Background: Cyanobacteriochromes (CBCRs), photoreceptors that sense red to near-UV light, were not previously reported in the cyanobacterium Microcoleus.
Results: The Microcoleus genome encodes seven CBCR proteins covalently attached to phycocyanobilin or phycoviolobilin.
Conclusion: Near-UV and violet CBCRs are enriched in Microcoleus, whereas red- and green-sensitive CBCRs are absent.
Significance: This is the first report of CBCRs in the Microcoleus genome.
Keywords: cyanobacteria, genomics, photobiology, photoreceptor, ultraviolet-visible spectroscopy (UV-Vis spectroscopy), Microcoleus, cyanobacteriochromes, nonheterocystous filamentous cyanobacterium
Abstract
Cyanobacteriochromes (CBCRs), which are exclusive to and widespread among cyanobacteria, are photoproteins that sense the entire range of near-UV and visible light. CBCRs are related to the red/far-red phytochromes that utilize linear tetrapyrrole (bilin) chromophores. Best characterized from the unicellular cyanobacterium Synechocystis sp. PCC 6803 and the multicellular heterocyst forming filamentous cyanobacteria Nostoc punctiforme ATCC 29133 and Anabaena sp. PCC 7120, CBCRs have been poorly investigated in mat-forming, nonheterocystous cyanobacteria. In this study, we sequenced the genome of one of such species, Microcoleus IPPAS B353 (Microcoleus B353), and identified two phytochromes and seven CBCRs with one or more bilin-binding cGMP-specific phosphodiesterase, adenylyl cyclase and FhlA (GAF) domains. Biochemical and spectroscopic measurements of 23 purified GAF proteins from phycocyanobilin (PCB) producing recombinant Escherichia coli indicated that 13 of these proteins formed near-UV and visible light-absorbing covalent adducts: 10 GAFs contained PCB chromophores, whereas three contained the PCB isomer, phycoviolobilin (PVB). Furthermore, the complement of Microcoleus B353 CBCRs is enriched in near-UV and violet sensors, but lacks red/green and green/red CBCRs that are widely distributed in other cyanobacteria. We hypothesize that enrichment in short wavelength-absorbing CBCRs is critical for acclimation to high-light environments where this organism is found.
Introduction
Photosynthetic organisms optimize their photosynthetic performance in response to changing light environments using a range of photosensory signaling systems. Unlike in higher plants, which possess phytochromes (Phys) that sense only red and far-red light, cyanobacteria possess proteins of the bilin-based Phy superfamily (1). All members of this superfamily contain bilin-binding GAF domains that associate with linear tetrapyrrole (bilin) chromophores (2–4). In Phys of plants, algae, fungi, and numerous bacteria, the GAF domain typically occurs as part of a knotted PAS (period circadian protein, ARNT, and single-minded protein domain)-GAF-PHY (phytochrome-specific) tridomain (5). In addition to canonical Phys, cyanobacteria have evolved knotless Phys that lack the N-terminal PAS domain (Cph2 type) and multicolored GAF-only cyanobacteriochromes (CBCRs)4 (1, 6).
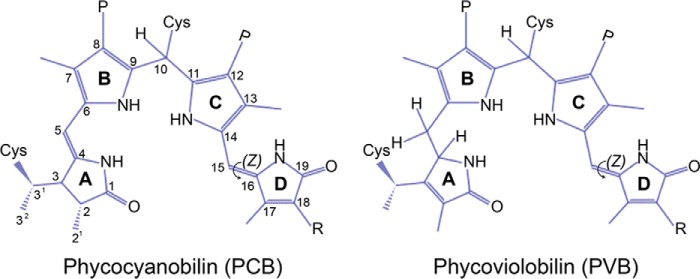
Because almost all CBCRs utilize PCB as their chromophore, CBCR spectral diversity is mostly due to the presence of one or more conserved Cys residues that form thioether linkages with the bilin chromophore (Fig. 1) and/or to substitutions within the bilin-binding pocket. One class of CBCRs with a single Cys linkage (single Cys CBCRs) comprises proteins with red/green (7–10) and green/red (11, 12) photocycles as described for AnPixJ, RGS, NpR6012g4, NpR3784, CcaS, and FdRcaE. A second class of CBCR subfamilies with a second Cys residue (dual Cys CBCRs) is often, but not exclusively, found within a conserved Asp/Glu-Xaa-Cys-Phe motif (DXCF-CBCRs) (13–16) or within a weakly conserved Cys-Xaa-Xaa-Arg/Lys motif in an insert sequence (insert Cys CBCRs) (17). One notable exception is the far-red/orange CBCR AM1_1557 from Acaryochloris marina that effectively binds biliverdin (BV), forming a photoreversible complex (18). This second Cys is capable of forming a thioether linkage at C10 of the PCB chromophore, which shortens the conjugated system and thereby blue-shifts the absorption spectrum. The stability of this second covalent linkage can be light-dependent, and the bond is often broken in one of the two photostates, as observed in Tlr0924 (13), TePixJ (19), and Tlr1999 (20) from Thermosynechococcus elongatus BP-1 (hereafter T. elongatus) and VO1 (NpF2164g3) from Nostoc punctiforme ATCC29133 (hereafter N. punctiforme) (17).5
FIGURE 1.
Phycocyanobilin and phycoviolobilin chromophores in dual Cys CBCRs in their protein-bound states. The canonical first Cys residues of Phys and CBCRs bind a linear tetrapyrrole chromophore at the 3′-position of the A-ring ethylidene group. The second thioether linkages to the C10 position of the bilin chromophore occur in dual Cys CBCRs where the second Cys is found within the DXCF motif (DXCF CBCRs) or within the insert region (insert-Cys CBCRs). The second linkage can be observed in either photostate (17). In some DXCF CBCRs, PCB is autocatalytically isomerized into PVB adduct, shortening the conjugated system due to loss of the 4,5-double bond (25). PCB adopts C5-Z,syn/C10-Z,syn/C15-Z,anti configuration in the parental dark state. A Z/E isomerization of the C15=C16 double bond between rings C and D occurs during photoconversion. P, propionate; R, ethyl.
In addition to the second Cys-mediated splitting of the tetrapyrrole π-conjugation, cyanobacteria have diversified their color sensing by adopting distinct tuning mechanisms including protochromism (12) and trapped-twist (21) of the PCB chromophore as observed in some green/red or red/green CBCRs. In some DXCF-CBCRs, the initially formed PCB adduct slowly isomerizes to PVB, which also absorbs shorter wavelengths. This occurs in the near-UV/green CBCR SyUirS (22) and in blue/green CBCRs such as TePixJ, NpR2903, and NpF1883g3 of N. punctiforme (19, 23–25). Thus, in contrast to Phys, these spectral tuning mechanisms contribute to the variety of photocycles found in CBCRs, which can detect light from the near-UV wavelengths through the entire visible light spectrum.
Light sensing and signaling in mat forming cyanobacteria from extreme environments such as saline lagoons, thermal springs, or soda lakes has not been extensively studied. Such habitats, which have high pH and contain high levels of carbonates, harbor unicellular (Rhabdoderma, Euhalothece, andSynechococcus), and multicellular filamentous (Microcoleus, Phormidium, and Mastigocladus) cyanobacteria (26). These halo- and alkali-philic organisms are considered to be representatives of ancient ecosystems; consequently, such organisms could provide insight into the early photosensory networks that contributed to cyanobacterial colonization of extreme environments on the planetary surface. Microcoleus B353, initially isolated from the soda Lake Khilganta located in the southeastern Transbaikal region, is a mat forming cyanobacteria (27). This alkaliphilic cyanobacterium shows both diurnal migration and light intensity dependent movement in natural mat communities, but little is presently known about the photobiology of this organism. This study was therefore undertaken to identify and characterize the bilin-based sensors found in this mat species.
Experimental Procedures
Strains and Culture
Microcoleus IPPAS B353 (Microcoleus B353), originally isolated from Lake Khilganta (Buryatiya, Russia; 50° 25′ N, 106° 53′ E, altitude 606°m) (28), was obtained from the Culture Collection of the Institute of Microbiology, Russian Academy of Sciences, Moscow, Russia. The strain was grown on S-solid media containing 2% agar or S-liquid media (29) at 28 °C under continuous white light (30 μmol m−2 s−1). For light sources, a UV-A lamp (Model XX-40, Spectroline, 355 ± 28.6 nm) was used for near-UV, a fluorescent lamp (OSRAM, 20W) for broad-spectrum white light, and LEDs controlled by a GFPR-1600C controller (Goodfeeling, Korea) for blue (GFBS320, λmax = 464 ± 23.2 nm), green (GFGS320, λmax =525 ± 32.7 nm), red (GFRS, λmax = 650 ± 19.4 nm), and far-red (GFFS320, λmax = 730 ± 19.4 nm) light.
Genome Sequencing, Scaffolding, and Annotation
Genomic DNA was isolated as described (30). The genome was sequenced on the Illumina HiSeq platform, using genome fragment libraries (paired end, 2K, 3K, 5K, and 10K), which generated a total of 279,654,542 reads (ncbi.nlm.nih.gov/bioproject/203668). Quality filtering was applied to the short reads using the Solexa QA software, followed by trimming to eliminate low-quality reads <20 bp using the program Dynamic Trim (31). After data trimming, length pairing of short reads was performed using Length Sort, eliminating short reads (length <25 bp), ultimately yielding a total of 22 Gb. Assemblies were generated from single- and paired-end data sets with varying k-mers (39∼97) using SOAP de novo version 1.05. The scaffolding software SSPACE version 1.0 was used for contig preparation and the 11 best scaffolds were chosen based on k-mer coverage depth analysis (∼4.8 Mb size) and by mapping raw data reads (paired-end) using the Bowtie software. Gap filling of the final scaffold was performed using Gap Closer software, which yielded the largest scaffold (4,857,012 bp in length). Remaining gaps were manually closed by sequencing PCR products using gap-neighboring primer sets. Genes in the scaffold were identified using RAST (Rapid Annotation using Subsystem Technology version 4.0). Gene annotation was performed using the UniProt Complete microbial proteomes DB (5,000,015 proteins) and BLASTX. Only predicted proteins with E value less than or equal to 1E-6 were used for annotation.
Photobiological Responses of Filamentous Fragments
Microcoleus B353 cells grown on S-solid media containing 2% agar for 2–3 weeks form clumps of tangled filament bundles of variable length and thickness. To obtain homogenous unrolled filaments of relatively short length, matted cells were suspended in S-liquid media and sonicated 10 times for 30-s intervals (2 W, Vibra Cell VC700, Sonics and Materials). After cell debris was removed by washing with fresh medium, vegetative filaments less than 100 μm in length adjusted to an A750 of 0.2, were used for further assessment of growth, pigmentation, and bundle formation under light of various colors and intensities (10, 30, and 60 μmol m−2 s−1 for white light and 5 μmol m−2 s−1 for near-UV, blue, green, red, and far-red light). Chlorophyll a, phycocyanin (PC), and carotenoid levels of cells grown on S-liquid media for 3 days were estimated using either whole cell spectra (32) or in 100% N,N-dimethylformamide extracts (33). Phototaxis was tested by culturing vegetative filaments on 0.5% agar containing S-solid media.
Expression and Purification of Recombinant His-tagged GAFs from BV- or PCB-producing Escherichia coli
Plasmids pPL-BV and pPL-PCB were used for BV or PCB biosynthesis in E. coli (34), respectively. GAF domains were amplified by PCR with appropriate primers (supplemental Table S1) using Microcoleus B353 genomic DNA as the template. Appropriately restriction-digested PCR fragments were then cloned into vector pBAD-MycHisC (Invitrogen) to yield the bacterial expression plasmid pBAD-GAF. E. coli LMG194 (Invitrogen) cells co-transformed with pBAD-GAF and pPL-BV or pPL-PCB were grown overnight at 37 °C in 5 ml of minimal media RM (34) containing 50 μg/ml of kanamycin and 200 μg/ml of ampicillin. Recombinant GAF proteins were isolated and further purified as previously described (22). Both photostates of recombinant GAFs were denatured by addition of final concentration of 8 m urea/HCl (pH 2.0) at room temperature in the dark (22, 35).
Spectrophotometric Analyses
Steady-state absorbance and fluorescence spectra were recorded at room temperature with a UV1601 spectrophotometer (Shimadzu) and an LS55 fluorescence spectrometer (PerkinElmer Life Sciences), respectively. Photointerconversion of recombinant GAFs was triggered by illumination of UV-A tube (Spectroline, 355 ± 28.6 nm) or halogen lamp (Osram LT05041) with various optical band pass filters (Edmund Optics, 442 ± 10, 480 ± 10, 520 ± 10, 580 ± 10, 610 ± 10, and 671 ± 10 nm). An electrically calibrated pyroelectric radiometer (ECPR, Model Rs5900, Laser Probe), which remained almost constant throughout the experiment (within 1%), was used to hold the fluence rate constant in each experiment.
SDS-PAGE and In-gel Zinc-dependent Fluorescence Assays
For zinc-dependent fluorescence assays (36), purified CBCR GAF proteins were prepared on a 12% (w/v) SDS-PAGE gel. Gels were soaked with 20 mm zinc acetate at room temperature for 30 min in the dark, and imaged for fluorescence under UV-B excitation (302 nm) using a Bio-Rad Gel Doc 2000 equipped with a blue filter (480BP). Gels were then stained with Coomassie Brilliant Blue R-250 (Bio-Rad).
Bioinformatics
For phylogenetic analyses of different cyanobacterial strains, 16S rRNA gene sequences of 59 species were downloaded from GenBankTM with Gloeobacter used as an outgroup to overcome constraints of morphological simplicity among cyanobacterial taxa (37, 38). After taxon sampling and outgroup assignment, sequence alignments were constructed using the program MUSCLE (39). Bayesian (38) and Maximum Likelihood (40) analyses were performed using datasets (60 taxa, 1088 sites, gaps excluded; 419 sites variable). To identify representatives of the Phy superfamily in the Microcoleus B353 genome, BLAST searches were performed using SyCph1 (Slr0473) and SyUirS (Slr1212) as query sequences. Twenty-three GAF domain-containing genes (E ≤ 1 × 10−7) were selected and their protein domain architectures were predicted using SMART. The resulting 41 GAF domain sequences were aligned with those of SyCph2, AnPixJg2, NpF2164g3 (VO1), NpR1597g2 (UB1), TePixJ, and FdIflAg3 using ClustalW. Twenty-three of these proteins from 11 candidate Phys and CBCRs were used for further biochemical characterization. Amino acids in close proximity to the PCB chromophore from AnPixJg2, used as the template (Protein Data Bank code 3W2Z), were drawn using PyMol and LigPlot+ (41).
To compare domain structures of Microcoleus B353 Phys and CBCRs with proteins whose biochemical or biological functions were previously characterized, full-length sequences of 15 ORFs from Synechocystis sp. PCC 6803 (Synechocystis 6803), Anabaena sp. PCC 7120 (Anabaena 7120), Fremyella diplosiphon, N. punctiforme, and T. elongatus were downloaded from NCBI. The numbers of single or dual Cys CBCR GAF domains were determined in 60 cyanobacterial genomes encompassing representative or reference genomes of all five sections of cyanobacteria. The strains used for this determination, along with their genome sizes, were as follows: Section I, A. marina MBIC 11017, 8.3 Mb; Chamaesiphon minutus PCC 6605, 6.7 Mb; Crocosphaera watsonii WH 8501, 6.2 Mb; Cyanobacterium aponinum PCC 10605, 4.1 Mb; Cyanobacterium stanieri PCC 7202, 3.1 Mb; Cyanobium gracile PCC 6307, 3.3 Mb; Dactylococcopsis salina PCC 8305, 3.7 Mb; Geminocystis herdmanii PCC 6308, 4.2 Mb; Gloeobacter kilaueensis JS1, 4.7 Mb; Gloeobacter violaceus PCC 7421, 4.6 Mb; Microcystis aeruginosa NIES-843, 5.8 Mb; Prochlorococcus marinus str. AS9601, 1.6 Mb; MIT 9201, 1.6 Mb; MIT 9211, 1.6 Mb; MIT 9215, 1.7 Mb; MIT 9301, 1.6 Mb; MIT 9302, 1.7 Mb; MIT 9312, 1.7 Mb; MIT 9313, 2.4 Mb; MIT 9515, 1.7 Mb; NATL2A, 1.8 Mb; P. marinus subsp. marinus str. CCMP 1375, 1.7 Mb; P. marinus subsp. pastoris str. CCMP 1986, 1.6 Mb; Prochlorothrix hollandica PCC 9006, 5.5 Mb; Rubidibacter lacunae KORDI 51–2, 4.1 Mb; S. elongatus PCC 7942, 2.7 Mb; and T. elongatus, 2.5 Mb; Section II: Chroococcidiopsis thermalis PCC 7203, 6.6 Mb; and Stanieria cyanosphaera PCC 7437, 5.5 Mb; Section III: Arthrospira platensis NIES-39, 6.7 Mb; Coleofasciculus chthonoplastes PCC 7420, 8.6 Mb; Crinalium epipsammum PCC 9333, 5.6 Mb; Kamptonema formosum PCC 6407, 6.8 Mb; Leptolyngbya boryana PCC 6306, 7.2 Mb; Lyngbya aestuarii BLJ, 6.8 Mb; Moorea producens 3L, 8.4 Mb; Nodosilinea nodulosa PCC 7104, 6.8 Mb; Oscillatoria acuminata PCC 6304, 7.8 Mb; Oscillatoria nigroviridis PCC 7112, 8.2 Mb; Planktothrix agardhii NIVA-CYA 126/8, 5.0 Mb; Pseudanabaena biceps PCC 7429, 5.4 Mb; Spirulina subsalsa PCC 9445, 5.3 Mb; and Trichodesmium erythraeum IMS101, 7.7 Mb; Section IV: Anabaena cylindrica PCC 7122, 7.0 Mb; A. variabilis ATCC 29413, 7.1 Mb; Aphanizomenon flos-aquae NIES-81, 5.8 Mb; Cylindrospermum stagnale PCC 7417, 7.6 Mb; Dolichospermum circinale AWQC310F, 4.4 Mb; Nodularia spumigena CCY9414, 5.4 Mb; “Nostoc azollae” 0708, 5.4 Mb; N. punctiforme PCC 73102, 9.0 Mb; Raphidiopsis brookii D9, 3.1 Mb; Richelia intracellularis HH01, 3.2 Mb; R. intracellularis RC01, 5.4 Mb; and Scytonema hofmanni PCC 7110, 12 Mb; Section V: Chlorogloeopsis fritschii PCC 6912, 7.7 Mb; Fischerella muscicola PCC 73103, 7.3 Mb; Fischerella thermalis PCC 7521, 5.4 Mb; Mastigocladopsis repens PCC 10914, 6.4 Mb; and Mastigocoleus testarum BC008, 15.8 Mb. DELTA-BLAST (Domain Enhanced Lookup Time Accelerated BLAST) search in the NCBI browser using SyCph1, AnPixJg2, and Mbr3854g4 as query sequences (E ≤ 1 × 10−10), and subsequent multiple alignment using ClustalW version 2 identified 555 GAFs containing at least the conserved first Cys motif as CH, CY, and CL. Excluding 66 canonical Phys-GAFs, 489 GAF domains were used to count the number of the insert-Cys CBCR GAFs in the respective genomes.
Results
Microcoleus B353 Genome Assembly and Annotation
Pairwise distance comparison of the 16S rRNA sequence of Microcoleus B353 with those of 60 previously validated cyanobacteria taxa (38) revealed <5% differences relative to Geitlerinema BBD HS217 (42). Furthermore, Maximum Likelihood and Bayesian tree of Microcoleus B353 were supported by a bootstrap value of 100 and a posterior probability of 1.0, respectively. Thus, Microcoleus B353 may be a sister species to Geitlerinema sp. BBD HS217, which diverged between 0.67 and 1.5 billion years ago (38). Therefore, Microcoleus B353 is likely far older than the unicellular freshwater cyanobacterium Synechocystis 6803, which presumably diverged between 0.36 to 0.96 billion years ago, and the heterocyst forming filamentous cyanobacterium Anabaena 7120, which diverged between 0.24 and 0.80 billion years ago (38). The genome size of Microcoleus B353 is ∼4.87 Mb, which is rather small relative to those of other filamentous cyanobacteria belonging to Sections III and IV (Fig. 2). Within the 4,867,089-bp genome (52% G + C content), 94 tRNAs, 12 rRNA operons, and 4,568 protein-coding genes were identified, with 94.4% of these protein-coding genes having predicted functions. Based on clusters of orthologous group categories (COGs), 45% of annotated genes were involved in molecular functions including catalytic and binding activities, followed by biological functions and cellular functions in descending order. A survey of 102 housekeeping genes universally present in bacteria (43) revealed that 100 of these genes were present; the exceptions, encoding ribosomal protein S10 (COG0051) and predicted ATPase of the PP-loop superfamily, which has been implicated in cell-cycle control (COG0037) were absent from the Microcoleus B353 genome (supplemental Table S2). Copy numbers for housekeeping genes correlate well with other sequenced cyanobacterial genomes. Circularization of the genome sequence was confirmed by paired end sequencing (http://112.220.192.2/cyano815MB/).
FIGURE 2.
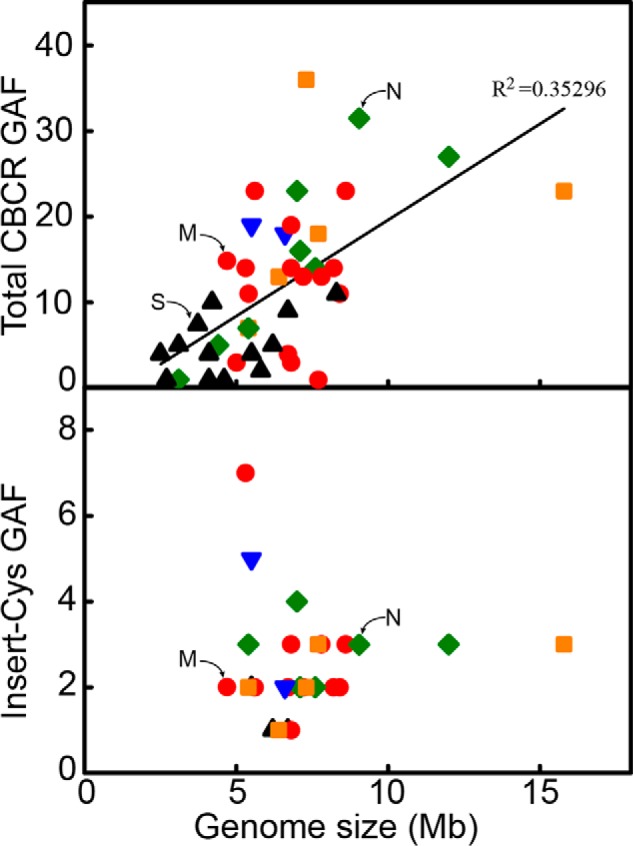
Genomic survey of putative bilin-binding GAF light sensors in 44 cyanobacterial species. Number of predicted bilin-binding GAFs as a function of genome sizes of various cyanobacterial lineages is plotted. Based on the organismal structure and pattern of development, cyanobacteria were divided into five sections (44). Section I (black triangle) consists of predominantly spheroidal, solitary, and colonial unicelluar cyanobacteria that reproduce by fission or by budding. Section II (blue inverted triangle) includes unicellular or pseudofilamentous forms that undergo multiple fissions to give rise to small daughter cells known as baeocytes. Sections III (red circle) and IV (green diamond) include uniseriate cyanobacterial filaments that lack cellular differentiation (III) or exhibit cellular differentiation into akinetes and heterocysts (IV). Section V (orange square) encompasses more complex heterocystous cyanobacterial filaments that exhibit true branching. Synechocystis 6803, N. punctiforme, and Microcoleus B353 are marked with S, N, and M, respectively.
Photobiological Responses of Microcoleus B353
Under continuous white light growth conditions, Microcoleus B353 grows in three-dimensional mat-like structures (Fig. 3, A and B). Mature vegetative cells are usually more than 20 μm long and divide longitudinally, forming nonheterocystous filaments up to >1-mm long. On solid media, many filaments assemble into large bundle strips that spread widely in a roughly semicircular arrangement, resulting in stacking of bundles to various extents. When the solid media surface is fully covered by bundle strips, cells in the upper layer start to clump, swirling out counterclockwise. In darkness, vegetative filaments elongate poorly and bundle-like structures still form, but to a lesser extent than in the light (Fig. 3C). Microcoleus B353 cells underwent unidirectional white light-induced movement (Fig. 3D) as observed in other filamentous cyanobacteria (44, 45). Neither types II nor III chromatic adaptation responses (46) were observed in Microcoleus B353; however, light quality- and intensity- dependent changes in photosynthetic pigment content, i.e. PC and carotenoid relative to chlorophyll a, phototactic movement, and filament stacking were observed (Table 1). Both carotenoid and PC levels were significantly higher under near-UV than under other colors of light. By contrast, red light was the most effective at inducing unidirectional movement. Stacking of bundle strips was also light quality dependent, with near-UV, red, and far-red being more effective than blue or green light. These photobiological responses hint that Microcoleus B353 retains photosensory and signaling machinery that allow it to adapt to changing light environments.
FIGURE 3.
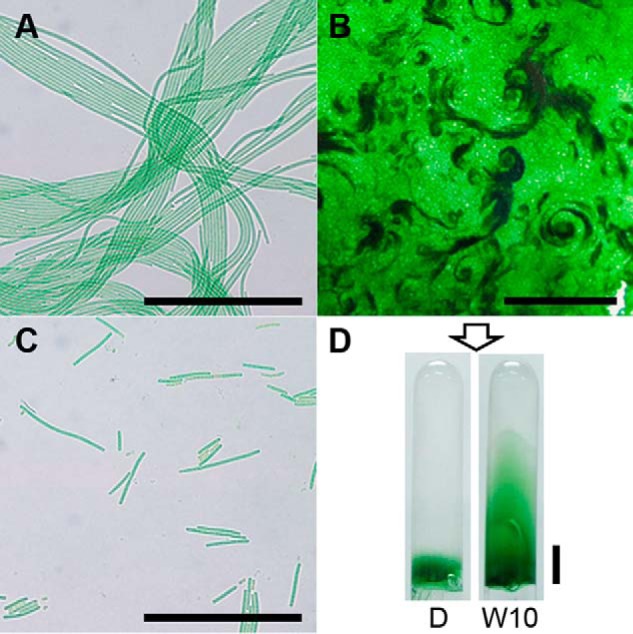
Microcoleus B353 grown under various light conditions on S-solid medium. A, filament bundles (<200 μm in length) consisting of several filaments aligned longitudinally move in rows both forward and backward directions. Bundle width and length increase during exponential growth. Scale bar = 100 μm. B, outermost filaments of bundles grow in random directions, whereas filaments inside bundles grow in parallel. As such, three-dimensional growth of filaments resulted in generation of several layers of bundle strips, forming a mat-like structure. Upper layers of mats form counterclockwise bundles with various thickness viewed during stationary growth phase. Scale bar = 1 cm. C, filaments incubated in the dark condition form few bundles, some of which become yellow in color. Scale bar = 100 μm. D, filaments of Microcoleus B353 move toward unidirectional white (W) light source and sometime penetrate through 0.5% S-solid media. Scale bar = 1 cm.
TABLE 1.
Photobiological responses of Microcoleus B353 in response to varying light colors and fluence rates
Homogenous filaments obtained by sonication were subjected to various light conditions (D, dark; UV, near-UV light; B, blue light; G, green light; R, red light; FR, far-red light; W, white light) for 3 days. Light fluence rates were adjusted to 5 μmol m−2 s−1 for UV, B, G, R, and FR, and 10 μmol m−2 s−1 for W. At 6-fold greater white light intensity (60 μmol m−2 s−1), PC/chlorophyll (Chl) and carotenoid (Car)/Chl ratios were 0.13 and 0.34, respectively. Extent of filament stacking was examined from a 1 cm2 area using ImageJ. Mean values (± S.E.) were obtained from five (pigment contents and movement) or 10 (filaments stacking) biological replicates.
| D | UV | B | G | R | FR | W | |
|---|---|---|---|---|---|---|---|
| PC/Chl | 0.14 ± 0.01 | 0.37 ± 0.01a | 0.24 ± 0.01a | 0.18 ± 0.01 | 0.19 ± 0.02 | 0.19 ± 0.01 | 0.22 ± 0.01 |
| Car/Chl | 0.18 ± 0.01 | 0.31 ± 0.01a | 0.19 ± 0.01 | 0.17 ± 0.01 | 0.18 ± 0.01 | 0.18 ± 0 | 0.19 ± 0.01 |
| Movement (cm) | 0.85 ± 0.14 | 1.64 ± 0.09 | 1.66 ± 0.09 | 2.62 ± 0.21a | 3.79 ± 0.18a | 0.93 ± 0.1 | 3.89 ± 0.36a |
| Filaments stacking (μm) | 3.52 ± 0.14 | 6.59 ± 0.67a | 4.18 ± 0.35 | 5.54 ± 0.49 | 8.86 ± 1.49a | 6.74 ± 0.74a | 26.02 ± 5.59a |
a Student's t test between dark- and light-samples (p < 0.01).
Phy Superfamily Photoreceptors of Microcoleus B353
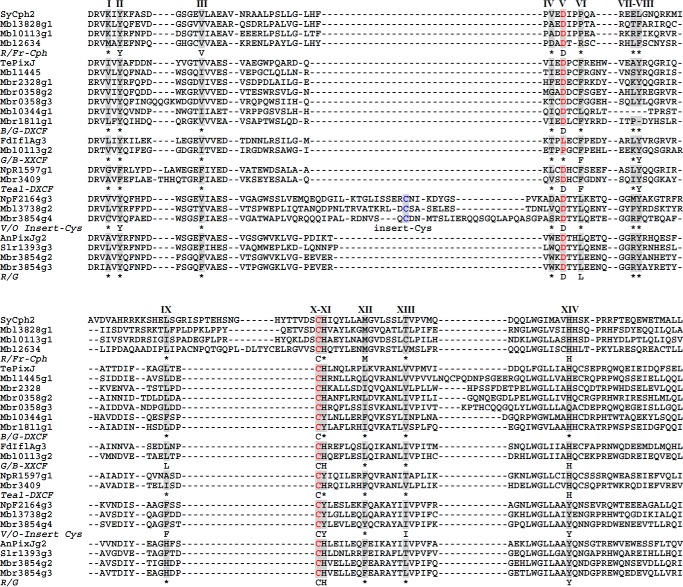
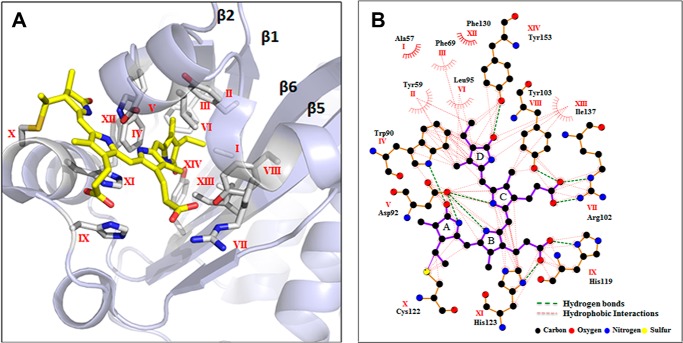
To identify the bilin photoreceptor complement in Microcoleus B353, we performed BLAST searches of its genome (http://112.220.192.2/cyano815MB/) using SyCph1 and SyUirS from Synechocystis 6803 as query sequences. This analysis yielded 11 proteins containing one Cph1-type Phy, two Cph2-type Phys, and eight CBCRs (Table 2). In addition to one canonical Cph1-type Phy (Mbl2634), we identified 22 other GAF domains among the remaining Cph2-type Phys and CBCRs; 14 domains exhibited fully conserved first Cys residues, whereas this residue was absent from eight GAFs (Mbl3828g2, Mbl0344g2, Mbl1445g2 and g3, Mbl3738g1, Mbr0358g1, Mbr1811g2, and Mbr3854g1). Besides the Cph1 (Mbl2634), four of the cysteine-containing GAFs were found in knotless Cph2-type Phys (Mbl0113g1 and Mbl3828g1) or in CBCRs (Mbr3854g2 and Mbr3854g3), all of which only contained the single Cys residue within a CH motif. The remaining 10 GAFs containing a second Cys residue were found in one Cph2 type Phy (Mbl0113g2) and nine CBCRs with second Cys residue located within either the DXCF motif (Mbl0344g1, Mbl1445g1, Mbr1811g1, Mbr2328, Mbr3409, Mbr0358g2, and Mbr0358g3) or the CXX(R/K) motif (Mbl3738g2 and Mbr3854g4) (Fig. 4). The latter contained CBCR subclass-specific residues within the chromophore binding, GAF-domain pocket (21, 47) that are indicated by Roman numerals I to XIV (Fig. 4). To gain insight into the chromophore binding pockets and chromophore-protein interactions in these new proteins, we leveraged crystal structures of GAF domains from SyCph1, SyCph2, and a red-absorbing dark state (Pr) of AnPixJg2, and a green-absorbing photoproduct (Pg) of TePixJ (3, 47, 48). Notably, we chose to use the AnPixJg2 structure (Protein Data Bank code 3W2Z) to map these amino acid residues onto the conserved GAF domain fold that surrounds the PCB chromophore (Fig. 5A) and to predict their side chain interactions with the PCB chromophore (Fig. 5B). Three key residues, including the Cys and His of the CH motif and the Asp at the DXCF motif, are shared by the vast majority of CBCRs in which the chromophore is covalently ligated to the Cys and lies between the His and Asp residues on the upper and lower sides, respectively (47). On the other hand, variable amino acid residues near the chromophore include the lid Trp in AnPixJ (47), the steric block Phe residues (21), and the insert Cys (17) have been proposed to be responsible for spectral diversity of red/green CBCRs and DXCF CBCRs, respectively.
TABLE 2.
Domain architectures of bilin-based sensors identified from Microcoleus B353
Color-coded GAF domains indicated their photocycles between dark and light states. Definitions: HK, histidine kinase with ATPase; REC, receiver domain; HPT, histidine phosphotransfer domain; HAMP, histidine kinase, adenylyl cyclases, methyl accepting proteins, and phosphatases; MCP, methyl accepting chemotaxis protein; GGDEF, diguanylate cyclase; EAL, diguanylate phosphodiesterase; CBS, cystathionine β-synthase; RD, regulatory domain.
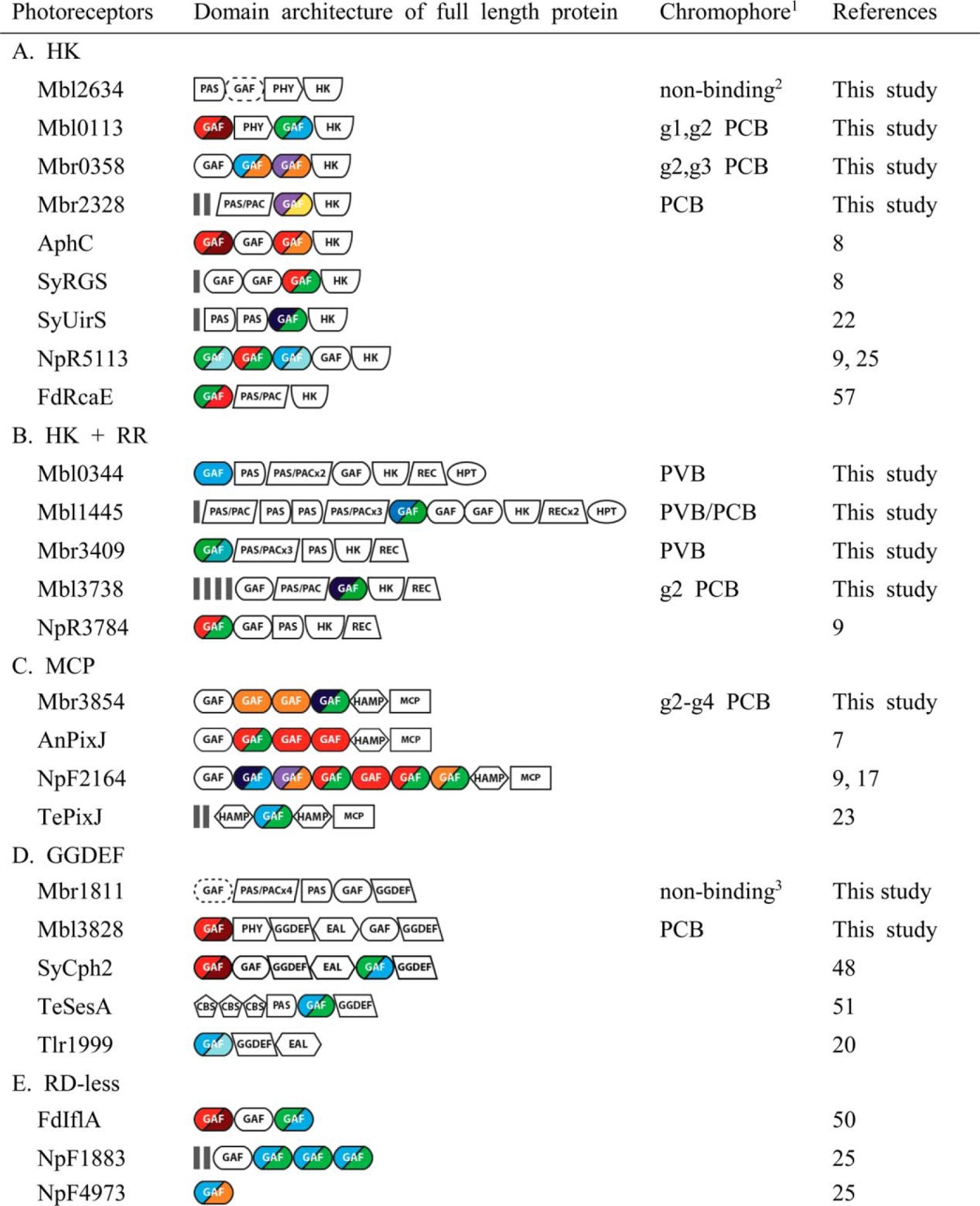
1 Chromophores were determined by acid denaturation studies.
2 This Cph1 GAF domain was non-chromophorylated when expressed in PCB producing E. coli since GAF-only domains of Cph1 and Phys do not fold in the absence of their N-terminal PAS domains (1).
3 This Mbr1811g1 contained a conserved first Cys, but was not chromophorylated when expressed in PCB producing E. coli.
FIGURE 4.
Multiple protein sequence alignment of bilin-binding GAFs from Microcoleus B353. CBCR GAF subclasses are clustered based on spectral properties of the nearest known relative. R/FR, red/far-red GAFs include Cph1 and knotless Cph2 phytochromes; B/G-DXCF, blue/green GAFs originally identified with 2nd Cys in Asp motif (DXCF) also includes blue/orange (Mbr0358g2), violet/orange (Mbr0358g3), violet/yellow (Mbr2328), and blue/blue (Mbl0344g1) CBCRs; teal-DXCF, GAFs with PVB chromophore contain teal absorbing photostate converted from green (green/teal, NpR5113g1) or blue (blue/teal, NpR1597g1, Tlr1999g1) light absorbing forms; G/B-XXCF, this subfamily consists of green/blue GAFs with Phe (Mbl0113g2, NpR5313g2) or Leu (FdIflAg3) variation in the Asp motif; V/O-Insert Cys, violet/orange GAFs with 2nd Cys residue located at the insert region include violet/orange (NpF2164g3), near-UV/blue (NpF2164g2 and NpR1597g2), and near-UV/green (Mbr3854g4 and Mbl3738g2) photocycles; R/G, red/green GAFs such as AnPixJg2 and Slr1393g3. Amino acid residues contacting (or near) the chromophore based on the three-dimensional structure of AnPixJ2 are marked with Roman numerals. Consensus residues are obtained from multiple sequence alignments from experimentally characterized CBCR GAFs. *, variable residues.
FIGURE 5.
Amino acid residues within the chromophore pocket of AnPixJg2 GAF. A, amino acids in the proximity of PCB chromophore of the GAF domain identified in the present study labeled using Roman numerals from I to XIV are mapped onto the AnPixJg2 structure (Protein Data Bank code 3W2Z). B, LigPlot+ presentation of the amino acids interacting with PCB chromophore via H-bonding or van der Waals interactions.
To characterize the bilin-binding and spectroscopic properties of these proteins, 23 Microcoleus GAF-only domains of one Cph1, four Cph2-type, and 18 CBCRs were expressed in BV- or PCB-producing E. coli. None were found to be chromophorylated by BV, yet 10 yielded PCB adducts exhibiting various photocycles including red/far-red (Mbl0113g1 and Mbl3828g1), green/blue (Mbl0113g2), blue/green (Mbl1445g1), violet/yellow (Mbr2328), green/teal (Mbr3409), blue/orange (Mbr0358g2), violet/orange (Mbr0358g3), or near-UV/green (Mbl3738g2 and Mbr3854g4) photocycles (Table 2; Fig. 6). None of these photoactive CBCR-GAFs exhibited noticeable thermal (dark) reversion of the photoproducts to their short wavelength absorbing dark states. By contrast with these well behaved adducts, the DXCF CBCR Mbl0344g1 yielded a PCB adduct mixture with a partially photoactive blue peak and a non-photoactive orange peak. In addition, the orange-absorbing PCB adducts of the R/G CBCRs, Mbr3854g2 and Mbr3854g3, were non-photoactive and fluorescent (Fig. 6). Unfortunately, we were unable to express a stable form of Mbr1811g1 despite the presence of conserved first Cys, as was also observed for NpR3572 from N. punctiforme (25). As expected for the GAF-only domain of Cph1 (Mbl2634) (1) and first Cys lacking GAFs, Mbl3828g2, Mbl0344g2, Mbl1445g2 and g3, Mbl3738g1, Mbr0358g1, Mbr1811g2, and Mbr3854g1 could also be expressed in PCB-expressing E. coli, but none were chromophorylated.
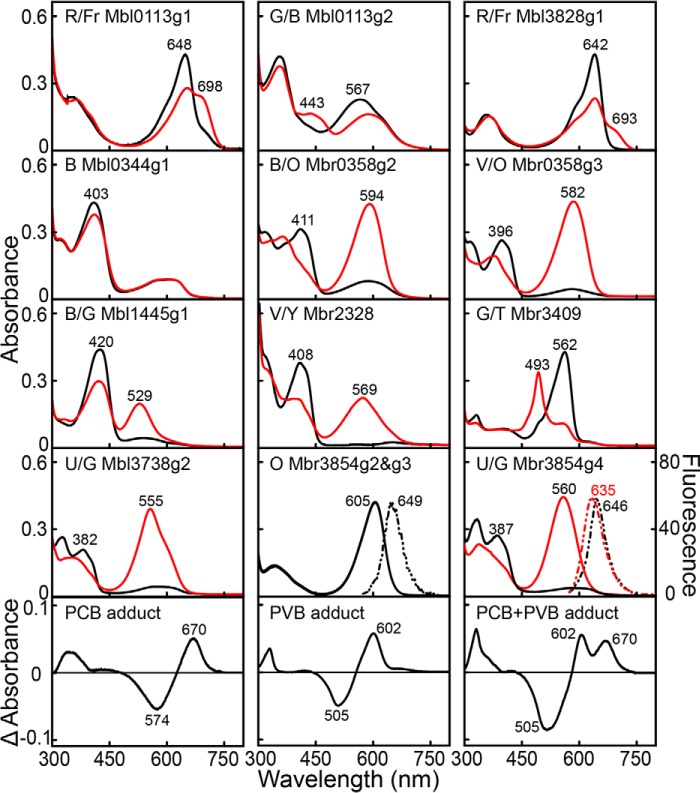
FIGURE 6.
Absorption spectra of Cph2- and CBCR-GAFs from Microcoleus B353. Black and red lines indicate absorption of dark- and light-adapted samples, respectively. Nonphotochromic orange light sensors Mbr3854g2 and g3 excited by green light showed identical fluorescence emission peaks at 649 nm. Near-UV and green absorbing form of Mbr3854g4 excited with near-UV (390 nm) or green (540 nm) light yielded fluorescence emission peaks at 646 or 635 nm, respectively. Light-minus-dark difference spectra of acid-denatured GAFs were divided into three subgroups based on chromophore adduct species: PCB (Mbl0113g1/g2, Mbr0358g2/g3, Mbr2328, Mbl3738g2, Mbl3828g1, Mbr3854g2/g3/g4), PVB (Mbl0344g1 and Mbr3409), and a mixture of PCB and PVB (Mbl1445g1). R, red; Fr, far-red; G, green; B, blue; O, orange; V, violet; Y, yellow; T, teal; U, near-UV.
Next, we assessed the structures of the bilin chromophores bound to recombinant GAFs by acid denaturation (35). Long wavelength absorbing photostates of all GAFs were denatured with acidic urea (8 m urea/HCl, pH 2.0) in the dark and then illuminated with white light. Light-minus-dark difference spectra of acid-denatured Mbl0113g1 and g2, Mbr0358g2 and g3, Mbr2328, Mbl3738g2, Mbl3828g1, and Mbr3854g2/g3/g4 (Fig. 6) were almost indistinguishable from those of 15Z-PCB and 15E-PCB from acid-denatured AphA of Anabaena 7120 (35). This indicated that these GAFs assembled with PCB in the 15Z configuration in the dark and that all were photoconverted to the 15E configuration. Difference spectra of acid-denatured Mbl0344g1 and Mbr3409 (Fig. 6) were almost identical to those of NpR1597g1 and NpR5113g1 of N. punctiforme, which contains PVB as chromophore (25). By contrast, Mbl1445g1 harbored a mixture of PCB and PVB as indicated by observations that its difference spectrum was similar to those of Tlr0924 and TePixJ of T. elongatus (24, 25), SyUirS (22) of Synechocystis 6803, and the N. punctiforme CBCRs NpR2903 and NpF1883g3 (25), although the relative concentrations of PCB and PVB varied among these proteins. Thus, the GAFs identified in this study revealed three groups of CBCRs harboring a single bilin population of PCB or PVB, or a mixture of PCB and PVB that photoconverts between the 15Z configuration in the dark-adapted states and the 15E configuration in the light-activated states.
Discussion
Our current knowledge of light sensors and signaling machinery in cyanobacteria is limited to a few species. In this study, we identified the complement of bilin-based sensors in the genome of an understudied mat-forming cyanobacterium, Microcoleus B353, isolated from a saline and alkaline environment. Through de novo genome analysis in conjunction with biochemical studies of recombinant GAF-containing ORFs, we identified nine genes encoding two knotless Phys and seven CBCRs in the Microcoleus B353 genome, half as many as in N. punctiforme (18 total: two Cph1s, three Cph2s, and 13 CBCRs). The Microcoleus complement is comparable with the seven bilin sensors (one Cph1, one Cph2, and five CBCRs) found in the genome of the unicellular cyanobacterium Synechocystis 6803 (Fig. 2). We also identified two knotless Phys, Mbl0113 and Mbl3828, which possess distinct domain structures (Table 2) and photochemical properties (Fig. 6) relative to SyCph2, the single knotless Phy in Synechocystis 6803 (48). These studies also expand our knowledge of both the diversity and spectral tuning of dual Cys CBCR GAFs. The two newly identified near-UV/green insert-Cys CBCRs, Mbr3854g4 and Mbl3738g2, add new members to the CBCR family that sense in the near-UV to violet range (17).
Similar domain architectures of these Microcoleus photoreceptors, based on SMART motif analysis, were almost indistinguishable from those other bilin photoreceptors previously characterized biochemically or biologically (Table 2). Seven of these proteins are typical bacterial two-component signal transduction proteins containing a His kinase (HK) domain, either alone or followed by a receiver (REC) domain, with or without a His phosphotransferase domain. The other two CBCR proteins, Mbr3854, and Mbl3828, lack an HK domain, and instead contain a methyl-accepting chemotaxis protein domain implicated in phototactic responses (14) or a diguanylate cyclase (GGDEF) domain involved in the generation of secondary messenger cGMP (49), respectively. However, CBCRs lacking signaling domains other than GAF(s), such as FdIflA (50), or containing multiple cystathione β-synthase domains, such as TeSesA (51), were not identified in this genome. Like the majority of CBCRs, the natural chromophore of these Phys and CBCR proteins is very likely to be PCB, rather than BV (18) or phycoerythrobilin (52). The Microcoleus genome harbors only PCB biosynthesis genes, which include two heme oxygenases (ho1, mbr4200, and mbr2519) and a phycocyanobilin:ferredoxin oxidoreductase (pcyA, mbr2212). Phycoerythrobilin biosynthetic genes coding for 15,16-dihydrobiliverdin:ferredoxin oxidoreductase (pebA) and phycoerythrobilin:ferredoxin oxidoreductase (pebB) are absent. The failure of all Microcoleus GAFs to bind BV in E. coli strongly argues against BV as a natural chromophore.
All CBCRs chromophorylated with PCB in the present study contained three key residues Asp/Cys and axial His/Tyr (V/X and XI positions, Figs. 4 and 5). As expected, the absence of the first Cys residue correlated with the lack of chromophore binding. However, our studies showed that the presence of the Asp residue in the Asp motif (position V) and the His residue adjacent to the first Cys (position XI) were not essential for chromophorylation and photointerconversion, as substitutions of Asp with Pro (Mbl0113g2) and His with Tyr (Mbl0344g1, Mbl3738g2, and Mbr3854g4) did not abolish PCB binding nor affect the photoactivity of Mbl3738g2 and Mbr3854g4 (Fig. 6). Other subclass-specific residues known to regulate spectral tuning of the two photostates are also well conserved in corresponding residues of GAFs characterized from Microcoleus B353. For instance, substitution of DXCF Asp to Pro in Mbl0113g2 correlates with green/blue sensitivity rather than the expected blue/green cycle of the canonical DXCF family. This photocycle is shared with those of NpR5313g2 (25), FdIflA (50), and a recently discovered family of DXCF-derived CBCRs in which the conserved Asp residue is replaced with a non-ionizable residue (53).
Red/green CBCR-specific residues equivalent to Arg-301 (position VII) and Tyr-302 (position VIII) in AnPixJ2 (47) are both conserved in the red fluorescent single Cys CBCRs Mbr3854g2/g3 and NpF2164g5 (9), and the replacement of VIII Tyr with Pro in Slr1393g3 did not alter the red/green photoactivity of this CBCR (54), so their role in red/green photochromism is unclear. Other lineage-specific amino acid substitutions for the XIV Tyr and the highly variable IX residue are predicted to exert spectral tuning-specific H-bonding and/or van der Waals interactions with the PCB chromophore (47). Furthermore, H-bonding networks mediated by the XI His and VII Arg residues are predicted to play important tuning roles (47). However, replacement of the IX Phe-525 of Slr1393g3 with Ala did not alter the spectrum of the variant (54). Taken together, these results fail to implicate a direct role of these residues in red/green CBCR chromophorylation or photointerconversion. Substitution variants W496I at position IV and R508N at position VII of Slr1393g3 displayed respective blue (25 nm)- and red (42 nm)-shifted photostate absorption maxima, suggesting involvement of these residues in photoproduct formation (47, 54). However, the position IV ”lid-Trp“ is absent in the known red/green CBCRs NpR6012g3 and NpR5113g2 and is dispensable in NpR6012g4 (9, 21). Indeed, the role of multiple Phe residues in red/green CBCR ”trapped twist“ tuning is now well established for such red/green photocycles (10, 21, 55, 56).
The biological function of these bilin photosensors from Microcoleus B353 remains unknown. It is possible that they regulate light color- and intensity-dependent responses of photosynthetic pigmentation, directional movement, and the extents of filament strip stacking (Fig. 3 and Table 1). We predict that some of these Phys and CBCRs are Microcoleus B353-specific, and play a sensing role in mat formation, which is not observed in unicellular Synechocystis 6803 or filamentous N. punctiforme. The two red/far-red knotless Phys in Microcoleus B353, Mbl0113 and Mbl3828, likely contribute to unilateral light-dependent movement or growth in view of the preferential sensitivity to red light. Although Mbl3828 possesses the identical domain organization as SyCph2, its second GAF is photochemically inert. Both N-terminal GGDEF and EAL domains contain well conserved GGDEF and EAL motifs, suggesting that only the N-terminal guanylate cyclase is red/far-red light regulated. We predict that c-di-GMP signaling in Microcoleus B353, presumably responsible for cell growth and phototactic movement responses, will be red/far-red dependent, rather than blue/green sensitive as seen in Synechocystis 6803 (48, 49).
Chromatic acclimation responses in the phycoerythrin-containing multicellular cyanobacteria, F. diplosiphon mediated by FdRcaE (57) and in N. punctiforme mediated by NpCcaS (58), as well as those in the unicellular cyanobacterium Synechocystis 6803 (SyCcaS) (11), which lacks phycoerythrin, are mediated by CBCRs that respond to red and green light. In Synechocystis 6803, light acclimation to green and red light is sensed by SyCcaS, which mediates transcriptional regulation of cpcG2 (11). An apparent ortholog of the transcriptional target of SyCcaS, i.e. cpcG2, is also found in the Microcoleus B353 genome (mbl2616). However, the absence of red/green or green/red photoreceptors suggest that an alternative light color sensing system involving near-UV (Mbl3738g2), violet (Mbr2328 and Mbr0358g3), or blue (Mbl1445g1, Mbr0358g2) light-sensing CBCRs participate in the regulation of the synthesis of photosynthetic pigments.
Microcoleus B353 contains two near-UV-absorbing CBCRs, Mbr3854 and Mbl3738, which to date appear to be rarely found in other cyanobacterial genomes. Mbr3854 is likely involved in unidirectional light-induced gliding motility based on the presence of a C-terminal methyl-accepting chemotaxis protein signaling motif (Table 2). This hypothesis is further strengthened by its gene context. The mbr3854 gene cluster contains patA (mbr3852), cheY (mbr3853), mcp (mbr3854), and cheA (mbr3855) genes that is very similar to those of SyPixJ in Synechocystis 6803 (59) and ptxD in N. punctiforme (45). The other near-UV/green sensor Mbl3738 may modulate near-UV-regulated pigmentation and/or mat formation responses because accumulation of carotenoid and filament bundle formation are enhanced under UV-A illumination (Table 1). However, the redox state of photosynthetic electron transport is known to mediate carotenoid accumulation, a response that is strongly dependent on light intensity in Microcoleus B353. Thus, the role of photoreceptors in this response may be minor as has been seen for Phy and cryptochromes in Synechocystis 6803 (60, 61).
The Microcoleus B353 genome contains two three-color sensing CBCRs, Mbr0358 and Mbr3854, similar to those found in other cyanobacteria such as Synechocystis 6803, Anabaena 7120, F. diplosiphon, and N. punctiforme (Table 2). Mbr0358 contains tandemly arranged repeats of three GAF domains in its photosensory module, quite similar with those of red/green RGS (encoded by slr1393) from Synechocystis 6803 and red/far-red and red/orange AphC (encoded by all2699) of Anabaena 7120 (8). Although the biological functions and underlying spectral tuning mechanisms of Mbr0358 are not yet defined, this dual light sensor could extend its light-sensing spectrum from violet to blue light within a single CBCR. Mbr3854 senses three different colors, i.e. near-UV, green and orange, but the biological significance remains unclear. Because light absorption by the orange-absorbing domains does not contribute to photochemistry, transmission of orange light information to the methyl-accepting chemotaxis protein output is unlikely to occur in this protein. In this regard, it is possible that PCB binding to the non-photoactive GAF2 and GAF3 domains could modulate signaling output by supporting progressive dimerization of tandem GAFs as proposed for TePixJ and NpF2164 (62). Light absorption by one GAF domain might also affect chromophorylation or photoconversion of another domain as suggested for the four-color sensor FdIflA from F. diplosiphon (50). Currently, it is not clear whether cooperative action as in NpF2164 or cross-activation as in FdIflA among multi-GAF domains are occurring in Mbr3854. It is also conceivable that the non-photoactive GAF2 and GAF3 domains of Mbr3854 reflect a spontaneous loss of chromophore binding as suggested as an alternative to antenna role in GAF3 and GAF4 of AnPixJ (7).
The absence of red/green or green/red sensors, and the abundance of near-UV and violet sensors, likely reflect the adaptation of this Microcoleus species to its high light environment. The enriched presence of short wavelengths light sensors is not a characteristic feature shared by all Section III cyanobacteria that encompasses uniseriate and undifferentiated filamentous cyanobacteria including Oscillatoria and Spirulina, but are widely distributed among all five cyanobacterial sections (Fig. 2). Thus, natural light environment of Microcoleus B353 rather than morphological characteristics appear to be relevant to the abundance of short wavelength-absorbing CBCRs. Microcoleus B353 forms microbial mats 10–15 mm in thick in Lake Khilganta (27) found in natural communities with other uni- and multicellular cyanobacteria and purple sulfur bacteria. Lake Khilganta is shallow (35–45 cm in depth) and Microcoleus B353 filaments reside in the outermost layers of the mats. The natural habitat of Microcoleus B353 therefore would expose this organism to a wide range of solar radiation unfiltered by other photoautotrophs. The Microcoleus B353 genome also lacks UV-B sensing UVR8 (63), near-UV intensity sensor SyUirS (22), and flavin-based blue light sensing photoreceptors with the light-oxygen-voltage domain, such as MPAC (ZP_05024462.1) from Microcoleus chthonoplastes PCC 7420 (64, 65) or the BLUF domain, such as Slr1694 from Synechocystis PCC 6803 (66, 67). It is therefore reasonable that such short wavelength near-UV and violet sensing CBCRs would provide an adaptive advantage to living in high light environments. We look forward to application of molecular genetic approaches to addressing specific biological roles of the new photosensors identified in the present study in relationship to photosynthetic pigmentation, phototactic motility, and biofilm formation in response to varying light colors and intensities.
Author Contributions
Y. I. P. designed the research; S. M. C., S. C. J., J. Y. S., E. V. K., N. A. P., B. W. L., S. W. J., and J. J. S. performed the research; S. M. C., B. S. P., S-B. C., J. J. S., and Y. I. P. wrote the paper. All authors discussed the results and commented on the manuscript.
Supplementary Material
Acknowledgments
We thank J. Clark Lagarias for helpful discussions and comments on the manuscript. Multiple sequence alignment of GAF domains was greatly improved thanks to discussions with N. C. Rockwell.
This work was supported by grants from the Next-Generation BioGreen 21 Program, Rural Development Administration Grant PJ011659, and the Advanced Biomass R&D Center (ABC) of Korea Grant NRF-2011-0031344, funded by the Ministry of Science, ICT, and Future Planning, Korea (to Y-I. P.). The authors declare that they have no conflicts of interest related to the contents of this article.

This article contains supplemental Tables S1 and S2.
Whole genome sequences, annotation information and detailed predicted functions of Microcoleus B353 are accessible via NCBI (http://www.ncbi.nlm.nih.gov/bioproject/203668).
In this study, we adopt the nomenclature for CBCRs to represent proteins with single or multiple bilin-binding GAF domains. Domain names are described as follows: GAF domains are numbered from the N terminus. For the Mbr3854 CBCR, there are four GAF domains. Thus, Mbr3854g1 is the most N-terminal of these domains. For cases where there is a single GAF domain, no number is assigned (e.g. Mbr2328). Throughout in this article, we used the following color-code: near-UV (UV), 300–395 nm; violet (V), 395–410 nm; blue (B), 410–480 nm; teal (T), 480–520 nm; green (G), 520–570 nm; yellow (Y), 570–580 nm; orange (O), 580–615 nm; red (R), 615–685 nm; far-red (FR), 685–750 nm.
- CBCR
- cyanobacteriochrome
- PC
- phycocyanin
- COG
- clusters of orthologous group
- PCB
- phycocyanobilin
- PVB
- phycoviolobilin.
References
- 1.Rockwell N. C., and Lagarias J. C. (2010) A brief history of phytochromes. Chemphyschem. 11, 1172–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wagner J. R., Brunzelle J. S., Forest K. T., and Vierstra R. D. (2005) A light-sensing knot revealed by the structure of the chromophore binding domain of phytochrome. Nature 438, 325–331 [DOI] [PubMed] [Google Scholar]
- 3.Essen L. O., Mailliet J., and Hughes J. (2008) The structure of a complete phytochrome sensory module in the Pr ground state. Proc. Natl. Acad. Sci. U.S.A. 105, 14709–14714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X., Kuk J., and Moffat K. (2008) Crystal structure of Pseudomonas aeruginosa bacteriophytochrome: photoconversion and signal transduction. Proc. Natl. Acad. Sci. U.S.A. 105, 14715–14720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Auldridge M. E., and Forest K. T. (2011) Bacterial phytochromes: More than meets the light. Crit. Rev. Biochem. Mol. Biol. 46, 67–88 [DOI] [PubMed] [Google Scholar]
- 6.Ikeuchi M., and Ishizuka T. (2008) Cyanobacteriochromes: a new superfamily of tetrapyrrole-binding photoreceptors in cyanobacteria. Photochem. Photobiol. Sci. 7, 1159–1167 [DOI] [PubMed] [Google Scholar]
- 7.Narikawa R., Fukushima Y., Ishizuka T., Itoh S., and Ikeuchi M. (2008) A novel photoactive GAF domain of cyanobacteriochrome AnPixJ that shows reversible green/red photoconversion. J. Mol. Biol. 380, 844–855 [DOI] [PubMed] [Google Scholar]
- 8.Chen Y., Zhang J., Luo J., Tu J. M., Zeng X. L., Xie J., Zhou M., Zhao J. Q., Scheer H., and Zhao K. H. (2012) Photophysical diversity of two novel cyanobacteriochromes with phycocyanobilin chromophores: photochemistry and dark reversion kinetics. FEBS J. 279, 40–54 [DOI] [PubMed] [Google Scholar]
- 9.Rockwell N. C., Martin S. S., and Lagarias J. C. (2012) Red/green cyanobacteriochromes: sensors of color and power. Biochemistry 51, 9667–9677 [DOI] [PubMed] [Google Scholar]
- 10.Rockwell N. C., Martin S. S., Gan F., Bryant D. A., and Lagarias J. C. (2015) NpR3784 is the prototype for a distinctive group of red/green cyanobacteriochromes using alternative Phe residues for photoproduct tuning. Photochem. Photobiol. Sci. 14, 258–269 [DOI] [PubMed] [Google Scholar]
- 11.Hirose Y., Shimada T., Narikawa R., Katayama M., and Ikeuchi M. (2008) Cyanobacteriochrome CcaS is the green light receptor that induces the expression of phycobilisome linker protein. Proc. Natl. Acad. Sci. U.S.A. 105, 9528–9533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirose Y., Rockwell N. C., Nishiyama K., Narikawa R., Ukaji Y., Inomata K., Lagarias J. C., and Ikeuchi M. (2013) Green/red cyanobacteriochromes regulate complementary chromatic acclimation via a protochromic photocycle. Proc. Natl. Acad. Sci. U.S.A. 110, 4974–4979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rockwell N. C., Njuguna S. L., Roberts L., Castillo E., Parson V. L., Dwojak S., Lagarias J. C., and Spiller S. C. (2008) A second conserved GAF domain cysteine is required for the blue/green photoreversibility of cyanobacteriochrome Tlr0924 from Thermosynechococcus elongatus. Biochemistry 47, 7304–7316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshihara S., Katayama M., Geng X., and Ikeuchi M. (2004) Cyanobacterial phytochrome-like PixJ1 holoprotein shows novel reversible photoconversion between blue- and green-absorbing forms. Plant Cell Physiol. 45, 1729–1737 [DOI] [PubMed] [Google Scholar]
- 15.Ma Q., Hua H. H., Chen Y., Liu B. B., Krämer A. L., Scheer H., Zhao K. H., and Zhou M. (2012) A rising tide of blue-absorbing biliprotein photoreceptors: characterization of seven such bilin-binding GAF domains in Nostoc sp. PCC7120. FEBS J. 279, 4095–4108 [DOI] [PubMed] [Google Scholar]
- 16.Rockwell N. C., Martin S. S., and Lagarias J. C. (2012) Mechanistic insight into the photosensory versatility of DXCF cyanobacteriochromes. Biochemistry 51, 3576–3585 [DOI] [PubMed] [Google Scholar]
- 17.Rockwell N. C., Martin S. S., Feoktistova K., and Lagarias J. C. (2011) Diverse two-cysteine photocycles in phytochromes and cyanobacteriochromes. Proc. Natl. Acad. Sci. U.S.A. 108, 11854–11859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Narikawa R., Nakajima T., Aono Y., Fushimi K., Enomoto G., Ni-Ni-Win, Itoh S., Sato M., and Ikeuchi M. (2015) A biliverdin-binding cyanobacteriochrome from the chlorophyll d-bearing cyanobacterium Acaryochloris marina. Sci. Rep. 5, 7950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishizuka T., Kamiya A., Suzuki H., Narikawa R., Noguchi T., Kohchi T., Inomata K., and Ikeuchi M. (2011) The cyanobacteriochrome, TePixJ, isomerizes its own chromophore by converting phycocyanobilin to phycoviolobilin. Biochemistry 50, 953–961 [DOI] [PubMed] [Google Scholar]
- 20.Enomoto G., Hirose Y., Narikawa R., and Ikeuchi M. (2012) Thiol-based photocycle of the blue and teal light-sensing cyanobacteriochrome Tlr1999. Biochemistry 51, 3050–3058 [DOI] [PubMed] [Google Scholar]
- 21.Rockwell N. C., Martin S. S., Gulevich A. G., and Lagarias J. C. (2014) Conserved phenylalanine residues are required for blue-shifting of cyanobacteriochrome photoproducts. Biochemistry 53, 3118–3130 [DOI] [PubMed] [Google Scholar]
- 22.Song J. Y., Cho H. S., Cho J. I., Jeon J. S., Lagarias J. C., and Park Y.-I. (2011) Near-UV cyanobacteriochrome signaling system elicits negative phototaxis in the cyanobacterium Synechocystis sp. PCC 6803. Proc. Natl. Acad. Sci. U.S.A. 108, 10780–10785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishizuka T., Shimada T., Okajima K., Yoshihara S., Ochiai Y., Katayama M., and Ikeuchi M. (2006) Characterization of cyanobacteriochrome TePixJ from a thermophilic cyanobacterium Thermosynechococcus elongatus strain BP-1. Plant Cell Physiol. 47, 1251–1261 [DOI] [PubMed] [Google Scholar]
- 24.Ishizuka T., Narikawa R., Kohchi T., Katayama M., and Ikeuchi M. (2007) Cyanobacteriochrome TePixJ of Thermosynechococcus elongatus harbors phycoviolobilin as a chromophore. Plant Cell Physiol. 48, 1385–1390 [DOI] [PubMed] [Google Scholar]
- 25.Rockwell N. C., Martin S. S., Gulevich A. G., and Lagarias J. C. (2012) Phycoviolobilin formation and spectral tuning in the DXCF cyanobacteriochrome subfamily. Biochemistry 51, 1449–1463 [DOI] [PubMed] [Google Scholar]
- 26.Sergeev V. N., Gerasimenko L. M., and Zavarzin G. A. (2002) The proterozoic history and present state of cyanobacteria. Mikrobiologiia 71, 725–740 [PubMed] [Google Scholar]
- 27.Kompantseva E. I., Sorokin DIu., Gorlenko V. M., and Namsaraev B. B. (2005) The phototrophic community found in lake Khilganta (an alkaline saline lake located in the southeastern Transbaikal region). Mikrobiologiia 74, 410–419 [PubMed] [Google Scholar]
- 28.Gerasimenko L. M., Mitiushina L. L., and Namsaraev B. B. (2003) Microcoleus mats from alkaliphilic and halophilic communities. Mikrobiologiia 72, 84–92 [PubMed] [Google Scholar]
- 29.Castenholz R. W., and Waterbury J. B. (1989) Bergey's Manual of Systematic Bacteriology, Vol. 3, pp. 1710–1727, Williams and Wilkins Co, Baltimore, MD [Google Scholar]
- 30.Williams J. G. (1988) Construction of specific mutations in photosystem II photosynthetic reaction center by genetic engineering methods in Synechocystis 6803. Methods Enzymol. 167, 766–778 [Google Scholar]
- 31.Cox M. P., Peterson D. A., and Biggs P. J. (2010) SolexaQA: at-a-glance quality assessment of Illumina second-generation sequencing data. BMC Bioinformatics 11, 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Myers J., Graham J. R., and Wang R. T. (1980) Light harvesting in Anacystis nidulans studied in pigment mutants. Plant Physiol. 66, 1144–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chamovitz D., Sandmann G., and Hirschberg J. (1993) Molecular and biochemical characterization of herbicide resistant mutants of cyanobacteria reveals that phytoene desaturation is a rate limiting step in carotenoid biosynthesis. J. Biol. Chem. 268, 17348–17353 [PubMed] [Google Scholar]
- 34.Gambetta G. A., and Lagarias J. C. (2001) Genetic engineering of phytochrome biosynthesis in bacteria. Proc. Natl. Acad. Sci. U.S.A. 98, 10566–10571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao K. H., Ran Y., Li M., Sun Y. N., Zhou M., Storf M., Kupka M., Böhm S., Bubenzer C., and Scheer H. (2004) Photochromic biliproteins from the cyanobacterium Anabaena sp. PCC 7120: lyase activities, chromophore exchange, and photochromism in phytochrome AphA. Biochemistry 43, 11576–11588 [DOI] [PubMed] [Google Scholar]
- 36.Berkelman T. R., and Lagarias J. C. (1986) Visualization of bilin-linked peptides and proteins in polyacrylamide gels. Anal. Biochem. 156, 194–201 [DOI] [PubMed] [Google Scholar]
- 37.Schirrmeister B. E., Antonelli A., and Bagheri H. C. (2011) The origin of multicellularity in cyanobacteria. BMC Evol. Biol. 11, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schirrmeister B. E., de Vos J. M., Antonelli A., and Bagheri H. C. (2013) Evolution of muticellularity coincided with increased diversification of cyanobacteria and the Great Oxidation Event. Proc. Natl. Acad. Sci. U.S.A. 110, 1791–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edgar R. C. (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang E. C., Boo G. H., Kim H. J., Cho S. M., Boo S. M., Andersen R. A., and Yoon H. S. (2012) Supermatrix data highlight the phylogenetic relationships of photosynthetic stramenopiles. Protist. 163, 217–231 [DOI] [PubMed] [Google Scholar]
- 41.Laskowski R. A., and Swindells M. B. (2011) LigPlot+: multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model 51, 2778–2786 [DOI] [PubMed] [Google Scholar]
- 42.Myers J. L., Sekar R., and Richardson L. L. (2007) Molecular detection and ecological significance of the cyanobacterial genera Geitlerinema and Leptolyngbya in black band disease of corals. Appl. Environ. Microbiol. 73, 5173–5182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Puigbò P., Wolf Y. I., and Koonin E. V. (2009) Search for a ”Tree of Life“ in the thicket of the phylogenetic forest. J. Biol. 8, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rippka R., Deruelles J., Waterbury J. B., Herdman M., and Stanier R. Y. (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 111, 1–61 [Google Scholar]
- 45.Campbell E. L., Hagen K. D., Chen R., Risser D. D., Ferreira D. P., and Meeks J. C. (2015) Genetic analysis reveals the identity of the photoreceptor for phototaxis in hormogonium filaments of Nostoc punctiforme. J. Bacteriol. 197, 782–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gutu A., and Kehoe D. M. (2012) Emerging perspectives on the mechanisms, regulation, and distribution of light color acclimation in cyanobacteria. Mol. Plant. 5, 1–13 [DOI] [PubMed] [Google Scholar]
- 47.Narikawa R., Ishizuka T., Muraki N., Shiba T., Kurisu G., and Ikeuchi M. (2013) Structures of cyanobacteriochromes from phototaxis regulators AnPixJ and TePixJ reveal general and specific photoconversion mechanism. Proc. Natl. Acad. Sci. U.S.A. 110, 918–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anders K., Gutt A., Gärtner W., and Essen L. O. (2014) Phototransformation of the red light sensor cyanobacterial phytochrome 2 from Synechocystis species depends on its tongue motifs. J. Biol. Chem. 289, 25590–25600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Savakis P., De Causmaecker S., Angerer V., Ruppert U., Anders K., Essen L. O., and Wilde A. (2012) Light-induced alteration of c-di-CMP level controls motility of Synechocystis sp. PCC 6803. Mol. Microbiol. 85, 239–251 [DOI] [PubMed] [Google Scholar]
- 50.Bussell A. N., and Kehoe D. M. (2013) Control of a four-color sensing photoreceptor by a two-color sensing photoreceptor reveals complex light regulation in cyanobacteria. Proc. Natl. Acad. Sci. U.S.A. 110, 12834–12839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Enomoto G., Nomura R., Shimada T., Ni-Ni-Win, Narikawa R., and Ikeuchi M. (2014) Cyanobacteriochrome SesA is a diguanylate cyclase that induces cell aggregation in Thermosynechococcus. J. Biol. Chem. 289, 24801–24809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun Y. F., Xu J. G., Tang K., Miao D., Gärtner W., Scheer H., Zhao K. H., and Zhou M. (2014) Orange fluorescent proteins constructed from cyanobacteriochromes chromophorylated with phycoerythrobilin. Photochem. Photobiol. Sci. 13, 757–763 [DOI] [PubMed] [Google Scholar]
- 53.Rockwell N. C., Martin S. S., and Lagarias J. C. (2015) Identification of DXCF cyanobacteriochrome lineages with predictable photocycles. Photochem. Photobiol. Sci. 14, 929–941 [DOI] [PubMed] [Google Scholar]
- 54.Xu X. L., Gutt A., Mechelke J., Raffelberg S., Tang K., Miao D., Valle L., Borsarelli C. D., Zhao K. H., and Gärtner W. (2014) Combined mutagenesis and kinetics characterization of the bilin-binding GAF domain of the protein Slr1393 from the cyanobacterium Synechocystis PCC 6803. ChemBioChem. 15, 1190–1199 [DOI] [PubMed] [Google Scholar]
- 55.Rockwell N. C., Martin S. S., Lim S., Lagarias J. C., and Ames J. B. (2015) Characterization of red/green cyanobacteriochrome NpR6012g4 by solution NMR spectroscopy: a protonated bilin ring system in both photostates. Biochemistry 54, 2581–2600 [DOI] [PubMed] [Google Scholar]
- 56.Rockwell N. C., Martin S. S., Lim S., Lagarias J. C., and Ames J. B. (2015) Characterization of red/green cyanobacteriochrome NpR6012g4 by solution nuclear magnetic resonance spectroscopy: A hydrophobic pocket for the C15-E,anti chromophore in the photoproduct. Biochemistry 54, 3772–3783 [DOI] [PubMed] [Google Scholar]
- 57.Kehoe D. M., and Grossman A. R. (1996) Similarity of a chromatic adaptation sensor to phytochrome and ethylene receptors. Science 273, 1409–1412 [DOI] [PubMed] [Google Scholar]
- 58.Hirose Y., Narikawa R., Katayama M., and Ikeuchi M. (2010) Cyanobacteriochrome CcaS regulates phycoerythrin accumulation in Nostoc punctiforme, a group II chromatic adapter. Proc. Natl. Acad. Sci. U.S.A. 107, 8854–8859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoshihara S., Geng X., and Ikeuchi M. (2002) pilG gene cluster and split pilL genes involved in pilus biogenesis, motility and genetic transformation in the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 43, 513–521 [DOI] [PubMed] [Google Scholar]
- 60.Ryu J. Y., Song J. Y., Lee J. M., Jeong S. W., Chow W. S., Choi S. B., Pogson B. J., and Park Y.-I. (2004) Glucose-induced expression of carotenoid biosynthesis genes in the dark is mediated by cytosolic pH in the cyanobacterium Synechocystis sp. PCC 6803. J. Biol. Chem. 279, 25320–25325 [DOI] [PubMed] [Google Scholar]
- 61.Ryu J. Y., Song J. Y., Chung Y. H., Park Y. M., Chow W. S., and Park Y.-I. (2010) Transcript accumulation of carotenoid biosynthesis genes in the cyanobacterium Synechocystis sp. PCC6803 during the dark-to-light transition is mediated by photosynthetic electron transport. Plant Biotechnol. Rep. 4, 149–155 [Google Scholar]
- 62.Lim S., Rockwell N. C., Martin S. S., Dallas J. L., Lagarias J. C., and Ames J. B. (2014) Photoconversion changes bilin chromophore conjugation and protein secondary structure in the violet/orange cyanobacteriochrome NpF2164g3. Photochem. Photobiol. Sci. 13, 951–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rizzini L., Favory J. J., Cloix C., Faggionato D., O'Hara A., Kaiserli E., Baumeister R., Schäfer E., Nagy F., Jenkins G. I., and Ulm R. (2011) Perception of UV-B by the Arabidopsis UVR8 protein. Science 332, 103–106 [DOI] [PubMed] [Google Scholar]
- 64.Krauss U., Minh B. Q., Losi A., Gärtner W., Eggert T., von Haeseler A., and Jaeger K. E. (2009) Distribution and phylogeny of light-oxygen-voltage-blue-light-signaling proteins in the three kingdoms of life. J. Bacteriol. 191, 7234–7242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Raffelberg S., Wang L., Gao S., Losi A., Gärtner W., and Nagel G. (2013) A LOV-domain-mediated blue-light-activated adenylate (adenylyl) cyclase from the cyanobacterium Microcoleus chthonoplastes PCC 7420. Biochem. J. 455, 359–365 [DOI] [PubMed] [Google Scholar]
- 66.Masuda S., Hasegawa K., Ishii A., and Ono T. A. (2004) Light-induced structural changes in a putative blue-light receptor with a novel FAD binding fold sensor of blue-light using FAD (BLUF): Slr1694 of Synechocystis sp. PCC 6803. Biochemistry 43, 5304–5313 [DOI] [PubMed] [Google Scholar]
- 67.Masuda S. (2013) Light detection and signal transduction in the BLUF photoreceptors. Plant Cell Physiol. 54, 171–179 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.