Abstract
Purpose
Bevacizumab improves survival in lung adenocarcinomas. The potential anti-tumor benefit of bevacizumab in squamous cell lung cancers (SQCLCs) is unknown because bevacizumab is contraindicated in patients with advanced SQCLC due to an increased risk of hemoptysis. The risk of hemoptysis may be eliminated in patients with resected SQCLCs. We evaluated the safety of adjuvant bevacizumab in patients with resected SQCLCs and other lung cancers at high risk of hemoptysis.
Methods
As part of a prospective, phase II trial, patients with lung cancers at high risk of hemoptysis (defined by SQCLC histology, tumor near the central blood vessels, or history of hemoptysis) were treated with adjuvant bevacizumab following neo-adjuvant chemotherapy and complete surgical resection. Bevacizumab 15 mg/kg was given once every 3 weeks for up to 1 year. Patients were followed for safety and survival.
Results
Thirteen patients with high-risk features were treated: 7 patients had SQCLC, 3 had central tumors, and 3 had previous hemoptysis. No hemoptysis of any grade was seen following treatment with bevacizumab. Five of 13 patients experienced grade 1 bleeding (epistaxis, gum bleeding). Hypertension and lymphopenia were seen.
Conclusions
In a cohort of patients with resected lung cancers at high risk of hemoptysis, including those with SQCLC, treatment with adjuvant bevacizumab did not result in hemoptysis of any grade.
Keywords: Non-small cell lung cancers, Squamous cell lung cancers, Bevacizumab, Adjuvant therapy, Hemoptysis, Clinical trial
Background
The dependence of solid tumor growth on the continuous formation of new blood vessels was initially hypothesized in the 1970s [1]. Angiogenesis has since been established as a hallmark of cancer [2]. At the physiologic heart of angiogenesis is a family of endothelial mitogens, vascular endothelial growth factors (VEGF), and their membrane-bound tyrosine kinase receptors (VEGFR) [3–5]. VEGF-A and its signaling through VEGFR-2 on endothelial cells are of particular importance [6–8]. Thus, the development of a monoclonal antibody targeting vascular endothelial growth factor (VEGF), bevacizumab (Genentech, South San Francisco, CA), was greeted with great optimism. Since then, studies have demonstrated clinical benefit when bevacizumab is combined with cytotoxic chemotherapy in advanced non-squamous non-small cell lung cancers (NSCLCs) [9, 10], leading to FDA approval for this indication in 2006.
Despite the benefit seen in non-squamous NSCLCs, the use of bevacizumab in squamous cell lung cancers (SQCLC) has been routinely avoided. This omission is rooted in an experience in a small cohort of patients treated with bevacizumab in the initial randomized phase II study of bevacizumab and chemotherapy in advanced NSCLCs [11]. In this study, 6 of 67 (9 %) patients suffered severe (grade ≥3) hemoptysis, which was fatal in four cases. The majority of severe hemoptysis was seen in the SQCLC group (4 of 13 SQCLC (31 %), compared to only 2 of 54 (4 %) patients with other histologies) [11]. SQCLC tumors have been subsequently excluded from most trials of bevacizumab in lung cancers. Notably, the rate of severe hemoptysis was lower in subsequent phase III and phase IV trials of bevacizumab in advanced NSCLCs which excluded squamous histologies (phase III: 2.3 % [9] and 1.2 % [10]; phase IV: 1 % [12]; compared to initial phase II: 9 % [11]).
The reasons for increased hemoptysis in patients with SQCLC treated with bevacizumab are unclear. Anatomically, the propensity of SQCLC to develop centrally near large blood vessels [13] and to cavitate [14] have been speculated as potential explanations. All 6 patients with severe hemoptysis on the initial phase II trial of bevacizumab in lung cancers had central tumors, and five had evidence of cavitation [11]. It may therefore be the structural characteristics associated with SQCLC rather than the inherent biology of its histology that predispose SQCLC patients to severe hemoptysis when treated with bevacizumab. A retrospective analysis of 877 patients with lung cancer found that rate of fatal hemoptysis was significantly higher in those with SQCLCs (7.4 %) versus those with adenocarcinomas (0.8 %) [15]. However, approximately half of all those with fatal hemoptysis had either centrally located (15/29) or cavitated disease (14/29). Univariate analysis demonstrated a significant correlation between hemoptysis and SQCLC histology, centrally located disease, as well as cavitation (p < 0.001, respectively), but no multivariate analysis was performed to determine whether SQCLC histology independently contributes risk. Of note, more recent reports have contested the predictive value of central tumor location or the presence of cavitation on the risk of hemoptysis in patients treated with bevacizumab [16]. Taken together, the etiology of the increased risk of hemoptysis for patients with SQCLC treated with bevacizumab remains unknown.
In an effort to determine whether the clinical benefit of bevacizumab in patients with advanced lung cancers could be translated to patients with resectable disease, we performed a phase II trial of neo-adjuvant bevacizumab, cisplatin, and docetaxel followed by adjuvant bevacizumab in patients with resectable lung cancers (BEACON, BEvacizumab And Chemotherapy for Operable NSCLC, NCT00130780). Arm A of this study included all patients with adenocarcinomas, and the results have been reported [17]. Patients deemed to be at high risk of severe hemoptysis with bevacizumab (SQCLC histology, history of gross hemoptysis, or large central tumors) received neo-adjuvant chemotherapy alone, but were eligible for adjuvant bevacizumab (Arm B). Adjuvant bevacizumab represents a unique setting to assess the risk of hemoptysis in patients with SQCLCs in whom the anatomic variables of central cavitating tumors are removed and an important opportunity to better understand the factors that contribute to the increased risk of hemoptysis.
Patients and methods
Patients
All patients in this IRB-approved study gave written informed consent prior to enrollment. Patients had pathologically confirmed squamous cell lung cancer or any histology with a large central tumor near significant blood vessels and/or history of hemoptysis. Patients with clinical stage IB–IIIA (T1-3N0-2M0) by American Joint Committee on Cancer Staging 6th edition were eligible. Pretreatment evaluation included chest CT, PET scan, brain MRI, and pathologic mediastinal staging (mediastinoscopy or endobronchial ultrasound) if clinically indicated. Patients were required to have a Karnofsky performance status of ≥70 %, adequate organ function, and deemed resectable by a thoracic surgeon. Patients were ineligible if they were receiving anti-coagulation, had a history of stroke or myocardial infarction within the past year, uncontrolled hypertension (HTN), non-healing wound/ulcer/fracture, hearing loss, or peripheral neuropathy >grade 1.
Study design
This was a single institution, open-label, phase II study. The primary endpoint for the overall study—pathologic downstaging following treatment with chemotherapy and bevacizumab—was not applied to patients in Arm B as they did not receive bevacizumab pre-operatively. The primary endpoint of Arm B was safety. Secondary endpoints including overall survival and relapse-free survival following resection. This study was approved by the Institutional Review Board and all patients signed informed consent.
Pre-operative treatment
Patients were treated with neo-adjuvant intravenous cisplatin (75 mg/m2) and docetaxel (75 mg/m2). Treatment was given sequentially on day 1 of a 21-day cycle. Patients underwent a CT scan following 2 cycles of therapy. If at least a 10 % reduction in bi-dimensional tumor volume was achieved, patients received two additional cycles of chemotherapy (4 cycles total).
Surgical resection
Following treatment, patients were re-evaluated for surgery by clinical examination, chest CT, PET scan, pulmonary function tests, and brain MRI. Surgical resection, if appropriate, occurred 3–8 weeks after chemotherapy.
Post-operative therapy
Adjuvant bevacizumab (15 mg/kg) was administered intravenously starting 42–56 days post-operatively and continued every 21 days for 1 year (up to 18 cycles). If post-operative radiotherapy was indicated (e.g., N2 nodal involvement or a positive resection margin), bevacizumab was delayed until 28–52 days following the completion of radiation. No cytotoxic chemotherapy was given postoperatively. Patients were evaluated every 3 weeks with the administration of bevacizumab. CT scans of the chest and upper abdomen were performed every 4 months for recurrence.
Following completion of adjuvant bevacizumab, patients were followed with history, physical examination, and CT scans every 4 months for the first year, every 6 months in years 2–3, and annually thereafter.
Outcomes analyses
Safety analysis of the Arm B cohort was pre-planned; toxicity was monitored and graded using National Cancer Institute Common Toxicity Criteria, version 3.0. Overall survival (OS) and disease-free survival (DFS) were estimated using the Kaplan–Meier method, with patients followed from time of surgery until death (in OS analysis) and until relapse or recurrence of disease, or death, whichever came first (in DFS analysis). Patients who did not experience the event of interest during study time were censored at the time of last known follow-up.
Results
Patients
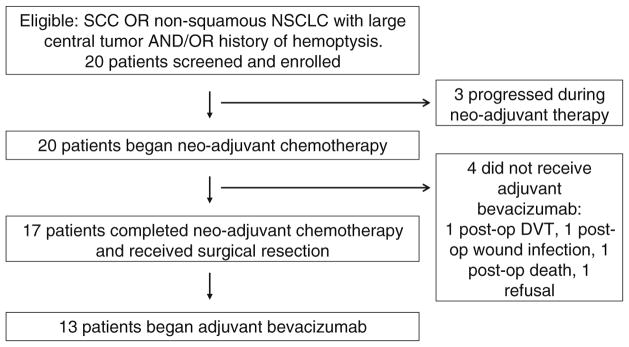
Twenty patients were enrolled between August 2005 and April 2011. Three patients progressed during neo-adjuvant chemotherapy and were taken off study. Of the 17 patients who completed neo-adjuvant chemotherapy and underwent surgical resection, 4 patients did not receive post-operative bevacizumab. Reasons for not receiving adjuvant bevacizumab included deep-vein thrombosis (DVT), wound infection, death, and patient refusal. The CONSORT diagram is shown in Fig. 1. The baseline characteristics of the 13 patients treated with post-operative bevacizumab are presented in Table 1.
Fig. 1.
CONSORT diagram
Table 1.
Baseline characteristics
| N | |
|---|---|
| Total # of patients | 13 |
| Sex | |
| Male | 7 (54 %) |
| Median age (range) | 61 (47–67) |
| Smoking status | |
| Current/former | 12 (92 %) |
| Never | 1 (7 %) |
| Histology | |
| Squamous cell carcinoma | 7 (54 %) |
| Adenocarcinoma | 6 (46 %) |
| Clinical stage at diagnosis | |
| IB | 2 (15.4 %) |
| IIA | 0 |
| IIB | 3 (23.1 %) |
| IIIA | 8 (61.5 %) |
| # of cycles of pre-operative chemotherapy | |
| 4 | 13 (100 %) |
| Pathologic stage at resection | |
| IA | 2 (15.4 %) |
| IB | 3 (23.1 %) |
| IIA | 2 (15.4 %) |
| IIB | 3 (23.1 %) |
| IIIA | 3 (23.1 %) |
| Resection status | |
| R0 | 10 (76.9 %) |
| R1 | 3 (23.1 %) |
| R2 | 0 |
| Post-operative radiation | |
| Yes | 5 (38.4 %) |
| No | 8 (61.5 %) |
Response to neo-adjuvant chemotherapy
Thirteen patients received neo-adjuvant chemotherapy with cisplatin and docetaxel and adjuvant therapy with bevacizumab. Pathologic response to neo-adjuvant chemotherapy was evaluated at the time of surgical resection. Seven of 13 (54 %) patients were downstaged (defined as an improvement in pathologic stage at the time of surgical resection compared to clinical staging at the time of diagnosis) following neo-adjuvant chemotherapy. The percent viable residual tumor was also assessed and ranged from 0 to 100 % (median 70 %). One patient had a complete pathologic response.
Bevacizumab compliance and toxicity
The median number of cycles received was 9 (range 2–18). Two patients completed all planned treatments. Reasons for early cessation of bevacizumab were toxicity (n = 4), disease recurrence (n = 2), and patient request (n = 5).
Toxicity experienced in the adjuvant bevacizumab treatment phase is summarized in Table 2. Five of 13 (38.5 %) patients experienced grade 1 bleeding (epistaxis, gum bleeding). One patient stopped therapy as a result of grade 1 epistaxis. No higher grade bleeding (grades 2–5) was reported in any patients. No patient developed hemoptysis of any grade.
Table 2.
Toxicity, all patients
| Toxicity | Any grade | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|
| Hyperglycemia | 13 (100 %) | 0 | 0 | 0 |
| Fatigue | 6 (46.2 %) | 0 | 0 | 0 |
| Hyponatremia | 6 (46.2 %) | 0 | 0 | 0 |
| Bleeding/epistaxis | 5 (38.5 %) | 0 | 0 | 0 |
| Anemia | 4 (30.8 %) | 0 | 0 | 0 |
| Transaminitis | 4 (30.8 %) | 0 | 0 | 0 |
| Lymphopenia | 3 (23.1 %) | 3 (23.1 %) | 0 | 0 |
| Rash | 3 (23.1 %) | 0 | 0 | 0 |
| Renal dysfunction | 3 (23.1 %) | 0 | 0 | 0 |
| Hypernatremia | 3 (23.1 %) | 0 | 0 | 0 |
| HTN | 2 (15.4 %) | 2 (15.4 %) | 0 | 0 |
| Hyperbilirubinemia | 2 (15.4 %) | 0 | 0 | 0 |
| Acute MI | 1 (7.7 %) | 0 | 0 | 1 (7.7 %) |
| Pneumonia | 1 (7.7 %) | 0 | 1 (7.7 %) | 0 |
| CVA | 1 (7.7 %) | 0 | 1 (7.7 %) | 0 |
| DVT | 1 (7.7 %) | 1 (7.7 %) | 0 | 0 |
| Pleural effusion | 1 (7.7 %) | 1 (7.7 %) | 0 | 0 |
| Syncope | 1 (7.7 %) | 1 (7.7 %) | 0 | 0 |
| Edema | 1 (7.7 %) | 0 | 0 | 0 |
| Nausea | 1 (7.7 %) | 0 | 0 | 0 |
Nine of 13 patients (69.2 %) experienced a grade 3+ adverse event. Two of these grade 3+ events, pneumonia and stroke, were unrelated to bevacizumab. The stroke predated the start of bevacizumab but was not detected until after therapy began. Grade 3 lymphopenia occurred in 3 patients (23.1 %), but no neutropenia was reported. One patient experienced a catheter-associated DVT. Grade 3 hypertension occurred in 2 patients (15.4 %).
Relapse-free and overall survival
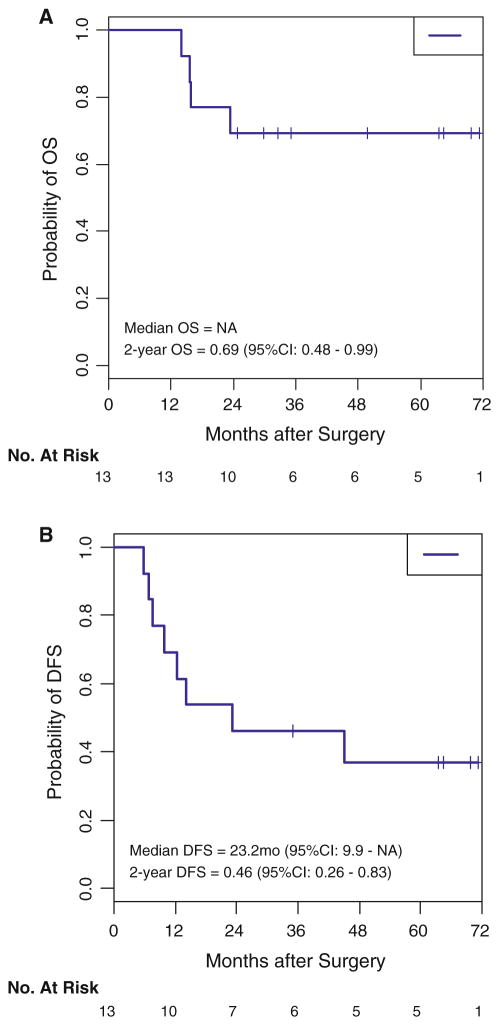
The median duration of follow-up after surgery was 32.5 months (range 14–71 months). Seven of 13 patients relapsed following surgery. Four of 13 have died, three of which were as a result of recurrent cancer. Median overall survival was not reached; 2-year overall survival was 69 % (95 % CI 48–99) (Fig. 2a). Median disease-free survival was 23 months (95 % CI 10—not reached); 2-year disease-free survival was 46 % (95 % CI 26–83) (Fig. 2b).
Fig. 2.
a Overall survival (OS). b Disease-free survival (DFS)
Table 3 reports the clinical characteristics, treatment course, toxicity, and survival data for all patients on study.
Table 3.
Individual patient characteristics, toxicity, and outcomes
| Patient # | Sex | Age | Ever smoker | Pk years | Histology | Pre-operative bevacizumab exclusion |
Clinical stage | # Cisplatin | # Docetaxel | Pathologic stage |
Pathologic downstage |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | F | 47 | Y | 47 | SQCLC | SQCLC | IIB | 4 | 4 | IA | Yes |
| 6 | M | 64 | Y | 42 | SQCLCa | SQCLC | IB | 4 | 4 | IB | No |
| 7 | M | 50 | Y | 32 | SQCLC | SQCLC | IB | 4 | 2 | IIB | No |
| 8 | F | 65 | Y | 50 | SQCLC | SQCLC | IIIA | 4 | 4 | IIIA | No |
| 10 | F | 62 | Y | 18 | SQCLC | SQCLC | IIB | 4 | 4 | IIA | Yes |
| 11 | M | 67 | Y | 20 | SQCLC | SQCLC | IIB | 4 | 4 | IIB | No |
| 17 | M | 53 | Y | 40 | SQCLC | SQCLC | IIIA | 4 | 4 | IIA | Yes |
| 3 | M | 62 | Y | 74 | ADC | Central | IIIA | 4 | 4 | IA | Yes |
| 4 | F | 64 | Y | 20 | ADC | Hemoptysis + central | IIIA | 2 | 4 | IIB | Yes |
| 12 | F | 61 | Y | 31 | ADC | Hemoptysis | IIIA | 4 | 4 | IB | Yes |
| 14 | M | 49 | Y | 15 | ADC | Central | IIIA | 4 | 4 | IB | Yes |
| 16 | M | 59 | N | – | ADC | Hemoptysis | IIIA | 3 | 4 | IIIA | No |
| 18 | F | 51 | Y | 20 | ADC | Central | IIIA | 4 | 4 | IIIA | No |
| Patient # | % viable tumor | Resection | PORT | # Bevacizumab | Reason bevacizumab stopped |
Any bleeding toxicity |
Grade 3+ toxicity | Grade 3+ toxicity attribution |
Time to relapse or recurrence (mo) |
Time to death (mo) |
Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 60 | 1 | N | 18 | Completed | 69.4 | |||||
| 6 | 40 | 0 | N | 14 | Pt request | 71.1 | |||||
| 7 | 90 | 0 | N | 6 | Recurrence | Grade 1 | 6.9 | 23.3 | 23.3 | ||
| 8 | 30 | 0 | N | 9 | Toxicity (HTN) | HTN (grade 3) | Definite | 63.3 | |||
| 10 | 95 | 1 | Y | 9 | Pt request | Grade 1 | Pleural effusion (grade 3) | Possible | 44.8 | 49.6 | |
| Lymphopenia (grade 3) | Possible | ||||||||||
| 11 | 80 | 0 | N | 2 | Toxicity (CVA—unrelated) | CVA (grade 4) | Unrelated | 5.7 | 15.8 | 15.8 | |
| 17 | 100 | 0 | Y | 12 | Toxicity (MI/death) | Grade 1 | PNA (grade 4) | Unrelated | 14.0 | 14.0 | |
| MI (grade 5) | Possible | ||||||||||
| 3 | 0 | 0 | N | 17 | Completed | Grade 1 | HTN (grade 3) | Definite | 64.2 | ||
| 4 | 30 | 0 | N | 8 | Recurrence | Syncope (grade 3) | Possible | 7.6 | 15.6 | 15.6 | |
| 12 | 70 | 1 | Y | 9 | Pt request | 35.0 | |||||
| 14 | 80 | 0 | N | 3 | Pt request | DVT (grade 3) | Probable | 9.8 | 32.5 | ||
| 16 | 40 | 0 | Y | 10 | Toxicity (G1 epistaxis) | Grade 1 | Lymphopenia (grade 3) | Possible | 12.4 | 29.6 | |
| 18 | 80 | 0 | Y | 5 | Pt request | Lymphopenia (grade 3) | Possible | 23.1 | 24.7 |
Pt #6 had SQCLC histology at the time of diagnosis, but predominantly ADC histology at the time of tumor resection
Post-operative bevacizumab in patients with squamous cell lung cancers
Seven of 13 patients treated with post-operative bevacizumab had squamous cell histologies. Patient #6 had SQCLC histology seen on diagnostic biopsy, although had predominantly adenocarcinoma histology at the time of resection [18].
Three of 7 patients with SQCLCs had disease-free and overall survival >5 years (#2, with clinical stage IIB; #6, with clinical stage IB; and #8, with clinical stage IIIA disease at diagnosis).
Patients received between 2 and 18 cycles of treatment. Three of 7 patients had grade 1 bleeding, but no higher grade bleeding was reported.
One patient completed all planned therapy. Three patients stopped therapy earlier than initially planned without dose-limiting toxicity.
The three other patients stopped treatment early due to toxicity, one of which was unrelated to bevacizumab.
Patient #11 developed a left visual field disturbance and ataxia two days following surgical resection. This was initially attributed to cataracts, and no imaging was performed. His symptoms improved and he began adjuvant bevacizumab, receiving 2 cycles. Thereafter, as his visual field disturbance persisted, he was evaluated by an ophthalmologist. Formal visual field testing revealed left homonymous hemianopsia. An MRI of the brain revealed a subacute-chronic right occipital lobe infarct. He was then taken off study. In retrospect, his stroke was felt to predate bevacizumab administration and was unrelated to bevacizumab.
Patient #8 stopped adjuvant therapy as a result of progressive hypertension after 9 cycles of bevacizumab. She has remains recurrence free more than 5 years since surgical resection.
Patient #17 received 5 cycles of adjuvant bevacizumab without complication. He was then noted to have a decreased ejection fraction on echocardiography (EF 40–45 %), which was further evaluated with a cardiac catheterization. Left ventricular EF by this method was 60 %, and only mild, non-obstructive coronary disease was seen. He returned to therapy with bevacizumab, completing cycles 6 through 8. Following cycle 8, we was hospitalized with fever and shortness of breath and diagnosed with pneumonia. He required transient mechanical ventilatory support but recovered with antibiotics. Repeat echocardiography was normal. He ultimately recovered enough to receive cycles 9–12 of bevacizumab. Following cycle 12, the patient was again hospitalized with dyspnea. An electrocardiogram revealed a non-ST segment elevation myocardial infarction with elevated cardiac enzymes. He was admitted, treated medically, but clinically deteriorated, and died 2 days after admission. An autopsy revealed diffuse atherosclerotic occlusive coronary disease and the cause of death was reported as acute myocardial infarction.
Discussion
This study is, to our knowledge, the first to address the safety of bevacizumab in patients with lung cancers at risk of developing life-threatening hemoptysis who have been rendered disease-free prior to the start of therapy. Class-specific toxicities of angiogenesis inhibitors such as bevacizumab, including venous thrombosis, rash, diarrhea, hypertension, and proteinuria, have been reported across many solid tumors. The risk of hemoptysis, however, is specific to lung cancers and, apparently, SQCLC in particular. Since the initial randomized phase II trial of chemotherapy with or without bevacizumab demonstrated an increased rate of hemoptysis in SQCLC [11], bevacizumab has been routinely avoided in patients with SQCLC. Nevertheless, it is not known whether patients with SQCLC are biologically predisposed to hemoptysis or if the clinical features associated with SQCLC, including central location and presence of cavitation, are the predisposing factors for this adverse event. This distinction is important, as bevacizumab in patients with SQCLC without structural predispositions to hemoptysis may be safe and potentially beneficial.
We found that in 13 patients who were initially deemed high risk for hemoptysis, 38 % experienced grade 1 bleeding (4 with epistaxis, 1 with gum bleeding), while no patients had any degree of hemoptysis during adjuvant treatment with bevacizumab.
Seven patients experienced grade 3 toxicities (lymphopenia, DVT, syncope, HTN, pleural effusion), each of which were possibly, probably, or definitely related to bevacizumab.
Two patients, both with SQCLCs, experienced grade 4–5 toxicities. One patient had a stroke which, in retrospect, predated treatment with bevacizumab and was unrelated to treatment. Another had grade 4 pneumonia, unlikely related to be bevacizumab, and grade 5 myocardial infarction possibly related to bevacizumab. The risk of myocardial infarction attributable to bevacizumab is uncertain. Across all indications, the rate of grade ≥3 arterial thromboembolic events (including myocardial infarction as well as angina and stroke) is 2.4 % compared to 0.7 % in the control arms [19], but no increase in myocardial infarction specifically was seen in a retrospective evaluation of 2,526 patients with stage IV colon cancer [20].
It is worth noting that strategies to safely incorporate bevacizumab in advanced SQCLC have been reported. The BRIDGE trial hypothesized that delayed integration of bevacizumab (after 2 cycles of chemotherapy) would allow initial cytoreduction and epithelial healing prior to starting bevacizumab and, therefore, reduce the risk of hemoptysis [21]. Only one patient had grade ≥3 hemoptysis (3.2 %, 90 % CI 0.3–13.5).
Although not reported in bevacizumab specifically, there may be safety concerns for the use of angiogenesis inhibitors in SQCLC that extend beyond hemoptysis. ESCAPE was a randomized, phase III trial of chemotherapy with or without sorafenib in advanced lung cancers [22]. SQCLC patients (223/926 enrolled, 24 %) receiving sorafenib had an increased risk of death compared to patients with SQCLC receiving placebo (HR 1.85 95 % CI 1.22–2.82). This was not attributable to hemorrhage as the rate of hemorrhage was the same across SQCLC patients regardless of treatment received. These data suggest that patients with SQCLC were harmed with sorafenib by heretofore unknown mechanisms.
The toxicity of angiogenesis inhibitors in patients with SQCLC treated with may not be uniform across this class of drugs. For example, an increased risk of hemoptysis and mortality was seen in patients with SQCLC treated with motesanib [23]. However, other studies of angiogenesis inhibitors in advanced lung cancers such as vandetanib [24–27] and cediranib [28, 29] have not reported increased toxicity in SQCLC patients.
Finally, it is important to mention that the biologic rationale for targeting VEGF in SQCLC has also been subject to some controversy. Two older studies suggested that intratumoral VEGF overexpression was a negative prognostic marker in SQCLC [30, 31], providing incentive to target VEGF therapeutically. A more recent meta-analysis was equivocal as to the prognostic relevance of VEGF in SQCLC [32]. Most recently, an analysis of VEGF, VEGFR-1, and VEGFR-2 reported higher composite expression in early stage SQCLCs to be associated with, contrary to earlier studies, improved prognosis [33].
In summary, our trial demonstrates that adjuvant bevacizumab did not lead to hemoptysis in a cohort of high-risk patients with lung cancers, 7 of whom had SQCLC, following curative surgical resection of their disease. These findings are consistent with previous notions that the risk of hemoptysis may be related to anatomic factors rather than inherent biology. In the absence of high-risk anatomic features, the risk of hemoptysis appears to drop substantially. We note that although the size of this cohort is small, it is the same (n = 13) as the cohort of patients with SQCLC who received chemotherapy and bevacizumab on the initial phase II trial in which the concern for hemoptysis originally arose. We eagerly await the results of the ECOG 1505 trial to more fully evaluate both the safety and the efficacy of bevacizumab in conjunction with chemotherapy in the adjuvant treatment of SQCLC and non-SQCLC.
Acknowledgments
This trial was supported by Genentech Inc. These data have been presented in part at the ASCO Annual Meetings in 2007, 2008, 2009 and at the IASLC World Conference on Lung Cancer 2011.
Footnotes
ClinicalTrials.gov Identifier: NCT00130780.
Conflict of interest Drs. Kris, Riely, and Krug have received consulting fees from Genentech/Roche. This study was sponsored by a research grant from Genentech. The authors have full control of the primary data and agree to allow the journal to review their data if requested.
Ethical Standards All human studies described in this manuscript have been approved by the Institutional Review Board of Memorial Sloan-Kettering Cancer Center. All human research has been performed in accordance with ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Contributor Information
Matthew D. Hellmann, Thoracic Oncology Service, Division of Solid Tumor Oncology, Department of Medicine, Memorial Sloan-Kettering Cancer Center, Weill Cornell Medical School, 300 East 66th Street, New York, NY 10065, USA
Jamie E. Chaft, Thoracic Oncology Service, Division of Solid Tumor Oncology, Department of Medicine, Memorial Sloan-Kettering Cancer Center, Weill Cornell Medical School, 300 East 66th Street, New York, NY 10065, USA
Valerie Rusch, Thoracic Service, Department of Surgery, Memorial Sloan-Kettering Cancer Center, Weill Cornell Medical School, New York, NY, USA.
Michelle S. Ginsberg, Department of Radiology, Memorial Sloan-Kettering Cancer Center, Weill Cornell Medical School, New York, NY, USA
David J. Finley, Thoracic Service, Department of Surgery, Memorial Sloan-Kettering Cancer Center, Weill Cornell Medical School, New York, NY, USA
Mark G. Kris, Thoracic Oncology Service, Division of Solid Tumor Oncology, Department of Medicine, Memorial Sloan-Kettering Cancer Center, Weill Cornell Medical School, 300 East 66th Street, New York, NY 10065, USA
Katharine A. R. Price, Thoracic Oncology Service, Division of Solid Tumor Oncology, Department of Medicine, Memorial Sloan-Kettering Cancer Center, Weill Cornell Medical School, 300 East 66th Street, New York, NY 10065, USA
Christopher. G. Azzoli, Thoracic Oncology Service, Division of Solid Tumor Oncology, Department of Medicine, Memorial Sloan-Kettering Cancer Center, Weill Cornell Medical School, 300 East 66th Street, New York, NY 10065, USA
Matthew G. Fury, Thoracic Oncology Service, Division of Solid Tumor Oncology, Department of Medicine, Memorial Sloan-Kettering Cancer Center, Weill Cornell Medical School, 300 East 66th Street, New York, NY 10065, USA
Gregory J. Riely, Thoracic Oncology Service, Division of Solid Tumor Oncology, Department of Medicine, Memorial Sloan-Kettering Cancer Center, Weill Cornell Medical School, 300 East 66th Street, New York, NY 10065, USA
Lee M. Krug, Thoracic Oncology Service, Division of Solid Tumor Oncology, Department of Medicine, Memorial Sloan-Kettering Cancer Center, Weill Cornell Medical School, 300 East 66th Street, New York, NY 10065, USA
Robert J. Downey, Thoracic Service, Department of Surgery, Memorial Sloan-Kettering Cancer Center, Weill Cornell Medical School, New York, NY, USA
Manjit S. Bains, Thoracic Service, Department of Surgery, Memorial Sloan-Kettering Cancer Center, Weill Cornell Medical School, New York, NY, USA
Camelia S. Sima, Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, Weill Cornell Medical School, New York, NY, USA
Nabil Rizk, Thoracic Service, Department of Surgery, Memorial Sloan-Kettering Cancer Center, Weill Cornell Medical School, New York, NY, USA.
William D. Travis, Department of Pathology, Memorial Sloan-Kettering Cancer Center, Weill Cornell Medical School, New York, NY, USA
Naiyer A. Rizvi, Thoracic Oncology Service, Division of Solid Tumor Oncology, Department of Medicine, Memorial Sloan-Kettering Cancer Center, Weill Cornell Medical School, 300 East 66th Street, New York, NY 10065, USA
Paul K. Paik, Email: paikp@mskcc.org, Thoracic Oncology Service, Division of Solid Tumor Oncology, Department of Medicine, Memorial Sloan-Kettering Cancer Center, Weill Cornell Medical School, 300 East 66th Street, New York, NY 10065, USA
References
- 1.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, Wu Y, Hicklin D, Zhu Z, Hackett NR, Crystal RG, Moore MA, Hajjar KA, Manova K, Benezra R, Rafii S. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 5.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 6.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling—in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 8.Koo PJ, Morgensztern D, Boyer JL, Herbst RS. Targeting vascular endothelial growth factor in patients with squamous cell lung cancer. J Clin Oncol. 2012;30:1137–1139. doi: 10.1200/JCO.2011.40.4053. [DOI] [PubMed] [Google Scholar]
- 9.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 10.Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, Leighl N, Mezger J, Archer V, Moore N, Manegold C. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27:1227–1234. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 11.Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM, Langer CJ, DeVore RF, 3rd, Gaudreault J, Damico LA, Holmgren E, Kabbinavar F. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22:2184–2191. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 12.Crino L, Dansin E, Garrido P, Griesinger F, Laskin J, Pavlakis N, Stroiakovski D, Thatcher N, Tsai CM, Wu YL, Zhou CC. Safety and efficacy of first-line bevacizumab-based therapy in advanced non-squamous non-small-cell lung cancer (SAiL, MO19390): a phase 4 study. Lancet Oncol. 2010;11:733–740. doi: 10.1016/S1470-2045(10)70151-0. [DOI] [PubMed] [Google Scholar]
- 13.Novotny WF, Holmgren E, Griffing S, Johnson D, De Vore R, Kabbinavar F. Identification of squamous cell histology and central, cavitary tumors as possible risk factors for pulmonary hemorrhage (PH) in patients with advanced NSCLC receiving bevacizumab (BV) Proc Am Soc Clin Oncol. 2001;20:1318. [Google Scholar]
- 14.Sandler AB, Schiller JH, Gray R, Dimery I, Brahmer J, Samant M, Wang LI, Johnson DH. Retrospective evaluation of the clinical and radiographic risk factors associated with severe pulmonary hemorrhage in first-line advanced, unresectable non-small-cell lung cancer treated with Carboplatin and Paclitaxel plus bevacizumab. J Clin Oncol. 2009;27:1405–1412. doi: 10.1200/JCO.2008.16.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller RR, McGregor DH. Hemorrhage from carcinoma of the lung. Cancer. 1980;46:200–205. doi: 10.1002/1097-0142(19800701)46:1<200::aid-cncr2820460133>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 16.Reck M, Barlesi F, Crino L, Henschke CI, Isla D, Stiebeler S, Spigel DR. Predicting and managing the risk of pulmonary haemorrhage in patients with NSCLC treated with bevacizumab: a consensus report from a panel of experts. Ann Oncol. 2012;23:1111–1120. doi: 10.1093/annonc/mdr463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaft JE, Rusch V, Ginsberg MS, Paik PK, Finley DJ, Kris MG, Price KAR, Azzoli CG, Fury MG, Riely GJ, Krug LM, Downey RJ, Bains MS, Sima CS, Rizk N, Travis WD, Rizvi NA. Phase II trial of neoadjuvant bevacizumab plus chemotherapy and adjuvant bevacizumab in patients with resectable non-squamous non-small cell lung cancers. J Thorac Oncol. 2013;8(8) doi: 10.1097/JTO.0b013e31829923ec. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaft JE, Rekhtman N, Ladanyi M, Riely GJ. ALK-rearranged lung cancer: adenosquamous lung cancer masquerading as pure squamous carcinoma. J Thorac Oncol. 2012;7:768–769. doi: 10.1097/JTO.0b013e31824c9485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Incorperated G; California SF, editor. Avastin (bevacizumab) intravenous infusion [package insert] 2011 http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/125085s225lbl.pdf.
- 20.Meyerhardt JA, Li L, Sanoff HK, Carpenter Wt, Schrag D. Effectiveness of bevacizumab with first-line combination chemotherapy for Medicare patients with stage IV colorectal cancer. J Clin Oncol. 2012;30:608–615. doi: 10.1200/JCO.2011.38.9650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hainsworth JD, Fang L, Huang JE, Karlin D, Russell K, Faoro L, Azzoli C. BRIDGE: an open-label phase II trial evaluating the safety of bevacizumab + carboplatin/paclitaxel as first-line treatment for patients with advanced, previously untreated, squamous non-small cell lung cancer. J Thorac Oncol. 2011;6:109–114. doi: 10.1097/JTO.0b013e3181f94ad4. [DOI] [PubMed] [Google Scholar]
- 22.Scagliotti G, Novello S, von Pawel J, Reck M, Pereira JR, Thomas M, Abrao Miziara JE, Balint B, De Marinis F, Keller A, Aren O, Csollak M, Albert I, Barrios CH, Grossi F, Krzakowski M, Cupit L, Cihon F, Dimatteo S, Hanna N. Phase III study of carboplatin and paclitaxel alone or with sorafenib in advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:1835–1842. doi: 10.1200/JCO.2009.26.1321. [DOI] [PubMed] [Google Scholar]
- 23.vScagliotti GV, Vynnychenko I, Park K, Ichinose Y, Kubota K, Blackhall F, Pirker R, Galiulin R, Ciuleanu TE, Sydorenko O, Dediu M, Papai-Szekely Z, Banaclocha NM, McCoy S, Yao B, Hei YJ, Galimi F, Spigel DR. International, randomized, placebo-controlled, double-blind phase III study of Motesanib Plus Carboplatin/Paclitaxel in Patients With Advanced Nonsquamous Non-Small-Cell Lung Cancer: MONET1. J Clin Oncol. 2012;30:2829–2836. doi: 10.1200/JCO.2011.41.4987. [DOI] [PubMed] [Google Scholar]
- 24.Herbst RS, Sun Y, Eberhardt WE, Germonpre P, Saijo N, Zhou C, Wang J, Li L, Kabbinavar F, Ichinose Y, Qin S, Zhang L, Biesma B, Heymach JV, Langmuir P, Kennedy SJ, Tada H, Johnson BE. Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer (ZODIAC): a double-blind, randomised, phase 3 trial. Lancet Oncol. 2010;11:619–626. doi: 10.1016/S1470-2045(10)70132-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Boer RH, Arrieta O, Yang CH, Gottfried M, Chan V, Raats J, de Marinis F, Abratt RP, Wolf J, Blackhall FH, Langmuir P, Milenkova T, Read J, Vansteenkiste JF. Vandetanib plus pemetrexed for the second-line treatment of advanced non-small-cell lung cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2011;29:1067–1074. doi: 10.1200/JCO.2010.29.5717. [DOI] [PubMed] [Google Scholar]
- 26.Natale RB, Thongprasert S, Greco FA, Thomas M, Tsai CM, Sunpaweravong P, Ferry D, Mulatero C, Whorf R, Thompson J, Barlesi F, Langmuir P, Gogov S, Rowbottom JA, Goss GD. Phase III trial of vandetanib compared with erlotinib in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2011;29:1059–1066. doi: 10.1200/JCO.2010.28.5981. [DOI] [PubMed] [Google Scholar]
- 27.Lee JS, Hirsh V, Park K, Qin S, Blajman CR, Perng RP, Chen YM, Emerson L, Langmuir P, Manegold C. Vandetanib Versus placebo in patients with advanced non-small-cell lung cancer after prior therapy with an epidermal growth factor receptor tyrosine kinase inhibitor: a randomized, double-blind phase III trial (ZEPHYR) J Clin Oncol. 2012;30:1114–1121. doi: 10.1200/JCO.2011.36.1709. [DOI] [PubMed] [Google Scholar]
- 28.Goss GD, Arnold A, Shepherd FA, Dediu M, Ciuleanu TE, Fenton D, Zukin M, Walde D, Laberge F, Vincent MD, Ellis PM, Laurie SA, Ding K, Frymire E, Gauthier I, Leighl NB, Ho C, Noble J, Lee CW, Seymour L. Randomized, double-blind trial of carboplatin and paclitaxel with either daily oral cediranib or placebo in advanced non-small-cell lung cancer: NCIC clinical trials group BR24 study. J Clin Oncol. 2010;28:49–55. doi: 10.1200/JCO.2009.22.9427. [DOI] [PubMed] [Google Scholar]
- 29.Laurie SA, Solomon BJ, Seymour L, Ellis PM, Goss GD, Shepherd FA, Boyer MJ, Arnold AM, Clingan P, Laberge F, Fenton D, Hirsh V, Zukin M, Stockler MR, Lee CW, Chen EX, Montenegro A, Ding K, Bradbury PA, Group NCT. A randomized double-blind trial of carboplatin plus paclitaxel (CP) with daily oral cediranib (CED), an inhibitor of vascular endothelial growth factor receptors, or placebo (PLA) in patients (pts) with previously untreated advanced non-small cell lung cancer (NSCLC): NCIC Clinical Trials Group study BR29. J Clin Oncol. 2012;30(suppl):7511. [Google Scholar]
- 30.Imoto H, Osaki T, Taga S, Ohgami A, Ichiyoshi Y, Yasumoto K. Vascular endothelial growth factor expression in non-small-cell lung cancer: prognostic significance in squamous cell carcinoma. J Thorac Cardiovasc Surg. 1998;115:1007–1014. doi: 10.1016/S0022-5223(98)70398-8. [DOI] [PubMed] [Google Scholar]
- 31.Volm M, Koomagi R, Mattern J. Prognostic value of vascular endothelial growth factor and its receptor Flt-1 in squamous cell lung cancer. Int J Cancer. 1997;74:64–68. doi: 10.1002/(sici)1097-0215(19970220)74:1<64::aid-ijc11>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 32.Zhan P, Wang J, Lv XJ, Wang Q, Qiu LX, Lin XQ, Yu LK, Song Y. Prognostic value of vascular endothelial growth factor expression in patients with lung cancer: a systematic review with meta-analysis. J Thorac Oncol. 2009;4:1094–1103. doi: 10.1097/JTO.0b013e3181a97e31. [DOI] [PubMed] [Google Scholar]
- 33.Pajares MJ, Agorreta J, Larrayoz M, Vesin A, Ezponda T, Zudaire I, Torre W, Lozano MD, Brambilla E, Brambilla C, Wistuba II, Behrens C, Timsit JF, Pio R, Field JK, Montuenga LM. Expression of tumor-derived vascular endothelial growth factor and its receptors is associated with outcome in early squamous cell carcinoma of the lung. J Clin Oncol. 2012;30:1129–1136. doi: 10.1200/JCO.2011.37.4231. [DOI] [PMC free article] [PubMed] [Google Scholar]