Background: PslG has been implicated in Psl biosynthesis.
Results: The structure of PslG was determined. PslG was shown to hydrolyze Psl, and in vitro and in vivo functional studies were performed.
Conclusion: PslG has a distinct endo-acting-like active site groove. Deletion of pslG did not impact Psl synthesis or biofilm formation.
Significance: Regulation of PslG is important for Psl biosynthesis and biofilm formation.
Keywords: bacterial genetics, biofilm, crystal structure, glycoside hydrolase, polysaccharide, Psl, PslG, exopolysaccharide biosynthesis
Abstract
A key component of colonization, biofilm formation, and protection of the opportunistic human pathogen Pseudomonas aeruginosa is the biosynthesis of the exopolysaccharide Psl. Composed of a pentameric repeating unit of mannose, glucose, and rhamnose, the biosynthesis of Psl is proposed to occur via a Wzx/Wzy-dependent mechanism. Previous genetic studies have shown that the putative glycoside hydrolase PslG is essential for Psl biosynthesis. To understand the function of this protein, the apo-structure of the periplasmic domain of PslG (PslG(31–442)) and its complex with mannose were determined to 2.0 and 1.9 Å resolution, respectively. Despite a domain architecture and positioning of catalytic residues similar to those of other family 39 glycoside hydrolases, PslG(31–442) exhibits a unique 32-Å-long active site groove that is distinct from other structurally characterized family members. PslG formed a complex with two mannose monosaccharides in this groove, consistent with binding data obtained from intrinsic tryptophan fluorescence. PslG was able to catalyze the hydrolysis of surface-associated Psl, and this activity was abolished in a E165Q/E276Q double catalytic variant. Surprisingly, P. aeruginosa variants with these chromosomal mutations as well as a pslG deletion mutant were still capable of forming Psl biofilms. However, overexpression of PslG in a pslG deletion background impaired biofilm formation and resulted in less surface-associated Psl, suggesting that regulation of this enzyme is important during polysaccharide biosynthesis.
Introduction
Pseudomonas aeruginosa is a ubiquitous, opportunistic Gram-negative pathogen capable of causing acute and chronic infections. During many infections, the bacteria switch from a planktonic mode of growth to form a matrix-encapsulated, surface-associated community known as a biofilm. The components of the biofilm vary but are generally composed of proteinaceous adhesins, nucleic acids, and exopolysaccharides that are functionally important in bacterial attachment, reducing antibiotic diffusion (1, 2), and providing a barrier against phagocytosis and the immune system (3). P. aeruginosa has the genetic capacity to produce three different exopolysaccharides, known as Psl, Pel, and alginate (4, 5). The Pel and Psl polysaccharides are utilized in non-mucoid isolates for biofilm formation. During airway colonization and infection of individuals with cystic fibrosis, P. aeruginosa undergoes phenotypic conversion from a non-mucoid to mucoid morphology that is associated with alginate production. Although alginate is the major exopolysaccharide constituent of mucoid biofilms, Pel and Psl remain functionally important (6, 7). Pel is composed of N-acetylgalactosamine and N-acetylglucosamine (82), and Psl is composed of a pentasaccharide-repeating unit of d-mannose, d-glucose, and l-rhamnose, which is distinct from other known polysaccharides (8). Together, Psl and Pel function to enhance adhesion (8, 9), and they play a significant role in the formation and maintenance of the biofilm architecture (1, 6, 10). Psl provides protection against the immune system (3) and is a first line of defense during the initial stages of biofilm development, especially toward attack by antibiotics (2). Patients recovering from P. aeruginosa infections have specific antibodies against Psl, demonstrating that the polysaccharide is clinically relevant during infection (11).
Despite the chemical diversity of bacterial exopolysaccharides, the means by which they are synthesized and exported from the bacterium have been categorized into three distinct mechanisms (12). In each mechanism, the proteins required to polymerize, modify, and export these exopolysaccharides are typically encoded on a single operon (13–15). One feature shared among many exopolysaccharide biosynthetic operons is the presence of a putative glycoside hydrolase (GH)6 or lyase. Examples include Escherichia coli BcsZ, Pseudomonas fluorescens WssD, P. aeruginosa AlgL, Listeria monocytogenes PssZ, the N-terminal domain of P. aeruginosa PelA, and the C-terminal domain of E. coli PgaB involved in cellulose, acetylated cellulose, alginate, the L. monocytogenes exopolysaccharide, Pel, and PNAG biosynthesis, respectively (16–21). The function of putative polysaccharide-degrading enzymes in exopolysaccharide biosynthetic operons is not intuitive or well defined; however, genetic deletions indicate that many are obligatory for polysaccharide biosynthesis (20, 22–25).
The Psl polysaccharide is synthesized by the proteins encoded by the psl (polysaccharide synthesis locus) operon (13), and in-frame deletion and complementation studies of the individual psl genes revealed that 11 of the 15 genes are required for Psl production and biofilm formation (8). Although the mechanistic details of Psl biosynthesis have not been fully defined, it is proposed to occur via a Wzx/Wzy-dependent mechanism similar to the E. coli group 1 capsular and extracellular polysaccharides (4). The Wzx/Wzy-dependent pathway utilizes a lipid acceptor to initiate polysaccharide synthesis and exports the polymer through a member of the OPX (outer membrane polysaccharide exporter) family (26). PslA is thought to function analogously to WbaP to provide a site for assembly of the oligosaccharide repeating unit onto an isoprenoid lipid carrier on the cytoplasmic face of the inner membrane (27). Based on structural homology modeling, PslD and PslE are proposed to be analogous in function to the OPX Wza, and tyrosine autokinase Wzc, respectively (4, 28), whereas PslJKL are proposed to constitute the polymerization and flippase machinery (4). Although the function of PslG is unknown, prior chromosomal deletion of pslG revealed its necessity for Psl synthesis and biofilm formation (8).
Herein, we present the P. aeruginosa PslG structure, the first protein in the Psl biosynthetic pathway to be structurally defined. PslG was located in the periplasm and anchored to the inner membrane by an N-terminal transmembrane domain. Structural alignment with members of the glycoside hydrolase family 39 (GH39) reveals a conserved orientation of the catalytic residues but a distinct difference in the overall structure of the active site groove. We demonstrate that, contrary to our previously published results (8), neither PslG nor its enzymatic activity appear to be required for Psl biosynthesis and biofilm formation. Overexpression of pslG led to a dose-dependent impairment of surface-associated Psl biosynthesis and biofilm formation, indicating that levels of PslG are critical for these processes.
Experimental Procedures
Bacterial strains, plasmids, and oligonucleotide primers used in this study are described in Table 1.
TABLE 1.
Bacterial strains and plasmids used in this study
Antibiotic resistance markers are abbreviated as follows: KanR, kanamycin resistance; GmR, gentamycin resistance; TetR, tetracycline resistance; CamR, chloramphenicol resistance.
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Strains | ||
| E. coli TOP10TM | F− mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 recA1 araD139 Δ(ara-leu) 7697 galU galK rpsL (Strr) endA1 nupG λ- | Invitrogen |
| E. coli BL21-CodonPlus® (DE3)-RP | F− ompT hsdS (rB− mB−) dcm+ Tetr galλ (DE3) endA [argU proL Camr] | Stratagene |
| P. aeruginosa PAO1 | WT | Ref. 79 |
| PAO1 Δpsl | Deletion of the psl promoter | Ref. 9 |
| PAO1 ΔpslG | pslG deletion in PAO1 | Ref. 8 |
| PAO1 PBADpsl | psl-araC-PBAD promoter replacement; expression of psl operon upon induction with l-arabinose | Ref. 9 |
| PAO1 PBADpsl ΔpelF | In-frame deletion of pelF in PBADpsl | This study |
| PAO1 PBADpsl ΔpelF ΔpslG | In-frame deletion of pslG in PBADpsl ΔpelF | This study |
| PAO1 PBADpsl ΔpelF ΔpslD | In-frame deletion of pslD in PBADpsl ΔpelF | This study |
| PAO1 PBADpsl ΔpelF pslGE165Q | Chromosomal mutation of PslG E165Q in PBADpsl ΔpelF | This study |
| PAO1 PBADpsl ΔpelF pslGE276Q | Chromosomal mutation of PslG E276Q in PBADpsl ΔpelF | This study |
| PAO1 PBADpsl ΔpelF pslGE165Q/E276Q | Chromosomal mutation of PslG E165Q/E276Q in PBADpsl ΔpelF | This study |
| Plasmids | ||
| pEX18Gm | Suicide cloning vector, GmR | Ref. 33 |
| pEX18Gm::ΔpelF | pelF deletion construct, GmR | This study |
| pEX18Gm::ΔpslG | pslG deletion construct, GmR | This study |
| pPSV39 | P. aeruginosa in trans overexpression plasmid, GmR | Ref. 80 |
| pPSV39::pslG(1–442) | PslG (1–442) overexpression vector | This study |
| pET28a | E. coli expression vector with N-terminal His6 tag, KanR | Novagen |
| pET28a::pslG(31–442) | PslG (31–442) overexpression vector | This study |
| pET28a::pslG(40–360) | PslG (40–360) overexpression vector | This study |
| pET28a::pslG(31–442) E165Q | PslG (31–442) containing E165Q mutation overexpression vector | This study |
| pET28a::pslG(31–442) E276Q | PslG (31–442) containing E276Q mutation overexpression vector | This study |
| pET28a::pslG(31–442) E165Q/E276Q | PslG (31–442) containing E165Q/E276Q mutation overexpression vector | This study |
Cloning, Expression, and Purification of PslG Constructs
The DNA sequence of pslG from P. aeruginosa PAO1 was previously deposited in GenBankTM under accession number AAG05625.1 (29). The TMHMM server version 2.0 (30) and SignalP version 4.1 (31) were used to determine whether PslG possesses a transmembrane helix or signal sequence, respectively. To obtain a soluble protein construct, the pslG gene, minus its predicted transmembrane segment, was amplified from genomic DNA by PCR using the primers GGGCATATGGAGATCCAGGTACTGAAG and GGGAAGCTTTCACTCCCAGACCAGCA to encompass amino acid residues 31–442. Introduced NdeI and HindIII restriction sites are underlined, and the gene was ligated into the pET28a (Novagen) expression vector encoding an N-terminal His tag. Site-directed mutagenesis to generate E165Q and E276Q variants was performed using the QuikChange® Lightning kit according to the prescribed protocol (Agilent Technologies). Constructs generated were verified by sequencing performed by ACGT DNA Technologies Corp. (Toronto, Canada).
E. coli BL21 (DE3) CodonPlus cells (Stratagene) were transformed with expression plasmids and grown in 2 liters of lysogeny broth (LB) containing 50 μg/ml kanamycin at 37 °C. When the A600 of the cell culture reached 0.5–0.6, protein expression was induced by the addition of isopropyl β-d-1-thiogalactopyranoside (IPTG) to a final concentration of 0.5 mm. The cells were incubated postinduction overnight at 18 °C with shaking at 200 rpm before being harvested by centrifugation at 5,000 × g for 30 min at 4 °C. Cell pellets were resuspended in 40 ml of buffer A (20 mm imidazole, 50 mm Tris-HCl, pH 7.5, 300 mm NaCl, 2% (v/v) glycerol) plus one SIGMAFASTTM protease inhibitor tablet, and the cells were lysed by at least three passes through an Emulsiflex C3 homogenizer at 100 megapascals (Avestin Inc.). The resulting cell debris was separated from soluble protein by centrifugation at 35,000 × g for 30 min. The supernatant was applied to 5 ml of nickel-nitrilotriacetic acid Superflow resin packed into a gravity column (Qiagen) pre-equilibrated with buffer A. The column was washed with 3 column volumes of buffer A, and the expressed protein was eluted with buffer A supplemented with 250 mm imidazole. The eluted fractions were pooled and dialyzed against 4 liters of buffer B (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 2% (v/v) glycerol) overnight at 4 °C. The His tag was removed by incubating the protein at 25 °C for 3 h with 1 unit of thrombin (Novagen) per 4 mg of protein. Untagged protein was separated from tagged protein by a second round of nickel affinity purification. The untagged protein was collected and buffer-exchanged into buffer B by size exclusion chromatography using a HiLoad 16/60 Superdex 200 gel filtration column (GE Healthcare). The purity of protein was judged to be >95% by SDS-PAGE, and the protein could be concentrated to 8–10 mg/ml and stored at 4 °C for more than 1 month without precipitation or degradation.
Strain Construction
Chromosomal deletion mutations were constructed using an unmarked, non-polar allelic replacement strategy (32). For the ΔpelF deletion allele, flanking regions of pelF were amplified from P. aeruginosa PAO1 genomic DNA and combined using splicing by overlap extension PCR. This PCR product was ligated into the suicide vector pEX18Gm (33), and the plasmid pEX18Gm::ΔpelF was verified by sequence analysis (Center for Applied Genomics). Allelic exchange plasmids were transferred into P. aeruginosa PAO1 PBADpsl (9) by biparental mating with E. coli SM10 (34). Single recombinant mutants were selected on Vogel-Bonner minimal medium containing 30 μg/ml gentamicin. Double recombinant mutants were selected on LB agar without NaCl containing 15% (w/v) sucrose and were confirmed by sequence analysis. The ΔpslG deletion allele was assembled, transferred into P. aeruginosa PAO1 ΔpelF PBADpsl, and incorporated into the chromosome as described above. Chromosomal point mutations were constructed using an unmarked, nonpolar allelic replacement strategy similar to that described above. A fragment excluding the first 200 base pairs from the 5′-end and the last 250 base pairs from the 3′-end of pslG was amplified from P. aeruginosa PAO1 genomic DNA, allowing for ∼300 base pairs on either side of the codons corresponding to Glu-165 and Glu-276 of PslG. This fragment was ligated into the suicide vector pEX18Gm (33), and the plasmid pEX18Gm::pslG was verified by sequence analysis (Center for Applied Genomics, Toronto, ON). The E165Q and E276Q point mutants were generated from pEX18Gm::pslG using the QuikChange® Lightning site-directed mutagenesis kit (Stratagene), and each plasmid was verified by sequence analysis. Allelic exchange plasmids were transferred into P. aeruginosa PAO1 PAO1 ΔpelF PBADpsl and incorporated into the chromosome as described above. Cloning of full-length pslG into the IPTG-inducible expression plasmid pPSV39 was completed to allow for in trans complementation in ΔpslG strains. All genomic constructs were verified by PCR amplification of the locus of interest with gene-specific primers, followed by DNA sequencing.
Subcellular Fractionation
Subcellular fractionation was adapted from a previously described procedure (35). Briefly, 1 liter of P. aeruginosa PAO1 culture grown overnight was harvested by centrifugation at 10,000 × g for 30 min. The pellet was resuspended in 5 ml of 0.2 m Tris-HCl, pH 8.0, 1 m sucrose, 1 mm EDTA, and 1 mg/ml lysozyme and incubated at room temperature for 5 min. At this time, 20 ml of distilled H2O was added, gently swirled to mix, and placed on ice for 20 min. The sample was centrifuged at 181,000 × g for 45 min at 4 °C, and the supernatant fraction was removed for periplasmic analysis. The pellet consisting of outer membranes and spheroplasts was resuspended in 50 ml of 10 mm Tris-HCl, pH 7.5, 5 mm EDTA, 1 mm DTT, and 7 μg/ml DNase I. The resulting suspension was homogenized using an Emulsiflex C3 at 100 megapascals. Unlysed cells were removed by centrifugation at 30,000 × g for 20 min. The supernatant was further centrifuged at 181,000 × g for 2 h. The supernatant consisted of the cytoplasmic fraction, and the pellet contained the membrane fraction. The pellet was resuspended in 25 ml of 50 mm Tris-HCl, pH 8.0, 2% (v/v) Triton X-100, and 10 mm MgCl2. The sample was centrifuged at 126,000 × g for 30 min at 4 °C, and the resulting supernatant contained the inner membrane fraction, whereas the pellet contained the outer membrane fraction. The pellet was again washed in 50 mm Tris-HCl, pH 8.0, 2% (v/v) Triton X-100, and 10 mm MgCl2 and centrifuged at 126,000 × g for 30 min at 4 °C. These steps were repeated until the pellet was clear, leaving only outer membranes.
Antibody Purification
Purified PslG(31–442) with the His tag removed was used to generate antiserum from rabbits using a 70-day standard protocol from Cedarlane Laboratories (Burlington, Canada). To purify the antibody, purified PslG(31–442) was separated at a concentration of 10 mg/ml by SDS-PAGE, transferred to a nitrocellulose membrane, and stained with Ponceau S. Membrane containing the PslG band was excised and washed with distilled H2O to remove the stain. The excised bands were cut into small pieces and blocked in 1× PBS, 0.1% (v/v) Tween 20, and 5% (w/v) skim milk powder for 1 h. The membrane was subsequently incubated with the recovered antiserum overnight at 4 °C. The membrane was washed three times for 15 min each in 1× PBS (pH ∼7.4) containing 0.1% Tween 20, followed by 15 min in 1× PBS. The membrane was incubated in 700 μl of 0.2 m glycine, pH 2.2, for 15 min to elute the antibody, and the solution was neutralized using 300 μl of 1 m K2HPO4. The resulting solution was dialyzed using 3,500 Da molecular mass cut-off dialysis tubing for 24 h against PBS. Glycerol was added 1:1 with the solution for antibody storage at −20 °C.
Crystallization, Data Collection, and Structure Solution
Purified PslG(31–442) minus its N-terminal His6 tag was concentrated to ∼8 mg/ml, and initial crystallization trials were performed using a Gryphon robot (Art Robbins) with 96-well 2-drop sitting drop Art Robbins Instruments Intelli-Plates® (Hampton Research) and the MCSG-1 to -4 sparse matrix screens (Microlytic). PslG(31–442) in the presence of 3 m mannose (PslG(31–442) + Man) was also screened for crystallization hits as described above. Protein (1 μl) was mixed with precipitant in a 1:1 ratio and equilibrated against 70 μl of precipitant using the sitting drop vapor diffusion method at 20 °C. Initial crystallization hits of the apoenzyme were obtained in the absence of the N-terminal His6 tag from condition 19, MCSG-3 suite (0.005 m cobalt chloride, 0.005 m magnesium chloride, 0.005 m nickel chloride, 0.1 m HEPES/NaOH, pH 7.5, 12% (w/v) polyethylene glycol (PEG) 3350) and condition 94, MCSG-4 suite (0.2 m zinc acetate, 0.1 m imidazole/HCl, pH 6.5, 10% (w/v) PEG 8000). Grid-optimized screens were generated by varying PEG concentration and different divalent transition metals in 48-well hanging-drop VDX plates (Hampton Research) using a 2-μl drop with a 1:1 protein/precipitant ratio. Diffraction quality crystals were grown using 1 mm CdCl2, 0.1 m HEPES, pH 7.0, 5% (w/v) PEG 3350 at 20 °C. An identical buffer supplemented with 2 mm CdCl2 and 3 m mannose was used in the crystallization of the mannose-containing structure.
Apo-PslG(31–442) crystals were cryoprotected for 10 s in precipitant solution supplemented with 25% (v/v) ethylene glycol prior to vitrification in liquid nitrogen. Diffraction data were collected at 100 K with a wavelength of 1.075 Å on beamline X29, National Synchrotron Light Source (Table 2). A high redundancy data set was generated by collecting 90 images with 2° oscillation at 95% beam attenuation and 720 images with 0.5° oscillation without beam attenuation on an ADSC Quantum-315 detector with a 250-mm crystal-to-detector distance and an exposure time of 0.3 s/image. All data were indexed, integrated, merged, and scaled using HKL2000 (36). Autosol (37) was used with the high redundancy data set to determine initial phases using the cadmium anomalous signal. A total of four experimental cadmium sites were identified and then used to generate a density-modified map. The resulting electron density map was of high quality and enabled PHENIX AutoBuild to build >95% of the protein. The remaining residues were built manually in COOT (38, 39) and alternated with refinement using PHENIX.REFINE (40). Translation/libration/screw groups were added to the refinement in PHENIX through the use of the TLSMD server (41, 42).
TABLE 2.
Summary of data collection and refinement statistics
Values in parentheses correspond to the highest resolution shell.
| PslG(31–442) | PslG(31–442) + mannose | |
|---|---|---|
| Data collection | ||
| Beamline | NSLS X29 | CLS 08ID-1 |
| Wavelength (Å) | 1.075 | 0.979 |
| Space group | P41212 | P41212 |
| Cell dimensions | ||
| a, b, c (Å) | 83.2, 83.2 163.40 | 83.6, 83.6 163.4 |
| α, β, γ (degrees) | 90, 90, 90 | 90, 90, 90 |
| Resolution (Å) | 50.00–2.00 (2.07–2.00) | 50.00–1.90 (1.97–1.90) |
| No. of reflections | 1,197,746 | 563,367 |
| No. of unique reflections | 39,575 | 46,642 |
| Redundancy | 30.3 (28.7) | 12.1 (12.5) |
| I/σI | 36.8 (6.1) | 36.7 (4.8) |
| Completeness (%) | 100 (100) | 100 (100) |
| Rmerge (%)a | 13.8 (65.6) | 10.7 (63.9) |
| Refinement | ||
| Rwork/Rfreeb | 14.9/18.8 | 15.5/18.7 |
| No. of atoms | ||
| Protein | 3346 | 3341 |
| Cadmium | 4 | 4 |
| Chlorine | 1 | 1 |
| Ligands | 16 | 52 |
| Water | 387 | 355 |
| Average B-factors (Å2)c | ||
| Protein | 23.2 | 27.1 |
| Cadmium | 57.6 | 51.5 |
| Chlorine | 52.8 | 65.0 |
| Ligands | 46.8 | 73.1 |
| Water | 32.7 | 37.2 |
| Root mean square deviations | ||
| Bond lengths (Å) | 0.007 | 0.007 |
| Bond angles (degrees) | 1.03 | 1.12 |
| Ramachandran plotc | ||
| Total favored (%) | 97.3 | 96.8 |
| Total allowed (%) | 100 | 100 |
| Coordinate error (Å)d | 0.16 | 0.16 |
| Protein Data Bank code | 5BX9 | 5BXA |
a Rmerge = ΣΣ|I(k) − 〈I〉|/ΣI(k), where I(k) and 〈I〉 represent the diffraction intensity values of the individual measurements and the corresponding mean values. The summation is over all unique measurements.
b Rwork = Σ‖Fo| − k|Fc‖/|Fo|, where Fo and Fc are the observed and calculated structure factors, respectively. Rfree is the sum extended over a subset of reflections (5%) excluded from all stages of the refinement.
c As calculated using MolProbity (81).
d Maximum likelihood-based coordinate error, as determined by PHENIX (39).
PslG(31–442) + Man crystals were not cryoprotected prior to vitrification in liquid nitrogen, and any residual ice was removed prior to data collection through liquid nitrogen washing and annealing. Diffraction data were collected at 100 K with a wavelength of 0.979 Å on beamline 08ID-1 at the Canadian Light Source (Table 2). A total of 180 images were collected with 1° oscillation without beam attenuation on a Rayonix MX300 CCD detector with an exposure time of 1.0 s/image. All data were indexed, integrated, merged, and scaled using HKL2000 (36). The structure was determined by molecular replacement with PHENIX AutoMR (39) using apo-PslG as a search model. Refinement was completed as described for the apo-PslG structure. All structure figures were generated using the PyMOL molecular graphics system (DeLano Scientific) (43), and quantitative electrostatics were calculated using PDB2PQR (43, 44) and APBS (45). Amino acid conservation was calculated using the Consurf server (46). Programs used for crystallographic data processing and analysis were accessed through SBGrid (47).
Psl Dot Blot Assay
Overnight arabinose-induced 2-ml cultures of P. aeruginosa Δpel PBADpsl and pslG chromosomal point mutants pslGE165Q, pslGE276Q, and pslGE165Q/E276Q were normalized for an A600 of 1.0 and centrifuged at 21,000 × g for 2 min. The supernatant was removed, and the cell pellet was resuspended in 1 ml of 1× PBS to wash the cells and then centrifuged again at 21,000 × g for 2 min. Psl dot blots to demonstrate the presence of Psl production were then performed as previously described (8).
Confocal Microscopy
All biofilms were grown overnight at room temperature in ibidi uncoated μ-Slide VI0.4 flow cell chambers (ibidi, Martinsfried, Germany). The channels were inoculated with 200 μl of culture with an A600 of 0.5 and grown in LB without salt supplemented with 0.5% l-arabinose. Biofilms were washed three times with sterile PBS and then stained with FITC-conjugated hippeastrum hybrid amaryllis (EY Laboratories, San Mateo, CA) lectin at 100 μg/ml for 2 h at 4 °C. The biofilms were then washed and fixed with 4% paraformaldehyde. Fluorescent images were acquired using an Olympus FV1000 filter confocal system using a ×20 LUCPLFLN, numerical aperture 0.45 objective lens (Olympus America, Melville, NY). Images were analyzed and constructed using Olympus Fluoview version 03.01 software.
Microtiter Dish Biofilm Assay
Overnight cultures were diluted 1:100 in LB without salt, and the cultures were incubated statically in flat bottom polystyrene plates (Thermo Fisher Scientific, catalog no. 243656) for 20 h at 25 °C to allow for biofilm formation. To eliminate edge effects, ∼200 μl of sterile water was placed in all outside wells, and the plate was sealed with parafilm. After incubation, non-adherent cells and media were removed by thoroughly washing the plate with deionized water. The wells were stained with 150 μl of 0.1% (w/v) crystal violet for 10 min, followed by rinsing with water. The remaining dye was solubilized by the addition of 200 μl of 95% (v/v) ethanol and left for 10 min, after which time the absorbance was measured at 595 nm using a SpectraMax M2 spectrophotometer from Molecular Devices (Sunnyvale, CA). The amount of biofilm is proportional to the absorbance from staining with crystal violet (48).
Preparation of Surface-associated Psl
P. aeruginosa strain WFPA801 (9) was grown overnight in 500 ml of Jensen's medium with the addition of 0.2% l-arabinose. Biomass was collected by centrifugation at 27,000 × g, and the resultant bacterial pellets were suspended in 0.9% NaCl. Cell surface-associated polymers were detached by mild sonication using a Sonic Dismembrator 100 probe sonicator (Fisher) with three cycles of 1-min sonication and 1-min rest at 50% power. The released material was collected by centrifugation at 27,000 × g for 1 h. The bacterial pellets were suspended and extracted twice more, and the supernatants were combined and lyophilized. To separate the cell-associated polysaccharides from the rest of the crude matrix extract, the dried pellets were suspended in cold 75% ethanol (v/v) and precipitated at 4 °C overnight. The precipitate was collected by centrifugation at 27,000 × g for 1 h, and the supernatant was discarded. The precipitate was again suspended in cold 95% ethanol (v/v) and dislodged from the side of the centrifuge tube using a metal spatula. The suspension was centrifuged as described previously, and the supernatant was discarded. Pelleted material was washed once more with cold 95% ethanol (v/v), followed by one wash with cold absolute ethanol. The resulting pellet was allowed to air dry at room temperature overnight. To completely remove any residual nucleic acid or protein contaminates, the crude polysaccharides were suspended in a minimal volume of sterile, endotoxin-free water. DNase I and RNase A were added to a final concentration of 0.1 mg/ml. The samples were incubated overnight at 37 °C with agitation. Following nucleic acid digestion, proteinase K (Qiagen, Valencia, CA) was added to a final concentration of 0.1 mg/ml and incubated overnight at 37 °C with agitation. The exopolysaccharides were transferred to a 3,500 Da molecular mass cut-off Slide-A-Lyzer dialysis cassette (Pierce) and dialyzed for three complete exchanges using sterile water at 4 °C prior to lyophilization.
Enzyme Assays
To probe for glycoside hydrolase activity, synthetic substrates 4-nitrophenyl β-d-glucopyranoside, 4-nitrophenyl β-d-mannopyranoside, and 4-nitrophenyl β-d-rhamnopyranoside (Sigma-Aldrich) were dissolved in DMSO to a final concentration of 2 mm and were added to a reaction containing 40 μg of PslG(31–442) in 100 mm HEPES buffer, pH 7.0, in a total reaction volume of 100 μl. Reactions were initiated by the addition of substrate and allowed to proceed for 120 min. Reaction progress was monitored in real time at 405 nm for the appearance of 4-nitrophenyl. Hydrolysis of isolated surface-associated Psl polysaccharide was determined by measuring an increase in reducing ends using the 3-methyl-2-benzothiazolinonehydrazone assay as described previously (49). Standard end point reactions contained 1.6 mg/ml purified Psl polysaccharide and 10 μg of PslG(31–442) in a total volume of 100 μl of 1× PBS, pH 7.4, at 25 °C. Following 20 h of incubation, the reactions were quenched by the addition of equal volumes of 0.5 m NaOH and 3-methyl-2-benzothiazolinonehydrazone/DTT solution to that of the reaction. The samples were heated at 80 °C for 15 min, after which time 2 volumes of 0.5% FeNH4(SO)4·12H2O, 0.5% sulfamic acid in 0.25 n HCl was added. The samples were diluted by one-third in distilled H2O, and the absorbance of each sample was determined at 620 nm. The hydrolysis of the substrate in the absence of enzyme, in 1× PBS buffer and signal from the enzyme were determined and subtracted from enzyme-catalyzed reactions. A calibration curve for glucose was obtained under the reaction conditions and used to calculate the concentration of newly formed reducing ends. The protein concentration of each enzyme variant was determined using the Pierce BCA protein assay kit from Thermo Scientific (Rockford, IL).
Monosaccharide-binding Assay
The binding of l-rhamnnose, d-glucose, and d-mannose to PslG(31–442) was monitored by intrinsic protein fluorescence quenching. Fluorescence measurements were carried out at 20 °C in a quartz cuvette (type no. 115F-QS; Hellma Analytics) using a PTI QuantaMaster 80 steady-state fluorimeter (Photon Technology International), with a 4-nm bandwidth for both excitation and emission and a speed of 2 nm/s. Fluorescence spectra were collected between 300 and 400 nm with an excitation wavelength (λex) of 283 nm, with a peak emission wavelength (λem) of 332 nm used for the calculation of the dissociation constant. Fluorescence data were collected by the addition of varying concentrations of sugar monomers into 400 μl of 1 μm PslG(31–442) in 20 mm Tris-HCl (pH 7.0) and 150 mm NaCl. Each mixture was incubated for >3 min before spectra were collected. Varying concentrations between 0.1 m and 1.5 m were collected for all rhamnose, glucose, and mannose. Titrations were corrected for ligand fluorescence and inner filter effect. All ligands had linear fluorescence over the concentration range used in this study and did not exceed 5% of PslG(31–442) fluorescence.
Results
PslG Is an Inner Membrane-associated Family 39 Glycoside Hydrolase
Glycoside hydrolases are grouped into families based on amino acid sequence similarities that reflect structural features rather than substrate specificity (50). Examination of the CAZy database indicates that PslG is a member of the GH39 family (51). Further bioinformatics analysis with the TMHMM server (30) suggests that PslG is anchored to the inner membrane by a single N-terminal transmembrane domain composed of residues 5–24 and contains a soluble periplasmic domain from residue 25 to 442 (Fig. 1A). To determine the subcellular localization of PslG, membrane fractionation was carried out using P. aeruginosa strain PAO1. The inner membrane protein PilP was used as a control (52) to demonstrate successfully fractionation of soluble and membrane components (Fig. 1B). Utilizing antibodies generated against PslG(31–442), PslG was found to be enriched in the inner membrane fraction. A smaller percentage of the PslG was also found in the periplasmic fraction. The lower molecular weight PslG observed in the periplasm is probably produced by the cleavage of the transmembrane domain (3 kDa), generating a soluble protein. Fractionation supports the results of the TMHMM prediction, and the presence of PslG in the periplasmic fraction orients the soluble domain to the periplasmic side of the inner membrane.
FIGURE 1.

PslG is an inner membrane-associated protein. A, the TMHMM server predicts PslG to contain a single pass N-terminal transmembrane domain. B, Western blot analysis of the cytoplasmic (C), inner membrane (IM), and periplasmic (P) fractions using α-PslG and α-PilP. The inner membrane lipoprotein PilP was utilized as a control.
The structure of PslG(31–442) Reveals a Unique Groove
To gain structural insight into the function of PslG, a construct encoding the entire predicted soluble domain, residues 31–442 (PslG(31–442)), was expressed and purified to homogeneity. After removal of the His-tag, PslG(31–442) was subjected to crystallization trials, and protein crystals were obtained in the presence of divalent transition metal ions, including Ni2+, Cu2+, Co2+, Zn2+, and Cd2+. Diffraction data were collected to 2.0 Å on a crystal grown in the presence of 1 mm CdCl2 (Table 2). Four cadmium ions are bound to the protein, resulting in metal-mediated symmetrization to form a crystal lattice and allowing the structure to be determined using cadmium single-wavelength anomalous diffraction. PslG crystallized in space group P41212 with one molecule in the asymmetric unit. This is consistent with analytical size exclusion chromatography that indicates that PslG is a monomer in solution. Refinement produced a final model with an Rwork and Rfree of 14.9 and 18.8%, respectively.
PslG(31–442) is a two-domain protein with an N-terminal (β/α)8 barrel fold and C-terminal β-sandwich fold (Fig. 2A). The putative catalytic domain has a canonical (β/α)8 fold with six large loops (L1–L6) present on one face of the fold (Fig. 2B). These large loops contain small secondary structural elements, which function to both connect the α-helices and β-sheets of the (β/α)8 fold and form a ∼32-Å-long, sickle-shaped groove on one side of the molecule (Fig. 2C). The β-sandwich domain is composed of one small β-strand preceding (residues 33–35) and six β-strands (residues 360–442) following the (β/α)8 catalytic domain. Electrostatic surface potential analysis indicates that PslG(31–442) is predominantly electronegative with distinct patches of acidic electronegative residues localized in the center of the groove (Fig. 3A). This groove is lined with several conserved surface-exposed aromatic residues, Tyr-114, Phe-208, Tyr-239, Phe-319, and Tyr-335, which probably facilitate the proper binding orientation of the polysaccharide for catalysis (Fig. 3B).
FIGURE 2.
Structure of PslG. A, overall structure of PslG(31–442) shown in a schematic representation with the canonical α-helixes and β-strands that compose the (β/α)8-fold labeled and colored green and blue, respectively. Secondary structures that are not components of the (β/α)8-fold are colored in yellow, and the β-strands that form the β-sandwich domain are depicted in red. N and C termini are labeled accordingly. B, the six loops, L1–L6, that form the active site groove are shown in the same orientation as in A. The residues in each loop are shown. The active site groove is depicted as a gray surface. C, side view of the (β/α)8-fold and active site groove. The color scheme is as described for A.
FIGURE 3.
Surface representation of PslG reveals a conserved electronegative groove. A, electrostatic surface representation of the active site groove of PslG. Quantitative electrostatics are colored from red (−5 kT/e) to blue (+5 kT/e). B, surface representation of amino acid conservation in the groove. Inset, highly conserved residues in proximity to the catalytic residues Glu-165 and Glu-276. Electrostatics were generated in PyMOL using APBS (45), and amino acid conservation was calculated using the Consurf server (46).
Structural Comparison of PslG(31–442) Reveals Distinct Differences Relative to Other GH39 Members
GH family 39 utilizes a retaining catalytic mechanism requiring two glutamic acid residues that function as the acid/base and nucleophile (53). All prokaryotic GH39 members characterized to date exhibit β-xylosidase activity, whereas eukaryotic members display α-l-iduronidase activity (54). Structural alignment of PslG(31–442) using DaliLite (55) to other GH39 members indicates that the top two hits, β-xylosidases XynB1 from Geobacillus stearothermophilus (Protein Data Bank entry 2BS9) (56) and XynB from Thermoanaerobacterium saccharolyticum (Protein Data Bank entry 1UHV) exhibit only 19 and 15% sequence identity, respectively. Both proteins align with PslG with root mean square deviations of 3.7 Å over 357 Cα atoms (Fig. 4A). Examination of the active site of PslG(31–442) and other GH39 members reveals that residues Glu-165 and Glu-276, located in the center of the active site groove, superimpose with the acid/base and nucleophile residues implicated in catalysis in these proteins (57, 58) (Fig. 4B). PROPKA3 (59) predicts the pKa of PslG Glu-165 as 5.63 and the pKa of PslG Glu-276 as 3.33, supporting their role as the acid/base and nucleophile, respectively. Furthermore, several invariant residues, including Asn-164, His-237, and Tyr-239, of PslG are located at comparable positions in the active site of other GH39 members. Two notable differences observed in PslG are (i) that Phe-319 replaces a conserved tryptophan and (ii) the absence of a histidine residue that in other GH39 members is involved in substrate binding (Fig. 4B). Although residues in the active site superimpose well, closer examination of PslG reveals that the structure lacks several accessory motifs that are conserved among structurally characterized GH39 members. The absent motifs include a catalytic β-hairpin motif that decorates the (β/α)8-barrel fold as well as an α-helical subdomain and a long C-terminal extension, which is required for oligomerization (60). PslG and C. crescentus XynB2 both lack the C-terminal extension and are the only monomeric GH39 members characterized to date. The largest topographical difference between PslG(31–442) and the structurally characterized GH39 members is the formation of a sickle-shaped groove on one side of the molecule. Using CASTp, this groove has a calculated solvent-accessible volume of 856.1 Å3 (61, 62). All other GH39 members have a closed active site pocket formed from two structurally conserved loops. Variation in the PslG loop architecture alters the active site binding pocket to generate an extended groove in the following ways. In GH39 members, conserved phenylalanine (Phe-115) and glutamic acid (Glu-323) residues on L1 and L6 in XynB1, respectively, build the posterior end of the catalytic pocket. The glutamic acid forms hydrogen bonds with the 3′- and 4′-OH of the substrate or substrate analogs in holo-structures (Fig. 4A). Although L6 is similarly positioned in PslG(31–442), the glutamic acid is absent and instead is replaced by an aspartic acid (Asp-332) on an α-helix following the loop that orients perpendicularly into the groove. L1 in PslG is preceded by a small α-helix that results in the loop orienting perpendicularly relative to other members, generating a continuous groove.
FIGURE 4.

PslG(31–442) has a similar fold but distinct structural features from other GH39 members. A, superposition of PslG(31–442) (green) with GH39 family members G. stearothermophilus XynB1 (yellow; Protein Data Bank code 2BS9) and T. saccharolyticum XynB (blue; Protein Data Bank code 1UHV). The α-helical subdomain (α-HS), β-hairpin motif (β-HM), and C-terminal extension (CTE), which are common to GH39 members but absent in PslG, are highlighted in dashed ovals. The active site pocket of GH39 is formed by loops 1 and 6, and residues Glu-323, Phe-115, and Tyr-116 of XynB from T. saccharolyticum, depicted as sticks, build the posterior end of the active site. B, active site residues of PslG, XynB1, and XynB, depicted in sticks, with the same color scheme used in A. The proposed PslG catalytic residues Glu-165 and Glu-276 superimpose with the residues implicated in catalysis in the XynB1 and XynB structures. Numbering corresponds to PslG.
PslG(31–442) Binds Mannose Monosaccharides along the Electronegative Active Site Groove
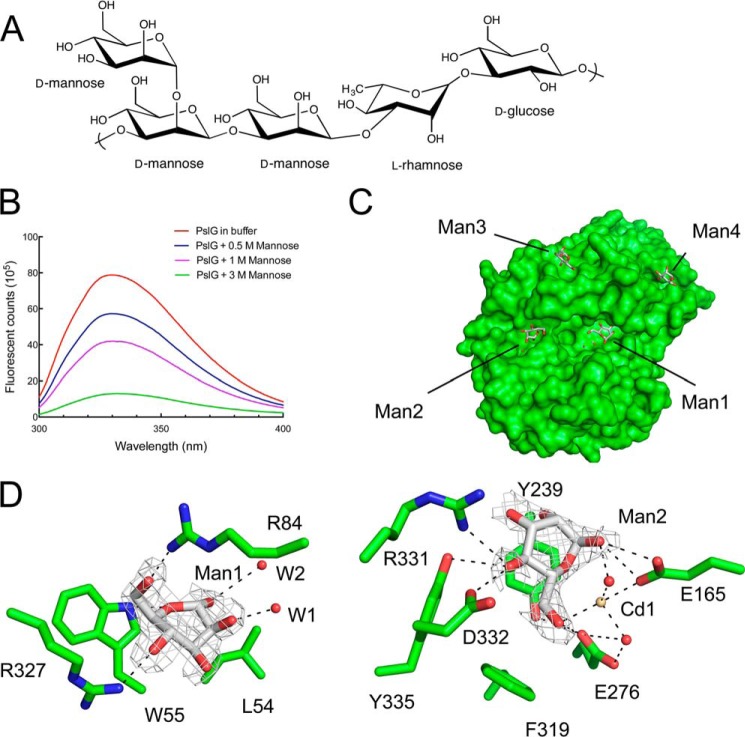
The large active site groove found in PslG appears to be well suited to accommodate the heterogeneous, branched Psl polysaccharide. The ∼32-Å-long groove would theoretically be able to accommodate between 12 and 15 saccharide units, depending on the conformation of the polysaccharide. Attempts to crystalize PslG in the presence of Psl by mutating the putative catalytic residues Glu-165 and Glu-276 (see below) to both eliminate hydrolytic activity and remove the cadmium found in the active site resulted in poorly formed crystals that were not amenable to data collection. Thus, we used intrinsic tryptophan quenching to examine possible binding of d-glucose, d-mannose, and l-rhamnose, which are the monosaccharide components of the Psl polysaccharide (Fig. 5A). A titration of d-glucose and l-rhamnose at concentrations exceeding 1.5 m did not result in a significant fluorescence change; however, the addition of mannose resulted in a concentration-dependent quenching of PslG(31–442) fluorescence (Fig. 5B). Fluorescence quenching could not be saturated utilizing concentrations exceeding 1.5 m mannose.
FIGURE 5.
PslG(31–442) binds mannose monosaccharides. A, chemical structure of the Psl polysaccharide repeating pentamer. B, the intrinsic tryptophan fluorescence quenching observed when increasing concentrations of mannose were titrated into PslG(31–442). C, surface representation of the PslG(31–442) + mannose structure showing the location of the four monosaccharides bound to the protein. Two mannose sugars, Man1 and Man2, are located in the active site groove. D, |Fo − Fc| difference density omit map for PslG mannose complex is shown as gray mesh and contoured at 2.5σ. Residues localized around each monosaccharide are depicted as green sticks. Hydrogen bonds are shown as black dashes.
To identify saccharide-binding sites, crystallization trials of PslG(31–442) were completed in the presence of 3 m mannose. Rescreening all crystallization conditions identified hits in the divalent metal ion conditions identical to those in the apo-protein. The PslG-mannose complex crystallized in space group P41212 with one molecule in the asymmetric unit, and diffraction data were collected to 1.9 Å (Table 2). The structure was solved by molecular replacement using the apo-structure as a search model. Four mannose monosaccharides, in addition to the four cadmium ions present in the apo-structure, were readily observable in an unbiased |Fo − Fc| electron density map. Two mannose monosaccharides are located in the PslG(31–442) active site groove ∼13 Å apart, suggesting that at least three saccharide units could fit between these binding sites (Fig. 5C). Modeling using the Psl pentameric repeat unit indicates that Man1 and Man2 are positioned at a distance that exceeds that of the three mannose residues in a single repeat unit of Psl. Therefore, these mannose residues would be part of adjacent repeat units when Psl is bound to PslG. One mannose (Man1) hydrogen-bonds with the indole nitrogen of Trp-55 (3.04 Å), the Nϵ of Arg-84, and the O6 (2.85 Å) and O5 (2.80 Å) of Arg-327, respectively (Fig. 5D). This is the only mannose that directly interacts with a tryptophan and is probably a major contributor to the intrinsic tryptophan fluorescence quenching observed in solution. The second active site mannose (Man2) is coordinated by a bound Cd2+ ion, the putative catalytic residues Glu-165 and Glu-276, and Arg-331 and Asp-332 (Fig. 5D). It is important to note that the poorly defined electron density for Man2 suggests that the monosaccharide may be bound in multiple conformations. Residues Trp-55 and Arg-84 interact with Man2 but exhibit nearly identical rotameric configurations between the apo- and holo-structures. The remaining monosaccharides are bound in small exterior pockets on the (β/α)8-barrel, which are formed by the loops on the top face of this domain (Fig. 5B).
PslG(31–442) Can Hydrolyze Isolated Psl Polysaccharide
To determine whether PslG is an active glycoside hydrolase, we first examined whether PslG(31–442) was capable of cleaving the synthetic substrates 4-nitrophenyl β-d-glucopyranoside, 4-nitrophenyl β-d-mannopyranoside, and 4-nitrophenyl β-d-rhamnopyranoside. Despite exhaustive attempts, these substrates could not be hydrolyzed by the enzyme. However, utilizing surface-associated Psl isolated from P. aeruginosa PAO1 PBADpsl as the substrate, wild-type PslG(31–442) exhibited glycoside hydrolase activity, as detected through an increase in reducing ends (Fig. 6A). Reducing ends were decreased 3–18-fold in the single-glutamic acid variants and eliminated in the PslG E165Q/E276Q double variant.
FIGURE 6.
PslG displays glycoside hydrolase activity that is not required for Psl biosynthesis and biofilm formation. A, reducing sugar assay of cell-associated Psl with PslG(31–442) and catalytic variants. Error bars, S.E. from triplicate experiments. B, crystal violet biofilm assay with P. aeruginosa PAO1 ΔpelF PBADpsl and pslGE165Q, pslGE276Q, and pslGE165Q/E276Q chromosomal variants. PAO1ΔpelF PBADpsl ΔpslD was utilized as a negative control. C, Psl dot blot for strains listed in B.
PslG Is Not Necessary for Psl Biosynthesis but Overexpression Leads to Defects in Biofilm Formation
Previous genetic deletions of pslG and genes encoding other hydrolytic enzymes have demonstrated that they are required for exopolysaccharide biosynthesis (8, 20, 22–25). To determine whether the glycoside hydrolase activity of PslG is required for Psl biosynthesis, chromosomal point mutations, pslGE165Q, pslGE276Q, and pslGE165Q/E276Q, were constructed in a PAO1 ΔpelF PBADpsl background using an unmarked, non-polar allelic replacement strategy. No significant differences in biofilm biomass were observed between PAO1 ΔpelF PBADpsl and chromosomal variants (Fig. 6B); however, more bacterial aggregation was observed in the pslG chromosomal variants. A Psl dot blot using α-Psl was employed to confirm the presence of the surface-associated polysaccharide in all cultures (Fig. 6C). Because the abolishment of catalytic activity was not consistent with the phenotype of the pslG genetic deletion, we attempted in trans complementation of the previously generated pslG deletion strain (PAO1 ΔpslG) (8) with a plasmid expressing the wild-type enzyme. Complementation was not successful regardless of the IPTG concentration utilized, suggesting that this genetic deletion may have impacted expression of genes downstream of pslG. Reexamination of this strain revealed that the PAO1 ΔpslG deletion may have eliminated a cis-acting regulatory element located 5′ of pslH. Therefore, we generated a pslG deletion in the PAO1 ΔpelF PBADpsl background to incorporate native sequences located 133 bp upstream of the pslH start codon. The absence of PslG in the PAO1 ΔpelF PBADpsl ΔpslG strain was confirmed using Western blot analysis. The strain was capable of forming Psl-dependent biofilms, as visualized using crystal violet (Fig. 7A) and confocal microscopy using hippeastrum hybrid amaryllis lectin staining (Fig. 7B). The results of the previously generated PAO1 ΔpslG are shown for comparison. The deletion of pslG also displayed similar aggregation as the catalytic point variants, as visualized using confocal microscopy (data not shown). To examine the effect of pslG overexpression, we complemented pslG in trans under the control of the lacZ promoter. A titration of IPTG revealed that overexpression of pslG led to the dose-dependent impairment of biofilm formation (Fig. 7C), resulting from a reduction in surface-associated Psl (Fig. 7D). Together, these data indicate that PslG glycoside hydrolase activity is not required for Psl biosynthesis but that overexpression leads to reduced Psl and biofilm formation.
FIGURE 7.

PslG is not required for Psl biosynthesis and biofilm formation. A, crystal violet biofilm assay and Western blot analysis using specific anti-PslG and RNA polymerase (RNAP) of wild-type and ΔpslG strains. RNA polymerase was utilized as a loading control in A and C. B, hippeastrum hybrid amaryllis lectin staining to probe for the presence of the Psl polysaccharide in PAO1 ΔpelF PBADpsl, PAO1 ΔpelF PBADpsl pslGE165Q/E276Q, PAO1 ΔpelF PBADpsl ΔpslG, and PAO1 ΔpslG. C, crystal violet biofilm assay and Western blot analysis biofilm assay for the in trans complementation of pslG in PAO1 ΔpelF PBADpsl ΔpslG strain. D, Psl dot blot to examine Psl levels upon overexpression of pslG. Error bars, S.E. from triplicate experiments.
Discussion
To better understand the biological role of PslG, we determined the structure of the protein and examined its function using both in vitro and in vivo assays. PslG is an inner membrane anchored protein whose periplasmic region contains a long carbohydrate-binding active site groove that is distinct from other GH39 members. The enzyme can hydrolyze surface-associated Psl, and residues Glu-165 and Glu-276 were implicated in catalysis. Mutation of these residues on the bacterial chromosome and our new ΔpslG strain demonstrated that neither PslG nor its enzymatic activity are required for Psl biosynthesis and biofilm formation; however, overexpression of PslG leads to diminished surface-associated Psl and biofilm formation.
The deep active site groove of PslG, lined with conserved surface-exposed aromatic residues, is analogous to the active site groove of BcsZ, which facilitates binding and catalysis of cellulose (16), and a carbohydrate-binding groove in the C-terminal domain of PgaB, a putative GH involved in PNAG biosynthesis (63). These features are common to processive, endo-acting enzymes (64, 65), and polysaccharide binding is observed in the grooves of both BcsZ and PgaB. In comparison, GH39 members, with the exception of PslG, exhibit exo-acting hydrolytic activity that arises as a direct consequence of an active site pocket that permits the correct orientation of the substrate to allow for hydrolysis of only the terminal sugar. PslG(31–442) exhibits low hydrolytic activity toward purified surface-associated Psl in solution but, comparable with BcsZ (16), exhibited no activity toward 4-nitrophenyl glycosides, which are substrates for exo-acting glycoside hydrolases. Low in vitro hydrolysis of carboxymethylcellulose by BcsZ could only be observed using Congo Red when the substrate was embedded in agar (16), and an ex vivo assay was required to demonstrate the glycoside hydrolase activity of L. monocytogenes PssZ (19). This depressed activity may be a consequence of the incompatibility between the intrinsically compact structure of long polysaccharides and the requirement to bind in a thermodynamically disfavored extended conformation across an active site groove. The existence of polysaccharide aggregates has been previously suggested to result in low hydrolysis for the endo-acting GHs ExoK and ExsH from Rhizobium meliloti and BcsZ (16, 66). In support of this hypothesis, BcsZ was more efficient at hydrolyzing in vitro synthesized cellulose in situ (67).
Although the presence of a carbohydrate-cleaving enzyme in an exopolysaccharide biosynthetic pathway is not intuitive, there are several possible functions, including (i) chaperon-like activity to fine tune levels of the polysaccharide in the periplasm of Gram-negative organisms, (ii) cleavage of the polysaccharide off the lipid carrier (Wzx/Wzy-dependent systems) or from the glycosyltransferase (synthase-dependent systems), or (iii) modulation of the chain length or the ratio of surface-associated versus cell-free polysaccharide to adjust to the needs of the cell/biological niche. Genetic deletions of these enzymes have varying phenotypes, suggesting that their function may be diverse across biosynthetic systems.
Periplasmic GHs in Gram-negative exopolysaccharide biosynthetic pathways are common, and the alginate lyase, AlgL, substantiates the role of a polysaccharide-cleaving enzyme in periplasmic maintenance. AlgL is proposed to be bifunctional, acting both as a lyase and an integral component of the trans-envelope alginate secretion complex (12). Deletion of algL presumably prevents complex assembly, resulting in polymer accumulation in the periplasm and cell rupture. To our knowledge, this is the only example where the absence of a polysaccharide-cleaving enzyme results in a lethal phenotype because deletion or mutation of catalytic residues of many GHs either impairs polysaccharide export or does not affect biosynthesis (20, 22–25). This difference may be attributed to the fact that each mannuronate residue in alginate carries a −1 charge, and retention of this anionic polymer in the periplasm would be detrimental to the proton motive force by reducing the membrane potential. The entrapment of neutral or positively charged exopolysaccharides would not exhibit the same effect on chemiosmosis.
Genetic deletion of pslG did not abrogate Psl biosynthesis and biofilm formation as described previously (8). This is consistent with the recent deletion of the extracellular GH PssZ involved in L. monocytogenes exopolysaccharide biosynthesis (19). Our data suggest that PslG is not required to control polysaccharide levels in the periplasm because compromising this critical function would presumably result in a significant defect in Psl biosynthesis. Additionally, PslG is unlikely to cleave the polysaccharide from a lipid carrier because PslG, like other endo-acting glycoside hydrolases, would result in the production of a non-recyclable lipid. We attribute the Psl-non-producing phenotype in the previously generated PAO1 ΔpslG strain (8) to the loss of a cis-acting regulatory element located 5′ of pslH that may have altered translation of pslH. We cannot eliminate the possibility that Psl and biofilm production in the new ΔpslG stain could arise from the functional redundancy of other glycoside hydrolases, a phenomenon that is well established (68–70). This may occur because the components of Psl, glucose, mannose, and rhamnose are present in core oligosaccharide and A-band lipopolysaccharide (LPS) (71–73) and thus may be susceptible to hydrolysis by the promiscuous activity of periplasmic GHs, such as transglycosidases. In support of this, Psl can be readily degraded by a cellulase that recognizes β1–3- and β1–4-linked glucans despite only one glucose molecule within the five-sugar repeat unit (10). Functional redundancy can also occur between biosynthetic pathways, as observed previously between Psl and LPS biosynthesis. Accordingly, pslB promoted A-band LPS synthesis in a ΔwbpW strain, whereas wbpW restored Psl production in a ΔpslB strain (8).
It is well established that many bacterial exopolysaccharides, including Psl and succinoglycan, produce both a low molecular weight and a high molecular weight polysaccharide (8, 74). An obvious candidate for the production of cell-free low molecular weight Psl is the hydrolysis of the high molecular weight Psl by the action of PslG. Such a mechanism would be similar to the biosynthesis of the low molecular weight succinoglycan, which occurs via the hydrolysis of the high molecular weight polysaccharide by the non-essential extracellular endoglycases (GH16) ExoK and ExsH (66). This low molecular weight form is beneficial but not absolutely required for this rhizobial species to establish symbiosis with its host plants (75, 76) and serves as a signaling molecule to facilitate root nodulation and bacterial update into these nodules (76). Psl also acts as a positive feedback-signaling molecule promoting the production of the intracellular secondary messenger c-di-GMP (77). Because elevated levels of c-di-GMP lead to increased production of Psl and other components of the biofilm, PslG may function to produce low molecular weight cell-free Psl involved in this signaling process.
It is important to note that, in addition to PslG, PssZ, and ExoK/ExsH, other examples of GHs in Wzx/Wzy-dependent pathways exist. For example, members of the Burkholderia cepacia complex encode a putative glycoside hydrolase (BceP) in the bce-II gene cluster required for the biosynthesis of cepacian (78). Although the protein has not been studied, it is proposed to localize to the periplasm or the outer membrane, raising the possibility that it processes the polysaccharide before and/or after export, depending on its cellular localization. Although it is tempting to speculate that these non-essential, endo-acting GHs may have an analogous function to PslG, the difference is that PslG is retained in the periplasm rather than in the outer membrane. Further insights into the biological functions of PslG will require the isolation of Psl from the strains generated in this study and the examination of the positive regulation of Psl signaling on ci-d-GMP levels in a pslG deletion strain.
Author Contributions
P. B., D. J. W., and P. L. H. designed the study and wrote the paper. P. B. designed the vectors for expression of the wild type and mutant proteins, purified the proteins, determined the structure, characterized all strains for biofilm and Psl production, and performed the in vitro enzymatic characterization of PslG and its mutant variants. G. B. W. was responsible for the creation of genetic deletion strains and chromosomal point mutations. P. J. H. completed confocal experiments and produced Psl substrate with M. J. P. H. R. was responsible for x-ray data collection and processing. D. J. L. was responsible for x-ray screening, refinement, and analysis. P. B., G. B. W., P. J. H., D. J. L., M. J. P., H. R., D. J. W., and P. L. H. analyzed the results. All authors approved the final version of the manuscript.
Acknowledgments
We thank Shaunivan Labiuk (Canadian Light Source) for assistance with data collection and Patrick Yip for technical assistance. Confocal images presented herein were generated using the instruments and services at the Campus Microscopy and Imagine Facility of Ohio State University.
This work was supported by Canadian Institutes of Health Research (CIHR) Grants 43998 and 13337 (to P. L. H.) and National Institutes of Health (NIH) Grant R01AI097511 (to D. J. W.). The National Synchrotron Light Source beamline X29A is supported by the United States Department of Energy Office of Biological and Environmental Research and the NIH National Center for Research Resources. Beamline 08ID-1 at the Canadian Light Source is supported by NSERC, the National Research Council of Canada, CIHR, the Province of Saskatchewan, Western Economic Diversification Canada, and the University of Saskatchewan. The authors declare that they have no conflicts of interest with the contents of this article.
The atomic coordinates and structure factors (codes 5BX9 and 5BXA) have been deposited in the Protein Data Bank (http://wwpdb.org/).
- GH
- glycoside hydrolase
- IPTG
- isopropyl β-d-1-thiogalactopyranoside
- Man
- mannose
- c-di-GMP
- cyclic dimeric guanosine monophosphate.
References
- 1.Colvin K. M., Gordon V. D., Murakami K., Borlee B. R., Wozniak D. J., Wong G. C., and Parsek M. R. (2011) The pel polysaccharide can serve a structural and protective role in the biofilm matrix of Pseudomonas aeruginosa. PLoS Pathog. 7, e1001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Billings N., Millan M., Caldara M., Rusconi R., Tarasova Y., Stocker R., and Ribbeck K. (2013) The extracellular matrix component Psl provides fast-acting antibiotic defense in Pseudomonas aeruginosa biofilms. PLoS Pathog. 9, e1003526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mishra M., Byrd M. S., Sergeant S., Azad A. K., Parsek M. R., McPhail L., Schlesinger L. S., and Wozniak D. J. (2012) Pseudomonas aeruginosa Psl polysaccharide reduces neutrophil phagocytosis and the oxidative response by limiting complement-mediated opsonization. Cell. Microbiol. 14, 95–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franklin M. J., Nivens D. E., Weadge J. T., and Howell P. L. (2011) Biosynthesis of the Pseudomonas aeruginosa extracellular polysaccharides, alginate, Pel, and Psl. Front. Microbiol. 2, 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryder C., Byrd M., and Wozniak D. J. (2007) Role of polysaccharides in Pseudomonas aeruginosa biofilm development. Curr. Opin. Microbiol. 10, 644–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma L., Wang S., Wang D., Parsek M. R., and Wozniak D. J. (2012) The roles of biofilm matrix polysaccharide Psl in mucoid Pseudomonas aeruginosa biofilms. FEMS Immunol. Med. Microbiol. 65, 377–380 [DOI] [PubMed] [Google Scholar]
- 7.Yang L., Hengzhuang W., Wu H., Damkiaer S., Jochumsen N., Song Z., Givskov M., Høiby N., and Molin S. (2012) Polysaccharides serve as scaffold of biofilms formed by mucoid Pseudomonas aeruginosa. FEMS Immunol. Med. Microbiol. 65, 366–376 [DOI] [PubMed] [Google Scholar]
- 8.Byrd M. S., Sadovskaya I., Vinogradov E., Lu H., Sprinkle A. B., Richardson S. H., Ma L., Ralston B., Parsek M. R., Anderson E. M., Lam J. S., and Wozniak D. J. (2009) Genetic and biochemical analyses of the Pseudomonas aeruginosa Psl exopolysaccharide reveal overlapping roles for polysaccharide synthesis enzymes in Psl and LPS production. Mol. Microbiol. 73, 622–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma L., Jackson K. D., Landry R. M., Parsek M. R., and Wozniak D. J. (2006) Analysis of Pseudomonas aeruginosa conditional psl variants reveals roles for the psl polysaccharide in adhesion and maintaining biofilm structure postattachment. J. Bacteriol. 188, 8213–8221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma L., Conover M., Lu H., Parsek M. R., Bayles K., and Wozniak D. J. (2009) Assembly and development of the Pseudomonas aeruginosa biofilm matrix. PLoS Pathog. 5, e1000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiGiandomenico A., Warrener P., Hamilton M., Guillard S., Ravn P., Minter R., Camara M. M., Venkatraman V., Macgill R. S., Lin J., Wang Q., Keller A. E., Bonnell J. C., Tomich M., Jermutus L., McCarthy M. P., Melnick D. A., Suzich J. A., and Stover C. K. (2012) Identification of broadly protective human antibodies to Pseudomonas aeruginosa exopolysaccharide Psl by phenotypic screening. J. Exp. Med. 209, 1273–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whitney J. C., and Howell P. L. (2013) Synthase-dependent exopolysaccharide secretion in Gram-negative bacteria. Trends Microbiol. 21, 63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson K. D., Starkey M., Kremer S., Parsek M. R., and Wozniak D. J. (2004) Identification of psl, a locus encoding a potential exopolysaccharide that is essential for Pseudomonas aeruginosa PAO1 biofilm formation. J. Bacteriol. 186, 4466–4475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman L., and Kolter R. (2004) Genes involved in matrix formation in Pseudomonas aeruginosa PA14 biofilms. Mol. Microbiol. 51, 675–690 [DOI] [PubMed] [Google Scholar]
- 15.Friedman L., and Kolter R. (2004) Two genetic loci produce distinct carbohydrate-rich structural components of the Pseudomonas aeruginosa biofilm matrix. J. Bacteriol. 186, 4457–4465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazur O., and Zimmer J. (2011) Apo- and cellopentaose-bound structures of the bacterial cellulose synthase subunit BcsZ. J. Biol. Chem. 286, 17601–17606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spiers A. J., Bohannon J., Gehrig S. M., and Rainey P. B. (2003) Biofilm formation at the air-liquid interface by the Pseudomonas fluorescens SBW25 wrinkly spreader requires an acetylated form of cellulose. Mol. Microbiol. 50, 15–27 [DOI] [PubMed] [Google Scholar]
- 18.Schiller N. L., Monday S. R., Boyd C. M., Keen N. T., and Ohman D. E. (1993) Characterization of the Pseudomonas aeruginosa alginate lyase gene (algL): cloning, sequencing, and expression in Escherichia coli. J. Bacteriol. 175, 4780–4789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Köseoğlu V. K., Heiss C., Azadi P., Topchiy E., Guvener Z. T., Lehmann T. E., Miller K. W., and Gomelsky M. (2015) Listeria monocytogenes exopolysaccharide: origin, structure, biosynthetic machinery and c-di-GMP-dependent regulation. Mol. Microbiol. 96, 728–743 [DOI] [PubMed] [Google Scholar]
- 20.Colvin K. M., Alnabelseya N., Baker P., Whitney J. C., Howell P. L., and Parsek M. R. (2013) PelA deacetylase activity is required for Pel polysaccharide synthesis in Pseudomonas aeruginosa. J. Bacteriol. 195, 2329–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X., Preston J. F. 3rd, and Romeo T. (2004) The pgaABCD locus of Escherichia coli promotes the synthesis of a polysaccharide adhesin required for biofilm formation. J. Bacteriol. 186, 2724–2734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Römling U. (2002) Molecular biology of cellulose production in bacteria. Res. Microbiol. 153, 205–212 [DOI] [PubMed] [Google Scholar]
- 23.Spiers A. J., Kahn S. G., Bohannon J., Travisano M., and Rainey P. B. (2002) Adaptive divergence in experimental populations of Pseudomonas fluorescens. I. Genetic and phenotypic bases of wrinkly spreader fitness. Genetics 161, 33–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bakkevig K., Sletta H., Gimmestad M., Aune R., Ertesvåg H., Degnes K., Christensen B. E., Ellingsen T. E., and Valla S. (2005) Role of the Pseudomonas fluorescens alginate lyase (AlgL) in clearing the periplasm of alginates not exported to the extracellular environment. J. Bacteriol. 187, 8375–8384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Itoh Y., Rice J. D., Goller C., Pannuri A., Taylor J., Meisner J., Beveridge T. J., Preston J. F. 3rd, and Romeo T. (2008) Roles of pgaABCD genes in synthesis, modification, and export of the Escherichia coli biofilm adhesin poly-β-1,6-N-acetyl-d-glucosamine. J. Bacteriol. 190, 3670–3680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cuthbertson L., Mainprize I. L., Naismith J. H., and Whitfield C. (2009) Pivotal roles of the outer membrane polysaccharide export and polysaccharide copolymerase protein families in export of extracellular polysaccharides in Gram-negative bacteria. Microbiol. Mol. Biol. Rev. 73, 155–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whitfield C. (2006) Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu. Rev. Biochem. 75, 39–68 [DOI] [PubMed] [Google Scholar]
- 28.Collins R. F., Beis K., Dong C., Botting C. H., McDonnell C., Ford R. C., Clarke B. R., Whitfield C., and Naismith J. H. (2007) The 3D structure of a periplasm-spanning platform required for assembly of group 1 capsular polysaccharides in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 104, 2390–2395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stover C. K., Pham X. Q., Erwin A. L., Mizoguchi S. D., Warrener P., Hickey M. J., Brinkman F. S., Hufnagle W. O., Kowalik D. J., Lagrou M., Garber R. L., Goltry L., Tolentino E., Westbrock-Wadman S., Yuan Y., Brody L. L., Coulter S. N., Folger K. R., Kas A., Larbig K., Lim R., Smith K., Spencer D., Wong G. K., Wu Z., Paulsen I. T., Reizer J., Saier M. H., Hancock R. E., Lory S., and Olson M. V. (2000) Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406, 959–964 [DOI] [PubMed] [Google Scholar]
- 30.Krogh A., Larsson B., von Heijne G., and Sonnhammer E. L. (2001) Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305, 567–580 [DOI] [PubMed] [Google Scholar]
- 31.Petersen T. N., Brunak S., von Heijne G., and Nielsen H. (2011) SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods 8, 785–786 [DOI] [PubMed] [Google Scholar]
- 32.Choi K. H., and Schweizer H. P. (2005) An improved method for rapid generation of unmarked Pseudomonas aeruginosa deletion mutants. BMC Microbiol. 5, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoang T. T., Karkhoff-Schweizer R. R., Kutchma A. J., and Schweizer H. P. (1998) A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212, 77–86 [DOI] [PubMed] [Google Scholar]
- 34.de Lorenzo V., and Timmis K. N. (1994) Analysis and construction of stable phenotypes in gram-negative bacteria with Tn5- and Tn10-derived minitransposons. Methods Enzymol. 235, 386–405 [DOI] [PubMed] [Google Scholar]
- 35.Salamitou S., Lemaire M., Fujino T., Ohayon H., Gounon P., Béguin P., and Aubert J. P. (1994) Subcellular localization of Clostridium thermocellum ORF3p, a protein carrying a receptor for the docking sequence borne by the catalytic components of the cellulosome. J. Bacteriol. 176, 2828–2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Otwinowski Z., and Minor W. (1997) Processing of x-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 [DOI] [PubMed] [Google Scholar]
- 37.Terwilliger T. C., and Berendzen J. (1999) Automated MAD and MIR structure solution. Acta Crystallogr. D Biol. Crystallogr. 55, 849–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Emsley P., and Cowtan K. (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 39.Adams P. D., Afonine P. V., Bunkoczi G., Chen V. B., Davis I. W., Echols N., Headd J. J., Hung L. W., Kapral G. J., Grosse-Kunstleve R. W., McCoy A. J., Moriarty N. W., Oeffner R., Read R. J., Richardson D. C., Richardson J. S., Terwilliger T. C., and Zwart P. H. (2010) PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Afonine P. V., Mustyakimov M., Grosse-Kunstleve R. W., Moriarty N. W., Langan P., and Adams P. D. (2010) Joint x-ray and neutron refinement with phenix.refine. Acta Crystallogr. D Biol. Crystallogr. 66, 1153–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Painter J., and Merritt E. A. (2006) Optimal description of a protein structure in terms of multiple groups undergoing TLS motion. Acta Crystallogr. D Biol. Crystallogr. 62, 439–450 [DOI] [PubMed] [Google Scholar]
- 42.Painter J., and Merritt E. A. (2006) TLSMD web server for the generation of multi-group TLS models. J. Appl. Crystallogr. 39, 109–111 [Google Scholar]
- 43.Dolinsky T. J., Czodrowski P., Li H., Nielsen J. E., Jensen J. H., Klebe G., and Baker N. A. (2007) PDB2PQR: expanding and upgrading automated preparation of biomolecular structures for molecular simulations. Nucleic Acids Res. 35, W522–W525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dolinsky T. J., Nielsen J. E., McCammon J. A., and Baker N. A. (2004) PDB2PQR: an automated pipeline for the setup of Poisson-Boltzmann electrostatics calculations. Nucleic Acids Res. 32, W665–W667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baker N. A., Sept D., Joseph S., Holst M. J., and McCammon J. A. (2001) Electrostatics of nanosystems: application to microtubules and the ribosome. Proc. Natl. Acad. Sci. U.S.A. 98, 10037–10041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ashkenazy H., Erez E., Martz E., Pupko T., and Ben-Tal N. (2010) ConSurf 2010: calculating evolutionary conservation in sequence and structure of proteins and nucleic acids. Nucleic Acids Res. 38, W529–W533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morin A., Eisenbraun B., Key J., Sanschagrin P. C., Timony M. A., Ottaviano M., and Sliz P. (2013) Collaboration gets the most out of software. eLife 2, e01456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Merritt J. H., Kadouri D. E., and O'Toole G. A. (2005) Growing and analyzing static biofilms. Curr. Protoc. Microbiol. 10.1002/9780471729259.mc01b01s00 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anthon G. E., and Barrett D. M. (2002) Determination of reducing sugars with 3-methyl-2-benzothiazolinonehydrazone. Anal. Biochem. 305, 287–289 [DOI] [PubMed] [Google Scholar]
- 50.Henrissat B., and Bairoch A. (1993) New families in the classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem. J. 293, 781–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lombard V., Golaconda Ramulu H., Drula E., Coutinho P. M., and Henrissat B. (2014) The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 42, D490–D495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ayers M., Sampaleanu L. M., Tammam S., Koo J., Harvey H., Howell P. L., and Burrows L. L. (2009) PilM/N/O/P proteins form an inner membrane complex that affects the stability of the Pseudomonas aeruginosa type IV pilus secretin. J. Mol. Biol. 394, 128–142 [DOI] [PubMed] [Google Scholar]
- 53.Vocadlo D. J., Wicki J., Rupitz K., and Withers S. G. (2002) A case for reverse protonation: identification of Glu160 as an acid/base catalyst in Thermoanaerobacterium saccharolyticum β-xylosidase and detailed kinetic analysis of a site-directed mutant. Biochemistry 41, 9736–9746 [DOI] [PubMed] [Google Scholar]
- 54.Bie H., Yin J., He X., Kermode A. R., Goddard-Borger E. D., Withers S. G., and James M. N. (2013) Insights into mucopolysaccharidosis I from the structure and action of α-l-iduronidase. Nat. Chem. Biol. 9, 739–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holm L., Kääriäinen S., Rosenström P., and Schenkel A. (2008) Searching protein structure databases with DaliLite v.3. Bioinformatics 24, 2780–2781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Czjzek M., Ben David A., Bravman T., Shoham G., Henrissat B., and Shoham Y. (2005) Enzyme-substrate complex structures of a GH39 β-xylosidase from Geobacillus stearothermophilus. J. Mol. Biol. 353, 838–846 [DOI] [PubMed] [Google Scholar]
- 57.Nieman C. E., Wong A. W., He S., Clarke L., Hopwood J. J., and Withers S. G. (2003) Family 39 α-l-iduronidases and β-d-xylosidases react through similar glycosyl-enzyme intermediates: identification of the human iduronidase nucleophile. Biochemistry 42, 8054–8065 [DOI] [PubMed] [Google Scholar]
- 58.Vocadlo D. J., MacKenzie L. F., He S., Zeikus G. J., and Withers S. G. (1998) Identification of Glu-277 as the catalytic nucleophile of Thermoanaerobacterium saccharolyticum β-xylosidase using electrospray MS. Biochem. J. 335, 449–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Olsson M., and Søndergaard C. R. (2011) PROPKA3: consistent treatment of internal and surface residues in empirical pKa predictions. J. Chem. Theory Comput. 7, 525–537 [DOI] [PubMed] [Google Scholar]
- 60.Santos C. R., Polo C. C., Corrêa J. M., Simão Rde C., Seixas F. A., and Murakami M. T. (2012) The accessory domain changes the accessibility and molecular topography of the catalytic interface in monomeric GH39 β-xylosidases. Acta Crystallogr. D Biol. Crystallogr. 68, 1339–1345 [DOI] [PubMed] [Google Scholar]
- 61.Dundas J., Ouyang Z., Tseng J., Binkowski A., Turpaz Y., and Liang J. (2006) CASTp: computed atlas of surface topography of proteins with structural and topographical mapping of functionally annotated residues. Nucleic Acids Res. 34, W116–W118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Binkowski T. A., Naghibzadeh S., and Liang J. (2003) CASTp: computed atlas of surface topography of proteins. Nucleic Acids Res. 31, 3352–3355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Little D. J., Li G., Ing C., DiFrancesco B. R., Bamford N. C., Robinson H., Nitz M., Pomès R., and Howell P. L. (2014) Modification and periplasmic translocation of the biofilm exopolysaccharide poly-β-1,6-N-acetyl-d-glucosamine. Proc. Natl. Acad. Sci. U.S.A. 111, 11013–11018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Payne C. M., Baban J., Horn S. J., Backe P. H., Arvai A. S., Dalhus B., Bjørås M., Eijsink V. G., Sørlie M., Beckham G. T., and Vaaje-Kolstad G. (2012) Hallmarks of processivity in glycoside hydrolases from crystallographic and computational studies of the Serratia marcescens chitinases. J. Biol. Chem. 287, 36322–36330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Purushotham P., and Podile A. R. (2012) Synthesis of long-chain chitooligosaccharides by a hypertransglycosylating processive endochitinase of Serratia proteamaculans 568. J. Bacteriol. 194, 4260–4271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.York G. M., and Walker G. C. (1998) The Rhizobium meliloti ExoK and ExsH glycanases specifically depolymerize nascent succinoglycan chains. Proc. Natl. Acad. Sci. U.S.A. 95, 4912–4917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Omadjela O., Narahari A., Strumillo J., Mélida H., Mazur O., Bulone V., and Zimmer J. (2013) BcsA and BcsB form the catalytically active core of bacterial cellulose synthase sufficient for in vitro cellulose synthesis. Proc. Natl. Acad. Sci. U.S.A. 110, 17856–17861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rolain T., Bernard E., Courtin P., Bron P. A., Kleerebezem M., Chapot-Chartier M. P., and Hols P. (2012) Identification of key peptidoglycan hydrolases for morphogenesis, autolysis, and peptidoglycan composition of Lactobacillus plantarum WCFS1. Microb. Cell Fact. 11, 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Heijenoort J. (2011) Peptidoglycan hydrolases of Escherichia coli. Microbiol. Mol. Biol. Rev. 75, 636–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Charaoui-Boukerzaza S., and Hugouvieux-Cotte-Pattat N. (2013) A family 3 glycosyl hydrolase of Dickeya dadantii 3937 is involved in the cleavage of aromatic glucosides. Microbiology 159, 2395–2404 [DOI] [PubMed] [Google Scholar]
- 71.Kocíncová D., and Lam J. S. (2011) Structural diversity of the core oligosaccharide domain of Pseudomonas aeruginosa lipopolysaccharide. Biochemistry 76, 755–760 [DOI] [PubMed] [Google Scholar]
- 72.Pier G. B. (2007) Pseudomonas aeruginosa lipopolysaccharide: a major virulence factor, initiator of inflammation and target for effective immunity. Int. J. Med. Microbiol. 297, 277–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rocchetta H. L., Burrows L. L., and Lam J. S. (1999) Genetics of O-antigen biosynthesis in Pseudomonas aeruginosa. Microbiol. Mol. Biol. Rev. 63, 523–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reinhold B. B., Chan S. Y., Reuber T. L., Marra A., Walker G. C., and Reinhold V. N. (1994) Detailed structural characterization of succinoglycan, the major exopolysaccharide of Rhizobium meliloti Rm1021. J. Bacteriol. 176, 1997–2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mendis H. C., Queiroux C., Brewer T. E., Davis O. M., Washburn B. K., and Jones K. M. (2013) The succinoglycan endoglycanase encoded by exoK is required for efficient symbiosis of Sinorhizobium meliloti 1021 with the host plants Medicago truncatula and Medicago sativa (Alfalfa). Mol. Plant Microbe Interact. 26, 1089–1105 [DOI] [PubMed] [Google Scholar]
- 76.Wang L. X., Wang Y., Pellock B., and Walker G. C. (1999) Structural characterization of the symbiotically important low-molecular-weight succinoglycan of Sinorhizobium meliloti. J. Bacteriol. 181, 6788–6796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Irie Y., Borlee B. R., O'Connor J. R., Hill P. J., Harwood C. S., Wozniak D. J., and Parsek M. R. (2012) Self-produced exopolysaccharide is a signal that stimulates biofilm formation in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U.S.A. 109, 20632–20636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ferreira A. S., Silva I. N., Oliveira V. H., Cunha R., and Moreira L. M. (2011) Insights into the role of extracellular polysaccharides in Burkholderia adaptation to different environments. Front. Cell. Infect. Microbiol. 1, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Holloway B. W. (1955) Genetic recombination in Pseudomonas aeruginosa. J. Gen. Microbiol. 13, 572–581 [DOI] [PubMed] [Google Scholar]
- 80.Rietsch A., Vallet-Gely I., Dove S. L., and Mekalanos J. J. (2005) ExsE, a secreted regulator of type III secretion genes in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U.S.A. 102, 8006–8011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen V. B., Arendall W. B. 3rd, Headd J. J., Keedy D. A., Immormino R. M., Kapral G. J., Murray L. W., Richardson J. S., and Richardson D. C. (2010) MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jennings L. K., Storek K. M., Ledvina H. E., Coulon C., Marmont L. S., Sadovskaya I., Secor P. R., Tseng B. S., Scian M., Filloux A., Wozniak D. J., Howell P. L., and Parsek M. R. (2015) Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosa biofilm matrix. Proc. Natl. Acad. Sci. U. S. A. 112, 11353–11358 [DOI] [PMC free article] [PubMed] [Google Scholar]