Abstract
Purpose: To compare the efficacy of Coupled Plasma Filtration and Adsorption (CPFA) plus Continuous Veno-Venous Haemofiltration (CVVH) versus CVVH alone as an adjunct treatment of sepsis in terms of haemodynamic stability, inotropic requirement and inflammatory mediators.
Design and Methods: Prospective randomized controlled trial involving septic patients with/without acute kidney injury (AKI) whom were randomized to receive CPFA + CVVH or CVVH alone. Haemodynamic parameters including inotropic requirements and inflammatory mediators [procalcitonin (PCT) and C reactive protein (CRP)] were measured.
Results: Twenty-three patients [CPFA + CVVH (n = 11), CVVH (n = 12)] were enrolled. Haemodynamic stability occurred earlier and sustained in the CPFA + CVVH group with an increase in diastolic blood pressure (p = 0.001 vs. p = 0.226) and mean arterial pressure (p = 0.001 vs. p = 0.575) at the end of treatment with no increment in inotropic requirement. Both groups had a reduction in PCT and CRP (CPFA + CVVH: p = 0.003, p = 0.026 and CVVH: p = 0.008, p = 0.071 respectively). The length of intensive care unit stay, hospital stay and 30 day outcomes were similar between the groups. There was an inverse association between serum albumin and CRP (p = 0.018). Serum albumin positively correlated with systolic blood pressure (p = 0.012) and diastolic blood pressure (p = 0.009). We found a trend between CRP and length of hospital stay (p = 0.056). Patients with a lower PCT at 24 h had a better outcome (survival) than those with a higher PCT (p = 0.045).
Conclusion: CPFA is a feasible, albeit expensive adjunctive extracorporeal treatment that may be superior to CVVH alone in the treatment of severe sepsis.
Keywords: acute kidney injury, CPFA, C reactive protein, CVVH, sepsis, multiorgan dysfunction
Introduction
Sepsis is one of the leading causes of death in critically ill patients with the Surviving Sepsis Campaign reporting mortality rates from 30.8 % to 37 % (Dellinger et al., 2004[15]). Local Malaysian data reported a 54 % mortality rate in patients with sepsis and septicaemia (Gillani et al., 2009[19]). Sepsis is characterized by an inflammatory systemic response with the activation of cytokines resulting in endothelial injury, vasodilatation and a reduction in blood pressure (Levy et al., 2003[29]; Cohen, 1926[12]).
Acute kidney injury (AKI) is common in a wide variety of critical care settings and its prevalence in sepsis ranges from 10 to 40 % (Bellomo et al., 2004[4]; Hoste et al., 2003[25]; Levy et al., 1996[28]). Although the presence of multi-organ dysfunction and other co-morbidities contribute to the high mortality in sepsis, AKI independently increases morbidity and mortality to nearly 75.2 % (Neveu et al., 1996[34]).
Continuous Renal Replacement Therapy (CRRT) has been shown to be effective in reducing circulating cytokine levels compared to haemodialysis (Bellomo et al., 1993[6]). De Vriesse et al. (1999[14]) reported that CRRT eliminates an appreciable amount of TNF-α and other pro-inflammatory cytokines. Hence, CRRT has been proposed as a therapeutic option for blood purification in sepsis (Bellomo and Ronco, 1999[3]).
Procalcitonin (PCT), a newer diagnostic biomarker of systemic bacterial infection has been shown to be a predictor of mortality in severely septic patient (Meynaar et al., 2011[33]). Although C reactive protein (CRP) is a well recognized marker of sepsis, studies have shown PCT to be superior (Luzzani et al., 2003[31]). CRRT has also been shown to reduce PCT (Dahaba et al., 2002[13]; Nishikura, 1999[35]).
Coupled Plasma Filtration and Adsorption (CPFA) is a novel extracorporeal blood purification therapy aimed at non-selectively reducing the circulating levels of both pro-inflammatory and anti-inflammatory mediators during sepsis and multi-organ dysfunction (Tetta et al., 1998[41], 2000[42]). In vitro studies have shown CPFA to be effective in adsorbing IL-1β, IL-6, IL-8, IL-10 and TNF-α amongst others (Bellomo et al., 2002[5]). CPFA has been shown to achieve early haemodynamic stability by better blood pressure measurements, reduced inotropic support requirement and an improvement in the immune response (Cesano et al., 2003[11]). Ronco et al. (2002[39]) in his prospective pilot study demonstrated an increment in mean arterial pressure (MAP) with a reduction in noradrenaline requirement and reduced serum TNF-α in patients treated with CPFA compared to Continuous Veno-Venous Haemodialysis (CVVH). CPFA has been shown to be superior to high volume haemofiltration (HVHF) in septic patients with multiple organ dysfunction syndromes (Hu et al., 2012[26]). However, no study has directly compared the benefits of CPFA in addition to CVVH compared to CVVH alone as an adjunctive treatment for sepsis.
At our centre, conventional CVVH and HVHF are routinely used as the extracorporeal treatment for critically ill patients with sepsis and AKI.
The main objective of this study was to compare CPFA + CVVH versus CVVH alone as an adjunctive treatment in septic patients in terms of:
Haemodynamic stability and reduction in inotropic requirement
Serum profile of inflammatory biomarkers of sepsis.
The secondary objective was to compare the duration of intensive care unit (ICU) stay, hospital stay and 30 day outcomes between the patients treated by CPFA + CVVH and CVVH alone.
Materials and Methods
This was a prospective randomized controlled clinical trial involving septic patients at our institution. The study was approved by the Hospital Research and Ethics Committee (FF 192-2011).
Inclusion and exclusion criteria
Patients had to have either severe sepsis or septic shock with/without AKI to be included. The criteria used for sepsis is the modified version by Bone et al. (1992[7]) and adopted by the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference. Patients had to be over 18 years of age but less than 75 years to be included in the study and able to give written informed consent. In the patients who were unable to consent, consent was obtained from their legal representative.
Our exclusion criteria included patients on more than 2 inotropes, history of cardiopulmonary resuscitation, prolonged ventilation or poor premorbid status. Patients were also excluded if they did not complete 24 h of treatment.
Study design
Patients referred for sepsis with or without AKI were assessed for eligibility. Those who fulfilled the criteria were randomized to CPFA + CVVH or CVVH alone using random number generator software (www.sealedenvelope.com) in a 1:1 fashion. The CPFA + CVVH group received CPFA for 4 - 8 h followed by CVVH for the remaining 24 h. The CVVH group received CVVH for 24 h. We collected serum for full blood count, urea, creatinine, electrolytes and albumin. Serum inflammatory markers (PCT and CRP) were taken at baseline and at 24 h (end of treatment).
Patients
All patients received standard supportive care including fluid resuscitation with colloids and crystalloids for hypotension and started on inotropes (noradrenaline and dopamine) to maintain a mean arterial pressure > 65mm Hg. Patients received broad spectrum antibiotics that were tailored to culture results and managed by the critical care physicians. A dual lumen 12F vascular catheter (Medcomp, Harleysville, USA) was inserted under ultrasound guidance using Seldinger technique. Placement of vascular access was either in the internal jugular vein or femoral vein.
Demographic, clinical and laboratory data were collected as part of the standard sepsis ICU management. SOFA (Sequential Organ Failure Assessment) and APACHE II (Acute Physiology and Chronic Health Evaluation) scores were used to assess severity of sepsis. All patients' were followed up to 30 days from recruitment.
Coupled plasma filtration and adsorption
CPFA is an extracorporeal blood purification therapy that incorporates three phases. The first phase is plasma filtration. Blood from the patient is driven through a filter allowing plasma components to pass through the membrane pores while blood cells are returned to the patient. The plasma then passes through a sorbent cartridge where a specific resin allows non-specific adsorption of pro-inflammatory and anti-inflammatory mediators and endotoxins. This is called 'purification'. After purification, the treated plasma is returned to the circulation. Thereafter, blood and purified plasma are passed through a haemofilter for dialysis of excess water and other small solutes. Adsorption is the accumulation of molecules on the surface of a sorbent material and depends on the membrane material, pH, ionic strength and pore size amongst other factors (Formica et al., 2007[17]). CPFA duration was until all the resin was adsorbed on the sorbent and at this point the CPFA pump was turned off and patients continued with CVVH. We did not replace the sorbent after it was fully adsorbed. CPFA was performed using the HF440 device (INFOMED, Geneva, Switzerland) with a blood flow rate of 180 ml/minute, ultrafiltration rate of 35 ml/kg/h and plasma flow rate equivalent to 20 % of blood flow rate. The haemofilter used for CPFA had a surface area of 1.4 m2 (Haemofilter DF-140, Infomed, Geneva, Switzerland). Ultrafiltration rate depended on the fluid status of the patient.
For the CVVH alone group, we used the Aquarius CVVH machine (Baxter, Illinois, USA) with the Aquamax haemofilter (Baxter, Illinois, USA) with a surface area of 1.5 m2. The prescribed ultrafiltration rate of CVVH was 35 ml/kg/h with a blood flow rate of 180 - 200 ml/min. CVVH was performed using bicarbonate replacement fluid. We did not routinely change the haemofilter within 24 h unless it clotted. If there was a clinical need to continue CVVH for more than 24 h, then CVVH was continued till the clinical condition improved.
Both groups of patient did not receive heparin as most of these patients had a coagulopathy due to sepsis. Regular saline flushes were used to prevent filter clotting.
Statistical analysis
Data was analyzed using the SPSS statistical package version 20.0 (SPSS Inc, Chicago, IL, USA). All data were tested for normality. Normally distributed numerical data were expressed as mean ± standard deviation. Non-normally distributed data were subjected to non parametric tests and reported as median with inter-quartile range (IQR). Students't-test was used for comparison of quantitative data. Chi-square χ2 test with Yates correction or Fishers' Exact test was used for qualitative data. Mann-Whitney U and Wilcoxon Signed Rank test were used if data was not normally distributed. Pearson correlation and Spearman Rank order were used to ascertain correlations. A p value of < 0.05 was considered statistically significant.
Results
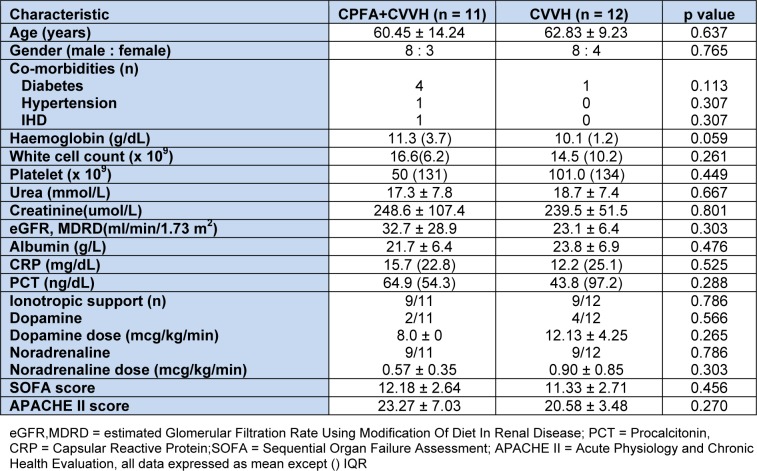
This study was carried out over a 6 month period between August 2011 and January 2012. A total of 41 patients were screened but only 26 met the inclusion criteria. All 26 patients consented to participate in the study. However, 3 patients who were recruited died shortly after randomization and within the first 8 h of treatment. Thus only 23 patients (CPFA + CVVH (n = 11), CVVH alone (n = 12)) who completed 24 h of treatment were included in the analysis. The baseline demographic and laboratory characteristics are shown on Table 1(Tab. 1).
Table 1. Table1: Baseline demographics of patients according to treatment groups.
Majority (13/23, 56.5 %) of the patients were suffering from either a community acquired or healthcare associated pneumonia. Other causes of sepsis included urinary tract infection (3/23, 13 %), intra-abdominal sepsis (2/23, 8.7 %), infective endocarditis (1/23, 4.3 %) and tuberculosis (1/23, 4.3 %). The causative organisms were gram negative organisms (9/23, 39.1 %), gram positive organisms (5/23, 21.7 %), no growth (4/23, 17.4 %), fungal (2/23, 8.7 %), mixed gram positive and gram negative (1/23, 4.3 %) and one patient each with dengue shock syndrome and tuberculosis.
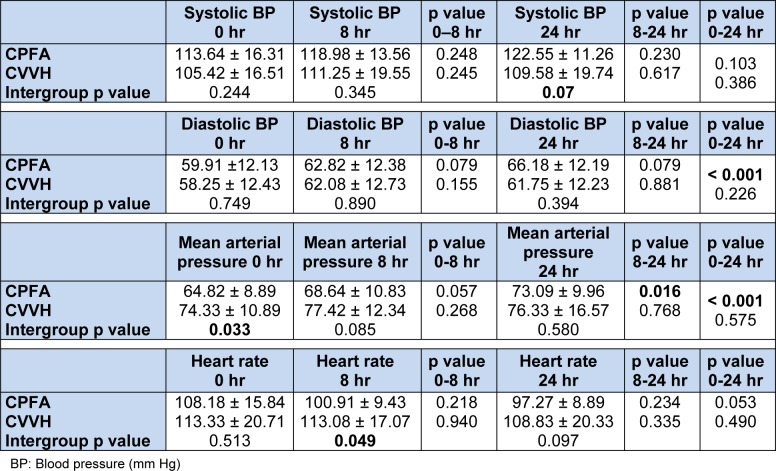
The blood pressure (BP) and haemodynamics are as shown on Table 2(Tab. 2) and Figure 1(Fig. 1). The inotropic agents used in this study were dopamine and noradrenaline. In the CPFA group 2 patients were on double inotropes where as in the CVVH group 4 patients were on double inotropes. The mean doses of dopamine at baseline in the CPFA + CVVH and CVVH alone group were 8.0 ± 0 and 12.13 ± 4.25 mcg/kg/min respectively (p = 0.265). At 24 h of treatment, the mean doses of dopamine were 12.00 ± 11.31 and 11.00 ± 10.52 mcg/kg/ min respectively (p = 0.919).
Table 2. Haemodynamic parameters of both groups.
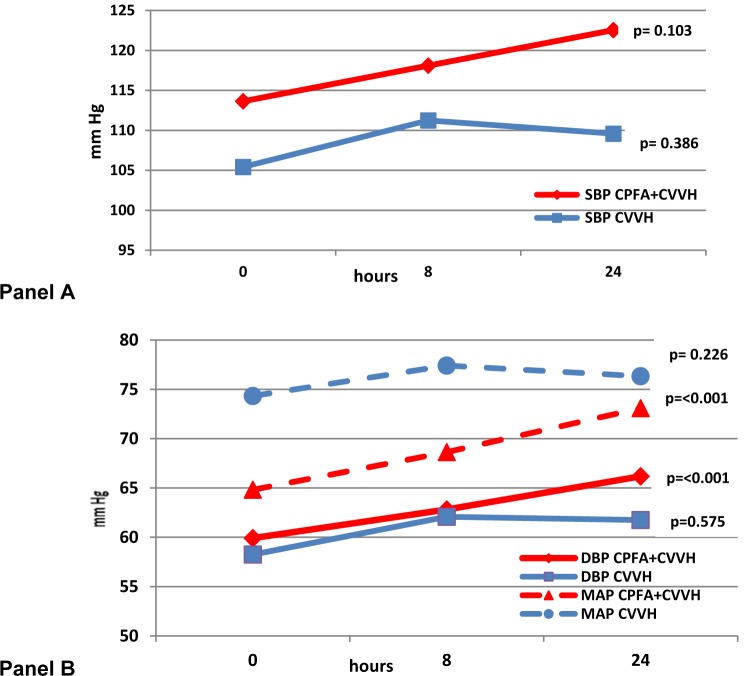
Figure 1. Systolic blood pressure (Panel A) and diastolic blood pressure and mean arterial pressure (Panel B) for both groups.
The mean dose of noradrenaline in the CPFA + CVVH and CVVH alone group at baseline was 0.57 ± 0.35 and 0.90 ± 0.85 mcg/kg/min respectively (p = 0.303). At the end of the study, the mean dose of noradrenaline was 0.69 ± 0.67 and 1.13 ± 1.18 mcg/min respectively (p = 0.343). There was no significant change in the dose of dopamine or noradrenaline (p = ns) in the CPFA arm. Similarly there was no change in the dose of dopamine and noradrenaline (p = ns) in the CVVH arm from the beginning to end of treatment.
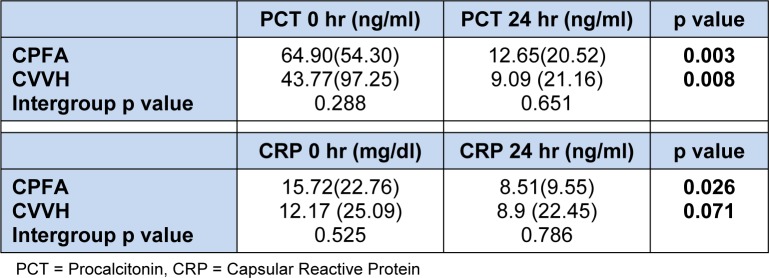
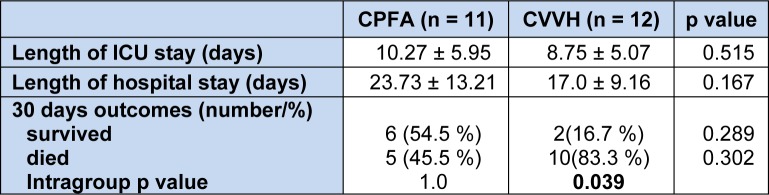
Pre and post treatment serum inflammatory markers are shown on Table 3(Tab. 3). Majority of the patients in both treatment groups had an ICU admission of less than 15 days and this shown on Table 4(Tab. 4) together with 30 days outcomes.
Table 3. Serum inflammatory mediators at the beginning and end of treatment.
Table 4. Length of ICU and hospital stay and outcomes.
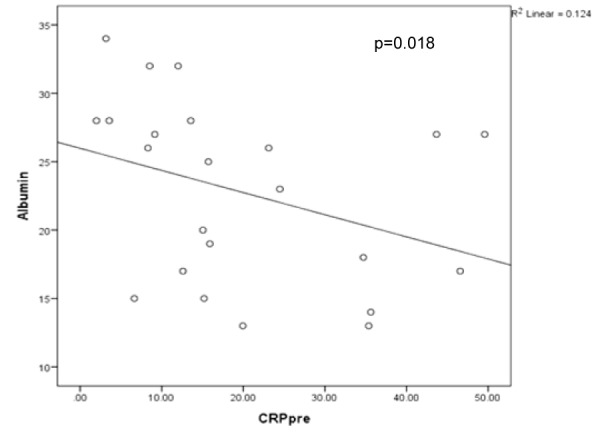
There was a strong inverse association between CRP and albumin (R² = -0.488, p = 0.018) and is demonstrated on Figure 2(Fig. 2). We found a trend between serum CRP and PCT (R2 = 0.354, p = 0.098). Serum CRP also had a trend to correlate with length of hospital stay (R2 = 0.404, p = 0.056) and length of ICU stay (R2 = 0.375, p = 0.078). CRP trended to an inverse relationship with both SBP (p = 0.085) and MAP (p = 0.059). SOFA and APACHE scores correlated well (p = 0.007). We found a strong correlation between serum albumin and systolic BP (R2 = 0.517, p = 0.012) and diastolic BP (R2 = 0.532, p = 0.009).
Figure 2. Correlation between CRP (mg/dl) and albumin (g/dL).
We explored various parameters with outcome but found no significant difference in APACHE scores/SOFA/CRP and PCT in those who survived or died at baseline. However, we found that those with a lower PCT at 24 h post treatment had a better outcome (survival) than those with a higher PCT (p = 0.045).
Discussion
Sepsis remains a major cause of morbidity and mortality (Oppert et al., 2008[36]). Despite a wider armamentarium of antibiotics to treat infection, mortality still remains high. This may be because of the other effects of sepsis leading to multiple organ dysfunction including AKI (Lopes et al., 2010[30]). Sepsis is associated with a release of both pro and anti inflammatory mediators that lead to endothelial damage, changes in immune and neuroendocrine systems, consequently resulting in multi-organ dysfunction syndrome (Annane et al., 2005[1]). There is emerging evidence that extracorporeal techniques remove inflammatory mediators and improve haemodynamic stability thereby proposed in the treatment of sepsis (Luzzani et al., 2003[31]; Klouche et al., 2002[27]). CPFA has been shown to restore immune function and haemodynamic stability and proposed as an adjunct therapy early in the course of sepsis and multi-organ dysfunction syndrome (Bellomo et al., 2002[5]; Ronco et al., 2002[39]).
The mean age of our overall study cohort was 61.70 ± 11.68 years and is in keeping with the literature whereby 26 to 51 % of patients admitted to ICU were elderly (Hennessy et al., 2005[22]). The mean estimated GFR in both groups was 27.7 ± 20.6 ml/min/1.73 m2 and CVVH was not instituted for AKI per se but for sepsis which tends to occur simultaneously (Bagshaw et al., 2008[2]). Despite randomization, patients in the CPFA + CVVH group had a trend towards a higher baseline serum haemoglobin compared to the CVVH group due to one patient in the CVVH group having an upper gastrointestinal bleed due to severe coagulopathy from sepsis. All patients received prophylactic proton pump inhibitors. We did not monitor platelets at the end of treatment as we did not use heparin and secondly in CPFA, no contact of blood cells occurs (including platelets) with the sorbent so unlikely to get treatment induced thrombocytopenia. (Ronco et al., 2003[38]) Both groups had high but comparable inflammatory mediators in keeping with sepsis. APACHE and SOFA scores were similar at baseline in both groups. Pneumonia accounted for the majority of sepsis in these patients.
Haemodynamic compromise is a major feature of severe sepsis and septic shock and in keeping with our study population, most of whom required inotropic support. We excluded patients who required more than two inotropes at baseline as these patients were unlikely to benefit from any additional intervention. Our study demonstrated that patients treated by CPFA + CVVH appeared to achieve an earlier and better haemodynamic stability compared to those in the CVVH only group.
The systolic BP was similar between the CPFA + CVVH and CVVH only group at baseline and increased with a trend to significance between them at the end of 24 h (p = 0.07). Both the diastolic BP and mean arterial pressure (MAP) were similar between both the groups but there was a significant improvement at the end of treatment in the CPFA + CVVH group (p < 0.001). The MAP was significantly lower in the CPFA + CVVH group than in the CVVH group at baseline but this reduced as the MAP improved significantly in the CPFA + CVVH group with both groups having similar MAP at the end of treatment. More importantly, there was no increased requirement of inotropes at 24 h of treatment in either group and some patients were weaned off their inotropes as their sepsis responded to multiple interventions including continuous renal replacement therapy. These results demonstrate the non selective adsorption of both pro and anti-inflammatory leads to a lower level of inflammatory mediators in the circulation, thereby reducing vasodilatation of the blood vessels and hence leading to an improvement in the blood pressure. Ronco et al. (2002[39]) demonstrated a significant improvement in MAP (p = 0.01), reduced noradrenaline requirement (p = 0.003) and reduced TNF-α (p = 0.009) in patients treated with CPFA compared to continuous veno-venous haemodialysis. Formica et al. (2007[17]) have also shown an increase in MAP, cardiac index, systemic venous resistance index, survival and a 72 % decline in CRP in septic shock patients treated with CPFA. Hu et al. (2012[26]) found CPFA to be superior to high volume haemofiltration with better in haemodynamic stability in patients with multi-organ dysfunction syndrome. Our study results concur with others in that CPFA offers better haemodynamic support (Ronco et al., 2002[39]; Formica et al., 2007[17], Hu et al., 2012[26]).
Although gram negative sepsis accounted for sepsis in 39 % of the patients, there were similar numbers of patients with gram negative sepsis in both groups. Lipopolysaccharide is the main component in gram negative bacterial cell wall and is involved in mediating the endotoxic shock of gram negative sepsis (Shimazu et al., 1999[40]). There is evidence from in vitro studies demonstrating CPFA to restore the leucocyte responsiveness to lipopolysaccharide and thereby reducing the inflammatory effects (Ronco et al., 2002[39]).
Serum PCT is a better marker for the identification of severe sepsis and predictor of outcome (Meynaar et al., 2011[33]). Serum PCT levels have been shown to be more sensitive and specific than CRP levels to differentiate bacterial from non-infective causes of inflammation as well as the ability to differentiate bacterial from viral cause of sepsis (Meisner et al., 1999[32]). However, at the time of randomization, no PCT levels were available thereby eliminating randomization bias. At the end of treatment, both groups had significant reduction of both PCT suggesting both modalities were efficacious in sepsis. Studies have shown an improvement in survival by removing pro-inflammatory mediators using haemofiltration (Hoffmann et al., 1995[24]; Hoffmann and Faist, 2001[23]). At the end of 24 h of treatment, serum CRP had decreased by 57 % in the CPFA group and this finding concurs with others (Formica et al., 2003[18]). The PCT levels had also reduced by about 75 % in the CPFA group. In our study we demonstrated that PCT at 24 h of treatment was a predictor of outcome and patients with a higher PCT were unlikely to survive. Studies have shown the correlation between PCT and morbidity and mortality (Harbarth et al., 2001[21]; Boussekey et al., 2005[9]; Castelli et al., 2006[10]).
We found no difference in terms of length of ICU or total hospital stay between the two groups. This may be due to two reasons. Firstly, we are comparing a 24 h session of CVVH treatment with or without a single cycle of CPFA sorbent and this on its own may be inadequate to significantly alter outcomes as there are multiple factors affecting the outcome rather than the treatment modality alone. Secondly, the sample size was small.
However, we did show a correlation between CRP and length of hospital stay which has been previously demonstrated (Prieto et al., 2008[37]). In our study we demonstrated a direct relationship of serum albumin with both systolic and diastolic BP and patients with low serum albumin had a lower BP. It is well recognized that a low serum albumin at sepsis presentation is a strong predictor of septic shock (Bossink et al., 2001[8]). Serum albumin is also strongly associated with the severity and outcome of sepsis in critically ill patients (Goldwasser and Feldman, 1997[20]).
We noted in the CVVH group there were a higher number of non survivors than survivors. However, we couldn't demonstrate any significance with parameters like SOFA score and APACHE II score with outcomes. This may be because we looked at these scores at baseline, whereas other studies have looked at serial SOFA scores over 48 h and up to 5 days (Ferreira et al., 2001[16]).
The strength of our study lies in the fact that it was a prospective, randomized control study and all patients were followed for the length of their ICU and hospital stays up to 30 days post treatment. The main drawback in our study was cost and technicalities of the new sorbents. Even though previous studies have been promising, this did not take into account of cost for each treatment. Due to the prohibitive costs of CPFA disposables, we could only afford to provide a single cycle of CPFA followed by CVVH per treatment whereas most studies provided about 4-8 cycles of CPFA per treatment session of 24 h.
Secondly, the initiation of CRRT in ICU was based on many factors apart from that of severe sepsis alone and included metabolic acidosis, oliguria, uraemia and fluid overload. No patient was referred sufficiently early in the course of sepsis with normal renal function and as majority of our patients had AKI, this may also explain the high mortality rate in this cohort. Thirdly, due to the small sample size, we could not ascertain the validity of 'significant' results. A larger prospective randomized controlled trial involving multiple centres is indicated to confirm these preliminary findings.
Conclusion
Our study showed that treatment with CPFA + CVVH compared with CVVH alone resulted in earlier and sustained haemodynamic stability. Both therapies significantly reduced the serum biomarkers of sepsis. These initial results do suggest that CPFA is indicated as the extracorporeal treatment of choice in selected patients with severe sepsis.
Acknowledgement
We would like to thank the Faculty of Medicine UKM for giving us a grant to carry out this research (FF-109-2011) and the Dean of UKM for allowing us to publish these data. We would also like to thank our nephrologists and haemodialysis staff who came to our aid day or night to help these critically ill septic patients.
References
- 1.Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet. 2005;365:63–78. doi: 10.1016/S0140-6736(04)17667-8. [DOI] [PubMed] [Google Scholar]
- 2.Bagshaw SM, George C, Bellomo R. Early acute kidney injury and sepsis: a multicentre evaluation. Crit Care. 2008;12:R47. doi: 10.1186/cc6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellomo R, Ronco C. Renal replacement therapy in the intensive care unit. Crit Care Resusc. 1999;1:13–24. [PubMed] [Google Scholar]
- 4.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute Dialysis Quality Initiative workgroup. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellomo R, Tetta C, Brendolan A, Ronco C. Coupled plasma filtration adsorption. Blood Purif. 2002;20:289–292. doi: 10.1159/000047022. [DOI] [PubMed] [Google Scholar]
- 6.Bellomo R, Tipping P, Boyce N. Continuous veno-venous hemofiltration with dialysis removes cytokines from the circulation of septic patients. Crit Care Med. 1993;21:522–526. doi: 10.1097/00003246-199304000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 8.Bossink AW, Groeneveld AB, Koffeman GI, Becker A. Prediction of shock in febrile medical patients with a clinical infection. Crit Care Med. 2001;29:25–31. doi: 10.1097/00003246-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Boussekey N, Leroy O, Georges H, Devos P, D'Escrivan T, Guery B. Diagnostic and prognostic values of admission procalcitonin levels in community-acquired pneumonia in an intensive care unit. Infection. 2005;33:257–263. doi: 10.1007/s15010-005-4096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castelli GP, Pognani C, Cita M, Stuani A, Sgarbi L, Paladini R. Procalcitonin, c-reactive protein, white blood cells and SOFA score in ICU: diagnosis and monitoring of sepsis. Minerva Anestesiol. 2006;72:69–80. [PubMed] [Google Scholar]
- 11.Cesano G, Livigni S, Vallero A, Olivieri C, Borca M, Quarello F, et al. Treatment of septic shock with the use of CPFA (associated plasma filtration and adsorption): impact on hemodynamics monitored with PiCCO. G Ital Nefrol. 2003;20:258–263. [PubMed] [Google Scholar]
- 12.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 13.Dahaba AA, Elawady GA, Rehak PH, List WF. Procalcitonin and proinflammatory cytokine clearance during continuous venovenous haemofiltration in septic patients. Anaesth Intensive Care. 2002;30:269–274. doi: 10.1177/0310057X0203000302. [DOI] [PubMed] [Google Scholar]
- 14.De Vriese AS, Colardyn FA, Philippe JJ, Vanholder RC, De Sutter JH, Lameire NH. Cytokine removal during continuous hemofiltration in septic patients. J Am Soc Nephrol. 1999;10:846–853. doi: 10.1681/ASN.V104846. [DOI] [PubMed] [Google Scholar]
- 15.Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, et al. Surviving sepsis campaign guidelines for management of severe sepsis and septic shock. Intensive Care Med. 2004;30:536–555. doi: 10.1007/s00134-004-2210-z. [DOI] [PubMed] [Google Scholar]
- 16.Ferreira FL, Bota DP, Bross A, Melot C, Vincent JL. Serial evaluation of the SOFA scores to predict outcome in critically ill patients. JAMA. 2001;286:1754–1758. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- 17.Formica M, Inguaggiato P, Bainotti S, Wratten ML. Coupled plasma filtration adsorption. Contrib Nephrol. 2007;156:405–410. doi: 10.1159/000102131. [DOI] [PubMed] [Google Scholar]
- 18.Formica M, Olivieri C, Livigni S, Cesano G, Vallero A, Maio M, et al. Hemodynamic response to coupled plasma filtration-adsorption in human septic shock. Intensive Care Med. 2003;29:703–708. doi: 10.1007/s00134-003-1724-0. [DOI] [PubMed] [Google Scholar]
- 19.Gillani WS, Sulaiman AS, Nejad FB. Antibiotic resistance and therapeutic management of sepsis in a Malaysian public hospital. Australas Med J. 2009;14:244–245. [Google Scholar]
- 20.Goldwasser P, Feldman J. Association of serum albumin and mortality risk. J Clin Epidemiol. 1997;50:693–703. doi: 10.1016/s0895-4356(97)00015-2. [DOI] [PubMed] [Google Scholar]
- 21.Harbarth S, Holeckova K, Froidevaux C, Pittet D, Ricou B, Grau GE, et al. Diagnostic value of procalcitonin, interleukin-6, and interleukin-8 in critically ill patients admitted with suspected sepsis. Am J Respir Crit Care Med. 2001;164:396–402. doi: 10.1164/ajrccm.164.3.2009052. [DOI] [PubMed] [Google Scholar]
- 22.Hennessy D, Juzwishin K, Yergens D, Noseworthy T, Doig C. Outcomes of elderly survivors of intensive care: a review of the literature. Chest. 2005;127:1764–1774. doi: 10.1378/chest.127.5.1764. [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann JN, Faist E. Removal of mediators by continuous hemofiltration in septic patients. World J Surg. 2001;25:651–659. doi: 10.1007/s002680020027. [DOI] [PubMed] [Google Scholar]
- 24.Hoffmann JN, Hartl WH, Deppisch R, Faist E, Jochum M, Inthorn D. Hemofiltration in human sepsis: evidence for elimination of immunomodulatory substances. Kidney Int. 1995;48:1563–1570. doi: 10.1038/ki.1995.448. [DOI] [PubMed] [Google Scholar]
- 25.Hoste EA, Lameire NH, Vanholder RC, Benoit DD, Decruyenaere JM, Colardyn FA. Acute renal failure in patients with sepsis in a surgical ICU: predictive factors, incidence, co morbidity, and outcome. J Am Soc Nephrol. 2003;14:1022–1030. doi: 10.1097/01.asn.0000059863.48590.e9. [DOI] [PubMed] [Google Scholar]
- 26.Hu D, Sun S, Zhu B, Mei Z, Wang L, Zhu S, et al. Effects of coupled plasma filtration adsorption on septic patients with multiple organ dysfunction syndrome. Ren Fail. 2012;34:834–9. doi: 10.3109/0886022X.2012.684553. [DOI] [PubMed] [Google Scholar]
- 27.Klouche K, Cavadore P, Portales P, Clot J, Canaud B, Béraud JJ. Continuous veno-venous hemofiltration improves hemodynamics in septic shock with acute renal failure without modifying TNF alpha and IL6 plasma concentrations. J Nephrol. 2002;15:150–157. [PubMed] [Google Scholar]
- 28.Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality. A cohort analysis. JAMA. 1996;275:1489–1494. [PubMed] [Google Scholar]
- 29.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;29:530–538. doi: 10.1007/s00134-003-1662-x. [DOI] [PubMed] [Google Scholar]
- 30.Lopes JA, Fernandes P, Jorge S, Resina C, Santos C, Pereira A, et al. Long-term risk of mortality after acute kidney injury in patients with sepsis: a contemporary analysis. BMC Nephrology. 2010;11:9. doi: 10.1186/1471-2369-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luzzani A, Polati E, Dorizzi R, Rungatscher A, Pavan R, Merlini A. Comparison of procalcitonin and C reactive protein as markers of sepsis. Crit Care Med. 2003;31:1737–1741. doi: 10.1097/01.CCM.0000063440.19188.ED. [DOI] [PubMed] [Google Scholar]
- 32.Meisner M, Tschaikowsky K, Palmaers T, Schmidt J. Comparison of procalcitonin (PCT) and C-reactive protein (CRP) plasma concentrations at different SOFA scores during the course of sepsis and MODS. Crit Care. 1999;3:45–50. doi: 10.1186/cc306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meynaar IA, Droog W, Batstra M, Vreede R, Herbrink P. In critically ill patients, serum procalcitonin is more useful in differentiating between sepsis and SIRS than CRP, Il-6, or LBP. Crit Care Res Pract. 2011;2011:594645. doi: 10.1155/2011/594645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neveu H, Kleinknecht D, Brivet F, Loirat P, Landais P. Prognostic factors in acute renal failure due to sepsis. Results of a prospective multicentre study. The French Study Group on Acute Renal Failure. Nephrol Dial Transplant. 1996;11:293–299. doi: 10.1093/oxfordjournals.ndt.a027256. [DOI] [PubMed] [Google Scholar]
- 35.Nishikura T. The clearance of procalcitonin (PCT) during continuous veno-venous hemodiafiltration (CVVHD) Intensive Care Med. 1999;25:1198–1199. doi: 10.1007/s001340051041. [DOI] [PubMed] [Google Scholar]
- 36.Oppert M, Engel C, Brunkhorst FM, Bogatsch H, Reinhart K, Frei U, et al. Acute renal failure in patients with severe sepsis and septic shock – a significant independent risk factor for mortality: results from the German Prevalence Study. Nephrol Dial Transplant. 2008;23:904–9. doi: 10.1093/ndt/gfm610. [DOI] [PubMed] [Google Scholar]
- 37.Prieto MF, Kilstein J, Bagilet D, Pezzotto SM. C-reactive protein as a marker of mortality in intensive care unit. Med Intensiva. 2008;32:424–430. doi: 10.1016/s0210-5691(08)75719-x. [DOI] [PubMed] [Google Scholar]
- 38.Ronco C, Brendolan A, d'Intini V, Ricci Z, Wratten ML, Bellomo R. Coupled plasma filtration adsorption: rationale, technical development and early clinical experience. Blood Purif. 2003;21:409–416. doi: 10.1159/000073444. [DOI] [PubMed] [Google Scholar]
- 39.Ronco C, Brendolan A, Lonnemann G, Bellomo R, Piccinni P, Digito A, et al. A pilot study of coupled plasma filtration with adsorption in septic shock. Crit Care Med. 2002;30:1250–1255. doi: 10.1097/00003246-200206000-00015. [DOI] [PubMed] [Google Scholar]
- 40.Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, et al. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–82. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tetta C, Cavaillon JM, Schulze M, Ronco C, Ghezzi PM, Camussi G, et al. Removal of cytokines and activated complement components in an experimental model of continuous plasma filtration coupled with sorbent adsorption. Nephrol Dial Transplant. 1998;13:1458–1464. doi: 10.1093/ndt/13.6.1458. [DOI] [PubMed] [Google Scholar]
- 42.Tetta C, Gianotti L, Cavaillon JM, Wratten ML, Fini M, Braga M, et al. Coupled plasma filtration-adsorption in a rabbit model of endotoxic shock. Crit Care Med. 2000;28:1526–1533. doi: 10.1097/00003246-200005000-00045. [DOI] [PubMed] [Google Scholar]