Abstract
Ferric pyrophosphate citrate (FPC) is a water-soluble iron salt administered via dialysate to supply iron directly to transferrin. The PRIME study tested whether treatment with FPC could reduce prescribed erythropoiesis-stimulating agent (ESA) use and maintain hemoglobin in hemodialysis patients. This 9-month, randomized, placebo-controlled, double-blind, multicenter clinical study included 103 patients undergoing hemodialysis 3–4 times weekly. The FPC group received dialysate containing 2 μmol/l of iron. The placebo group received standard dialysate. A blinded central anemia management group facilitated ESA dose adjustments. Intravenous iron was administered according to the approved indication when ferritin levels fell below 200 μg/l. The primary end point was the percentage change from baseline in prescribed ESA dose at end of treatment. Secondary end points included intravenous iron use and safety. At the end of treatment, there was a significant 35% reduction in prescribed ESA dose in FPC-treated patients compared with placebo. The FPC patients used 51% less intravenous iron than placebo. Adverse and serious adverse events were similar in both groups. Thus, FPC delivered via dialysate significantly reduces the prescribed ESA dose and the amount of intravenous iron needed to maintain hemoglobin in chronic hemodialysis patients.
Keywords: anemia, erythropoiesis-stimulating agent, ferric pyrophosphate citrate, hemodialysis, iron
Anemia is an inevitable complication in patients with chronic kidney disease who receive maintenance hemodialysis (CKD 5HD). This is primarily because of loss of renal erythropoietin production, chronic inflammation, and increased blood losses related to uremia and hemodialysis. The result is an iron loss of ∼5–7 mg per dialysis session.1, 2 Although erythropoietin deficiency and inflammatory suppression of erythropoiesis can be partly counteracted by erythropoiesis-stimulating agents (ESAs), increased erythroid iron requirements because of ESAs, together with ongoing blood losses, exceed the amount of iron that can be provided from adequate marrow iron stores.3 Furthermore, chronic inflammation suppresses the iron supply that is available from stores by stimulating hepatic production of hepcidin.3, 4 Hepcidin prevents efflux of iron from stores into plasma.5 Iron is then retained within reticuloendothelial (RE) macrophages in bone marrow, liver, and spleen. Plasma transferrin saturation (TSAT) falls, making iron inaccessible for red blood cell production.6 Intravenous (i.v.) iron is commonly administered in hemodialysis patients to replace dialysis-related blood losses and to overcome inflammatory sequestration of iron.
The i.v. iron products comprise ferric hydroxide cores within a carbohydrate shell and require uptake and processing by RE macrophages. With concurrent inflammation, a considerable proportion of iron derived from these iron carbohydrate complexes is sequestered within macrophages and is not readily available for transport to erythroid marrow for use in hemoglobin (Hgb) synthesis.7 Although approved and indicated for treatment of iron deficiency anemia, i.v. iron products are commonly used as a ‘maintenance' therapy to replace ongoing iron losses in hemodialysis patients. As a result, serum ferritin levels have increased from ∼400 to 800 μg/l over the past decade in the United States,8 leading to concerns about iron overload and consequent inflammation, oxidative stress, endothelial dysfunction, cardiovascular disease, immune dysfunction, and the risk of bacterial infections.9
Ferric pyrophosphate citrate (FPC) was first used to deliver iron via dialysate in 1999 and allowed maintenance of Hgb and iron balance while reducing the need for i.v. iron by ~80%.10 Rockwell Medical licensed FPC in 2002 and conducted pharmacology–toxicology studies and a phase 1–3 clinical program. FPC (Triferic) was approved by the US Food and Drug Administration (FDA) in January 2015 as the first iron product indicated to maintain Hgb in adult CKD 5HD patients.
FPC is added to liquid bicarbonate concentrate at the clinic. The bicarbonate concentrate with FPC subsequently mixes with acid concentrate and water in the hemodialysis machine to generate dialysate. FPC crosses the dialyzer membrane, enters the blood, donates its iron directly to transferrin, and is rapidly cleared from circulation. This provides for optimal iron utilization for erythropoiesis and avoids iron sequestration within RE macrophages.10, 11
The PRIME (Physiological Replenishment Iron Maintenance Equivalency) study tested the hypothesis that administration of FPC via dialysate during hemodialysis sessions would reduce prescribed ESA use and maintain Hgb in the recommended range in CKD 5HD patients.
RESULTS
Study population
A total of 108 patients were randomized; 52 received FPC, 51 received placebo, and 5 discontinued before receiving study drug. Baseline demographic and clinical characteristics, including amount of prescribed ESA per week at baseline and amount of i.v. iron administered ⩽6 weeks before study start, were similar between groups (Table 1). Most patients were male (61.2%) and white (61.2%); mean age was 59 years (range, 25–93 years).
Table 1. Baseline demographics and renal history.
| Characteristic | FPC, n=54a | Placebo, n=49a |
|---|---|---|
| Age (years), mean (s.d.) | 59.4 (12.4) | 58.5 (13.9) |
| Male sex | 31 (57.4%) | 32 (65.3%) |
| Race | ||
| White | 31 (57.4%) | 32 (65.3%) |
| Other | 23 (42.6%) | 17 (34.7%) |
| Posthemodialysis weight (kg), mean (s.d.) | 85.2 (17.4) | 83.5 (17.6) |
| Time since first hemodialysis (mo), mean (s.d.) | 47.6 (49.2) | 44.9 (63.7) |
| Prescribed ESA, mean (s.d.) | ||
| Epoetin (units/week) | 9625 (5644) | 9168 (5285) |
| Darbepoetin (μg/week) | — | 18.8 (8.8) |
| Intravenous iron in previous 6 weeks (mg), mean (s.d.) | 102.1 (128.6) | 96.4 (111.9) |
| Serum iron (μg/dl), mean (s.d.) | 66.6 (16.8) | 73.2 (23.1) |
| Serum transferrin (g/l), mean (s.d.) | 1.8 (0.3) | 1.8 (0.3) |
| Serum TSAT (%), mean (s.d.) | 26.7 (7.1) | 28.5 (7.6) |
| Serum ferritin (μg/l), mean (s.d.) | 625.8 (192.7) | 599.5 (226.5) |
| TIBC (μg/dl), mean (s.d.) | 226.9 (30.6) | 231.2 (38.4) |
| UIBC (μg/dl), mean (s.d.) | 160.4 (31.5) | 158.0 (31.5) |
| Relevant medical history | ||
| Diabetes | 34 (63.0%) | 36 (73.5%) |
| Cardiovascular disease | ||
| Congestive heart failure | 20 (37.0%) | 23 (46.9%) |
| Coronary artery disease | 14 (25.9%) | 26 (53.1%) |
| Myocardial infarction | 6 (11.1%) | 7 (14.3%) |
| Angina pectoris | 4 (7.4%) | 4 (8.2%) |
| Atrial fibrillation | 3 (5.6%) | 4 (8.2%) |
| Bradycardia | 3 (5.6%) | 3 (6.1%) |
Abbreviations: ESA, erythropoiesis-stimulating agent; FPC, ferric pyrophosphate citrate; TIBC, total iron binding capacity; TSAT, transferrin saturation; UIBC, unsaturated iron binding capacity.
Two patients who were randomized to the placebo group and who incorrectly received a dose of FPC in error were included in the FPC group in the safety analyses (safety population, FPC, n=54; placebo, n=49) but were analyzed in the placebo group in the efficacy analyses (modified intent-to-treat population, FPC, n=52; placebo, n=51).
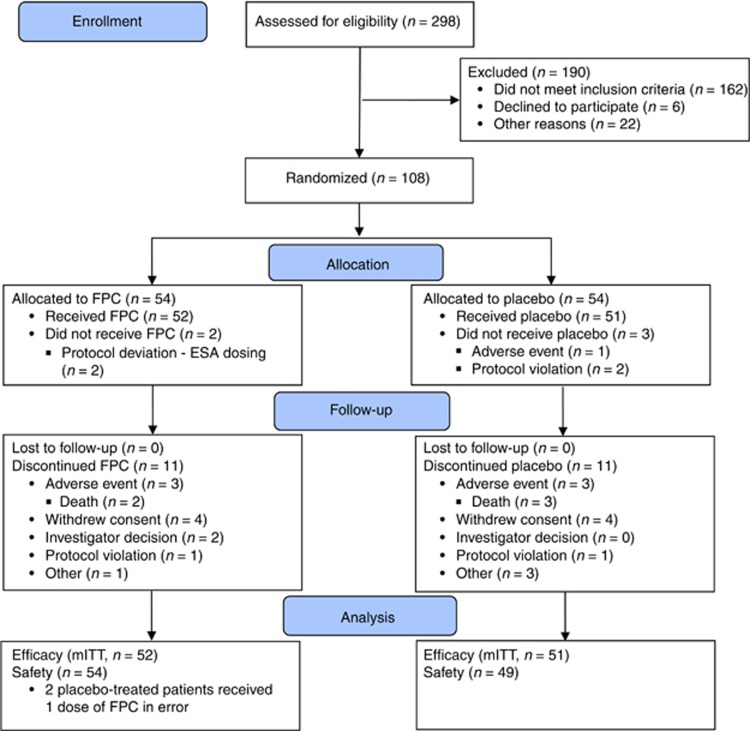
In all, 75% of randomized patients completed the study; 11 patients in each group discontinued prematurely. Reasons for discontinuation were similar between groups (Figure 1).
Figure 1.
Patient disposition—CONSORT diagram. ESA, erythropoiesis-stimulating agent; FPC, ferric pyrophosphate citrate; mITT, modified intent-to-treat.
Two patients randomized to placebo received one dose of FPC in error. These patients were included in the placebo group in the efficacy analyses (modified intent-to-treat population) and in the FPC group in the safety analyses (Figure 1). All patients, including those who prematurely discontinued from the study, were included in the efficacy and safety analyses.
Efficacy outcomes
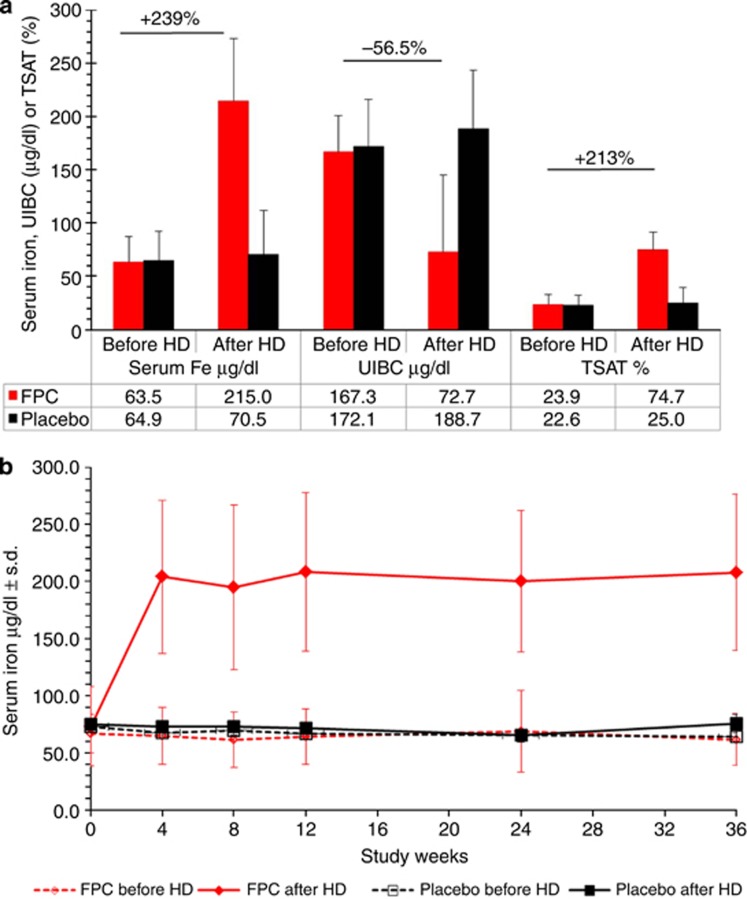
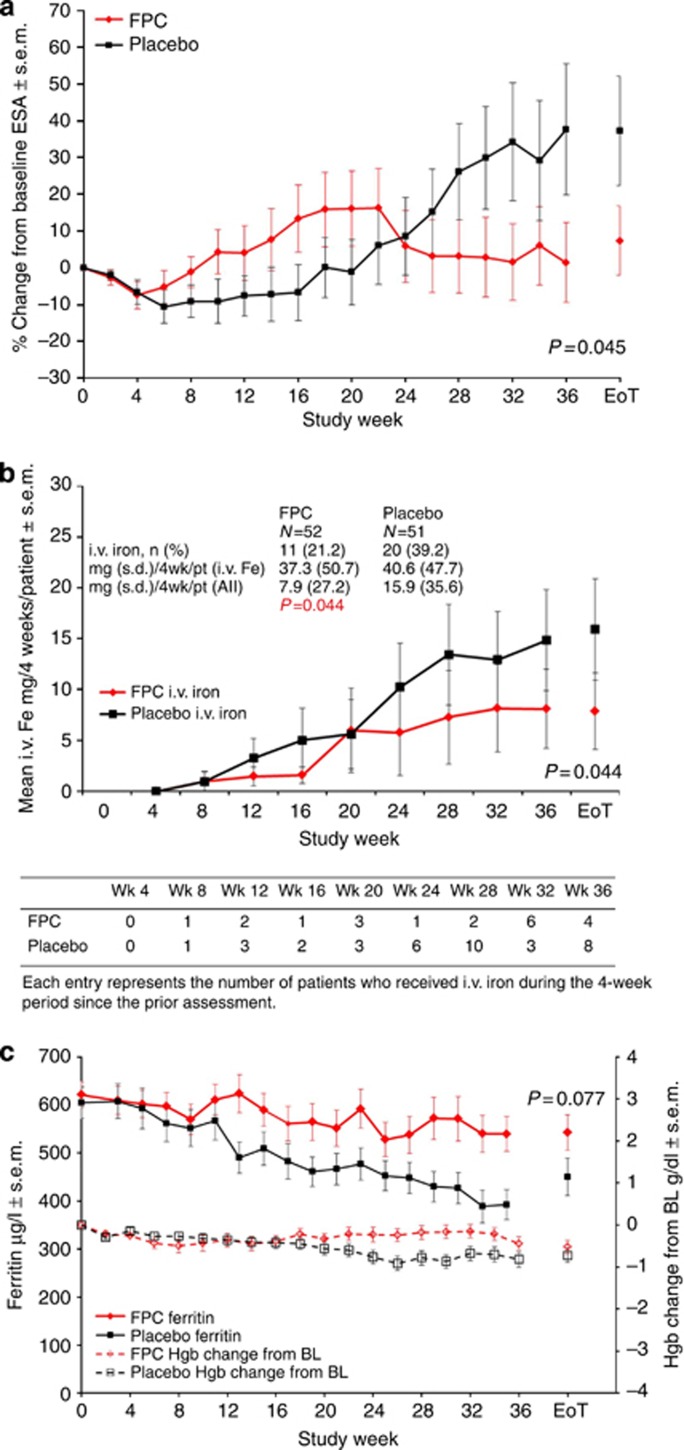
FPC delivered via dialysate reliably delivered iron to patients while significantly reducing prescribed ESA by 35% relative to placebo. Mean serum iron level during a single dialysis treatment increased in the FPC group from 63.5 μg/dl before dialysis to 215.0 μg/dl after dialysis (95% confidence interval (CI), 197.1–233.5) but did not change significantly in the placebo group (Figure 2a). The Hgb concentration was maintained at baseline levels in both groups. At the end of treatment (EoT), the ESA dose was not significantly changed from baseline in the FPC group (+4.9% 95% CI, −19.1 to 28.8) but had increased 39.8% (95% CI, 15.7–64.0) from baseline in the placebo group (Figure 3a). The least squares mean difference (FPC–placebo) in the prescribed ESA dose was 35% (95% CI, −69.1 to −0.8; P=0.045). A prespecified analysis of the ∼20% ESA-hyporesponsive patients in each group (baseline ESA >13,000 units/week) showed a 74.4% reduction in the prescribed ESA dose in the FPC group (not statistically significant because of small sample size).
Figure 2.
Serum iron parameters and measurements. Bars indicate s.d. (a) Serum iron parameters (total Fe, UIBC, and TSAT) before and after dialysis at the end of treatment. (b) Serum iron concentration before and after dialysis over the course of the study. Fe, iron; FPC, ferric pyrophosphate citrate; HD, hemodialysis; TSAT, transferrin saturation; UIBC, unsaturated iron binding capacity.
Figure 3.
Erythropoiesis-stimulating agent use, intravenous iron use, serum ferritin, and hemoglobin over time. Bars indicate s.e.m. (a) Prescribed ESA percentage change from baseline. (b) Number of patients receiving i.v. iron and i.v. iron administration over time. (c) Serum ferritin concentrations and change from baseline in hemoglobin concentration over time. BL, baseline; EoT, end of treatment; ESA, erythropoiesis-stimulating agent; Fe, iron; FPC, ferric pyrophosphate citrate; Hgb, hemoglobin; i.v., intravenous; pt, patient; wk, week.
In each group, 11 patients did not complete the 9-month study. Analysis of percentage change from baseline in prescribed ESA showed that the effect of FPC in these patients was similar to that in the entire study population (mean (s.d.): FPC, +27.5% (56.3%); placebo, +60.0% (98.7%)).
Fewer FPC-treated patients (11/52, 21.2%) than placebo-treated patients (20/51, 39.2%) required i.v. iron supplementation. Overall, the FPC group required 51% less i.v. iron supplementation (mean (s.d.), 7.9 (27.2) mg/4 weeks vs. 15.9 (35.6) mg/4 weeks; P=0.044; Figure 3b). Patients who required supplemental iron received similar amounts (mean, 37.3 mg/4 weeks for FPC vs. 40.6 mg/4 weeks for placebo).
Mean TSAT before and after dialysis increased from 23.9% (95% CI, 20.9–26.9%) to 74.7% (95% CI, 69.1–80.3%) at EoT in the FPC group but did not change significantly in the placebo group (22.6–25.0% Figure 2a). Mean serum iron levels increased from before dialysis to after dialysis in the FPC group. The mean incremental change in serum iron from before to after dialysis during the study was 102.3 μg/dl (95% CI, 93.8–110.8) and 2.9 μg/dl (95% CI, 0.4–5.4) in the FPC and placebo groups, respectively.
Serum iron results were consistent with previous studies showing that iron is rapidly cleared in FPC-treated patients and returns to baseline levels by the next dialysis session.10 Predialysis serum iron levels were not significantly different from those of placebo during the study (Figure 2b). Postdialysis serum iron values were similar at each observation over the study in the FPC group.
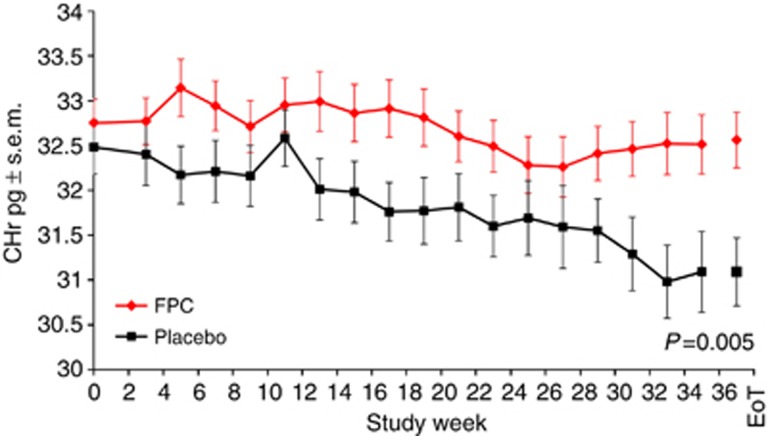
Reticulocyte hemoglobin levels were relatively maintained from baseline through EoT in the FPC group but showed a steady decline during the study in the placebo group (Figure 4).
Figure 4.
Change from baseline reticulocyte hemoglobin over time. Bars indicate s.e.m. CHr, reticulocyte hemoglobin; EoT, end of treatment; FPC, ferric pyrophosphate citrate.
At EoT, mean predialysis serum ferritin level was not significantly changed from baseline in the FPC group (−61.9 μg/l; 95% CI, −128.7 to 4.9) but had declined in the placebo group (−156.0 μg/l; 95% CI, −233.2 to −78.8; Figure 3c). The difference between groups (94.0 μg/l; 95% CI, −10.6 to 198.6) was not statistically significant at EoT (P=0.077).
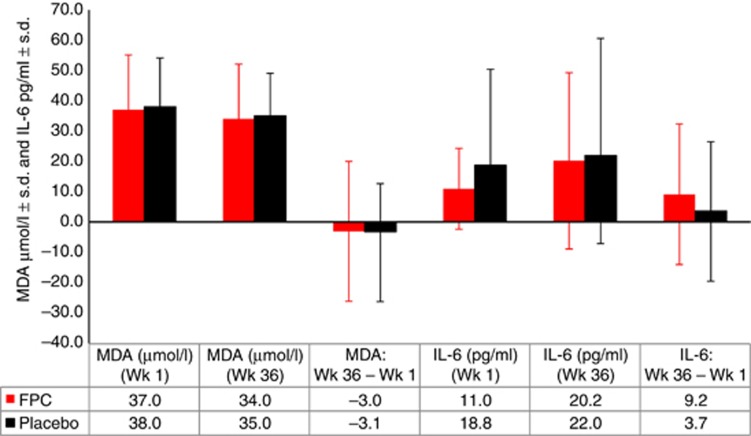
Measurement of markers of inflammation and oxidative stress over the study revealed no statistically significant differences between groups in predialysis interleukin-6 or malondialdehyde (Figure 5). Mean C-reactive protein levels were similar between groups at baseline (FPC, 92.0 nmol/l; placebo, 91.4 nmol/l). At EoT, mean C-reactive protein levels were 90.3 nmol/l and 128.2 nmol/l in the FPC and placebo groups, respectively; the difference between groups was not statistically significant (−37.9 nmol/l; 95% CI, −101.3 to 25.6).
Figure 5.
Biomarkers of inflammation and oxidative stress. Biomarkers of inflammation (interleukin-6) and oxidative stress (malondialdehyde) measured before dialysis at baseline and at week 36, and the difference between measurements. Bars indicate s.d. P is not significant for all comparisons. FPC, ferric pyrophosphate citrate; IL-6, interleukin-6; MDA, malondialdehyde; Wk, week.
Adverse events
Treatment-related treatment-emergent adverse events (TEAEs) were reported in 7.4% (4/54) and 6.1% (3/49) of the FPC-treated and placebo-treated patients, respectively. Serious TEAEs (FPC, 33.3% placebo, 40.8%) and TEAEs resulting in study discontinuation (FPC, 7.4% placebo, 2.0%) were assessed by investigators as unrelated to the study drug. Deaths (FPC, 2; placebo, 3) occurred only during the interdialytic interval and were not considered related to study drug.
Cardiac events were reported in similar percentages of patients (FPC, 25.9% placebo, 26.5% Table 2). Hemodialysis-induced symptoms (22.2% vs. 12.2%) and cough (22.2% vs. 6.1%) were reported more frequently in the FPC group (Table 3), occurred at various times during the treatment period, and were not attributed to study drug. Bradycardia was reported more frequently in the FPC group (13.0% vs. 4.1%), was transient, generally occurred with intradialytic hypotension, and was not considered related to study drug.
Table 2. Frequency of treatment-emergent adverse events by system organ class.
|
FPC (n=54) |
Placebo (n=49) |
|||
|---|---|---|---|---|
| MedDRA system organ classa | Events, n | Patients, n (%) | Events, n | Patients, n (%) |
| Any TEAE | 559 | 50 (92.6) | 651 | 46 (93.9) |
| Blood and lymphatic system disorders | 6 | 4 (7.4) | 2 | 2 (4.1) |
| Cardiac disorders | 28 | 14 (25.9) | 37 | 13 (26.5) |
| Ear and labyrinth disorders | 1 | 1 (1.9) | 2 | 2 (4.1) |
| Endocrine disorders | 2 | 2 (3.7) | 1 | 1 (2.0) |
| Eye disorders | 3 | 2 (3.7) | 7 | 4 (8.2) |
| Gastrointestinal disorders | 57 | 28 (51.9) | 75 | 27 (55.1) |
| General disorders and administration site conditions | 32 | 19 (35.2) | 24 | 18 (36.7) |
| Immune system disorders | 1 | 1 (1.9) | 4 | 3 (6.1) |
| Infections and infestations | 34 | 21 (38.9) | 31 | 24 (49.0) |
| Injury, poisoning, and procedural complications | 219 | 35 (64.8) | 267 | 33 (67.3) |
| Investigations | 9 | 7 (13.0) | 14 | 10 (20.4) |
| Metabolism and nutrition disorders | 26 | 17 (31.5) | 26 | 20 (40.8) |
| Musculoskeletal and connective tissue disorders | 33 | 14 (25.9) | 51 | 17 (34.7) |
| Neoplasms benign, malignant, and unspecified (including cysts and polyps) | 3 | 2 (3.7) | 1 | 1 (2.0) |
| Nervous system disorders | 36 | 14 (25.9) | 40 | 20 (40.8) |
| Psychiatric disorders | 9 | 6 (11.1) | 9 | 7 (14.3) |
| Renal and urinary disorders | 1 | 1 (1.9) | 2 | 2 (4.1) |
| Reproductive system and breast disorders | 0 | 0 (0.0) | 1 | 1 (2.0) |
| Respiratory, thoracic, and mediastinal disorders | 37 | 19 (35.2) | 32 | 15 (30.6) |
| Skin and subcutaneous tissue disorders | 8 | 6 (11.1) | 12 | 9 (18.4) |
| Vascular disorders | 14 | 13 (24.1) | 13 | 10 (20.4) |
Abbreviations: FPC, ferric pyrophosphate citrate; TEAE, treatment-emergent adverse event.
Verbatim terms were coded using the Medical Dictionary for Regulatory Activities (MedDRA), version 14.1. Patients who experienced more than one event within a System Organ Class are counted only once in that System Organ Class.
Table 3. Treatment-emergent adverse events reported in >10% of patients in either group.
|
FPC,
n=54 |
Placebo,
n=49 |
|||
|---|---|---|---|---|
| Preferred terma | Events, n | Patients, n (%) | Events, n | Patients, n (%) |
| Any event | 559 | 50 (92.6) | 651 | 46 (93.9) |
| Procedural hypotension | 145 | 18 (33.3) | 184 | 20 (40.8) |
| Hemodialysis-induced symptom | 19 | 12 (22.2) | 13 | 6 (12.2) |
| Cough | 15 | 12 (22.2) | 3 | 3 (6.1) |
| Diarrhea | 14 | 10 (18.5) | 17 | 8 (16.3) |
| Nausea | 12 | 8 (14.8) | 11 | 7 (14.3) |
| Fluid overload | 9 | 8 (14.8) | 6 | 6 (12.2) |
| Dyspnea | 9 | 8 (14.8) | 11 | 5 (10.2) |
| Headache | 16 | 7 (13.0) | 16 | 8 (16.3) |
| Asthenia | 11 | 7 (13.0) | 3 | 3 (6.1) |
| Bradycardia | 15 | 7 (13.0) | 3 | 2 (4.1) |
| Vomiting | 7 | 6 (11.1) | 10 | 8 (16.3) |
| Upper respiratory tract infection | 8 | 6 (11.1) | 4 | 4 (8.2) |
| Arteriovenous fistula site complication | 7 | 4 (7.4) | 17 | 10 (20.4) |
| Pain in extremity | 4 | 4 (7.4) | 10 | 9 (18.4) |
| Dizziness | 9 | 4 (7.4) | 6 | 5 (10.2) |
| Muscle spasms | 12 | 3 (5.6) | 15 | 5 (10.2) |
| Pyrexia | 4 | 3 (5.6) | 5 | 5 (10.2) |
| Arthralgia | 2 | 2 (3.7) | 7 | 6 (12.2) |
| Hypersensitivity/anaphylaxisb | 0 | — | 0 | — |
Abbreviation: FPC, ferric pyrophosphate citrate.
Verbatim terms were coded using the Medical Dictionary for Regulatory Activities (MedDRA), version 14.1. Patients who experienced the same event more than once are counted only once for that event.
No events related to study drug were reported.
The incidence of intradialytic hypotension was similar in the FPC and placebo groups, occurring during 4.7% and 5.5% of dialysis sessions, respectively (see Supplementary Table S1 online). The severity of intradialytic hypotension was also similar between the FPC and placebo groups: intradialytic hypotension episodes meeting adverse event reporting criteria and requiring intervention occurred in 2.0% and 2.2% of dialysis sessions, respectively (see Supplementary Tables S1, S2 and S3 online). Two patients in each group developed procedural hypotension that was considered possibly related to treatment. No hypersensitivity reactions to study drug were reported. Five patients (FPC, 2; placebo, 3) received blood transfusions.
DISCUSSION
FPC is a new FDA-approved iron replacement product that significantly reduces ESA requirements while maintaining Hgb in hemodialysis patients. The unique mode of action of FPC enables effective erythropoiesis by donating iron directly to transferrin, thereby bypassing the hepcidin block to iron efflux from the RE system that leads to iron sequestration and a functional iron deficiency in uremic patients.
The objective of this controlled study was to demonstrate that FPC can reduce the prescribed ESA requirement when ESA doses are administered in accordance with a protocol-specified ESA algorithm. FPC-treated patients required 35% less ESA than placebo-treated patients at EoT (Figure 3a) to maintain Hgb in the target range of 9.5–11.5 g/dl (Figure 3c) (P=0.045).
Iron was reliably delivered via dialysate in the FPC group as evidenced by the consistent increases in serum iron levels and TSAT from before to after dialysis. The decline in unsaturated iron binding capacity concomitant with the rise in serum iron and TSAT indicates that FPC iron is rapidly donated to transferrin (Figure 2a). After dialysis, FPC iron was cleared rapidly from the circulation as indicated by maintenance of predialysis serum iron at baseline levels (Figure 2b). This is consistent with previous studies showing an apparent half-life of 1.1–2.2 h and a return of serum iron to baseline levels by the next dialysis.10 The dialytic rise in serum iron increases the proportion of diferric transferrin and efficiency of iron delivery to the erythron because the diferric molecule has a higher affinity for the transferrin receptor and carries twice as much iron as the monoferric form.12 A dialysate FPC dose of 2 μmol/l delivers ∼5–7 mg of iron during the course of each dialysis treatment, thereby replacing the ongoing iron losses and maintaining iron sufficiency as reflected by maintenance of reticulocyte hemoglobin, a marker of iron delivery to the erythron (Figure 4), and ferritin levels (Figure 3c) relative to placebo. Serum ferritin levels did not increase in the FPC group (Figure 3c), indicating that FPC does not increase body iron stores despite being administered at each hemodialysis session. Markers of inflammation and oxidative stress showed no increase in the FPC group relative to placebo.
The PRIME study included patients who were initially iron replete (baseline ferritin, 200–1000 μg/l). Consequently, i.v. iron could be withheld until iron deficiency developed, as indicated by serum ferritin of 200 μg/l. Approximately twice as many placebo-treated patients as FPC-treated patients received i.v. iron (20 vs. 11), and placebo-treated patients received approximately twice as much i.v. iron during the study (total, 7304 vs. 3790 mg; Figure 3b).
Overall, the findings in the PRIME study indicate that FPC can effectively deliver iron and maintain Hgb levels without increasing iron stores while significantly reducing the need for ESAs (Figure 3a). The ESA requirement of the FPC group progressively decreased from 6 months forward relative to the placebo group (Figure 3a), suggesting that the 9-month study duration likely captured a partial effect of the potential of FPC to reduce ESA use. The ESA-sparing benefit of FPC is likely related to its direct donation of iron to transferrin with every hemodialysis treatment, allowing it to overcome the hepcidin-induced RE-block and functional iron deficiency that occur with uremic inflammation. The rapid and direct binding of FPC iron to transferrin is in contrast to i.v. iron where the iron-carbohydrate complexes undergo uptake by the RE system because of their particulate nature and are subject to sequestration in states of inflammation. The carbohydrate moiety of i.v. iron may also be responsible for the risk of hypersensitivity reactions. The higher ESA use in the placebo group is unlikely to be the result of iron depletion because the average EoT serum ferritin level was ∼450 μg/l in the placebo group, indicative of sufficient iron stores in the liver.13 Furthermore, studies have demonstrated that in hemodialysis patient populations with serum ferritin of 300–400 μg/l, more permissive use of i.v. iron that leads to further increase in serum ferritin may not always increase Hgb or reduce ESA use.14, 15 The ESA ‘sparing' effect of i.v. iron in hemodialysis patients may result from progressively reducing the fraction of subjects in the population with functional iron deficiency as the RE system is overloaded with iron.
The nature and frequency of TEAEs and serious TEAEs were similar in the FPC and placebo groups and consistent with those expected in hemodialysis patients. In particular, no hypersensitivity reactions to study drug or increase in the frequency or severity of infections, cardiac events, or intradialytic hypotension was observed in the FPC group. The increased incidence of cough and bradycardia in the FPC group was not attributed to FPC by the investigators and has not been observed in any other FPC trials. The mortality rate was similar in the FPC and placebo groups.
Regular administration of FPC did not worsen the underlying concomitant inflammatory state present in uremic patients as demonstrated by the lack of change in C-reactive protein, interleukin-6, and malondialdehyde levels, relative to placebo, over the study period. This may be related to pyrophosphate binding the iron(III) in FPC tightly in the circulation while promoting iron transfer rapidly and specifically to transferrin.11, 16 Furthermore, pyrophosphate is known to be an antioxidant.17
In conclusion, FPC delivered via the hemodialysate regularly with every hemodialysis treatment is a novel maintenance iron therapy that can maintain Hgb levels while reducing ESA use, without overloading iron stores in CKD 5HD patients.
MATERIALS AND METHODS
The PRIME study was conducted at 23 study locations in the United States from January 2011 to January 2013. The study was approved by an institutional review board at each site, and written informed consent was obtained from all patients or an authorized representative. The study was designed and conducted by the sponsor (Rockwell Medical, Wixom, MI) in collaboration with the principal investigators. All authors had full access to the data, reviewed and edited the manuscript, and assume responsibility for the integrity of the data.
Study population
Patients ⩾18 years old who had received chronic maintenance hemodialysis for ⩾4 months and were currently using an arteriovenous fistula or graft were enrolled. At screening, patients were required to be adequately dialyzed (Kt/V>1.2) and to have stable ESA doses, Hgb of 9.5–12.0 g/dl, serum ferritin of 200–1000 μg/l, and TSAT of 15–40%. Exclusion criteria included concomitant infection or active inflammatory disorder, use of >600 mg i.v. iron ⩽6 weeks before randomization, changes in ESA dose ⩽4 weeks before randomization, blood transfusion ⩽12 weeks before randomization, active bleeding, chronic active hepatitis, or scheduled surgery. Patients with chronic hepatitis who had hepatic transaminases <2 times the upper limit of normal were not excluded.
Study methods and interventions
This was a 9-month, prospective, randomized, double-blind, placebo-controlled, multicenter clinical trial (see ‘Randomization, Blinding, Sample Size, and Study Assessments' in Supplementary Appendix online). The study was exploratory and was not prospectively powered to demonstrate ESA sparing by FPC. A post hoc power calculation demonstrated the study had the ability to detect a 20% difference between groups with 80% power.
Patients were randomized in a 1:1 ratio to receive dialysate containing FPC (2 μmol/l (110 μg/l) iron) or standard dialysate (placebo) at every dialysis session. Randomization was stratified by baseline ESA dose. The blind was maintained by providing the study drug in containers with premixed FPC or placebo liquid bicarbonate concentrates.
The study comprised a 2-week screening period, a 36-week treatment period, and a 1-week follow-up period. Predialysis Hgb was measured weekly; serum iron, ferritin, and TSAT were measured every other week. Postdialysis serum iron, unsaturated iron binding capacity, transferrin, and calculated TSAT were assessed every 4 weeks through week 12 and then every 12 weeks.
Intravenous iron and changes in ESA dose, type, and administration route were prohibited during weeks 1 through 4, except for ESA dose reductions that were required to manage high Hgb levels. Beginning at week 5, i.v. iron could be administered and the ESA dose could be administered or adjusted to maintain Hgb from 9.5 to 11.5 g/dl. An independent, blinded, central anemia management center facilitated adherence to protocol-specified anemia management (see ‘Iron Management and Monitoring of Iron Status' and Tables S5 and S6 in Supplementary Appendix online). Study drug was withheld and replaced by the standard bicarbonate used in the dialysis center if patients had a systemic infection requiring administration of antibiotics (see ‘Criteria and Procedures for Study Drug Withholding' in Supplementary Appendix online). Study drug was resumed after discontinuation of antibiotics.
Safety monitoring included monthly analysis of laboratory parameters and close monitoring of patients during dialysis for symptoms and signs of potential iron toxicity (see Supplementary Table S4 online; protocol is available at the journal website).
Outcomes
The primary efficacy outcome was the percentage change from baseline to EoT in the ESA dose required to maintain Hgb in the target range, adjusted for baseline Hgb. All ESA doses were converted to standard epoetin units. The key secondary outcomes included the amount of supplementary i.v. iron. Exploratory analyses included predialysis measurement of markers of inflammation and oxidative stress at weeks 1 and 36.
Safety and tolerability were determined by the incidence and severity of TEAEs, and clinically significant changes in physical examinations, vital sign measurements, and laboratory tests. Iron parameters were assessed for comparison of predialysis with postdialysis serum iron and unsaturated iron binding capacity and baseline to EoT predialysis ferritin levels between groups.
Statistical analysis
Statistical comparisons were performed using two-sided tests at the α=0.05 significance level. Results were not adjusted for multiple comparisons.
The modified intent-to-treat population (randomized patients who received ⩾1 dose of study drug and had any postbaseline ESA dose information) was used for the efficacy analyses. Safety analyses were conducted using the safety analysis population (all randomized patients who received study drug).
Primary end point analysis was the percentage change in prescribed ESA dose from baseline to EoT (average of last 2 weeks of randomized treatment), performed using a one-way analysis of covariance model with percentage change from baseline in ESA as the response variable, treatment as the factor, and baseline Hgb as a covariate. Continuous variable secondary end points were summarized descriptively and analyzed using Wilcoxon rank-sum tests.
The number and percentage of patients with TEAEs were summarized. Intradialytic hypotension and hypersensitivity reactions were summarized by group and 4-week intervals.
All analyses were produced using SAS statistical software version 9.1.3 or higher (SAS Institute, Cary, NC).
Acknowledgments
This research was funded by Rockwell Medical (ClinicalTrials. gov identifier NCT01286012). Editorial and writing assistance was provided by Lillian L Neff (Innovative Analytics, Kalamazoo, MI).
Footnotes
Table S1. Summary of incidence of intradialytic hypotension: safety population.
Table S2. Treatment-emergent adverse events of intradialytic hypotension.
Table S3. Symptoms and interventions associated with treatment-emergent adverse events of intradialytic hypotension.
Randomization, blinding, sample size, and study assessments.
Table S4. Schedule of assessments.
Iron management and monitoring of iron status.
Table S5. Intravenous iron administration algorithm.
Table S6. ESA dose adjustment algorithm.
Criteria and procedures for study drug withholding.
Supplementary material is linked to the online version of the paper at http://www.nature.com/ki
AG, VL, RP, and CG are employees of Rockwell Medical, the sponsor of the study. AG holds the rights to the patent on parenteral delivery of FPC, including via the dialysis solutions, and a pending patent on FPC sparing the need for ESAs. AG, RP, and CG hold a patent pending for FPC-sparing ESA usage in ESA-hyporesponsive hemodialysis patients. TAI received grant funding from Rockwell Medical (study sponsor) during the conduct of the study for measurement of inflammatory and oxidative stress markers. AB received grants from Rockwell Medical (study sponsor) during the conduct of the study. AB also received grants from Akebia and Fibrogen and consulting fees from Amgen, Fibrogen, Bayer, and Pharmacosmos. He is currently an employee of Fibrogen.
Supplementary Material
References
- 1Babitt JL, Lin HY. Mechanisms of anemia in CKD. J Am Soc Nephrol 2012; 23: 1631–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2Sargent JA, Acchiardo SR. Iron requirements in hemodialysis. Blood Purif 2004; 22: 112–123. [DOI] [PubMed] [Google Scholar]
- 3Goodnough LT, Nemeth E, Ganz T. Detection, evaluation, and management of iron-restricted erythropoiesis. Blood 2010; 116: 4754–4761. [DOI] [PubMed] [Google Scholar]
- 4Zumbrennen-Bullough K, Babitt JL. The iron cycle in chronic kidney disease (CKD): from genetics and experimental models to CKD patients. Nephrol Dial Transplant 2014; 29: 263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Ganz T. Hepcidin and iron regulation, 10 years later. Blood 2011; 117: 4425–4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Thomas DW, Hinchliffe RF, Briggs C et al. Guideline for the laboratory diagnosis of functional iron deficiency. Br J Haematol 2013; 161: 639–648. [DOI] [PubMed] [Google Scholar]
- 7Auerbach M, Coyne D, Ballard H. Intravenous iron: from anathema to standard of care. Am J Hematol 2008; 83: 580–588. [DOI] [PubMed] [Google Scholar]
- 8Fishbane S, Mathew AT, Wanchoo R. Intravenous iron exposure and outcomes in patients on hemodialysis. Clin J Am Soc Nephrol 2014; 9: 1837–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Vaziri ND. Understanding iron: promoting its safe use in patients with chronic kidney failure treated by hemodialysis. Am J Kidney Dis 2013; 61: 992–1000. [DOI] [PubMed] [Google Scholar]
- 10Gupta A, Amin NB, Besarab A et al. Dialysate iron therapy: infusion of soluble ferric pyrophosphate via the dialysate during hemodialysis. Kidney Int 1999; 55: 1891–1898. [DOI] [PubMed] [Google Scholar]
- 11Gupta A, Crumbliss AL. Treatment of iron deficiency anemia: are monomeric iron compounds suitable for parenteral administration? J Lab Clin Med 2000; 136: 371–378. [DOI] [PubMed] [Google Scholar]
- 12Huebers HA, Finch CA. The physiology of transferrin and transferrin receptors. Physiol Rev 1987; 67: 520–582. [DOI] [PubMed] [Google Scholar]
- 13Rostoker G, Griuncelli M, Loridon C et al. Hemodialysis-associated hemosiderosis in the era of erythropoiesis-stimulating agents: a MRI study. Am J Med 2012; 125: 991–9 e1. [DOI] [PubMed] [Google Scholar]
- 14Kosch M, Bahner U, Bettger H et al. A randomized, controlled parallel-group trial on efficacy and safety of iron sucrose (Venofer) vs iron gluconate (Ferrlecit) in haemodialysis patients treated with rHuEpo. Nephrol Dial Transplant 2001; 16: 1239–1244. [DOI] [PubMed] [Google Scholar]
- 15United States Renal Data SystemUSRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, 2013. [Google Scholar]
- 16Cowart RE, Swope S, Loh TT et al. The exchange of Fe3+ between pyrophosphate and transferrin. Probing the nature of an intermediate complex with stopped flow kinetics, rapid multimixing, and electron paramagnetic resonance spectroscopy. J Biol Chem 1986; 261: 4607–4614. [PubMed] [Google Scholar]
- 17Cervato G, Viani P, Gatti P et al. Further studies on the antioxidant role of pyrophosphate in model membranes. Chem Phys Lipids 1993; 66: 87–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.