Abstract
Background:
Tilia amurensis (Tiliacese) has been used for anti-tumor and anti-inflammatory in Korea, China, and Japan.
Objective:
In this study, we isolated five compounds from T. amurensis and determined whether protected neuronal cells against glutamate-induced oxidative stress in HT22 cells.
Materials and Methods:
Compounds were isolated using chromatographic techniques including silica gel, Sephadex LH-20 open column and high performance liquid chromatography analysis, and evaluated neuroprotective effect in HT22 cells by 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay.
Results:
β-D-fructofuranosyl α-D-glucopyranoside (1), (-)-epicatechin (2), nudiposide (3), lyoniside (4), and scopoletin (5) were isolated by bioactivity-guided fractionation from the ethyl acetate fraction of T. amurensis. Among them, (-)-epicatechin, nudiposide, lyoniside, and scopoletin had significant neuroprotective activities against glutamate-injured neurotoxicity in HT22 cells.
Conclusion:
These results demonstrated that compound two, three, four, and five have a pronounced protective effect against glutamate-induced neurotoxicity in HT22 cells.
Keywords: Bioactivity compounds, glutamate excitotoxicity, HT22 cell, neuroprotection, tilia amurensis
INTRODUCTION
Alzheimer's disease (AD) is the progressive neurodegenerative disease that impaired memory and learning function. AD is a most common form of dementia in people over the age of 65.[1] Pathogenesis of AD is characterized by extracellular deposits of amyloid-beta and the intracellular neurofibrillary tangles in the brain.[2]
The hippocampus is a major component of the brains of humans and other mammals. It belongs to the limbic system and plays important roles in long-term memory and spatial navigation.[3] In AD, the hippocampus is one of the first regions of the brain to suffer damage; memory problems and disorientation appear among the first symptoms. Oxidative stress induced from reactive oxygen species (ROS) like hydrogen peroxide, superoxide is considered a risk factor in the incidence and progression of cognitive declines that occur during normal cerebral aging and dementia.[4] In addition, oxidative stress plays a critical role in neurodegenerative processes, like AD.[5,6,7,8,9,10,11,12]
Glutamate acts as an excitatory neurotransmitter in the central nervous system and is important in regards to memory.[13] High level of glutamate toxicity is a major contributor to pathological cell death within the nervous system and appears to be mediated by ROS. Oxidative glutamate toxicity is initiated by high concentrations of extracellular glutamate that inhibit cystine uptake onto the cells, followed by the depletion of intracellular cysteine and the loss of glutathione.[14,15] HT22 cells have been used as a useful in vitro model for studying the mechanism of oxidative glutamate toxicity. Because immortalized neuronal HT22 cells, originating from mouse hippocampus, lack functional ionotropic glutamate receptors, thus excluding excitotoxicity as a cause for glutamate triggered cell death.[16,17,18,19,20]
Tilia amurensis (Tiliacese) is commonly known as a bee tree and widely distributed in East Asian countries such as Korea, China, and Japan. The flowers of this tree have been used for removal of a fever in Korea, and its leaves have also been traditionally used to treat cancer in Korea. Previous chemical studies of this species have demonstrated the presence of coumarin, flavonoid, lignin, and triterpene. Recently, anti-tumor, anti-inflammatory, and topoisomerase I and II inhibitory activity were reported.[21,22] However, study on the neuroprotective activity has not been reported. In our previous study, we evaluated neuroprotective effect of four compounds, epicatechin, nudiposide, lyoniside and scopoletin from T. amurensis, and established simultaneous determination of compounds using high performance liquid chromatography (HPLC)-diode-array detection (DAD). Simultaneous determination has shown in previous manuscript.[23]
In this study, we implemented to isolate five compounds from T. amurensis and evaluated the neuroprotective effect of these compounds on glutamate-induced neurotoxicity in mouse hippocampal HT22 cells.
MATERIALS AND METHODS
General experimental procedures
1H (500 MHz) and 13C (125 MHz) one-dimensional-nuclear magnetic resonance (NMR), COSY, and heteronuclear single quantum coherence two-dimensional-NMR were recorded on a Bruker Avance 600 spectrometer and varian unity inova spectrometer with a cryoprobe at 500 MHz and 125 MHz, respectively. Column chromatography was conducted with silica gel and Sephadex LH-20. Thin-layer chromatography (TLC) plates were prepared with silica gel F254. TLC plates (Silica-gel 60 F254) and silica gel (70–230 mesh) were purchased from Merck. Sephadex LH-20 (bead size 25–100 μM) was purchased from Sigma. Analysis was performed on the HPLC system Dionex Ultimate 3000 with a Shiseido C18 column (5 μm, 4.60 mm I.D. ×250 mm). HPLC separations were performed on Gilson preparative HPLC system with C18 YMC hydrosphere (250 mm × 20 mm I.D. S-5 μm). Optical density was measured on a TECAN A-5002 ELISA reader.
Plant materials
The woods of T. amurnesis (Tiliacea) were purchased from Kyungdong traditional herbal market in Seoul, Korea and were identified by Dr. Young Bae Seo, a professor of the College of Oriental Medicine, Daejeon University, Korea. A voucher specimen has been deposited at the Kangwon National University in Chuncheon, Korea (NO. CJ0087).
Extraction and isolation
Woods of T. amurensis (5.4 kg) were extracted using 80% methanol (MeOH) (3 × 2 L) by ultrasonication-assisted extraction. The MeOH solution was evaporated to residue. The residue suspended in distilled water and then partitioned with n-hexane, chloroform (CHCl3), ethyl acetate (EtOAc) and n-butanol to yield a hexane (24.68 g), CHCl3 (18.05 g), EtOAc (55.67 g), and normal butanol (n-BuOH) (133.11 g) fractions, respectively. The EtOAc fraction was passed chromatographic process in a silica gel column (90 × 10 cm, 70–230 mesh) and eluted with a gradient of n-hexane/EtOAc (100:1 → 0:1, v/v) to obtain three fractions, denoted as A– C. Fraction C was conducted by passage over a silica gel column (90 × 5 cm, 70–230 mesh), eluted with EtOAc/MeOH (10:1 → 1:3, v/v) to give four fractions (C1–C4). Compound one (78.8 mg) was obtained as a crystal from the fraction C3. The crystal was collected and washed with MeOH.
Fraction C was subjected to silica gel column eluted with EtOAc/MeOH (10:1 → 1:3, v/v) to give four fractions (C1–C4). Fraction C1 was once chromatographed on a silica gel column (90 × 5 cm, 70–230 mesh), eluted with CHCl3/MeOH (80:1 → 0:1, v/v) to give 12 fractions (C1a-C1l). Compound two (270.7 mg) was obtained from fraction C1j as a crystal. Compound five (8.9 mg) was obtained from C1e fraction by silica gel column chromatography eluted with CHCl3/MeOH (100:1–0:1, v/v). Fraction C2 was separated by Sephadex LH-20 (eluent; 100% MeOH) to give four fraction (C2a-C2d). Once again, C2a was separated by Sephadex LH-20 (eluent; 100% MeOH) to give four fraction (C2a2-C2a4). Compounds three (7.9 mg) and four (8.5 mg) were obtained from fraction C2a2 by preparative HPLC on a C18 YMC hydrosphere (250 mm × 20 mm I.D. S-5 μm) with acetonitrile/water.
Cell culture and measurement of cell viability
Cell viability was investigated using previous described method.[24] Immortalized mouse hippocampal cell line, HT22 cells were provided by Seoul National University, Korea. The cells were cultured in DMEM supplemented with 10% FBS, 1% penicillin/streptomycin, 2 mg/mL NaHCO3 and 15 mM HEPES and were maintained in a humidified incubator with 5% CO2 at 37°C. Cells were subcultured once every 2 days. For assessment of cell viability, 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay were used. The cells were seeded in 48-well plates at a concentration of 3.14 × 103 cells/well. After 23 h of incubation at 37°C under humidified atmosphere, the cells were treated with various concentrations of the samples and were further incubated for 1 h. Then, glutamate was added to the cell culture medium, and the cells were incubated for an additional 24 h. Thereafter, MTT solution (1 mg/mL) was applied to the cells. After 3 h of incubation, culture media was removed and the formazan crystals in each well were dissolved in dimethyl sulfoxide, and the absorbance was measured via micro plate reader at a wavelength of 570 nm. The relative cell viability was evaluated in accordance with the quantity of MTT converted to the insoluble formazan. The neuroprotective activity of samples was investigated by relative protection ratio (%).
Statistical analysis
Data were evaluated for statistical significance by analysis of variance, tested using a computerized statistical package. Data are expressed as the mean ± standard deviation (SD). The confidence level for statistical significance was set at a probability value of 0.05.
RESULTS AND DISCUSSION
In this study, we isolated five compounds from the MeOH extract of T. amurensis and evaluated neuroprotective effect against glutamate neurotoxicity in HT22 cells using MTT assay. We established simultaneous determination of neuroprotective four compounds in T. amurensis by HPLC-DAD in our previous study.[23]
Neuroprotective effect of compounds is presented in this manuscript and simultaneous determination has shown in previous manuscript. Subsequent phytochemical research coupled with bioactivity was conducted to find the active compounds responsible for the neuroprotective activity of T. amurensis. In the MTT assay, cell viability determined protective effect against glutamate-induced oxidative injury. Neuroprotective activity was calculated by the relative protection value (%). We performed the measurement of neuroprotection of partitioned fractions (hexane, CHCl3, EtOAc and n-BuOH). The EtOAc fraction showed most potent neuroprotective activity on HT22 cell treated glutamate at a concentration of 100 μg/mL (35.24%, P < 0.01) [Figure 1]. Based on this result, we obtained five compounds by further fractionation and separation of the EtOAc fraction by several chromatographic methods.
Figure 1.
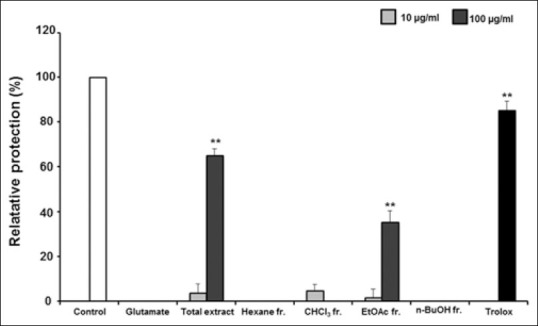
The neuroprotective activity of Tilia amurensis methanol extract and four partitioned fractions (hexane, chloroform, ethyl acetate, and normal butanol) against glutamate-induced cell death in HT22 cells. Data are mean ± standard deviation * P < 0.05 compared with glutamate treated cells
Five compounds were identified as β-D-fructofuranosyl α-D-glucopyranoside (1), epicatechin (2), nudiposide (3), lyoniside (4), and scopoletin (5) by the direct comparison of their physicochemical and spectroscopic data with those previously reported [Figure 2].[25,26,27,28,29]
Figure 2.
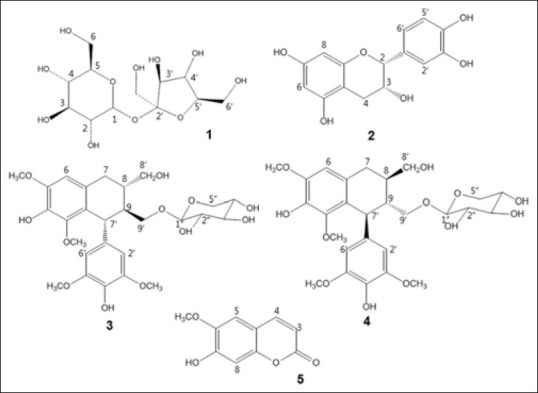
Chemical structures of compounds isolated from ethyl acetate fraction of Tilia amurensis: β-D-fructofuranosyl α-D-glucopyranoside (1), epicatechin (2), nudiposide (3), lyoniside (4), and scopoletin (5)
Five compounds were investigated for their protective activity against glutamate-induced neurotoxicity in HT22 cells. The relative neuroprotection of compounds is exhibited in Figure 3. Among them, epicatechin (2), nudiposide (3), lyoniside (4), and scopoletin (5) showed significant neuroprotective activities at concentrations ranging from 10, 50, and 100 μM in the dose-dependent manner. Four compounds increased HT22 cell density reduced by glutamate [Figure 4]. Neuroprotective activities of epicatechin, nudiposide, and lyoniside, scopoletin were firstly reported in this manuscript.
Figure 3.
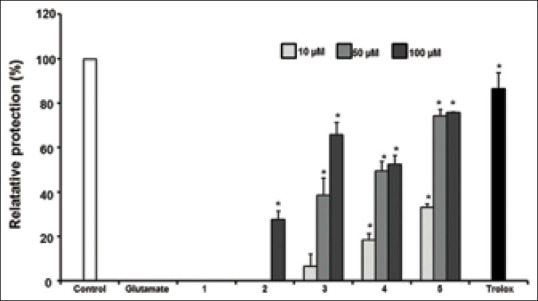
The protective activity of compounds isolated from the ethyl acetate fraction of Tilia amurensis wood against glutamate-induced neurotoxicity in HT22 cells: β-D-fructofuranosyl α-D-glucopyranoside (1), epicatechin (2), nudiposide (3), lyoniside (4), and scopoletin (5). Data are mean ± standard deviation * P < 0.05 compared with glutamate treated cells
Figure 4.
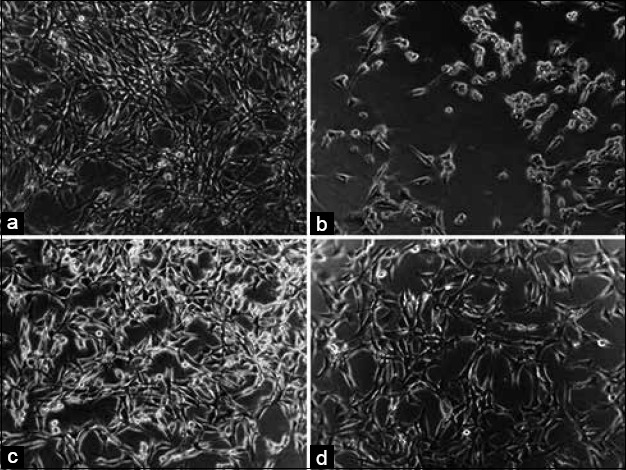
Neuroprotective effect of scopoletin against glutamate-induced cell death in HT22 cells. (a) control group, (b) glutamate treated group, (c) glutamate + trolox treated group, (d) glutamate + scopoletin (100 μM) treated group
Scopoletin (5) (74.10% at 50 μM and 75.70% at 100 μM; P < 0.05) showed the most potent activity against glutamate-induced neurotoxicity. The potency of scopoletin is similar with that of trolox, a positive control. It was reported that scopoletin increased rat retinal neuron cells at the high concentration and also showed anti-cholinesterase activity.[30,31]
Therefore, scopoletin may be a good candidate of drug development for treatment of AD.
CONCLUSION
We isolated five compounds from T. amurensis and evaluated the neuroprotective effect of five compounds on glutamate-induced oxidative stress in HT22 cells in this study. Epicatechin, nudiposide, lyoniside and scopoletin significantly protected neuronal cells, and these results suggest that epicatechin, nudiposide, lyoniside and scopoletin are correlated with neuroprotective effect of T. amurensis.
Further study is required to understand the bio mechanism of neuroprotective effect of four compounds.
ACKNOWLEDGMENT
This study is supported by Kangwon National University.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Swerdlow RH. Brain aging, Alzheimer's disease, and mitochondria. Biochim Biophys Acta. 1978;23:229–33. doi: 10.1016/j.bbadis.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swerdlow RH. Pathogenesis of Alzheimer's disease. Clin Interv Aging. 2007;2:347–59. [PMC free article] [PubMed] [Google Scholar]
- 3.Heo SJ, Cha SH, Kim KN, Lee SH, Ahn G, Kang DH, et al. Neuroprotective effect of phlorotannin isolated from Ishige okamurae against H2O2 -induced oxidative stress in murine hippocampal neuronal cells, HT22. Appl Biochem Biotechnol. 2012;166:1520–32. doi: 10.1007/s12010-012-9545-7. [DOI] [PubMed] [Google Scholar]
- 4.Butterfield DA, Howard B, Yatin S, Koppal T, Drake J, Hensley K, et al. Elevated oxidative stress in models of normal brain aging and Alzheimer's disease. Life Sci. 1999;65:1883–92. doi: 10.1016/s0024-3205(99)00442-7. [DOI] [PubMed] [Google Scholar]
- 5.Abeti R, Duchen MR. Activation of PARP by oxidative stress induced by ß-amyloid: Implications for Alzheimer's disease. Neurochem Res. 2012;37:2589–96. doi: 10.1007/s11064-012-0895-x. [DOI] [PubMed] [Google Scholar]
- 6.Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90:7915–22. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–95. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 8.Halliwell B. Reactive oxygen species and the central nervous system. J Neurochem. 1992;59:1609–23. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- 9.Tan S, Sagara Y, Liu Y, Maher P, Schubert D. The regulation of reactive oxygen species production during programmed cell death. J Cell Biol. 1998;141:1423–32. doi: 10.1083/jcb.141.6.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satoh T, Lipton SA. Redox regulation of neuronal survival mediated by electrophilic compounds. Trends Neurosci. 2007;30:37–45. doi: 10.1016/j.tins.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Satoh T, Enokido Y, Kubo T, Yamada M, Hatanaka H. Oxygen toxicity induces apoptosis in neuronal cells. Cell Mol Neurobiol. 1998;18:649–66. doi: 10.1023/A:1020633919115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aruoma OI. Free radicals, oxidative stress and anti-oxidants in human health and disease. J Am Oil Chem Soc. 1998;75:199–212. doi: 10.1007/s11746-998-0032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kornbuber J, Wiltfang J. The role of glutamate in dementia. J Neural Transm. 1998;53:277–87. doi: 10.1007/978-3-7091-6467-9_24. [DOI] [PubMed] [Google Scholar]
- 14.Tan S, Wood M, Maher P. Oxidative stress induces a form of programmed cell death with characteristics of both apoptosis and necrosis in neuronal cells. J Neurochem. 1998;71:95–105. doi: 10.1046/j.1471-4159.1998.71010095.x. [DOI] [PubMed] [Google Scholar]
- 15.Tan S, Schubert D, Maher P. Oxytosis: A novel form of programmed cell death. Curr Top Med Chem. 2001;1:497–506. doi: 10.2174/1568026013394741. [DOI] [PubMed] [Google Scholar]
- 16.Fukui M, Song JH, Choi J, Choi HJ, Zhu BT. Mechanism of glutamate-induced neurotoxicity in HT22 mouse hippocampal cells. Eur J Pharmacol. 2009;617:1–11. doi: 10.1016/j.ejphar.2009.06.059. [DOI] [PubMed] [Google Scholar]
- 17.Braun S, Liebetrau W, Berning B, Behl C. Dexamethasone-enhanced sensitivity of mouse hippocampal HT22 cells for oxidative stress is associated with the suppression of nuclear factor-kappaB. Neurosci Lett. 2000;295:101–4. doi: 10.1016/s0304-3940(00)01603-7. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Li L, Suo WZ. HT22 hippocampal neuronal cell line possesses functional cholinergic properties. Life Sci. 2009;84:267–71. doi: 10.1016/j.lfs.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Cho N, Choi JH, Yang H, Jeong EJ, Lee KY, Kim YC, et al. Neuroprotective and anti-inflammatory effects of flavonoids isolated from Rhus verniciflua in neuronal HT22 and microglial BV2 cell lines. Food Chem Toxicol. 2012;50:1940–5. doi: 10.1016/j.fct.2012.03.052. [DOI] [PubMed] [Google Scholar]
- 20.Fukui M, Zhu BT. Mithchondrial superoxide dismutase SOD2, but not cytosolic SOD1, palys a critical role in protection against glutamate-induced oxidative stress and cell death in HT22. Free Radic Biol. 2012;48:821–30. doi: 10.1016/j.freeradbiomed.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim KH, Moon E, Kim SY, Choi SU, Lee KR. Lignan constituents of Tilia amurensis and their biological evaluation on antitumor and anti-inflammatory activities. Food Chem Toxicol. 2012;50:3680–6. doi: 10.1016/j.fct.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Choi JY, Seo CS, Zheng MS, Lee CS, Son JK. Topoisomerase I and II inhibitory constituents from the bark of Tilia amurensis. Arch Pharm Res. 2008;31:1413–8. doi: 10.1007/s12272-001-2125-y. [DOI] [PubMed] [Google Scholar]
- 23.Lee B, Weon JB, Yun BR, Lee J, Eom MR, Ma CJ. Simultaneous determination of four neuroprotective compounds of Tilia amurensis by high performance liquid chromatography coupled with diode array detector. Pharmacogn Mag. 2014;10:195–9. doi: 10.4103/0973-1296.137353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weon JB, Lee B, Yun BR, Lee J, Ma CJ. Neuroprotective effects of 4,5-dimethoxypyrocatechol isolated from Cynanchum paniculatum on HT22 cells. Pharmacogn Mag. 2014;10:161–4. doi: 10.4103/0973-1296.131028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehta BK, Sharma U, Agrawal S, Pandit V, Joshi N, Gupta M. Isolation and characterization of new compounds from seeds of Nigella sativa. Med Chem Res. 2008;17:462–73. [Google Scholar]
- 26.Sudjaroen Y, Haubner R, Würtele G, Hull WE, Erben G, Spiegelhalder B, et al. Isolation and structure elucidation of phenolic antioxidants from Tamarind (Tamarindus indica L.) seeds and pericarp. Food Chem Toxicol. 2005;43:1673–82. doi: 10.1016/j.fct.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 27.Fuchino H, Satoh T, Tanaka N. Chemical evaluation of Betula species in Japan. I. Constituents of Betula ermanii. Chem Pharm Bull. 1995;43:1937–42. [Google Scholar]
- 28.Lee MK, Sung SH, Lee HS, Cho JH, Kim YC. Lignan and neolignan glycosides from Ulmus davidiana var.japonica. Arch Pharm Res. 2001;24:198–201. doi: 10.1007/BF02978256. [DOI] [PubMed] [Google Scholar]
- 29.Kim SS, Lee CK, Kang SS, Jung HA, Choi JS. Chlorogenic acid, an antioxidant principle from the aerial parts of artemisia iwayomogi that acts on 1,1-diphenyl-2-picrylhydrazyl radical. Arch Pharm Res. 1997;20:148–54. doi: 10.1007/BF02974002. [DOI] [PubMed] [Google Scholar]
- 30.Orhan I, Tosun F, Sener B. Coumarin, anthroquinone and stilbene derivatives with anti-cholinesterase activity. J Biosci. 2008;63:366–70. doi: 10.1515/znc-2008-5-610. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Sheng YM, Meng XL, Long Y. Effect of caffeic acid, seopoletin and scutellarin on rat retinal neurons in vitro. Zhongguo Zhong Yao Za Zhi. 2005;30:907–9. [PubMed] [Google Scholar]


