Abstract
Background:
The majority of Achillea species are the most important native economic plants of Anatolia. They include highly bioactive compounds, so they have therapeutic applications.
Objective:
In the present study, the aim was to investigate in vitro anti-oxidant, cytotoxic and pro-apoptotic effects of Achillea teretifolia Willd extracts (Turkish name: Beyaz civanperÇemi).
Materials and Methods:
The anti-oxidant potential of the extracts was analyzed by the free radical 1,1-diphenyl-2-picryl-hydrazyl (DPPH) and total phenolic content methods. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay was used to detect cytotoxicity of the extracts onhuman prostate cancer cell lines (DU145 and PC-3) and human gingival fibroblast (HGF) cells. mRNA expression levels of pro-apoptotic (bax, caspase-3) and anti-apoptotic (bcl-2) genes were measured by quantitative real-time polymerase chain reaction.
Results:
The results showed that extracts exhibited a remarkable DPPH scavenging activity, and total phenolic content of the methanol extract was higher than that of the water extract. As time and concentration were increased, the methanol extract exhibited a more powerful cytotoxic effect on prostate cancer cells. In prostate cancer cells, the levels of mRNA expression of the bax and caspase-3 genes were significantly up-regulated (P < 0.05), whereas the expression of bcl-2 was down-regulated (P < 0.05). In HGF cells, there were no cytotoxic effect and apoptosis induction triggered by the extracts.
Conclusion:
The methanol extract had more powerful anti-oxidant, cytotoxic and pro-apoptotic effects than the water extract. The extracts could be good anti-oxidant sources, and they might include anti-cancer compounds triggering the cytotoxicity and the apoptosis on prostate cancer cells.
Keywords: Achillea teretifolia Willd, anti-oxidant potential, bax, bcl-2, caspase-3, cytotoxic activity
INTRODUCTION
Achillea L. (civanperçemi; Turkish name) is a medicinal plant genus which has been used since ancient times. It possesses diversity all around the world. The majority of the Achillea species are important native economic plants of Anatolia. They have therapeutic applications due to containing highly bioactive compounds.[1] In Turkey, herbal teas prepared from some Achillea species are used in folk medicine for abdominal pain, diarrhea, and flatulence as well as a diuretic and emmenagog. Several Achillea species are also used as pharmaceuticals, cosmetic products, fragrances, food sources and for plant protection in agriculture.[2,3] Some Achillea extracts exhibit pharmacologic activities such as anti-oxidant,[4] antimicrobial,[5] wound healing,[2] anthelmintic,[6] antidiabetic,[7] anti-inflammatory,[8] antineoplastic,[9] antihypertensive, and antihyperlipidemic properties.[10] Some investigations about anti-oxidant and cytotoxic effects of Achillea species have been performed. The extracts or essential oils of Achillea wilhelmsii C. Koch,[11] Achillea micrantha Willd.,[12] Achillea millefolium L.,[13] Achillea pannonica Scheele,[14] and Achillea fragrantissima (Forssk.) Sch. Bip.[15] are natural anti-oxidant sources. A. millefolium L.,[16] Achillea clavennae L.,[17] Achillea talagonica Bioss.,[18] A. wilhelmsii C. Koch,[6] A. fragrantissima[19] are important species with cytotoxic effects. Their anti-oxidant potential and anticancer effects result from bioactive compounds including caffeic and p-coumaric acid,[13] achillinin A guaianolide,[16] flavonol centaureidin,[17] santoflavon,[18] and saponin.[6]
The Achillea genus has 49 species (58 taxa) occurring in five sections and 24 of them are endemic in Turkey.[20] Achillea ketenoglui H. Duman, Achillea milliana H. Duman, Achillea hamzaoglui Arabacı and Budak and Achillea sivasica Çelik and Akpulat are the last described as local endemics.[20,21,22,23,24] Achillea teretifolia Willd is confined to the Irano-Turanian region and is a species endemic to Turkey.[21] Although many Achillea species are known to be used in folk remedies, there is limited information about this endemic plant in the literature.
The objective of the present study was to investigate the in vitro anti-oxidant, cytotoxic, and pro-apoptotic effects of A. teretifolia in order to reveal the pharmacological properties of this plant. The water and methanol solvents in different polarities were chosen to compare radical scavenging potentials, phenolic contents, cytotoxic and apoptotic effects of the plant extracts.
MATERIALS AND METHODS
Chemicals and reagents
Folin-Ciocalteu's reagent and methanol (Merck) were purchased from Merck Co. (Darmstadt, Germany). 1,1-diphenyl-2-picrylhydrazyl (DPPH), dimethyl sulfoxide (DMSO), gallic acid, 3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and butylated hydroxytoluene (BHT) were obtained from Sigma Chemical Co. (St. Louis, MO, USA). The 90% RPMI 1640 medium, 2 mM L-glutamine, fetal bovine serum (FBS), penicillin-streptomycin, trypsin-EDTA solution, and Dulbecco's Modified Eagle Medium (DMEM) were obtained from Gibco BRL (Grand Island, NY, USA). All reagents were of analytical grade.
Collection of plant material
Achillea teretifolia was collected during flowering time (June 2011–2012) at an elevation of 1200 m from the steppe and the rocky side of Hüseyin Gazi Mountain in Ankara by Prof. Dr. Mecit Vural, Prof. Dr. Leyla Açık and Assistant Prof. Dr. Elif Burcu Bali. The plant material was identified by plant taxonomist Prof. Dr. Mecit Vural, in the Department of Biology, University of Gazi, Ankara, Turkey. Voucher specimens (A. teretifolia [99A053]) were stored in the Herbarium of “GAZI,” Faculty of Science.
Extraction of methanol and water extract
The whole plant (flowers, leaves, and stem) was dried at room temperature during 1-week. The dried plant was powdered, and the methanol extract was obtained by maceration of the powdered plant with absolute methanol (1 L/200 g) for 2 weeks at room temperature. The solvent was refreshed for every 2 days. The extract was filtered using Whatman filter paper number 1, and the filtrate was then evaporated under reduced pressure and dried using a rotary evaporator at 45°C. The water extract was obtained by maceration of the powdered plant with distilled water at room temperature overnight (1 L/50 g). The resulting crude extract was filtered and lyophilized down to dry powder. The dried extracts were stored in labeled sterile screw-capped bottles at +4°C for further testing. In anti-oxidant assays, each of the extracts was prepared with dilutions from 1 mg/ml stock solutions in methanol or water. In cytotoxic assays, stock of the methanol extract was dissolved in 10% DMSO and diluted in a cultured medium. The final concentration of DMSO was 0.1%. The water extract was dissolved in a cultured medium.
Anti-oxidant activity
1,1-diphenyl-2-picryl-hydrazyl (DPPH) Assay
The free radical scavenging activity of A. teretifolia extracts was determined by the DPPH method.[25] Briefly, 1 ml of 0.1 mM methanolic solution of DPPH was added to a test tube with 1 ml of the extracts at different concentrations (50–100−150–200−250 µg/ml). The reaction mixture was shaken vigorously and incubated for 30 min at room temperature in the dark. Thirty minutes later, the absorbance was measured at 517 nm against a blank by a spectrophotometer. The inhibition of radical scavenging activity in percent (I%) was calculated using the following equation:
I% = (Acontrol − Asample)/Acontrol × 100
Where Acontrol is the absorbance value of the control solution (containing all reagents except the test compound), and Asample is the absorbance of the test solution. A 50% inhibition the half maximal inhibitory concentration (IC50) value of the extract concentration was calculated from the graph plotting inhibition percentage against extract concentration. The assays were carried out in triplicate. The synthetic BHT was used as a reference anti-oxidant compound. The DPPH solution was kept in the dark and used freshly in every experiment.
Total phenolic contents of the extracts
The total phenolic content of the extracts was determined by using the Folin–Ciocalteu reagent using gallic acid as a standard, with a slight modifications.[26] The extract solutions (100 µl) were mixed with 200 µl of 50% Folin–Ciocalteu reagent. The mixture was allowed to react for 3 min, and 1 ml aqueous solution of 2% Na2 CO3 was added and shaken slightly. After 1 h of incubation at room temperature, the absorbance of each mixture was measured at 760 nm. The same procedure was also applied to the standard solutions of the gallic acid. The total phenolic content of the extracts was expressed in µg gallic acid equivalents per mg of the extracts.
Cytotoxic activity
Cell cultures
The human cell lines, including DU145 (androgen-insensitive prostate cancer cells) and PC-3 (androgen-sensitive prostate cancer cells), were obtained as a donation from the Research Center of Gulhane Military Medical Faculty, and gingival fibroblast cells were obtained from 20-year-impacted teeth of healthy volunteers after informed consent and approval by the local ethics committee. The prostate cancer and gingival fibroblast cell lines were cultured in RPMI-1640 and DMEM, respectively. Each medium was supplemented with 10% FBS, 100 units/ml penicillin and 100 µg/ml streptomycin. All cells were incubated at 37°C and in a humidified atmosphere of 95% air and 5% CO2.
Cytotoxic effects of the extracts against human prostate cancer and normal gingival fibroblast cells
The cytotoxic effects of the extracts were determined by an MTT assay.[27,28] In this assay, the cells were seeded into 96-well plates (400 mm3 cells/well) in 100 µl of the medium and incubated at 37°C with 5% CO2 for 24 h. Then, the cells were treated with the extracts (ranging from 20 to 100 µg/ml) or without the extracts (vehicle control, 0.1% DMSO) and incubated 24 h and 48 h for each cell line. After the incubation, 20 µl of MTT solution was added into each well and incubated for 4 h at 37°C. The supernatant was removed and replaced with 100 µl of DMSO. The optical density of the wells was measured with a microplate spectrophotometer reader (EIA Reader, ELX800, Biotek Instruments, Burlington, VT). The stock solution of the extracts was serially diluted with growth medium. The aqueous extract was dissolved in media. The same procedure was repeated for human gingival fibroblast (HGF) cells.
RNA extraction and cDNA synthesis
For the detection of mRNA gene expression levels, DU145, PC-3, and gingival fibroblast cells in 6-well plates were precultured in the media containing 10% FBS for 12 h, and incubated with IC50 value of Achillea extracts for 24 h. Total RNA was isolated using a High Pure RNA Isolation Kit (Roche Diagnostics GmbH, Mannheim, Germany), according to the manufacturer's recommendation. The quality and quantity of the total RNA were assessed by Nanodrop spectrophotometer (ND-1000, Thermo Fisher Scientific Inc., MA, USA) and RNA was kept at −80°C for further analysis. cDNA was synthesized using a Transcriptor First Strand cDNA Synthesis Kit (Roche Diagnostics) from aliquots of the total RNA (1 µg) and the combination of anchored-oligo and random hexamer primers.
Real-time polymerase chain reaction
Commercially available real-time ready assays[29] were carried out using forward, reverse primers (8 pmol) for Bax, Bcl-2 and caspase- 3 genes and fiuorescently labeled hydrolysis probes (4 pmol) from Universal Probe Library (Roche Applied Science). The gene expression analysis was performed with real-time polymerase chain reaction (RT-PCR) reactions in a final volume of 20 µl using 15 µl lightcycler 480 probes master and 5 µl diluted sample. The following thermal cycling conditions were applied on the Lightcycler 480 Instrument: First DNA was denatured at 95°C for 10 min. Then, DNA was amplified at 60°C for 30 s. Finally, the signal detection occurred at 72°C for 1 s and cooling at 40°C for 30 s. The Lightcycler completed the cycles 45 times. The negative controls lacking template RNA were included in each experiment. The human GAPDH gene was used as a housekeeping reference gene to normalize expression levels between the samples.
Statistical analysis
All the analyses were performed at least in triplicate. The results were expressed as the mean ± the standard deviation. One-way analysis of the variance was used in multiple group comparisons using the SPSS 11.0 software package. Differences with a value of P < 0.05, P < 0.01 and P < 0.001 were considered statistically significant.
RESULTS
Anti-oxidant effects of Achillea teretifolia extracts
The in vitro free radical scavenging effect of A. teretifolia extracts is shown in Figure 1. The free radical scavenging effect of the methanol extract (IC50: 42.3 ± 0.8 µg/ml) was higher than that of the water extract (IC50: 62.5 ± 0.5 µg/ml). The DPPH scavenging ability of the extracts was lower than that of synthetic anti-oxidant BHT (IC50: 27.5 ± 0.2 µg/ml). The radical scavenging activity of test samples was in the following order: BHT > the methanol extract > the water extract. In addition to the DPPH effect, the total phenolic content of the extracts was evaluated with the Folin– Ciocalteu reagent. The phenolic content of the water and methanol extracts was 34.45 ± 1.74 μg GAE/mg extract and 55.60 ± 1.25 μg GAE/mg extract, respectively. In vitro anti-oxidant results indicated that the methanolic extract including higher total phenolic compounds possessed a more powerful free radical scavenging effect than the water extract. Furthermore, there was a relationship between total phenolic content and IC50 values of DPPH scavenging effects of the extracts. According to the Pearson's correlation test, there was a negative correlation (99.2%) between the amount of phenolics in the extracts and their IC50 values.
Figure 1.

Dose-dependent scavenging activity of the extracts and the standart butylated hydroxytoluene on1,1-diphenyl-2-picryl-hydrazyl inhibition.
Achillea teretifolia extracts only exhibited the cytotoxicity on DU145 and PC-3 cells
In DU145 cells, the methanol extract reduced the cancer cell viability in a dose-dependent manner with calculated IC50 values of 0.40 ± 0.05 mg/ml and 0.14 ± 0.04 mg/ml for 24 and 48 h, respectively. It also inhibited the PC-3 cell proliferation in a dose-dependent manner with calculated IC50 values of 0.35 ± 0.06 mg/ml and 0.17 ± 0.07 mg/ml, respectively [Figure 2]. There was no statistical difference (P > 0.05) between the inhibition of the methanol extract on DU145 and PC-3 cell proliferation. The cytotoxic effect of the methanol extract on PC-3 cells is shown in Figure 3a–c. The water extract also exhibited the same cytotoxic effect on DU145 (IC50: 1.30 ± 0.05 mg/ml) and PC-3 (IC50: 1.30 ± 0.03 mg/ml) cells over 24 h [Figure 4]. According to these results, the methanol extract possessed a more powerful cytotoxic effect on DU145 and PC-3 cells than the water extract. The extracts exhibited significant dose (0.05–1.4 mg/ml) and time-dependent (24 and 48 h) cytotoxicity on prostate cancer cell proliferation (P < 0.05), but no cytotoxic effects on HGF cells [Figures 5 and 6].
Figure 2.
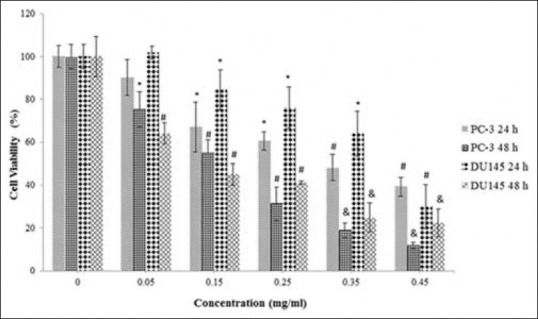
The cytotoxicity of the methanol extract on the proliferation of DU145 and PC-3 cells. The cells were treated with various concentrations (0.05–0.45 mg/ml) of the extract in a culture medium for 24 h and 48 h. Each value represents the mean ± standard error mean (n = 6). The superscripts show statistical differences obtained separately at different concentrations and time compared to their controls shown in figure as *P < 0.05, #P < 0.01, and &P < 0.001
Figure 3.
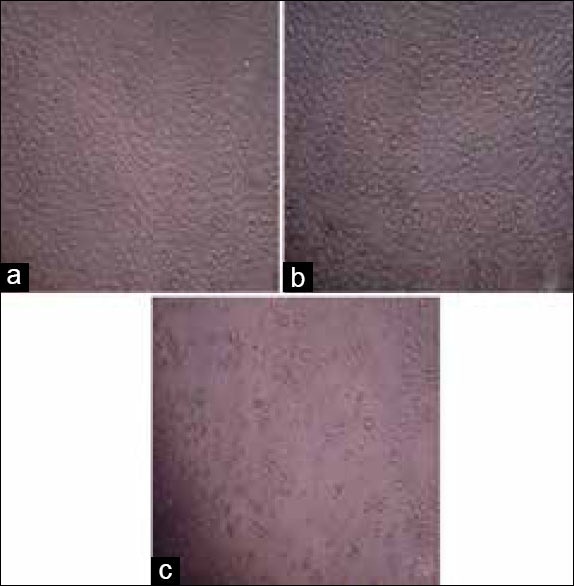
Morphological changes of PC-3 cells treated or not treated with the methanol extract at different concentrations for 24 h under inverted microscope. (a) Control (b) PC-3 cells treated with the methanol extract at 0.05 mg/ml and (c) PC-3 cells treated with the methanol extract at 0.45 mg/ml
Figure 4.
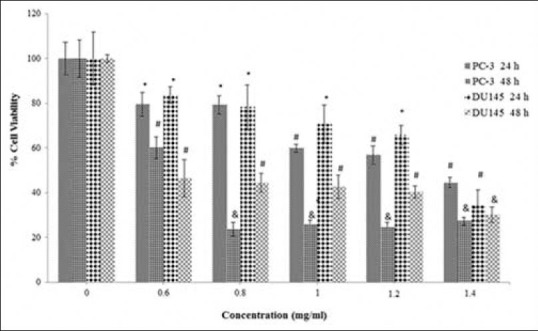
The cytotoxicity of the water extract on the proliferation of DU145 and PC-3 cells. The cells were treated with various concentrations (0.6–1.4 mg/ml) of the extract in a culture medium for 24 h and 48 h. Each value represents the mean ± standard error mean (n = 6). The superscripts show statistical differences obtained separately at different concentrations and time compared to their controls shown in figure as *P < 0.05, #P < 0.01, and &P < 0.001
Figure 5.
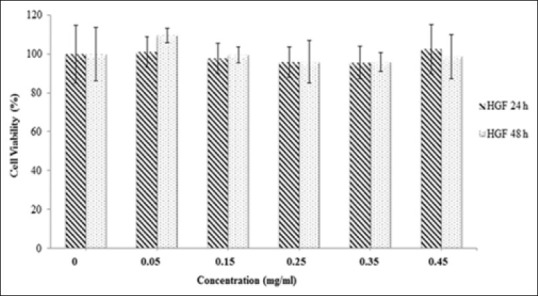
The cytotoxicity of the methanol extract on the proliferation of human gingival fibroblast cells. The cells were treated with various concentrations (0.05–0.45 mg/ml) of the extract in a culture medium for 24 h and 48 h. Each value represents the mean ± standard error mean (n = 6). Compared to the group treated with the methanol extract, there was no statistical difference (P > 0.05) with untreated group for 24 h and 48 h
Figure 6.
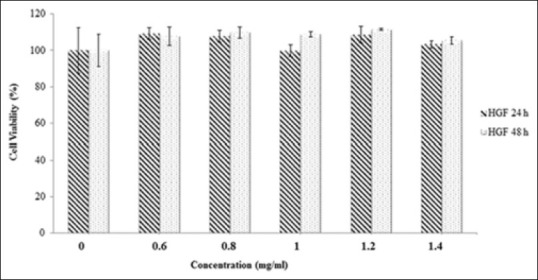
The cytotoxicity of the water extract on the proliferation of human gingival fibroblast cells. The cells were treated with various concentrations (0.6–1.4 mg/ml) of the extract in a culture medium for 24 h and 48 h. Each value represents the mean ± standard error mean (n = 6). Compared to the group treated with the methanol extract, there was no statistical difference (P > 0.05) with untreated group for 24 h and 48 h
The extracts trigger the apoptosis by suppressing bcl-2 expression as well as activating caspase- 3 and bax genes in cancer cells, but not in normal cells
To detect apoptotic effects of the methanol and water extracts, the expression levels of anti-apoptotic (bcl-2) and pro-apoptotic (bax and caspase-3) genes in prostate cancer and HGF cells were compared using quantitative RT-PCR. When exposing PC-3 and DU145 cells to the extracts, the mRNA expression levels of bax and caspase-3 were significantly up-regulated (P < 0.01 or P < 0.001), whereas the expression level of bcl-2 was significantly down-regulated (P < 0.01 or P < 0.001) as shown in Figures 7 and 8. These findings showed that both of the extracts induced the apoptosis by suppressing bcl-2 expression and activating caspase-3 and bax genes in prostate cancer cells. In DU145 cells exposed to the methanol extract, expression of caspase-3 and bax genes was significantly (P < 0.05) higher than in PC-3 cells; otherwise, expression of the bcl-2 gene in DU145 cells was lower than in PC-3. Furthermore, the methanol extract exhibited more powerful apoptosis induction than the water extract. As shown in Figure 9, there was no significant change in the expression levels of bax, bcl-2 and caspase-3 genes in HGF cells. Therefore, apoptosis induction triggered by the extracts was not observed.
Figure 7.
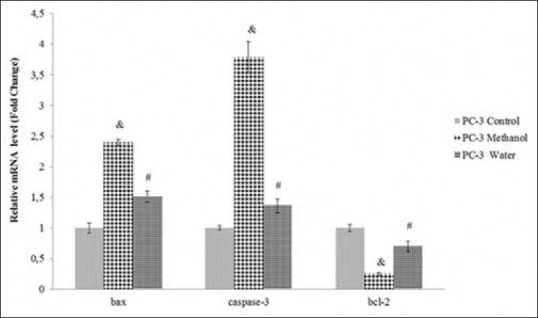
Relative mRNA levels of bax, bcl-2 and caspase-3 genes in PC-3 cells treated or untreated with the methanol (IC50:0.40 ± 0.05 mg/ml) or the water extract (IC50:1.30 ± 0.03 mg/ml) for 24 h. The data are expressed relative to values for untreated control cells and represent the mean ± standard deviation (n = 3, #P < 0.01, and &P < 0.001)
Figure 8.

Relative mRNA levels of bax, bcl-2 and caspase-3 genes in DU145 cells treated or untreated with the methanol (IC50:0.40 ± 0.05 mg/ml) or the water extract (IC50:1.30 ± 0.05 mg/ml) for 24 h. The data are expressed relative to values for untreated control cells and represent the mean ± standard deviation (n = 3, #P < 0.01, and &P < 0.001)
Figure 9.
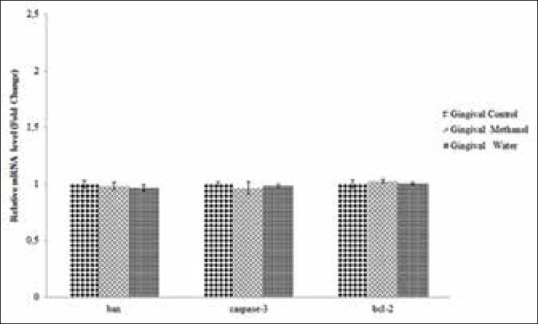
Relative mRNA levels of bax, bcl-2 and caspase- 3 genes in gingival fibroblast cells treated or untreated with the methanol (the half maximal inhibitory concentration [IC50]:0.40 ± 0.05 mg/ml) or the water extract (IC50: 1.30 ± 0.05 mg/ml) for 24 h. The data are expressed relative to values for untreated control cells and represent the mean ± standard deviation
DISCUSSION
Highly reactive phenolic compounds have an ideal structural chemistry containing hydroxyl groups for free radical scavenging activities.[30,31,32] They act as anti-oxidants by neutralizing excess free radicals and protecting the cells against their toxic effects. The accumulation of free radicals in the body also plays an important role in the pathogenesis of cancer, autoimmune disorders, cataracts, rheumatoid arthritis, and cardiovascular and neurodegenerative diseases.[31,33,34] Their effects in biological systems have drawn attention from many areas. In the present study, the DPPH free radical scavenging abilities of the water and methanol extracts were tested. The scavenging ability of the extracts was lower than those of BHT, which is appropriate for some reports about Achillea species;[35,36] however, the extracts exhibited remarkable scavenging activity. These results are in agreement with previous reports of this species that there was a dose-dependent DPPH scavenging activity.[4,35,37] It was reported that the inhibition effect of DPPH scavenging on infusion prepared at 1 mg/ml from A. teretifolia was 28.70 ± 0.55%;[38] however, in our study, it was 91.10 ± 0.48% in a treatment of 250 µg/ml water extract. Our result demonstrated that the DPPH inhibition effect of the water extract prepared without heat treatment was significantly higher than the effect of an infusion. Sultana et al.[39] reported that thermal processing conditions may cause the loss of natural anti-oxidants because heat treatment may accelerate their oxidation and other degenerative reactions. Thus, these results are in agreement with the findings of Sultana et al.[39]
There are different extraction processes prepared with different solvents such as water, methanol, ethanol or acetone.[40] In this study, methanol and water solvents were chosen for extraction due to their good solubility and availability. The anti-oxidant activity of the extracts including these polar solvents was compared. There was a significant positive correlation between DPPH radical scavenging activity and phenolic content, showing that the presence of phenolic compounds in the extracts contribute significantly to their anti-oxidant potential. These results are also in agreement with previous reports about Achillea species which found that DPPH scavenging activity is related to phenolic content.[38,41,42]
Achillea species have been widely used as a folk medicine for treatment of different cancers and warts.[43] Previous reports have demonstrated that several Achillea species and their isolated compounds exhibit cytotoxic activity on numerous human cell lines, including nonsmall-cell lung cancer (A549, RERF-LC-kj and QG-90), cervix epithelial carcinoma (HeLa), myelogenous leukemia (K562), breast adenocarcinoma (MCF-7, MDA-MB-231, MDA-MB-468) glioblastoma multiform (T98G), squamous carcinoma (A431), prostatic adenocarcinoma (PC-3), Caucasian gastric adenocarcinoma (AGS), colorectal adenocarcinoma (SW742), lung carcinoma (SKLC6), melanoma (A375, Fem-X), and liver hepatoma (PLC/PRF/5) cell lines.[9,16,17,35,44,45,46,47] It was reported that A. teretifolia species exhibited antineoplastic potential and promising antitumoral activity against MDA-MB-231 and MDA-MB-468 cell lines.[9] In the present study, prostate cancer cells were tested to detect the cytotoxic and apoptotic effects of the extracts of A. teretifolia.
The cytotoxicity results indicated that the methanol extract had a moderate cytotoxic effect on prostate cancer cells like in the literature.[46] The methanol extract possessing higher phenolic content than the water extract also showed more effective cytotoxic activity on cancer cells in the same period. There are some reports which demonstrate the relationships between cytotoxicity and anti-oxidant activity.[48,49,50,51] The results of the present study support these previous results.
Bcl-2 (anti-apoptotic) and Bax (pro-apoptotic) are members of the Bcl-2 family of genes and both control the activation of caspases.[52] Caspases, a subclass of cysteine proteases, are a family of proteins that are central effectors of apoptosis and their activation is also a hallmark of apoptosis. As a member of the caspases family, caspase- 3 is known as the crucial executioner caspase.[53,54] In the present study, the mRNA expression levels of bax and caspase-3 were significantly up-regulated (P < 0.05) after exposure of DU145 and PC-3 cells to the methanol and water extracts, whereas the expression of bcl-2 was down-regulated (P < 0.05).
In mammalian cells, overexpression of Bax leads to caspase activation and apoptosis.[55] As demonstrated in the literature,[56] overexpression of Bax also results in apoptosis as evidenced by caspase activation in prostate cancer cells. In the present study, mRNA expression of bax and caspase-3 genes in prostate cancer cells treated with the extracts was significantly (P < 0.05) increased. In contrast, there was no bax and caspase-3 gene activation in HGF cells treated with the extracts compared to a control.
Therefore, the apoptotic induction did not occur in normal cells after exposure to the extracts. This is a good result showing that the extracts do not contain compounds inducing or suppressing apoptosis in HGF cells.
Bcl-2 is expressed at high levels in advanced prostate cancer which can prevent more upstream molecules from inducing apoptosis.[56] In the present study, the expression of bcl-2 in prostate cancer cells, especially DU145 cells treated with the methanol extract, was down-regulated by Achillea extract treatment, whereas in HGF cells, its expression was not significantly suppressed by this treatment. These results indicate that the extracts, especially the methanol extract with high phenolic content, may include effective anticancer compounds inducing cytotoxicity and apoptosis in only cancer cells. Therefore, these compounds may suppress the proliferation of prostate cancer cells by causing the down-regulation of bcl-2 expression, and up-regulation of caspase-3 and bax expression.
CONCLUSION
This is the first report demonstrating the potential anti-oxidant, cytotoxic and pro-apoptotic effects of A. teretifolia extracts on DU145 and PC-3 cancer cells. Although further investigations about the extracts in animal models with prostate cancer are needed for additional understanding of in vivo activity, our in vitro results show that the extracts, especially methanol extract, could be anti-oxidant sources and might include anti-cancer agents for the treatment of prostate cancer.
ACKNOWLEDGMENTS
We would like to thank Owen M. Britton, Northern Virginia Community College, USA, for his assistance in editing the article. This work was funded by the Scientific Research Project Fund of Gazi University (Grant number: 05/2012-66).
Footnotes
Source of Support: This work was funded by the Scientific Research Project Fund of Gazi University (Grant number: 05/2012-66)
Conflict of Interest: Authors declare that there is no conflict of interest regarding the content of this article.
REFERENCES
- 1.Saeidnia S, Gohari A, Mokhber-Dezfuli N, Kiuchi F. A review on phytochemistry and medicinal properties of the genus Achillea. Daru. 2011;19:173–86. [PMC free article] [PubMed] [Google Scholar]
- 2.Akkol EK, Koca U, Pesin I, Yilmazer D. Evaluation of the wound healing potential of Achillea biebersteinii Afan. (Asteraceae) by in vivo excision and incision models. Evid Based Complement Alternat Med 2011. 2011:474026. doi: 10.1093/ecam/nep039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warwick SI, Black L. The biology of Canadian weeds: 52. Achillea millefolium L. S.L. Can J Plant Sci. 1982;62:163–82. [Google Scholar]
- 4.Ardestani A, Yazdanparast R. Anti-oxidant and free radical scavenging potential of Achillea santolina extracts. Food Chem. 2007;104:21–9. [Google Scholar]
- 5.Karaalp C, Yurtman AN, Yavasoglu NU. Evaluation of anti-microbial properties of Achillea L.flower head extracts. Pharm Biol. 2009;47:86–91. [Google Scholar]
- 6.Ali N, Shah SW, Shah I, Ahmed G, Ghias M, Khan I. Cytotoxic and anthelmintic potential of crude saponins isolated from Achillea wilhelmsii C. Koch and Teucrium stocksianum Boiss. BMC Complement Altern Med. 2011;11:106. doi: 10.1186/1472-6882-11-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conforti F, Loizzo MR, Statti GA, Menichini F. Comparative radical scavenging and antidiabetic activities of methanolic extract and fractions from Achillea ligustica ALL. Biol Pharm Bull. 2005;28:1791–4. doi: 10.1248/bpb.28.1791. [DOI] [PubMed] [Google Scholar]
- 8.Küpeli E, Orhan İ, Küsmenoğlu Ş, Yeşilada E. Evaluation of anti-inflammatory and anti-nociceptive activity of five anatolian Achillea species. Turk J Pharm Sci. 2007;4:89–99. [Google Scholar]
- 9.Turan M, Sökmen A, Karadayı K, Polat ZA, Şen M. Anti-neoplastic properties of some plant extracts which are endemic in Sivas. Cumhuriyet Med J. 2010;32:9–18. [Google Scholar]
- 10.Asgary S, Naderi GH, Sarrafzadegan N, Mohammadifard N, Mostafavi S, Vakili R. Antihypertensive and antihyperlipidemic effects of Achillea wilhelmsii. Drugs Exp Clin Res. 2000;26:89–93. [PubMed] [Google Scholar]
- 11.Souri E, Amin G, Dehmobed-Sharifabadi A, Nazifi A, Farsam H. Anti-oxidative activity of sixty plants from Iran. Iran J Pharm Res. 2004;3:55–9. [Google Scholar]
- 12.Nickavar B, Kamalinejad M, Haj-Yahya M, Shafaghi B. Comparison of the free radical scavenging activity of six Iranian Achillea species. Pharm Biol. 2006;44:208–12. [Google Scholar]
- 13.Wojdyło A, Oszmianski J, Czemerys R. Anti-oxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007;105:940–9. [Google Scholar]
- 14.Bozin B, Mimica-Dukic N, Bogavac M, Suvajdzic L, Simin N, Samojlik I, et al. Chemical composition, antioxidant and antibacterial properties of Achillea collina Becker ex Heimerl s.l. and A. pannonica Scheele essential oils. Molecules. 2008;13:2058–68. doi: 10.3390/molecules13092058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tarawneh KA, Irshaid F, Jaran AS, Ezealarab M, Khleifat KM. Evaluation of anti-bacterial and anti-oxidant activities of methanolic extracts of some medicinal plants in northern part of Jordan. J Biol Sci. 2010;10:325–32. [Google Scholar]
- 16.Li Y, Zhang ML, Cong B, Wang SM, Dong M, Sauriol F, et al. Achillinin A, a cytotoxic guaianolide from the flower of Yarrow, Achillea millefolium. Biosci Biotechnol Biochem. 2011;75:1554–6. doi: 10.1271/bbb.110234. [DOI] [PubMed] [Google Scholar]
- 17.Trifunovic S, Vajs V, Juranic Z, Zizak Z, Tesevic V, Macura S, et al. Cytotoxic constituents of Achillea clavennae from Montenegro. Phytochemistry. 2006;67:887–93. doi: 10.1016/j.phytochem.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 18.Saeidnia S, Moradi-Afrapoli F, Gohari AR, Malmir M. Cytotoxic flavonoid from Achillea talagonica Bioss. J Med Plants Res. 2009;8:52–6. [Google Scholar]
- 19.Sathiyamoorthy P, Lugasi-Evgi H, Schlesinger P, Kedar I, Gopas J, Pollack Y, et al. Screening for cytotoxic and anti-malarial activities in desert plants of the Negev and Bedouin market plant products. Pharm Biol. 1999;37:188–95. [Google Scholar]
- 20.Güner A, Akyıldırım B, Alkayış MF, Çıngay B, Kanoğlu SS, Özkan AM. Türkçe bitki adları. In: Güner A, Aslan S, Ekim T, Vural M, Babaç MT, et al., editors. Türkiye Bitkileri Listesi (Damarlı Bitkiler) İstanbul: Nezahat Gökyiğit Botanik Bahçesi ve Flora Araştırmaları Derneği Yayını; 2012. [Google Scholar]
- 21.Huber-Morath A. In: Achillea L. Flora of Turkey and the East Aegean Islands. Davis PH, editor. Edinburgh: Edinburgh University Press; 1975. pp. 224–52. [Google Scholar]
- 22.Duman H. In: Achillea L. Flora of Turkey and the East Aegean Islands. Güner A, özhatay N, Ekim T, Başer KH, editors. Edinburgh: Edinburgh University Press; 2000. pp. 158–9. [Google Scholar]
- 23.Çelik N, Akpulat HA. Achillea sivasica (Asteraceae: Sect. Babounya (DC.) O. Hoffm.), a new species from Inner Anatolia Turkey. Kew Bull. 2008;63:485–9. [Google Scholar]
- 24.Arabacı T, Budak U. Achillea hamzaoglui (Asteraceae), a new species from Turkey. Ann Bot Fennici. 2009;46:459–63. [Google Scholar]
- 25.Blois MS. Anti-oxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–200. [Google Scholar]
- 26.Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–58. [Google Scholar]
- 27.Ferrari M, Fornasiero MC, Isetta AM. MTT colorimetric assay for testing macrophage cytotoxic activity in vitro. J Immunol Methods. 1990;131:165–72. doi: 10.1016/0022-1759(90)90187-z. [DOI] [PubMed] [Google Scholar]
- 28.Ural AU, Avcu F, Candir M, Guden M, Ozcan MA. In vitro synergistic cytoreductive effects of zoledronic acid and radiation on breast cancer cells. Breast Cancer Res. 2006;8:R52. doi: 10.1186/bcr1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roche Real Time Ready Configurator. Turkey: Real Time Ready qPCR Assay Design and Configuration Portal Content. [Last cited on 2012 Jul 25]. Available from: http://www.roche.appliedscience.com/sis/realtimeready/index.jsp .
- 30.Robards K, Prenzler PD, Tucker G, Swatsitang P, Glover W. Phenolic compounds and their role in oxidative processes in fruits. Food Chem. 1999;66:401–36. [Google Scholar]
- 31.Rice-Evans CA, Miller NJ, Paganga G. Anti-oxidant properties of phenolic compounds. Trends Plant Sci. 1997;2:152–9. [Google Scholar]
- 32.Paganga G, Miller N, Rice-Evans CA. The polyphenolic content of fruit and vegetables and their antioxidant activities. What does a serving constitute? Free Radic Res. 1999;30:153–62. doi: 10.1080/10715769900300161. [DOI] [PubMed] [Google Scholar]
- 33.Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci. 2008;4:89–96. [PMC free article] [PubMed] [Google Scholar]
- 34.Ravikumar YS, Mahadevan KM, Kumaraswamy MN, Vaidya VP, Manjunatha H, Kumar V, et al. Antioxidant, cytotoxic and genotoxic evaluation of alcoholic extract of Polyalthia cerasoides (Roxb.) bedd. Environ Toxicol Pharmacol. 2008;26:142–6. doi: 10.1016/j.etap.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Kundakovic T, Stanojkovic T, Juranic Z, Kovacevic N. Cytotoxic and antioxidant activity of Achillea alexandri-regis. Pharmazie. 2005;60:319–20. [PubMed] [Google Scholar]
- 36.Turkoglu I, Turkoglu S, Celik S, Kahyaoglu M. Anti-oxidant and anti-microbial activities of Turkish endemic Achillea species. Afr J Microbiol Res. 2010;4:2034–42. [Google Scholar]
- 37.Barıs O, Gulluce M, Sahin F, Ozer H, Kılıc H, Ozkan H, et al. Biological activities of the essential oil and methanol extract of Achillea biebersteinii Afan. (Asteraceae) Turk J Biol. 2006;30:65–73. [Google Scholar]
- 38.Konyalioglu S, Karamenderes C. Screening of total flavonoid, phenol contents and anti-oxidant capacities of some Achilea L. species growing in Turkey. Acta Pharm Turc. 2004;46:163–70. [Google Scholar]
- 39.Sultana B, Anwar F, Ashraf M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules. 2009;14:2167–80. doi: 10.3390/molecules14062167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gong Y, Liu X, He WH, Xu HG, Yuan F, Gao YX. Investigation into the antioxidant activity and chemical composition of alcoholic extracts from defatted marigold (Tagetes erecta L.) residue. Fitoterapia. 2012;83:481–9. doi: 10.1016/j.fitote.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 41.Giorgi A, Bombelli R, Luini A, Speranza G, Cosentino M, Lecchini S, et al. Antioxidant and cytoprotective properties of infusions from leaves and inflorescences of Achillea collina Becker ex Rchb. Phytother Res. 2009;23:540–5. doi: 10.1002/ptr.2679. [DOI] [PubMed] [Google Scholar]
- 42.Asgarirad H, Pourmorad F, Hosseinimehr SJ, Saeidnia S, Ebrahimzadeh MA, Lotfi F. In vitro anti-oxidant analysis of Achillea tenuifolia. Afr J Biotechnol. 2010;9:3536–41. [Google Scholar]
- 43.Hartwell J. Plants used against cancer. J Nat Prod. 1968;31:71–170. [Google Scholar]
- 44.Abu-Dahab R, Afifi F. Anti-proliferative activity of selected medicinal plants of Jordan against a breast adenocarcinoma cell line (MCF-7) Sci Pharm. 2007;75:121–36. [Google Scholar]
- 45.Csupor-Löffler B, Hajdú Z, Zupkó I, Réthy B, Falkay G, Forgo P, et al. Antiproliferative effect of flavonoids and sesquiterpenoids from Achillea millefolium s.l. on cultured human tumour cell lines. Phytother Res. 2009;23:672–6. doi: 10.1002/ptr.2697. [DOI] [PubMed] [Google Scholar]
- 46.Maggi F, Bramucci M, Cecchini C, Coman MM, Cresci A, Cristalli G, et al. Composition and biological activity of essential oil of Achillea ligustica all. (Asteraceae) naturalized in central Italy: Ideal candidate for anti-cariogenic formulations. Fitoterapia. 2009;80:313–9. doi: 10.1016/j.fitote.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 47.Ghavami G, Sardari S, Shokrgozar MA. Anti-cancerous potentials of Achillea species against selected cell lines. J Med Plants Res. 2010;4:2411–7. [Google Scholar]
- 48.Cai Y, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74:2157–84. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Allahghadri T, Rasooli I, Owlia P, Nadooshan MJ, Ghazanfari T, Taghizadeh M, et al. Antimicrobial property, antioxidant capacity, and cytotoxicity of essential oil from cumin produced in Iran. J Food Sci. 2010;75:H54–61. doi: 10.1111/j.1750-3841.2009.01467.x. [DOI] [PubMed] [Google Scholar]
- 50.Hou J, Sun T, Hu J, Chen S, Cai X, Zou G. Chemical composition, cytotoxic and anti-oxidant activity of the leaf essential oil of Photinia serrulata. Food Chem. 2007;103:355–8. [Google Scholar]
- 51.Sulaiman GM, Hussien NN, Marzoog TR, Awad HA. Phenolic content, anti-oxidant, anti-microbial and cytotoxic activities of ethanolic extract of Salix alba. Am J Biochem Biotechnol. 2013;9:41–6. [Google Scholar]
- 52.Vandaele L, Goossens K, Peelman L, Van Soom A. mRNA expression of Bcl-2, Bax, caspase-3 and -7 cannot be used as a marker for apoptosis in bovine blastocysts. Anim Reprod Sci. 2008;106:168–73. doi: 10.1016/j.anireprosci.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 53.Crow MT, Mani K, Nam YJ, Kitsis RN. The mitochondrial death pathway and cardiac myocyte apoptosis. Circ Res. 2004;95:957–70. doi: 10.1161/01.RES.0000148632.35500.d9. [DOI] [PubMed] [Google Scholar]
- 54.Jin Z, El-Deiry WS. Overview of cell death signaling pathways. Cancer Biol Ther. 2005;4:139–63. doi: 10.4161/cbt.4.2.1508. [DOI] [PubMed] [Google Scholar]
- 55.Reed JC, Jurgensmeier JM, Matsuyama S. Bcl-2 family proteins and mitochondria. Biochim Biophys Acta. 1998;1366:127–37. doi: 10.1016/s0005-2728(98)00108-x. [DOI] [PubMed] [Google Scholar]
- 56.Lowe SL, Rubinchik S, Honda T, McDonnell TJ, Dong JY, Norris JS. Prostate-specific expression of Bax delivered by an adenoviral vector induces apoptosis in LNCaP prostate cancer cells. Gene Ther. 2001;8:1363–71. doi: 10.1038/sj.gt.3301531. [DOI] [PubMed] [Google Scholar]


