Abstract
Early mortality is high in hemodialysis (HD) patients, but little is known about early cardiovascular event (CVE) rates after HD initiation. To study this we analyzed data in the AROii cohort of incident HD patients from over 300 European Fresenius Medical Care dialysis centers. Weekly rates of a composite of CVEs during the first year and monthly rates of the composite and its constituents (coronary artery, cerebrovascular, peripheral arterial, congestive heart failure, and sudden cardiac death) during the first 2 years after HD initiation were assessed. Of 6308 patients that started dialysis within 7 days, 1449 patients experienced 2405 CVEs over the next 2 years. The first-year CVE rate (30.2/100 person-years; 95% CI, 28.7–31.7) greatly exceeded the second-year rate (19.4/100; 95% CI, 18.1–20.8). Composite CVEs were highest during the first week with increased risk compared with the second year, persisting until the fifth month. Except for sudden cardiac death, temporal patterns of rates for all CVE categories were very similar, with highest rates during the first month and a high-risk period extending to 4 months. Higher or lower cumulative weekly dialysis dose, lower blood flow, and lower net ultrafiltration during dialysis were associated with CVE during the high-risk period, but not during the post high-risk period. Thus, the incidence of CVE in the first weeks after HD initiation is much higher than during subsequent periods which raises concerns that HD initiation may trigger CVEs.
Keywords: cardiovascular disease, cardiovascular events, chronic kidney disease, hemodialysis initiation
In patients with chronic kidney disease (CKD) the first months on hemodialysis (HD) have been identified as a high-risk period for patient survival.1 In both the US2, 3, 4, 5, 6, 7 and Europe,7, 8, 9 increased mortality during the first 3 months after dialysis initiation, compared with subsequent periods, has been observed. The underlying reasons remain poorly understood. Early dialysis withdrawal may contribute to early mortality,3 but withdrawal practice varies significantly across countries and overall accounts for a comparatively small proportion of early death.7 Among other causes of death, up to 40% have been attributed to cardiovascular (CV) disease8, 9 and a large study found the greatest difference between mortality rates during the first 3 months and during the remainder of the first year on dialysis for CV-related deaths.3 Thus, study of CV events (CVE) after dialysis initiation should lead to a better understanding of the causes of early mortality, risk prediction, and preventive strategies in high-risk patients. However, there is a paucity of data on CVE rates following HD initiation. In particular, it is not known whether the time to event varies for specific CVE categories, whether CVE risk factors vary for different CVE categories, and whether CVE risk factors differ for early and subsequent periods on dialysis.
The clinical circumstances of dialysis initiation vary, making analysis of the early time period after starting dialysis challenging. Although many patients will initiate HD in an outpatient setting, others will have their first dialysis during a hospitalization period and will be subsequently referred to dialysis centers after variable time periods. Thus, important clinical events may easily be missed in registries of chronic HD patients. In fact, because of these circumstances and additional administrative reasons, many large registries do not capture clinical data on the first months on HD.10 Smaller studies, on the other hand, may have been of insufficient size to provide reliable rate estimates for different CVEs,11, 12 although they have been important in characterizing early mortality after dialysis initiation.
Utilizing data collected prospectively as part of the ARO (Analyzing data, Recognizing excellence, and Optimizing outcomes) CKD research initiative,13 we studied the occurrence of CVE after dialysis initiation in a large cohort of incident HD patients at over 300 Fresenius Medical Care dialysis centres in Europe (FME; ‘AROii'). Inclusion criteria for the current study were carefully chosen, and different scenarios for patients initiating HD within and outside the FME network were considered to reliably capture early events.
RESULTS
Study population
The AROii cohort includes 11,244 incident patients presenting to FME centers in 14 European countries and Turkey. Patients most frequently initiated HD on the day of study enrollment (n=3974) or within a week of this date (n=2270), whereas ~40% (n=4882) initiated HD at an earlier time point and a small proportion at later time points (n=118; see Supplementary Figure S1 online). For the purpose of the current study we chose to exclude patients with a dialysis vintage of ⩾7 days (n=4569) and patients who did not initiate dialysis within 365 days of admission to FME (n=21). Furthermore, we excluded sequentially patients with no dialysis data (n=484) or with a history of kidney transplantation (n=83), thus resulting in a study cohort of 6308 patients. Within this study cohort 4005 patients (63%) initiated dialysis within FME facilities and the remainder initiated dialysis elsewhere.
Patient characteristics
A total of 2405 CVEs were reported in 1449 patients (23% of the study cohort) within 2 years after initiation of dialysis. The characteristics of the study cohort stratified by the occurrence of at least one CVE in the 2-year study period are described in Table 1. Patients who experienced events in the first 2 years after dialysis initiation tended to be older and were more frequently male. They more often had vascular or diabetic causes for their CKD, and this pattern was repeated in their pre-dialysis clinical history, with a greater prevalence of the constituent CVE and diabetes observed. Also the percentage of former smokers was higher in those experiencing a CVE. Conversely, no geographical differences were apparent, and patients were similar with regard to social relationship and current smoking status.
Table 1. Characteristics of the study cohort.
| Factor | CVE within 2 years of HD initiation |
All (n=6308) | |
|---|---|---|---|
| Yes (n=1449) | No (n=4859) | ||
| Region, n (%) | |||
| West | 742 (51.2) | 2908 (59.8) | 3650 (57.9) |
| East | 707 (48.8) | 1951 (40.2) | 2658 (42.1) |
| Age (years) | |||
| Mean±s.d. | 69.0±11.8 | 63.1±15.0 | 64.5±14.5 |
| Gender, n (%) | |||
| Female | 552 (38.1) | 1995 (41.1) | 2547 (40.4) |
| Male | 895 (61.8) | 2860 (58.9) | 3755 (59.5) |
| Missing | 2 (0.1) | 4 (0.1) | 6 (0.1) |
| Marital status, n (%) | |||
| In a relationship | 796 (54.9) | 2744 (56.5) | 3540 (56.1) |
| Not in a relationship | 388 (26.8) | 1099 (22.6) | 1487 (23.6) |
| Missing | 265 (18.3) | 1016 (20.9) | 1281 (20.3) |
| Smoking status, n (%) | |||
| Non-smoker | 514 (35.5) | 1787 (36.8) | 2301 (36.5) |
| Former | 321 (22.2) | 742 (15.3) | 1063 (16.9) |
| Current | 111 (7.7) | 373 (7.7) | 484 (7.7) |
| Missing | 503 (34.7) | 1957 (40.3) | 2460 (39.0) |
| Body mass index (kg/m2) | |||
| Mean±s.d. | 27.1±15.0 | 26.7±11.2 | 26.8±12.3 |
| Missing, n (%) | 373 (25.7) | 1633 (33.6) | 2006 (31.8) |
| Pre-dialysis disease history, n (%) | |||
| CAE | 310 (21.4) | 522 (10.7) | 832 (13.2) |
| CBE | 172 (11.9) | 270 (5.6) | 442 (7.0) |
| CHFE | 150 (10.4) | 281 (5.8) | 431 (6.8) |
| PAE | 160 (11.0) | 244 (5.0) | 404 (6.4) |
| SCE | 3 (0.2) | 4 (0.1) | 7 (0.1) |
| Diabetes | 535 (36.9) | 1274 (26.2) | 1809 (28.7) |
| Atrial fibrillation | 68 (4.7) | 150 (3.1) | 218 (3.5) |
| Hypertension | 721 (49.8) | 2257 (46.4) | 2978 (47.2) |
| Dyslipidemia | 131 (9.0) | 360 (7.4) | 491 (7.8) |
| Chronic kidney disease etiology, n (%) | |||
| Hypertension/vascular | 302 (20.8) | 711 (14.6) | 1013 (16.1) |
| Glomerulonephritis | 89 (6.1) | 500 (10.3) | 589 (9.3) |
| Diabetic nephropathy | 439 (30.3) | 972 (20.0) | 1411 (22.4) |
| Tubulointerstitial | 147 (10.1) | 514 (10.6) | 661 (10.5) |
| Polycystic kidney disease | 50 (3.5) | 324 (6.7) | 374 (5.9) |
| Miscellaneous/other | 378 (26.1) | 1599 (32.9) | 1977 (31.3) |
| Invalid/missing | 44 (3.0) | 239 (4.9) | 283 (4.5) |
Abbreviations: CAE, coronary artery event; CBE, cerebrovascular event; CHFE, congestive heart failure event; CVE, composite cardiovascular event; HD, hemodialysis; PAE, peripheral arterial event; SCE, sudden cardiac event.
NB Tercile and quartile cut-offs supplied in Supplementary Table S5 online.
The characteristics of patients starting dialysis in FME facilities and those starting dialysis elsewhere are shown in Supplementary Table S1 online. With the exception of geography (a higher proportion of FME facilities in Eastern Europe are hospital based, hence the greater opportunity for initiation therein) and diabetes prevalence (which may in turn reflect geography), the populations were broadly similar.
Multiple events and mortality
Of the 1449 patients experiencing events, 905 (62.5%) experienced a single event during the study period. The distribution of first constituent events was broadly similar to all constituent events (coronary artery event, CAE: 26.5% vs. 28.8% peripheral arterial event, PAE: 18.3% vs. 24.8% congestive heart failure event, CHFE: 23.9% vs. 20.0% cerebrovascular event, CBE: 17.7% vs. 19.6%), with the exception of sudden cardiac event, SCE (13.6% vs. 6.9%). This was largely due to the exceptionally high mortality experienced by this patient group, with 114 of 123 patients (92.7%) dying within 7 days of a first SCE (mostly consisting of sudden cardiac death events) compared with 191 of 782 patients (24.4%) experiencing other first constituent events. The number of events experienced by the 544 patients with multiple events ranged from 2 to 12, but most patients experienced 2 or 3 (with these values forming the interquartile range). As expected, associated mortality was lower, with 188 deaths occurring within 7 days of the 956 subsequent events (19.7%), but subsequent SCE events were still temporally associated with high mortality (29/40; 72.5%). When considered sequentially, subsequent events fell within the same constituent event class in 522 instances (54.6%), but only the same ICD-10 block in 374 instances (39.1%), and both shared the same ICD-10 code in 289 instances (30.2%). The median time between a subsequent event and its predecessor was 19 days (interquartile range 1–114 days); when the subsequent event occurred on the same day (n=216) it was a code from the same event class in 77 instances (35.6%), the same block in 33 instances (15.3%), but never the same code, possibly due to the fact that a specific code on 1 day was only counted once.
Rates of CVE
Table 2 shows first- and second-year rates of composite CVE and five different CVE categories for all study patients and for those initiating dialysis within or outside FME. The first-year rate of composite CVE was 30.2 per 100 person-years (PYs) and the second-year rate was 19.4 per 100 PYs. Individual CVE constituent rates were generally higher during the first year compared with the second year. In patients initiating HD outside FME, the composite CVE rate was lower. This difference was largely driven by differences in the more common CAE and CHFE, but the event rates of the three other categories also tended to be lower in those initiating outside FME.
Table 2. Rates of composite CVE and CVE constituents during the first and second year in patients overall as well as in those initiating dialysis within Fresenius Medical Care dialysis centers in Europe (FME) and outside FME facilities (Non-FME).
| Groups of Patients, Events, Person-years at Risk and Rate per 100 Person-years (Rate (95% CI)) |
|||||||
|---|---|---|---|---|---|---|---|
| Subset | Event | First Year |
Second Year |
||||
| Events | Person-years | Rate (95% CI) | Events | Person-years | Rate (95% CI) | ||
| All | CVE | 1627 | 5396.6 | 30.2 (28.7–31.7) | 796 | 4108.1 | 19.4 (18.1–20.8) |
| CAE | 478 | 5396.6 | 8.9 (8.1–9.7) | 218 | 4108.1 | 5.3 (4.6–6.1) | |
| CHFE | 348 | 5396.6 | 6.5 (5.8–7.2) | 136 | 4108.1 | 3.3 (2.8–3.9) | |
| PAE | 418 | 5396.6 | 7.8 (7.0–8.5) | 183 | 4108.1 | 4.5 (3.8–5.2) | |
| CBE | 288 | 5396.6 | 5.3 (4.7–6.0] | 187 | 4108.1 | 4.6 (3.9–5.3) | |
| SCE | 95 | 5396.6 | 1.8 (1.4–2.2) | 72 | 4108.1 | 1.8 (1.4–2.2) | |
| FME | CVE | 1129 | 3392.6 | 33.3 (31.4–35.3) | 563 | 2561.8 | 22.0 (20.2–23.9) |
| CAE | 346 | 3392.6 | 10.2 (9.2–11.3) | 141 | 2561.8 | 5.5 (4.6–6.5) | |
| CHFE | 241 | 3392.6 | 7.1 (6.2–8.1) | 104 | 2561.8 | 4.1 (3.3–4.9) | |
| PAE | 300 | 3392.6 | 8.8 (7.9–9.9) | 152 | 2561.8 | 5.9 (5.0–7.0) | |
| CBE | 182 | 3392.6 | 5.4 (4.6–6.2) | 120 | 2561.8 | 4.7 (3.9–5.6) | |
| SCE | 60 | 3392.6 | 1.8 (1.4–2.3) | 46 | 2561.8 | 1.8 (1.3–2.4) | |
| Non-FME | CVE | 498 | 2004.0 | 24.9 (22.7–27.1) | 233 | 1546.3 | 15.1 (13.2–17.1) |
| CAE | 132 | 2004.0 | 6.6 (5.5–7.8) | 77 | 1546.3 | 5.0 (3.9–6.2) | |
| CHFE | 107 | 2004.0 | 5.3 (4.4–6.5) | 32 | 1546.3 | 2.1 (1.4–2.9) | |
| PAE | 118 | 2004.0 | 5.9 (4.9–7.1) | 31 | 1546.3 | 2.0 (1.4–2.9) | |
| CBE | 106 | 2004.0 | 5.3 (4.3–6.4) | 67 | 1546.3 | 4.3 (3.4–5.5) | |
| SCE | 35 | 2004.0 | 1.8 (1.2–2.4) | 26 | 1546.3 | 1.7 (1.1–2.5) | |
Abbreviations: CAE, coronary artery event; CBE, cerebrovascular event; CHFE, congestive heart failure event; CI, confidence interval; CVE, composite cardiovascular event; FME, Fresenius Medical Care dialysis centers in Europe; PAE, peripheral arterial event; SCE, sudden cardiac event; FME and Non-FME refers to the initiation of dialysis healthcare environment.
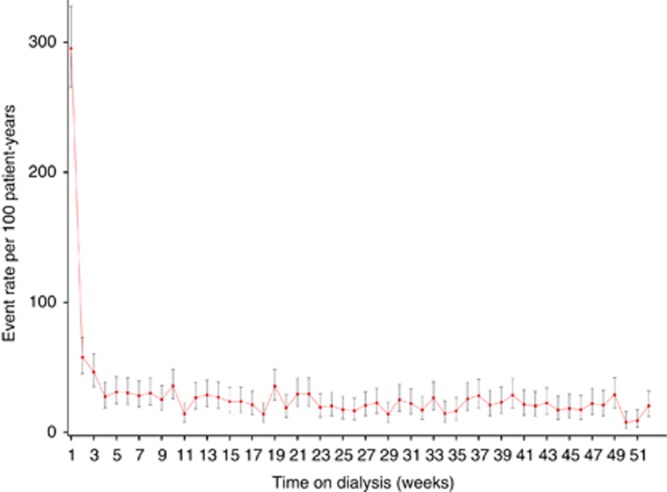
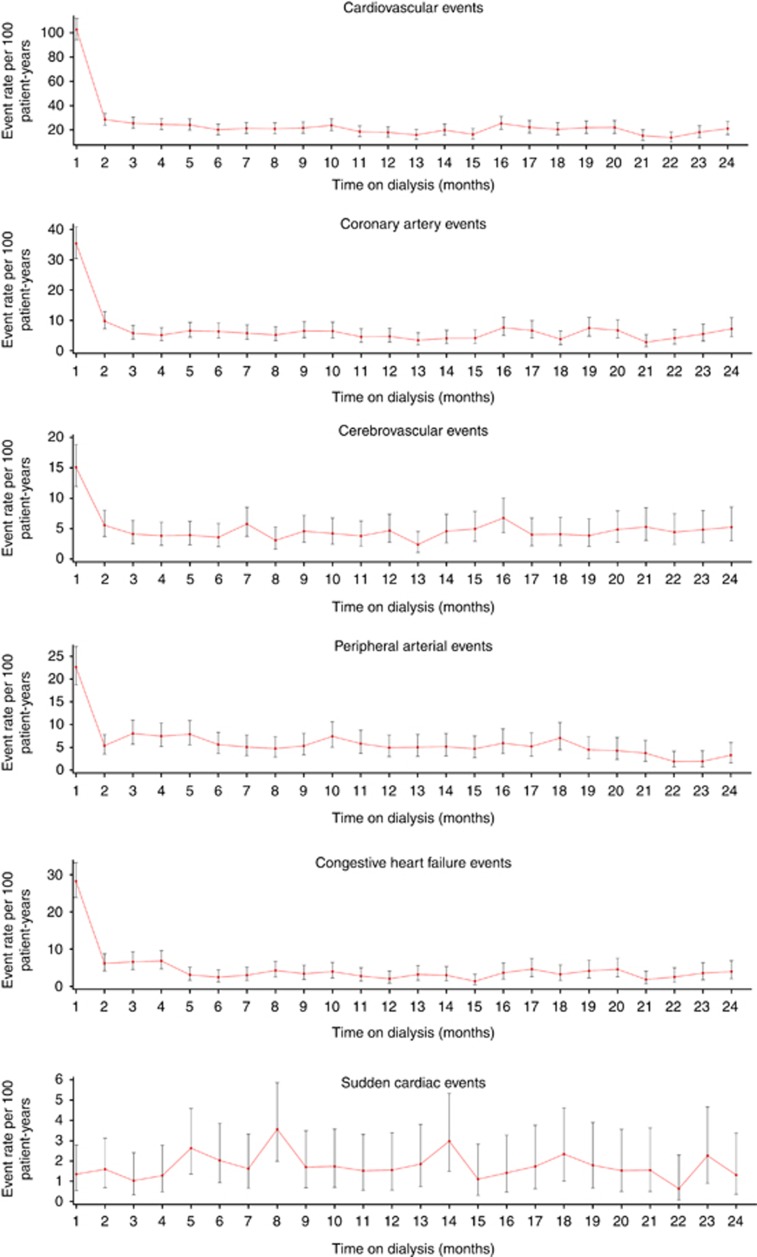
Figure 1 shows weekly composite CVE rates during the first year after HD initiation. The CVE rate peaked sharply during week 1, when it was more than 5-fold higher than during week 2 (rate ratio (RR) 5.10 (95% confidence interval (CI), 3.95–6.59)) and subsequently declined until week 4 to achieve a relatively stable level during the remainder of the first year. Monthly rates for the composite CVE and the five different CVE constituents are illustrated in Figure 2. The temporal pattern was very similar for CAE, CHFE, PAE, and CBE, all of which showed highest incidence rates during the first month. The event rate during month 1 was highest for CAE (35.4 per 100 PY), followed by CHFE (28.2 per 100 PY), PAD (22.6 per 100 PY), and CBE (15.1 per 100 PY). To define periods of elevated risk (high-risk period), monthly event rates during year 1 were related to the average event rate during year 2 (Table 3). Although the overall CVE high-risk period was sustained until month 5 after dialysis initiation, the risk for CBE and PAE was increased during the first month, but not the second month, the risk for CAE was increased during the first 2 months, and the risk for CHFE until month 4. The event RR was particularly high for CHFE, partly because the CHFE event rate during the second year was lower than that for several other CVEs. The risk for PAE was increased again during months 3–5 and 10. In contrast to other CVE categories, the incidence of SCE followed a different pattern, with no early peak and a significant increase compared with year 2 only in month 8.
Figure 1.
Weekly event rates for the composite cardiovascular events.
Figure 2.
Monthly event rates for the composite cardiovascular events and constituent events.
Table 3. High-risk periods for composite CVE and constituent events.
| Month | Rate ratio (95% confidence interval) |
|||||
|---|---|---|---|---|---|---|
| CVE | CAE | CHFE | PAE | CBE | SCE | |
| 1 | 5.32 (4.76–5.94) | 6.73 (5.52–8.20) | 8.57 (6.78–10.84) | 5.09 (4.03–6.42) | 3.28 (2.52–4.27) | 0.79 (0.36–1.71] |
| 2 | 1.47 (1.23–1.76) | 1.85 (1.36–2.52) | 1.87 (1.27–2.76) | 1.21 (0.80–1.81) | 1.21 (0.81–1.80) | 0.92 (0.44–1.92) |
| 3 | 1.32 (1.09–1.60) | 1.10 (0.74–1.63) | 2.00 (1.36–2.94) | 1.81 (1.28–2.55) | 0.90 (0.57–1.42) | 0.60 (0.24–1.48) |
| 4 | 1.27 (1.04–1.54) | 0.97 (0.64–1.48) | 2.07 (1.41–3.04) | 1.67 (1.17–2.40] | 0.83 (0.51–1.35] | 0.74 (0.32–1.71) |
| 5 | 1.25 (1.02–1.52) | 1.25 (0.85–1.83) | 0.93 (0.54–1.61) | 1.77 (1.24–2.53) | 0.86 (0.53–1.39) | 1.53 (0.83–2.82) |
| 6 | 1.04 (0.83–1.29) | 1.20 (0.81–1.78) | 0.75 (0.41–1.39) | 1.27 (0.83–1.92) | 0.78 (0.47–1.31) | 1.18 (0.59–2.36) |
| 7 | 1.10 (0.89–1.36) | 1.10 (0.72–1.66) | 0.91 (0.52–1.61) | 1.14 (0.73–1.78) | 1.26 (0.83–1.91) | 0.94 (0.43–2.04) |
| 8 | 1.08 (0.87–1.35) | 0.99 (0.64–1.54) | 1.30 (0.79–2.12) | 1.07 (0.67–1.69) | 0.67 (0.38–1.18) | 2.07 (1.18–3.61) |
| 9 | 1.12 (0.90–1.39) | 1.24 (0.83–1.86) | 1.03 (0.59–1.79) | 1.20 (0.77–1.87) | 1.00 (0.62–1.61) | 0.99 (0.45–2.14) |
| 10 | 1.23 (1.00–1.52) | 1.22 (0.82–1.84) | 1.20 (0.72–2.02) | 1.67 (1.14–2.46) | 0.92 (0.56–1.51) | 1.01 (0.46–2.19) |
| 11 | 0.96 (0.75–1.22) | 0.87 (0.54–1.40) | 0.85 (0.46–1.56) | 1.31 (0.85–2.02) | 0.83 (0.49–1.40) | 0.88 (0.38–2.03) |
| 12 | 0.93 (0.72–1.18) | 0.89 (0.55–1.44) | 0.63 (0.31–1.29) | 1.11 (0.69–1.78) | 1.02 (0.63–1.65) | 0.90 (0.39–2.08) |
Abbreviations: CAE, coronary artery event; CBE, cerebrovascular event; CHFE, congestive heart failure event; CVE, composite cardiovascular event; PAE, peripheral arterial event; SCE, sudden cardiac event.
Boldface data represent the high-risk periods, during which the event rate remained significantly higher than the average event rate of the second year.
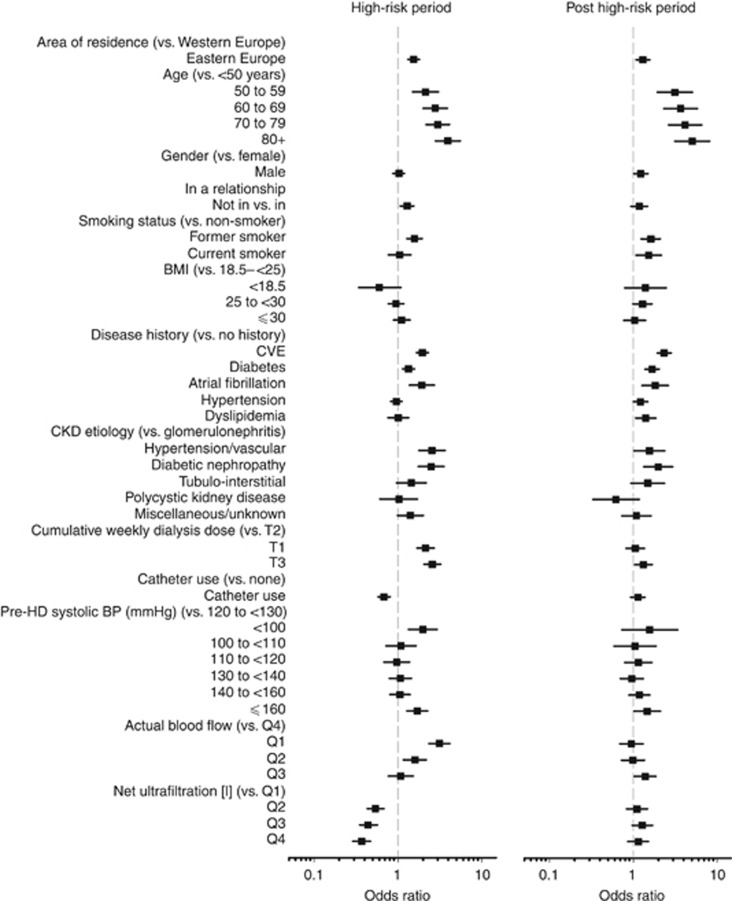
Risk factor analysis
Figure 3 shows the results of univariate analyses testing the association between demographic findings, patient history, clinical findings, and dialysis parameters for the high-risk and post high-risk periods (actual data in Supplementary Table S2 online). For most parameters we found consistency between the direction of association during the high-risk and the post high-risk period. Factors that were associated with increased risk during both periods included increasing age, treatment in Eastern Europe, former smoking, history of CVE, diabetes, or atrial fibrillation, as well as diabetic and vascular nephropathy as underlying disease. On the other hand, several dialysis session–related parameters were associated with either increased or decreased risk during the high-risk period, but not during the post high-risk period. Low weekly dialysis dose, low pre-dialysis blood pressure, and lower blood flow were associated with increased risk during the high-risk period only, whereas catheter use and higher net ultrafiltration were associated with reduced risk during the high-risk period only. The results for the constituent CVE were generally consistent with the composite CVE, but with less statistical power to detect significant differences (Supplementary Table S3 online).
Figure 3.
Factors associated with composite cardiovascular events in the high-risk (left) and post high-risk (right) periods. Univariate analysis (actual data shown in Supplementary Table S2 online). BMI, body mass index; CVE, cardiovascular events; HD, hemodialysis.
Multivariate analysis showed the independent association between age, patient history, and dialysis parameters with CVE in the high-risk period (Table 4; detailed data in Supplementary Table S2 online). Low or high cumulative weekly dialysis dose, high pre-dialysis systolic blood pressure, lower blood flow, and lower net ultrafiltration were associated with high early CVE risk. Only traditional CV risk factors (increasing age, smoking, and CVE history) were independently associated with CVE outside the high-risk period.
Table 4. Factors associated with composite CVE in the high-risk and post high-risk periods (summarized multivariate analysis; actual data shown in Supplementary Table S1 online).
| Group | High-risk period | Post high-risk period |
|---|---|---|
| Demographic | Increasing age | Increasing age |
| Former smoker | Former smoker | |
| Current smoker | ||
| Clinical | History of cardiovascular event | History of cardiovascular event |
| History of atrial fibrillation | ||
| CKD etiology of hypertension/vascular disorders | ||
| CKD etiology of diabetic nephropathy | ||
| Dialysis | Higher or lower cumulative weekly dialysis dose | |
| High pre-dialysis systolic blood pressure | ||
| Lower actual blood flow | ||
| Lower net ultrafiltration |
Abbreviations: CKD, chronic kidney disease; CVE, cardiovascular event.
Additional analyses were undertaken to examine whether the association between dialysis parameters and CVE in the high-risk period was driven by differences in patients initiating dialysis in FME facilities or elsewhere (Supplementary Figure S2 online) or by history of CVE (Supplementary Figure S3 online). Despite restricted patient populations, results were generally consistent, suggesting that these factors were not major drivers for our findings.
As we did not have data on pre-dialysis care, we used information on the use of graft and/or fistulas during the early dialysis period (30 days) as a surrogate for a planned dialysis start. The proportion of patients experiencing an event was lower among those patients initiating within FME in whom graft/fistula use was documented compared with those in whom this was not the case (20.1% vs. 29.7%). However such a difference was not found in patients initiating outside FME (20.7% vs. 20.9%). Moreover, the monthly CVE pattern in the FME and non-FME ‘graft/fistula' and ‘no graft/fistula' patient groups was similar, suggesting that a planned start cannot prevent an increase in events soon after HD initiation.
DISCUSSION
The prevalence of most manifestations of CVD increases with declining renal function and is particularly high in patients on dialysis.9, 14, 15 The current study shows that the immediate period following dialysis initiation is a very high CV risk period, characterized by a much higher rate of major CVE than the remainder of the first 2 years. Incidence rates that were at least 3- and up to 8-fold higher than during the second year on dialysis were found for vascular events affecting coronary, cerebral, and peripheral arterial beds as well as heart failure during the first month on dialysis. Analysis of the composite CVE rate, which allowed a more granular analysis of temporal trends showed, in fact, that the first week of HD was associated with maximum CVE risk.
Although, to our knowledge, no previous studies have determined CVE rates after dialysis onset, our findings are consistent with several investigations that have highlighted an increased mortality during the first year on dialysis4, 9, 16 and in particular during the first 902, 17 or 1203, 7 days. Although data on peak mortality rates are somewhat variable, one large cohort study of over 300,000 patients observed highest mortality rates within the first 2 weeks,5 which corroborates our notion of an early high-risk period. Multiple causes may contribute to early mortality on dialysis, but the majority are considered of CV origin,3, 6 which is also consistent with our observation of elevated CVE rates after dialysis initiation. Several traditional risk factors, which we found to be associated with CVE in the early phase after dialysis initiation, such as age, smoking history, and CVE history were also identified to be associated with early mortality in HD patients.2, 3 Although we observed a substantial overall mortality rate of 20% within 1 week following a CVE, the majority of observed CVEs were not immediately fatal, indicating a substantial increase in morbidity early after dialysis initiation.
Theoretically, at least two different reasons may underlie the increased rate of CVE following the onset of dialysis. First, deterioration of renal function and/or the signs and symptoms resulting in a decision to initiate dialysis may be prodromal signs of the subsequent event. This appears possible, e.g., for CHF events, but is less plausible for vascular events, such as CBE, PAE, or CAE. The second and alternative explanation would be that the initiation of dialysis triggers CVE. In fact, the HD procedure itself has been recognized to induce myocardial stunning and contractile dysfunction,18, 19 endothelial dysfunction,20 oxidative stress,21 and inflammation.22 Additional evidence suggests a direct temporal association of CVE with the dialysis procedure. Thus, about one-third of all strokes in dialysis patients were found to occur during or shortly after HD treatment.12, 23 Mortality rates and CVE admission rates in a large US study of dialysis patients were found to be associated with the dialysis schedule, with highest rates on the day following the long interval.24 Irrespective of what causes temporal associations between dialysis and CVE, additional factors must play a role that increases the sensitivity during the first weeks and months of dialysis. Although a decline in mortality rates may partly be explained by the fact that high-risk patients die early and less severely ill patients survive, similar considerations do not sufficiently explain why the incidence rates for a variety of different CVEs decline in parallel over time. The majority of associations between risk factors and CVE were similar for the high-risk and the post high-risk period, and we found little evidence for factors that are only associated with CVE during the high-risk period. For some aspects of CVD history at baseline and specific etiologies of kidney disease, multivariate analysis showed associations only during the early high-risk period, and it is plausible that these factors lose their relevance with time. In contrast to an early peak observed for other CVE constituents, SCE was the only type of event that followed a different pattern with no clear trend in incidence rates during the first 2 years. We cannot explain this difference but it is important to note that the accuracy of SCE diagnosis is likely to be lower than that for other CVEs.15
Our study has several limitations. Primarily, overall event rates were lower in patients initiating outside of FME facilities. The reasons for this difference remain speculative, but, given that many events occur within the first week after dialysis initiation, it is possible that fatal and non-fatal events occurred in patients initiating HD outside FME prior to possible capture in the study, thus resulting in a healthier sub-cohort (survival bias). Other differences related to patient selection and treatment, however, cannot be excluded. Despite this uncertainty, we chose to conduct further analysis with the whole study cohort, rather than only those patients initiating within FME, recognizing that this may lead to an underestimation of event rates. Sensitivity analyses, examining the effect of initiating within FME facilities or elsewhere, showed that the inclusion of these patients had a minimal impact on our risk factor analysis. Furthermore, CVEs were assessed on the basis of ICD-10 codes and were not adjudicated. In particular, distinguishing between heart failure and volume overload is inherently difficult. We also do not have data on several parameters that may have implications for the interpretation of CVEs, including first presentation to nephrologists, the level of renal function at dialysis initiation, the specific indication for starting dialysis, or residual renal function.25 As patients were enrolled at presentation to a dialysis provider, we are unable to compare event rates after dialysis initiation with event rates during the pre-dialysis period. The AROii study represents a random sample drawn from European FME centers to minimize selection bias, but the findings may not be generalizable for patients under the care of other providers. The strength of the study includes the prospective establishment of an incident cohort of dialysis patients, the continued coverage of patients with <7 days of dialysis vintage,26 the broad geographic distribution across several European countries, the assessment of fatal and non-fatal events, and the possibility to assess and compare the incidence rates of a whole spectrum of CVEs over time.
Although our findings clearly show that the early period after dialysis initiation should be recognized as a high-risk period that mandates increased surveillance, the optimal mode of patient care during this period remains unclear. We observed robust associations between CVE during the early high-risk period with some dialysis-related parameters, including higher and lower weekly dialysis dose, low actual blood flow, and low net ultrafiltration. The latter observations might suggest intensifying dialysis regimens early after dialysis initiation rather than gradually increasing treatment intensity over time. However, the association does not necessarily imply causality, and, despite being statistically independent, these parameters might also be surrogates for patients with compromised CV function, in whom neither a higher dialysis blood flow nor a higher rate of ultrafiltration could be achieved. We were surprised to observe that catheter use was associated with reduced risk during the high-risk period, as previous studies have consistently shown that catheter use is associated with early mortality.3, 6 A large portion of catheter-associated mortality is due to infections,27, 28 and the association with CV morbidity may be less strong. In any case, the finding was not confirmed in multivariate analysis, suggesting that it is due to confounding by other factors. Unfortunately, uncertainty also extends to acute management of CV complications and secondary prevention in patients on HD.15, 29 Of interest, it has been reported that a dedicated program of intensive patient education, evaluation, and early clinical intervention in patients new to dialysis reduced mortality within the first 120 days by 31% in a case–control study,30 suggesting that a significant improvement in prognosis is feasible.
Finally, our findings also have to be considered in the context of an ongoing debate about the optimal timing of dialysis initiation and alternative treatment strategies. Prognosis of patients on dialysis is inversely associated with the level of residual renal function at dialysis initiation;31 however, strong confounders of this association have been identified.32 Moreover, controlled studies and interventional trials in aggregate favor a later rather than an earlier dialysis initiation.33, 34 There is an increasing appeal for shared decision making for dialysis initiation, based on evaluation of risks, benefits, quality of life impact, and prognosis.35, 36 Based on our findings we believe that an increased risk for CVE after dialysis initiation should be taken into account in such deliberations.
MATERIALS AND METHODS
The ARO CKD research initiative began in 2007, with the purpose of improving HD patient outcomes in Europe through better understanding of patient morbidity and risk factors.37 The second ARO cohort, AROii, comprises adult subjects presenting at 1 of over 300 participating FME facilities in 14 European countries and Turkey between 1 January 2007 and 31 December 2009. Closed-cohort by design, AROii comprises incident HD patients (<183 days since commencing HD) with no history of renal transplantation. Data, comprising detailed patient-level information on medical and drug history, and longitudinal records of biochemical measurements and medications, are captured electronically via the validated FME European Clinical Database and supplied on a quarterly basis. All ethical and regulatory obligations concerning the use of patient data are met at each participating site. The patient population was restricted further in the current study to include patients with a dialysis vintage of <7 days on admission to an FME clinic, or who initiated within 365 days of admission. All patients were required to have at least one dialysis session in FME facilities.
Initial descriptive statistics were analyzed to describe the study population at baseline and the distributions of the study confounders. Continuous variables were described using mean and s.d., median, 25th and 75th percentiles, and minimum and maximum values. Skewed variables were described using a median and range, or categorized into meaningful categories at which point categorical data analysis was applied. Categorical data were reported as counts and frequencies.
The primary end point for analyses, the composite CVE, comprised fatal or non-fatal constituent CAE, CBE, PAE, CHFE, and SCE based on ICD-10 coded comorbidities data (Supplementary Table S4 online). Crude event rates were calculated for each week (for the composite CVE) and month of dialysis vintage (for all outcomes) during the first 2 years. In each instance, patients accrued time at risk from the beginning of the month of interest until they experienced the event of interest or were censored (lost-to-follow-up (>45 days without continuous dialysis at an FME facility), renal transplantation, or the end of that month, whichever came first). The event rate, p̂, was estimated as:
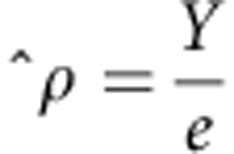 |
where Y is the observed number of events, and e is the exposure period in PY.38 Exact 95% Poisson CIs were derived and presented as:
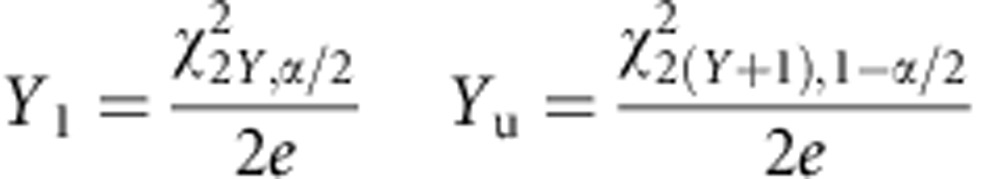 |
where Y is the observed number of events, Yl and Yu are lower and upper CIs for Y, respectively, and X2v,α is the χ2 quartile for upper tail probability α(0.05) on v degrees of freedom.39 An objective approach was taken to define the high-risk period, where RRs were calculated for each month in the first year using the second-year rate as the reference time period. The high-risk period was defined as the period during which the risk was significantly elevated as compared with the second year. In other words, the high-risk period extended to the point where the lower CI for the RR included one. The post high-risk period represented the period from the end of the high-risk period until the end of the first year. RRs were calculated on the basis of the formula:
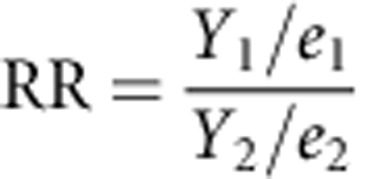 |
where Y1 and Y2 are the number of events in any given month and the second year, respectively, and e1 and e2 are the PYs at-risk for the same time points; 95% CIs for the RR were calculated on the basis of the formula log(RR)±1.96 × SE[log(RR)], where SE[log(RR)] (the standard error of the log RR) was calculated40 as:
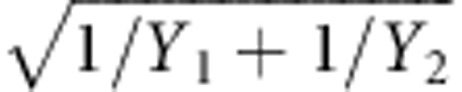 |
Logistic regression models were fitted to the data to determine separately potential predictors of events in high-risk and post high-risk periods. Patients experiencing the outcome of interest (coded ‘1' in the outcome variable) were compared with patients who did not (coded ‘0' in the outcome variable) as of the end of the period, with odds ratios and 95% CIs calculated for each explanatory variable (Table 1). Multivariate logistic regression was applied to control for confounding. Potential predictors—those associated with the outcome at the 95% level—were added and removed from the model in a stepwise manner, where 10% and 5% significance levels were used to determine variable entry and retention, respectively. Logistical regression models rather than a Cox proportional hazards model–based approach were used, as patients were defined by their outcome (rather than exposure). Moreover, regression coefficients obtained from both approaches are similar, when—as in our study—the follow-up time is short.41
Acknowledgments
The sponsors were responsible for data collection (FME) and data management (FME, Amgen); they provided resources for statistical and epidemiological analysis and participated in the interpretation of data and preparation of the manuscript (Amgen). Every step of development of the project, from design and scientific conduct of the study, through interpretation of the data, to preparation, review, and approval of the manuscript, was led by authors who are also members of the ARO Steering Committee. Results and their interpretations were discussed by all members of the ARO Steering Committee at plenary meetings twice a year. The ARO CKD Research Initiative is a joint observational research commitment from Amgen and Fresenius Medical Care (Europe), fully funded by Amgen (Europe) GmbH, Zug, Switzerland.
Author Contributions
We are grateful to the participating FME centers for collecting the data. Part of this study was presented as a preliminary communication at the Annual Meeting of the American Society of Nephrology, Atlanta, November 2013.
Footnotes
Figure S1. Patient's dialysis vintage on recruitment to the AROii cohort. Insert shows the hemodialysis peri–initiation period in detail.
Figure S2. Factors associated with composite CVE in the high–risk period by (FME) initiation status (univariate analysis).
Figure S3. Factors associated with composite CVE in the high–risk period by history of CVE (univariate analysis).
Table S1. Characteristics of the study population by initiation in a Fresenius Medical Care European (FME) facility or elsewhere.
Table S2. Factors associated with composite CVEs in the high-risk and post high-risk periods (univariate and multivariate analysis). Estimates where 95% confidence intervals do not include one are emboldened.
Table S3. Factors associated with the constituent events in the high-risk and post high-risk periods (univariate analysis). Estimates where 95% confidence intervals do not include one are emboldened. Depending on the limited number of observed sudden cardiac events (SCEs), the analysis is not provided for this constituent event category.
Table S4. Cardiovascular disease event codes.
Table S5. Quartile (25%, 50% & 75%) and Tercile (33% & 67%) cut-offs applied in the study.
Supplementary material is linked to the online version of the paper at http://www.nature.com/ki
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. K-UE, FK, PS, SDA, DCW, ALdF, and JF received consultancy fees from Amgen. IAG, MF, and SR are full-time Amgen employees. DM is a full-time FME employee.
Contributor Information
on behalf of the ARO Steering Committee:
P Aljama, S Anker, T B Drueke, K -U Eckardt, J Floege, A de Francisco, F Kronenberg, I C Macdougall, J Malyszko, G Schernthaner, P Stenvinkel, D C Wheeler, B Molemans, and B Canaud
Supplementary Material
References
- 1Noordzij M, Jager KJ. Increased mortality early after dialysis initiation: a universal phenomenon. Kidney Int 2014; 85: 12–14. [DOI] [PubMed] [Google Scholar]
- 2Soucie JM, McClellan WM. Early death in dialysis patients: risk factors and impact on incidence and mortality rates. J Am Soc Nephrol 1996; 7: 2169–2175. [DOI] [PubMed] [Google Scholar]
- 3Bradbury BD, Fissell RB, Albert JM et al. Predictors of early mortality among incident US hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Clin J Am Soc Nephrol 2007; 2: 89–99. [DOI] [PubMed] [Google Scholar]
- 4Collins AJ, Foley RN, Gilbertson DT et al. The state of chronic kidney disease, ESRD, and morbidity and mortality in the first year of dialysis. Clin J Am Soc Nephrol 2009; 4(Suppl 1): S5–11. [DOI] [PubMed] [Google Scholar]
- 5Chan KE, Maddux FW, Tolkoff-Rubin N et al. Early outcomes among those initiating chronic dialysis in the United States. Clin J Am Soc Nephrol 2011; 6: 2642–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Lukowsky LR, Kheifets L, Arah OA et al. Patterns and predictors of early mortality in incident hemodialysis patients: new insights. Am J Nephrol 2012; 35: 548–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Robinson BM, Zhang J, Morgenstern H et al. Worldwide, mortality risk is high soon after initiation of hemodialysis. Kidney Int 2014; 85: 158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Khan IH, Catto GR, Edward N et al. Death during the first 90 days of dialysis: a case control study. Am J Kidney Dis 1995; 25: 276–280. [DOI] [PubMed] [Google Scholar]
- 9de Jager DJ, Grootendorst DC, Jager KJ et al. Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA 2009; 302: 1782–1789. [DOI] [PubMed] [Google Scholar]
- 10Foley RN, Collins AJ. The USRDS: what you need to know about what it can and can't tell us about ESRD. Clin J Am Soc Nephrol 2013; 8: 845–851. [DOI] [PubMed] [Google Scholar]
- 11Rognant N, Alamartine E, Aldigier JC et al. Impact of prior CKD management in a renal care network on early outcomes in incident dialysis patients: a prospective observational study. BMC Nephrol 2013; 14: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12Sozio SM, Armstrong PA, Coresh J et al. Cerebrovascular disease incidence, characteristics, and outcomes in patients initiating dialysis: the choices for healthy outcomes in caring for ESRD (CHOICE) study. Am J Kidney Dis 2009; 54: 468–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13Floege J, Gillespie IA, Kronenberg F et al. Development and validation of a predictive mortality risk score from a European hemodialysis cohort. Kidney Int 2015; doi:10.1038/ki.2014.419. [DOI] [PMC free article] [PubMed]
- 14Matsushita K, van der Velde M, Astor BC et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Chronic Kidney Disease Prognosis, Consortium. Lancet 2010; 375: 2073–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15Herzog CA, Asinger RW, Berger AK et al. Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2011; 80: 572–586. [DOI] [PubMed] [Google Scholar]
- 16Innes A, Rowe PA, Burden RP et al. Early deaths on renal replacement therapy: the need for early nephrological referral. Nephrol Dial Transplant 1992; 7: 467–471. [PubMed] [Google Scholar]
- 17Khan IH, Campbell MK, Cantarovich D et al. Survival on renal replacement therapy in Europe: is there a 'centre effect'? Nephrol Dial Transplant 1996; 11: 300–307. [DOI] [PubMed] [Google Scholar]
- 18McIntyre CW, Burton JO, Selby NM et al. Hemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin J Am Soc Nephrol 2008; 3: 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Selby NM, McIntyre CW. The vicious cycle of dialysis-induced cardiac injury–are dynamic changes in diastolic function involved? Am J Kidney Dis 2013; 62: 442–444. [DOI] [PubMed] [Google Scholar]
- 20Weronika OF, Alicja P, Ewa B et al. Hemodialysis-induced changes in the blood composition affect function of the endothelium. Hemodial Int 2014; 18: 650–656. [DOI] [PubMed] [Google Scholar]
- 21Pupim LB, Himmelfarb J, McMonagle E et al. Influence of initiation of maintenance hemodialysis on biomarkers of inflammation and oxidative stress. Kidney Int 2004; 65: 2371–2379. [DOI] [PubMed] [Google Scholar]
- 22Carrero JJ, Stenvinkel P. Inflammation in end-stage renal disease–what have we learned in 10 years? Semin Dial 2010; 23: 498–509. [DOI] [PubMed] [Google Scholar]
- 23Toyoda K, Fujii K, Fujimi S et al. Stroke in patients on maintenance hemodialysis: a 22-year single-center study. Am J Kidney Dis 2005; 45: 1058–1066. [DOI] [PubMed] [Google Scholar]
- 24Foley RN, Gilbertson DT, Murray T et al. Long interdialytic interval and mortality among patients receiving hemodialysis. N Engl J Med 2011; 365: 1099–1107. [DOI] [PubMed] [Google Scholar]
- 25Rognant N, Laville M. Early mortality in dialysis and adequacy of predialysis renal care: the picture is more complex than we thought. Kidney Int 2014; 86: 238–240. [DOI] [PubMed] [Google Scholar]
- 26Foley RN, Chen SC, Solid CA et al. Early mortality in patients starting dialysis appears to go unregistered. Kidney Int 2014; 86: 392–398. [DOI] [PubMed] [Google Scholar]
- 27Collins AJ, Foley RN, Herzog C et al. US Renal Data System 2010 Annual Data Report. Am J Kidney Dis 2011; 57: e239–e252. [DOI] [PubMed] [Google Scholar]
- 28Pisoni RL, Arrington CJ, Albert JM et al. Facility hemodialysis vascular access use and mortality in countries participating in DOPPS: an instrumental variable analysis. Am J Kidney Dis 2009; 53: 475–491. [DOI] [PubMed] [Google Scholar]
- 29Pun PH, Lehrich RW, Honeycutt EF et al. Modifiable risk factors associated with sudden cardiac arrest within hemodialysis clinics. Kidney Int 2011; 79: 218–227. [DOI] [PubMed] [Google Scholar]
- 30Wingard RL, Chan KE, Lazarus JM et al. The "right" of passage: surviving the first year of dialysis. Clin J Am Soc Nephrol 2009; 4(Suppl 1): S114–S120. [DOI] [PubMed] [Google Scholar]
- 31Wright S, Klausner D, Baird B et al. Timing of dialysis initiation and survival in ESRD. Clin J Am Soc Nephrol 2010; 5: 1828–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32Lassalle M, Labeeuw M, Frimat L et al. Age and comorbidity may explain the paradoxical association of an early dialysis start with poor survival. Kidney Int 2010; 77: 700–707. [DOI] [PubMed] [Google Scholar]
- 33Susantitaphong P, Koulouridis I, Balk EM et al. Effect of frequent or extended hemodialysis on cardiovascular parameters: a meta-analysis. Am J Kidney Dis 2012; 59: 689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34Susantitaphong P, Altamimi S, Ashkar M et al. GFR at initiation of dialysis and mortality in CKD: a meta-analysis. Am J Kidney Dis 2012; 59: 829–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Schell JO, Da Silva-Gane M, Germain MJ. Recent insights into life expectancy with and without dialysis. Cur Opin Nephrol Hypertens 2013; 22: 185–192. [DOI] [PubMed] [Google Scholar]
- 36Williams AW. Older adults with CKD and acute kidney failure: do we know enough for critical shared decision making? J Am Soc Nephrol 2014; 25: 5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37de Francisco AL, Kim J, Anker SD et al. An epidemiological study of hemodialysis patients based on the European Fresenius Medical Care hemodialysis network: results of the ARO study. Nephron Clin Pract 2011; 118: c143–c154. [DOI] [PubMed] [Google Scholar]
- 38Woodward M. Epidemiology : Study Design and Data Analysis. 2nd edn. USA edn. Chapman & Hall/CRC: Boca Raton, FL, London, 2005. [Google Scholar]
- 39Ulm K. A simple method to calculate the confidence interval of a standardized mortality ratio (SMR). Am J Epidemiol 1990; 131: 373–375. [DOI] [PubMed] [Google Scholar]
- 40Shahar E. Estimating the Rate Ratio. In: The Logic and Math of Causal Inquiry: A Guide for the Perplexed. University of Arizona: Arizona, 2007. http://www.u.arizona.edu/~shahar/book/Chapter%2017.pdf. [Google Scholar]
- 41Symons MJ, Moore DT. Hazard rate ratio and prospective epidemiological studies. J Clin Epidemiol 2002; 55: 893–899. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.