic medicinal plants. Int J Curr Pharm Res. 2012;4:3. [Google Scholar]
- 113.Njagi JM, Ngugi MP, Kibiti CM, Ngeranwa NJ, Njagi EN, Njue MW, et al. Hypoglycemic effects of Caesalpinia volkensii on alloxan-induced diabetic mice. Asian J Pharm Clin Res. 2012;5:69–74. [Google Scholar]
- 114.Diatewa M, Samba CB, Assah TC, Abena AA. Hypoglycemic and antihyperglycemic effects of diethyl ether fraction isolated from the aqueous extract of the leaves of Cogniauxia podoleana baill on in normal and alloxan induced diabetic rats. J Ethnopharmacol. 2004;92:229–32. doi: 10.1016/j.jep.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 115.Piero NM, Murugi NJ, Kibiti MC, Ngeranwa JJ, NjueMW, Maina D, et al. Hypoglycemic activity of some Kenyan plants traditionally used to manage diabetes mellitus in eastern province. J Diabetes Metab. 2011;2:155. [Google Scholar]
- 116.Olaokun OO, McGaw LJ, Eloff JN, Naidoo V. Evaluation of the inhibition of carbohydrate hydrolysing enzymes, antioxidant activity and polyphenolic content of extracts of ten African Ficus species (Moraceae) used traditionally to treat diabetes. BMC Complement Altern Med. 2013;13:94. doi: 10.1186/1472-6882-13-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Njagi JM, Ngugi MP, Kibiti CM, Ngeranwa NJ, Njagi EN, Njue MW, et al. Evaluation of anti-diabetic effects of Kleinia squarrosa on alloxanized diabetic mice. Asian J Biochem Pharm Res. 2012;2:2. [Google Scholar]
- 118.Haraguchi H, Ohmi I, Kubo I. Inhibition of aldose reductase by maesanin and related p-benzoquinone derivatives and effects on other enzymes. Bioorg Med Chem. 1996;4:49–53. doi: 10.1016/0968-0896(95)00162-x. [DOI] [PubMed] [Google Scholar]
- 119.Budinsky A, Wolfram R, Oguogho A, Efthimiou Y, Stamatopoulos Y, Sinzinger H. Regular ingestion of Opuntia robusta lowers oxidation injury. Prostaglandins Leukot Essent Fatty Acids. 2001;65:45–50. doi: 10.1054/plef.2001.0287. [DOI] [PubMed] [Google Scholar]
- 120.Alarcon-Aguilar FJ, Jimenez-Estrada M, Reyes-Chilpa R, Roman-Ramos R. Hypoglycemic effect of extracts and fractions from Psacalium decompositum in healthy and alloxan-diabetic mice. J Ethnopharmacol. 2000;72:21–7. doi: 10.1016/s0378-8741(00)00202-6. [DOI] [PubMed] [Google Scholar]
- 121.Gray AM, Flatt PR. Insulin-releasing and insulin-like activity of the traditional anti-diabetic plant Coriandrum sativum (coriander) Br J Nutr. 1999;81:203–9. doi: 10.1017/s0007114599000392. [DOI] [PubMed] [Google Scholar]
- 122.El-Missiry MA, El Gindy AM. Amelioration of alloxan induced diabetes mellitus and oxidative stress in rats by oil of Eruca sativa seeds. Ann Nutr Metab. 2000;44:97–100. doi: 10.1159/000012829. [DOI] [PubMed] [Google Scholar]
- 123.El-Hilaly J, Hmammouchi M, Lyoussi B. Ethnobotanical studies and economic evaluation of medicinal plants in Taounate province (Northern Morocco) J Ethnopharmacol. 2003;86:149–58. doi: 10.1016/s0378-8741(03)00012-6. [DOI] [PubMed] [Google Scholar]
- 124.Vuksan V, Sievenpiper JL, Koo VY, Francis T, Beljan-Zdravkovic U, Xu Z, et al. American ginseng (Panax quinquefolius L) reduces postprandial glycemia in nondiabetic subjects and subjects with type 2 diabetes mellitus. Arch Intern Med. 2000;160:1009–13. doi: 10.1001/archinte.160.7.1009. [DOI] [PubMed] [Google Scholar]
- 125.Maobe MA, Nyarango RM. Fourier transformer infra-red spectrophotometer analysis of Urtica dioica medicinal herb used for the treatment of diabetes, malaria and pneumonia in Kisii region, Southwest Kenya. World Appl Sci J. 2013;21:1128–35. [Google Scholar]
- 126.Mehri A, Ranjbar SH, Larijani B, Abdollahi M. A systemic review of efficancy and safety of Urtica dioica in the treatment of diabetes. Int J Pharm. 2011;7:161–70. [Google Scholar]
- 127.Glombitza KW, Mahran GH, Mirhom YW, Michel KG, Motawi TK. Hypoglycemic and antihyperglycemic effects of Zizyphus spina-christi in rats. Planta Med. 1994;60:244–7. doi: 10.1055/s-2006-959468. [DOI] [PubMed] [Google Scholar]
- 128.Edwin J, Balakrishnan S, Dharam J, Chadra J. Diabetes and herbal medicines. Int J Pharmacol Ther. 2008;7:97–106. [Google Scholar]
- 129.Cummings E, Hundal HS, Wackerhage H, Hope M, Belle M, Adeghate E, et al. Momordica charantia fruit juice stimulates glucose and amino acid uptakes in L6 myotubes. Mol Cell Biochem. 2004;261:99–104. doi: 10.1023/b:mcbi.0000028743.75669.ab. [DOI] [PubMed] [Google Scholar]
- 130.Tanira MO. Anti-diabetic medicinal plants: A review of the present status and future directions. Int J Diabetes Mellit. 1994;2:15–22. [Google Scholar]
- 131.Bnouham M, Ziyyat A, Mekhfi H, Tahri A, Legssyer A. Medicinal plants with potential antidiabetic activity-A review of ten years of herbal medicine research (1990-2000) Int J Diabetes Metab. 2006;14:1–25. [Google Scholar]
- 132.Onderoglu S, Sozer S, Erbil KM, Ortac R, Lermioglu F. The evaluation of long-term effects of cinnamon bark and olive leaf on toxicity induced by streptozotocin administration to rats. J Pharm Pharmacol. 1999;51:1305–12. doi: 10.1211/0022357991776886. [DOI] [PubMed] [Google Scholar]
- 133.Grover JK, Yadav S, Vats V. Medicinal plants of India with anti-diabetic potential. J Ethnopharmacol. 2002;81:81–100. doi: 10.1016/s0378-8741(02)00059-4. [DOI] [PubMed] [Google Scholar]