Publisher's Note: There is an Inside Blood Commentary on this article in this issue.
Key Points
Factor V and protein S are required for sepsis mortality reduction and suppression of inflammatory gene expression by activated protein C.
The R506Q mutation (Leiden mutation) abrogates the anti-inflammatory cofactor function of factor V for activated protein C.
Abstract
The key effector molecule of the natural protein C pathway, activated protein C (aPC), exerts pleiotropic effects on coagulation, fibrinolysis, and inflammation. Coagulation-independent cell signaling by aPC appears to be the predominant mechanism underlying its highly reproducible therapeutic efficacy in most animal models of injury and infection. In this study, using a mouse model of Staphylococcus aureus sepsis, we demonstrate marked disease stage–specific effects of the anticoagulant and cell signaling functions of aPC. aPC resistance of factor (f)V due to the R506Q Leiden mutation protected against detrimental anticoagulant effects of aPC therapy but also abrogated the anti-inflammatory and mortality-reducing effects of the signaling-selective 5A-aPC variant that has minimal anticoagulant function. We found that procofactor V (cleaved by aPC at R506) and protein S were necessary cofactors for the aPC-mediated inhibition of inflammatory tissue-factor signaling. The anti-inflammatory cofactor function of fV involved the same structural features that govern its cofactor function for the anticoagulant effects of aPC, yet its anti-inflammatory activities did not involve proteolysis of activated coagulation factors Va and VIIIa. These findings reveal a novel biological function and mechanism of the protein C pathway in which protein S and the aPC-cleaved form of fV are cofactors for anti-inflammatory cell signaling by aPC in the context of endotoxemia and infection.
Introduction
Natural aPC exerts pleiotropic effects on coagulation, fibrinolysis, and inflammation (for review, see Esmon,1 Mosnier and Griffin,2 and Dahlbäck and Villoutreix3) that are predominantly mediated by 2 mechanistically discernable pathways: (1) aPC degrades activated coagulation factor (f)Va and fVIIIa to curtail the formation of thrombin by the plasma coagulation system and (2) when bound to the endothelial protein C receptor (EPCR, ProcR) or the integrin αMβ2 (Mac1)(CD11b/CD18), aPC becomes competent for activating the G protein–coupled protease activated receptor (PAR) 1, 2, or 3. PAR activation and crosstalk with other receptors mediate anti-inflammatory and cytoprotective effects of aPC in innate immune cells, vascular endothelium, and other cells (for review, see Weiler,4 Mosnier et al,5 and Dahlbäck and Villoutreix6). In animal models of endotoxemia and sepsis, the cell signaling activity of aPC on vascular endothelium and innate immune cells is the predominant therapeutic mechanism of action, and recombinant variants of aPC that are largely devoid of anticoagulant activity but retain signaling function confer vasoprotection, inhibit inflammation, and reduce mortality.7-9 Although the incidence of microvascular coagulopathies in patients with severe sepsis provides a compelling rationale for the benefits of aPC-mediated anticoagulation, the contribution of the latter to the outcome of aPC therapy in animal studies is less well characterized. In mouse models of sterile endotoxemia, the hyperantithrombotic E149A-aPC variant with diminished signaling activities lacked efficacy,10 but the effects of this aPC variant on survival of bacterial infection are unknown. The anticoagulant activity of aPC requires 2 nonenzymatic cofactors: protein S (pS) and fV. The fV cofactor form is generated by cleavage of intact procofactor V by aPC at R506 and facilitates (in cooperation with pS) the proteolytic degradation of fVa and fVIIIa. The mechanistic and structural details of these interactions are only partially characterized, and may involve an as-yet-hypothetical trimolecular complex of aPC-cleaved fV, pS, and aPC (reviewed in Dahlbäck and Villoutreix,3 Castoldi and Rosing,11 and Cramer and Gale12). The naturally occurring R506Q Leiden mutation in fV (fV Leiden) largely abrogates the anticoagulant functions of aPC not only by rendering fVa partially refractory to aPC proteolysis but also by preventing the formation of the anticoagulant cofactor form of fV. Notably, aPC resistance of fV Leiden may modulate the host response to infection in humans and mice: among patients enrolled in the placebo arm of the Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) trial, heterozygous fV Leiden carriers showed significantly reduced mortality,13 and a similar survival advantage of heterozygous Leiden carriers was documented in mice harboring the fV R504Q mutation (equivalent to the human R506Q mutation) that were challenged with endotoxin,13 gram-positive (Staphylococcus aureus), or gram-negative infection (Yersinia pestis).14 Such favorable effects associated with aPC resistance of fV Leiden support the somewhat counterintuitive notion that partial suppression of the endogenous protein C anticoagulant pathway may support the resolution of infection. The effect of fV Leiden on the outcome of therapy with therapeutically administered aPC has not been investigated.
The objective of the current study was to examine the relative contributions of aPC’s anticoagulant and signaling functions to its efficacy in mouse models of septic peritonitis. We found that the anticoagulant and signaling activities of aPC exert striking disease stage–specific beneficial and detrimental effects and identified novel, coagulation-independent functions of fV and pS as essential anti-inflammatory cofactors for the mortality-reducing effects of signaling-selective aPC variants.
Materials and methods
Animals
Wild-type C57BL/6N mice were from Charles River Laboratories (Wilmington, MA). fV Leiden, fVIII-deficient, and PAR4-deficient mice have been described earlier.15-17 Mice expressing the R38E-PAR2 variant were derived from a homozygous knockin mouse generated via homologous recombination in C57BL/6N embryonic stem cells (W.R., unpublished data, 2013). All other strains were backcrossed onto the C57BL/6N background (≥12 generations). Animal experiments were in adherence to National Institutes of Health guidelines on the use of laboratory animals and approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee.
Chemicals and reagents
Recombinant murine and human aPC variants,10,18-20 plasma-derived human fV, fVa, and fV Leiden,21 and recombinant human fV variants22 have been described previously. Escherichia coli O55:B5 lipopolysaccharide (LPS) and hirudin (Sigma-Aldrich, St Louis, MO), anti-mouse pS antibodies (clone sc-27027; Santa Cruz Biotechnology, Santa Cruz, CA), and fV- and fVIII-depleted human plasma (Innovative Research, Novi, MI) were from commercial sources. Normal mouse plasma was obtained by puncture of the vena cava of heparin-anticoagulated wild-type mice (intraperitoneal infusion of unfractionated heparin 30 minutes prior to blood drawing), followed by centrifugation (2000g) for 20 minutes at 4°C. Prior to use as culture supplement, plasma was incubated for 45 minutes at 56°C to minimize complement-mediated effects. Anti-mouse tissue factor (TF) antibody 21E10 was characterized earlier.23 Active-site blocked fVIIai (Dansyl-Glu-Gly-Arg-fVIIa) and nematode anticoagulant protein (Nap)5 and NapC2 were obtained from Corvas International (San Diego, CA). Calf thymus histone H3 was from Roche Diagnostics (Indianapolis, IN). SLIGRL PAR2 agonist peptide was purchased from Sigma-Aldrich. The 14E11 antibody was described earlier.24 Anti-human fV monoclonal antibody AHV-514625 was obtained from Haematologic Technologies (Essex Junction, VT).
Endotoxemia and sepsis models
Log-phase cultures of S aureus (American Type Culture Collection 25923) were injected intraperitoneally at a predetermined median lethal dose (LD50) inoculum of ∼108 bacteria. LPS was injected intraperitoneally. aPC and variants were diluted in phosphate-buffered saline and administered through IV injection. Survival was quantitated via log-rank analysis of Kaplan-Meier plots.
Cell culture
Bone marrow dendritic cells (BMDCs) were prepared from bone marrow cultures in the presence of granulocyte macrophage–colony-stimulating factor as described previously.26 Expression of dendritic and monocyte cell surface markers (CD11c, CD11b, F4/80) in the resulting adherent cell populations was ascertained by fluorescence-activated cell sorting, indicating ∼85% homogeneity with respect to these markers and ∼15% resembling F4/80POS monocytic cells. RAW264.7 cells (American Type Culture Collection TIB-71) and BMDCs were seeded at 0.75 × 106 cells per well in 6-well culture dishes, grown overnight in Dulbecco’s modified Eagle medium to 70% to 80% confluence, and washed with phosphate-buffered saline. To each well, 3 mL of medium containing various reagents was added exactly as described earlier.27 All culture media contained hirudin (2 U/mL) to inhibit potential thrombin activity.
TF activity
TF procoagulant activity on cells was determined by 2-stage chromogenic assay using recombinant mouse fVIIa (Novo Nordisk, Malov, Denmark), human fX (Enzyme Research Laboratories, South Bend, IN), and Spectrozyme FXa (Sekisui Diagnostics, Stamford, CT) as described previously.28
Gene expression analysis
Real-time polymerase chain reaction with gene-specific oligonucleotide primers for Irf8, Peli1, Ccl22, and Malt1; and RNA hybridization on Affymetrix mouse genome 430 plus 2.0 arrays were conducted exactly as described previously.27 Image data were analyzed with Affymetrix GeneChip Operating Software and normalized with Robust Multichip Analysis (www.bioconductor.org). The statistical significance of differential gene expression was derived through a Student t test, and false-discovery rates were determined with Significance Analysis of Microarrays software. Data were analyzed using Microsoft Excel and the upstream regulator tool of the Ingenuity Pathway Analysis software package (Ingenuity Systems, Redwood City, CA). Microarray data can be found in the Gene Expression Omnibus database, accession number GSE8328; raw data are found in data sets GSM1407132 through GSM1407141.
Results
aPC variants with altered anticoagulant vs signaling function show sepsis stage–specific effects
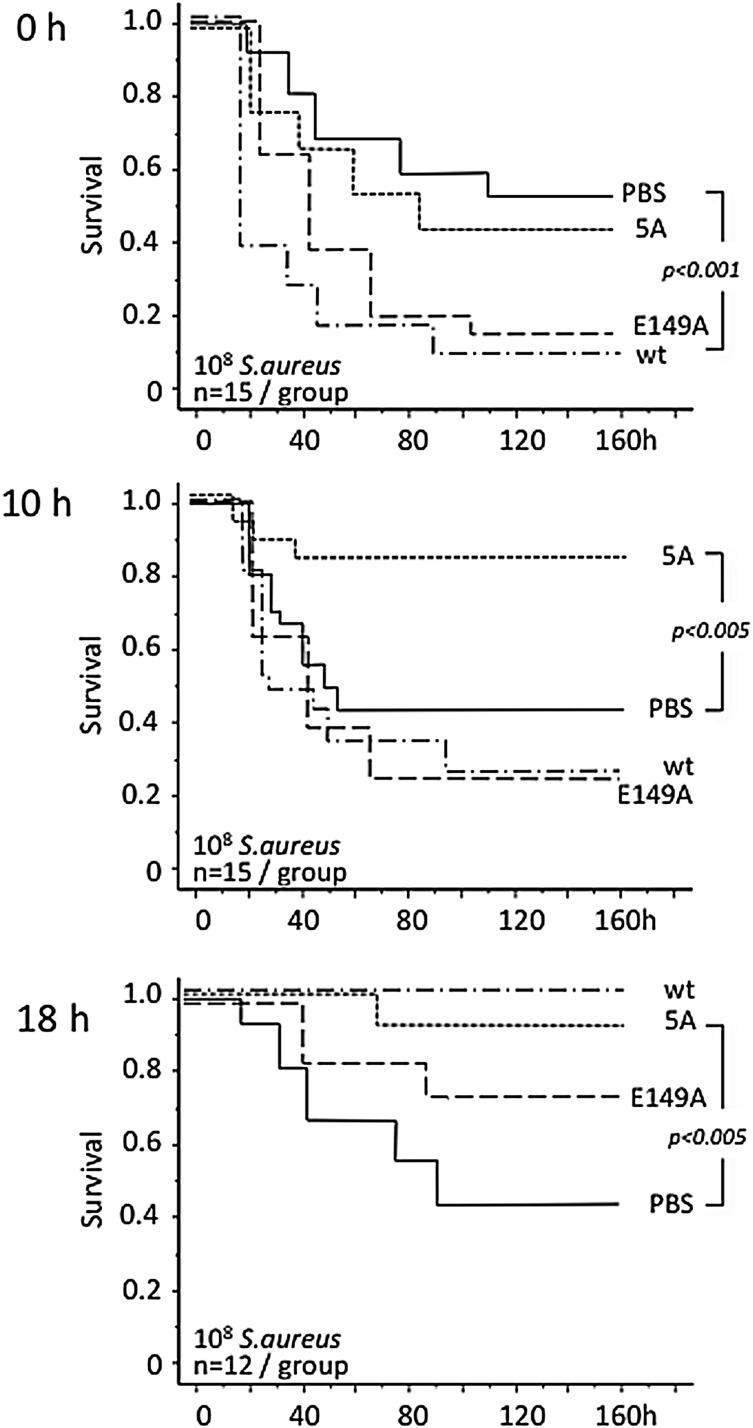
We first investigated potential disease stage–specific effects of therapy with wild-type aPC and signaling- and anticoagulant-selective aPC variants in mice subjected to lethal infection with live S aureus. Bolus administration of wild-type aPC <30 minutes after infection increased lethality; it had no effect on survival when given 10 hours after infection but afforded significant mortality reduction when administered 18 hours after infection (Figure 1). The hyperanticoagulant E149A-aPC variant reduced survival when given at the onset of infection, did not affect outcomes when administered 10 hours after infection, and moderately enhanced survival when given 18 hours after infection. The signaling-selective 5A-aPC variant lacked deleterious effects upon early administration, was already effective in intermediate-sepsis stages (10 hours), and retained efficacy upon late administration (18 hours).
Figure 1.
Sepsis stage–related response to infusion of aPC variants. Gram-positive peritonitis was induced in the indicated numbers of wild-type mice by intraperitoneal infection with ∼108 live S aureus bacteria. Wild-type (wt) mouse aPC, or the recombinant mouse aPC variants 5A-aPC (5A; signaling-selective) and E149A-aPC (E149A; hyperantithrombotic) were administered by IV injection at 150 μg/kg body weight within 30 minutes after infection (0 hours) and at 10 or 18 hours after infection. Significance of differences in cumulative 7-day survival was determined by Mantel-Cox log-rank analysis of Kaplan-Meyer survival plots. PBS, phosphate-buffered saline.
Adverse effects of aPC are mediated by its anticoagulant activity toward fV
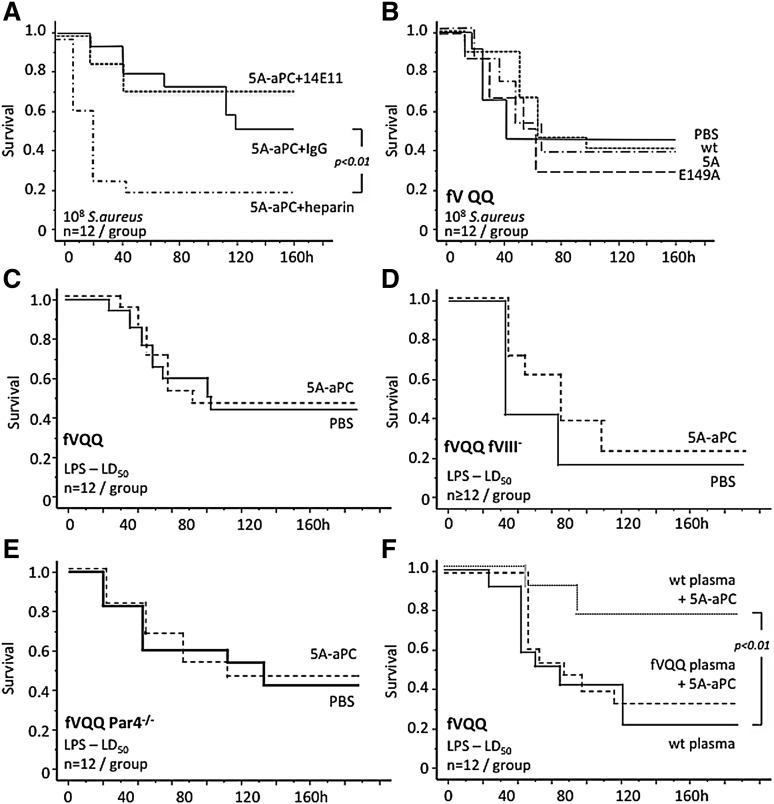
The above-mentioned data implied that both the adverse effects of wild-type aPC in early sepsis and aPC’s lack of efficacy in intermediate sepsis stages were associated with its anticoagulant activity. Subcutaneous administration of unfractionated heparin together with intravenous 5A-aPC at the onset of infection reduced survival after S aureus infection. In contrast, intraperitoneal infusion (<30 minutes after infection) of the 14E11 antibody that inhibits fXI-dependent intrinsic coagulation amplification without compromising hemostasis24 together with intravenous 5A-aPC did not cause lethality (Figure 2A). Resistance of fVa to cleavage by aPC secondary to homozygous carriership of the fV Leiden allele prevented the mortality caused by early administration of wild-type aPC or the hyperanticoagulant E149A-aPC variant, indicating that adverse aPC effects in early disease stages may be attributed to its anticoagulant activity toward fVa (Figure 2B).
Figure 2.
aPC resistance of fV Leiden modifies responsiveness to aPC therapy. (A) Survival of wild-type mice after intraperitoneal inoculation of live bacteria. Within 30 minutes of infection, mice received 150 μg/kg of IV 5A-aPC together with intraperitoneal 200 U/kg of unfractionated heparin, 30 mg/kg of mouse 14E11 monoclonal antibody, or 30 mg/kg of nonimmune mouse immunoglobulin G (IgG). (B) Homozygous fV Leiden mice (fV QQ) were infected with live S aureus and, within 30 minutes, received an IV injection of wild-type aPC (wt), 5A-aPC (5A), or E149A-aPC (E149A; 150 μg/kg body weight) dissolved in 200 μL of PBS, or vehicle alone. Homozygous fV Leiden mice (fVQQ) (C), fVQQ mice with superimposed hemophilia A (fVQQ fVIII−) (D), and fVQQ mice with superimposed PAR4 deficiency (fVQQ Par4−/−) (E) received intraperitoneal LPS (40 mg/kg) and, within 30 minutes, received an IV injection of 5A-aPC (150 μg/kg body weight) dissolved in 200 μL of PBS, or vehicle alone. (F) Survival of LPS-treated fVQQ mice infused within 30 minutes after LPS challenge with mouse 5A-aPC dissolved in 200 μL of plasma from wt or fVQQ mice, or with 200 μL of wild-type plasma alone. Significance of differences in cumulative 7-day survival was determined by Mantel-Cox log-rank analysis of Kaplan-Meyer survival plots.
aPC-resistant fV Leiden abrogates the therapeutic efficacy of the signaling-selective 5A-aPC variant
Although aPC resistance of fV R504Q prevented the deleterious aPC effects, early administration of wild-type aPC or 5A-aPC did not reduce mortality after S aureus infection (Figure 2B). To determine whether the lack of 5A-aPC efficacy in S aureus–infected fV Leiden mice was a model- or pathogen-specific effect, we examined the outcome of 5A-aPC therapy in homozygous Leiden mice subjected to sterile endotoxemia. As in bacterial infection, 5A-aPC lacked efficacy in LPS-challenged homozygous Leiden carriers (Figure 2C). Homozygous fV Leiden mice with superimposed deficiency of fVIII (hemophilia A), which blunts inflammation-induced thrombin generation29,30 (see supplemental Figure 1, available on the Blood Web site), remained refractory to 5A-aPC therapy of endotoxemia (Figure 2D). Likewise, 5A-aPC had no effect on endotoxemia survival in homozygous Leiden mice with superimposed deficiency of the thrombin receptor PAR4, which renders platelets unresponsive to thrombin31 (Figure 2E). Coinfusion of 5A-aPC and 200 μL of wild-type plasma (containing normal fV) restored the ability of 5A-aPC to reduce mortality of homozygous Leiden mice, whereas coinfusion of 5A-aPC and plasma from homozygous Leiden mice, or transfusion with 200 μL of wild-type plasma alone, had no effect (Figure 2F). Taken together, these findings suggested that the lack of therapeutic efficacy of aPC in homozygous Leiden mice was not due to exaggerated thrombosis or thrombin generation but was caused by some other inhibitory effect of the fV R504Q mutation on the protective mechanism engaged by the signaling-selective 5A-aPC variant.
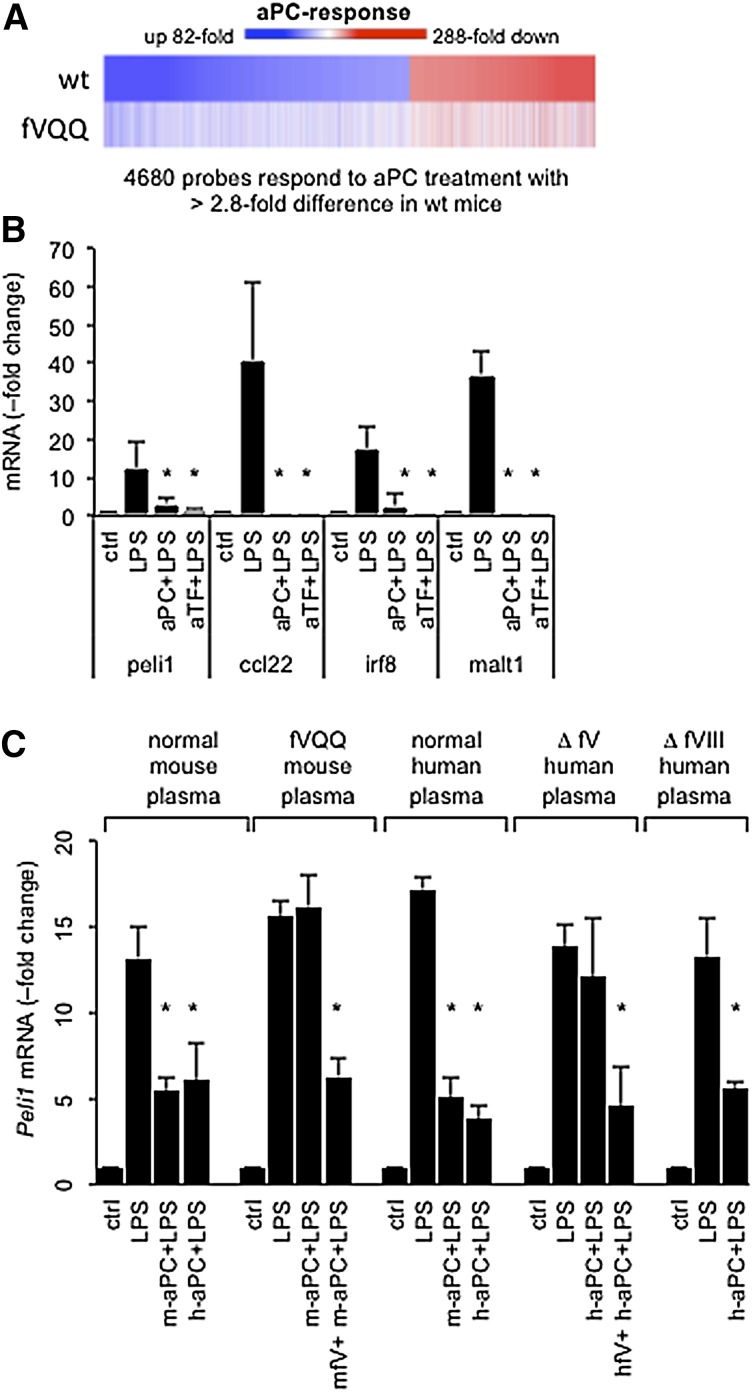
aPC resistance of fV Leiden suppresses in vitro regulation of inflammatory gene expression by aPC
In normal mice, aPC infusion elicits a profound suppression of the LPS-induced transcriptional response in spleen dendritic cells.7 In LPS-challenged homozygous Leiden mice, 5A-aPC failed to elicit the full gene expression signature seen in wild-type mice (Figure 3A). Of 4680 probe sets regulated >2.8-fold by 5A-aPC in wild-type mice, only 14% (666) were regulated to the same extent in homozygous fV Leiden mice (supplemental Table 2). We next compared the gene expression response of the murine myelomonocytic RAW264.7 cell line exposed to LPS and 5A-aPC in the presence of plasma from normal and homozygous Leiden mice (supplemental Table 2). Analysis of differentially regulated genes (>twofold) with the upstream regulator software tool (Ingenuity Systems) indicated that 5A-aPC inhibited in an fV-dependent manner inflammatory signaling triggered by LPS (P = 1.3 × 10−92), Toll-like receptor 4 (P = 2.6 × 10−71), Toll-like receptor 3 (P = 3.7 × 10−68), Toll-interleukin 1 receptor domain–containing adapter-inducing interferon-β (TRIF) (P = 1.3 × 10−66), and interferon γ (P = 8.8 × 10−65). For example, in the presence of normal plasma, but not homozygous Leiden plasma, aPC reduced >20-fold the LPS-induced expression of messenger (m)RNAs encoding interferon-inducible guanylate binding proteins (Gbp1, Gbp2, Gbp3, Gbp6, Gvin1, lfi47, Igtp, ligp1, Tgtp1), and by more than fivefold the abundance of mRNAs encoding Ccl22, Irf8, Malt1, and Peli1 (supplemental Table 3). Of note, this aPC- and fV-dependent gene signature was congruent with the LPS-induced transcriptional program that is mediated by the EPCR-dependent activation of PAR2 by the ternary TF-fVIIa-fXa complex.27 This finding indicated that the fV-sensitive aPC mechanism involved the suppression of EPCR-dependent inflammatory TF signaling. Quantitative real-time polymerase chain reaction analysis confirmed that 5A-aPC suppressed the LPS-induced expression of Peli1, Ccl22, Malt1, and Irf8 in BMDCs to the same extent as function-blocking anti-mouse TF antibodies (Figure 3B). In RAW264.7 (RAW) cells cultured with normal mouse or human plasma, mouse and human wild-type aPC suppressed the LPS-induced expression of Peli1 to near-baseline levels, whereas expression of this biomarker in fV-depleted human plasma remained unresponsive to aPC (Figure 3C). Supplementation of homozygous Leiden mouse plasma with 2 μg of recombinant normal mouse fV, or reconstitution of fV-depleted human plasma with plasma-derived normal human fV, restored responsiveness to aPC. Regulation by LPS and aPC occurred normally in human fVIII-depleted plasma (Figure 3C).
Figure 3.
aPC resistance of fV Leiden suppresses regulation of inflammatory gene expression by aPC. (A) Heat map depicts the regulation of 4680 genes that are responsive to aPC therapy in spleen dendritic cells collected by fluorescence-activated cell sorting 16 hours after LPS (LD50) challenge from a wild-type (wt) mouse as compared to a homozygous fV Leiden mouse (fVQQ). Equal amounts of total RNA were pooled from 5 individual animals per genotype and analyzed by hybridization to gene arrays. Colors indicate the response (fold change up or down) compared to LPS-challenged mice not receiving aPC. Individual genes are sorted according to their aPC response in wild-type mice. (B) Peli1, Ccl22, Irf8, and Malt1 mRNA levels were measured by quantitative real-time PCR analysis in RAW cells cultured with 10% normal mouse plasma (ctrl) and cells treated for 3 hours with 100 ng/mL of LPS, 100 ng/mL of LPS plus 100 ng/mL of mouse aPC (aPC+LPS), or 100 ng/mL of LPS plus 5 μg/mL of anti-mouse TF antibodies (aTF+LPS). Data represent the average ± standard deviation from ≥3 independent experiments with triplicate measurements of each sample. RNA levels in control cultures were arbitrarily set to “1.” *P < .05 by pairwise comparison with LPS-induced levels (Student 2-tailed t test). (C) Pellino-1 (Peli1) mRNA, measured 3 hours after LPS (100 ng/mL) addition in response to human aPC (h-aPC) or mouse aPC (m-aPC) in the presence of (10% vol/vol) pooled normal mouse or human plasma, or pooled normal human plasma depleted of fV (Δ fV) or fVIII (Δ fVIII). Recombinant mouse fV and plasma-derived human fV were added at 100 ng/mL. *P < .05 by pairwise comparison with LPS-induced levels (Student 2-tailed t test).
These findings showed that the fV-dependent in vivo effect of aPC on LPS-induced gene expression could be reproduced under in vitro conditions where neither thrombosis nor thrombin generation play a role. The failure of aPC to suppress LPS-induced inflammatory TF signaling in the absence of fV, and the normal aPC response in the absence of fVIII, ruled out (1) that the inhibitory effect of aPC involved its anticoagulant activity, mediated by inhibition of fV- and fVIII-dependent thrombin generation, and (2) that cleavage of fV/fVa or fVIII/fVIIIa by aPC eliminated some unknown, coagulation-independent inhibitory effect of these factors on anti-inflammatory aPC functions.
fV mediates inhibition of inflammatory TF signaling by aPC
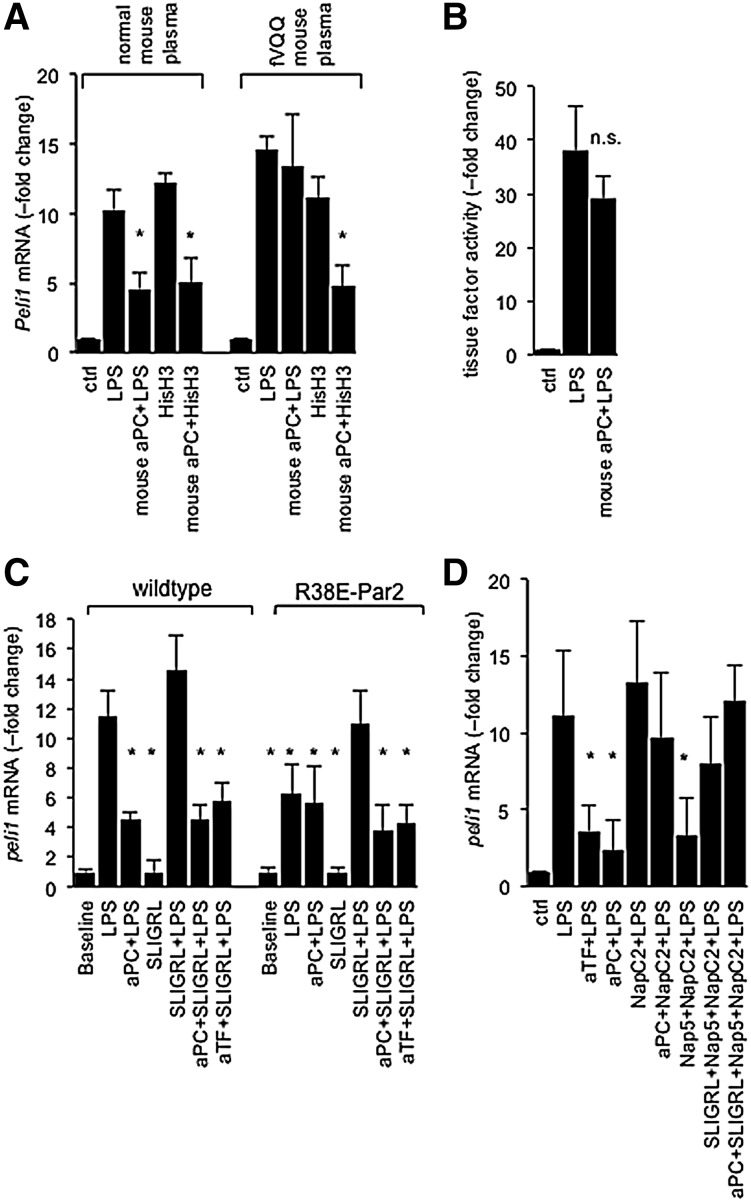
One candidate pathway by which aPC might suppress the above-mentioned LPS-triggered interferon gene signature in an fV-dependent manner, but independent of its anticoagulant activity, is the proteolytic degradation of extracellular histones.32 As reported earlier,27 calf thymus histones induced the expression of Peli1, Irf8, Ccl22, and Malt1 mRNA in RAW cells, but 5A-aPC suppressed histone-induced Peli1 mRNA levels to the same extent in the presence of wild-type and homozygous Leiden plasma (Figure 4A). 5A-aPC also did not diminish the extent of LPS-elicited TF activity on RAW cells, ruling out that fV enabled the downregulation of TF expression33 (Figure 4B). To clarify whether 5A-aPC inhibited Peli1 induction by proteolytic “disarming” of PAR2 via cleavage at R38 in the PAR2 extracellular domain, we tested BMDCs derived from mice homozygous for the R38E mutation that renders PAR2 resistant to cleavage by both fXa and aPC.34-36 As shown earlier,27 LPS-induced Peli1 expression in R38E-PAR2 cells was blunted but was restored by the PAR2 agonist SLIGRL. aPC inhibited Peli1 induction by LPS/SLIGRL in R38E-PAR2 cells to a similar extent as in wild-type cells, ruling out a role for R38 cleavage (Figure 4C). NapC2 blocks TF-fVIIa-fXa procoagulant activity but stabilizes a conformation of the TF-fVIIa-fXa complex that is conducive to PAR2 activation.37,38 NapC2 abolished the ability of aPC to inhibit Peli1 induction by either LPS alone, or by the combination of LPS, SLIGRL, and the fXa–active-site inhibitor Nap539 (Figure 4D). The fV-dependent suppression of LPS-induced gene expression by aPC, therefore, did not involve cleavage of histones, cleavage of PAR2 at R38, or the inhibition of TF procoagulant activity. Rather, the inhibitory effect of aPC was abolished by stabilization of the TF-fVIIa-fXa complex with NapC2, irrespective of whether PAR2 was activated proteolytically via fXa or nonproteolytically by the direct PAR2 agonist SLIGRL in the presence of the formed but proteolytically blocked TF complex. This finding indicated that aPC inhibited TF-EPCR-PAR2 signaling in an fV-dependent manner by destabilizing the signaling-competent conformation of the EPCR-TF-fVIIa-fXa complex.
Figure 4.
aPC inhibits PAR2 activation by the ternary TF-fVIIa-fXa complex. (A) aPC suppresses histone-induced Peli1 expression independent of fV. Quantitative PCR measurement of Peli1 mRNA in RAW cells treated for 3 hours with the indicated reagents (100 ng/mL of LPS; LPS plus 100 ng/mL of mouse 5A-aPC [mouse aPC+LPS]; 30 μg/mL of calf thymus histone H3 [HisH3]; 30 μg/mL of HisH3 plus 100 ng/mL of mouse 5A-aPC [mouse aPC+HisH3]). *P = .05 by Student t test, compared to cells treated with LPS alone. (B) TF activity in was measured in RAW cells via the rate of fXa generation in a 2-stage amidolytic assay for fXa activity at baseline and after 3 hours of exposure to LPS or LPS plus 5A-aPC (mouse aPC+LPS) in the presence of normal plasma. At this time, cells were washed and incubated with recombinant mouse fVII and fX. Data are corrected for background activity (no fVII) and represent the average ± standard deviation of 6 independent experiments, with triplicate measurement per sample. n.s., not significant. (C) Peli1 mRNA regulation by LPS in BMDCs prepared from wild-type mice or a knockin mouse strain expressing the cleavage-resistant R38E-PAR2 variant. Data represent the average ± standard deviation from 3 independent experiments. *P < .05 by Student 2-tailed t test, as compared to LPS-treated wild-type cells. (D) RAW cells were incubated for 3 hours with LPS (100 ng/mL) and the indicated reagents (anti-murine TF antibody [aTF], 5 μg/mL; mouse 5A-aPC [aPC], 100 ng/mL; Nap5, 500 nM; NapC2, 500 nM; and SLIGRL, 20 μM), and Peli1 mRNA was quantitative by real-time PCR analysis. Data represent the average ± standard deviation from 6 independent experiments. *P < .05 by Student t test, compared to cells treated with LPS alone. PCR, polymerase chain reaction.
Inhibition of TF-EPCR-PAR2 signaling by aPC requires pS and the fV B domain
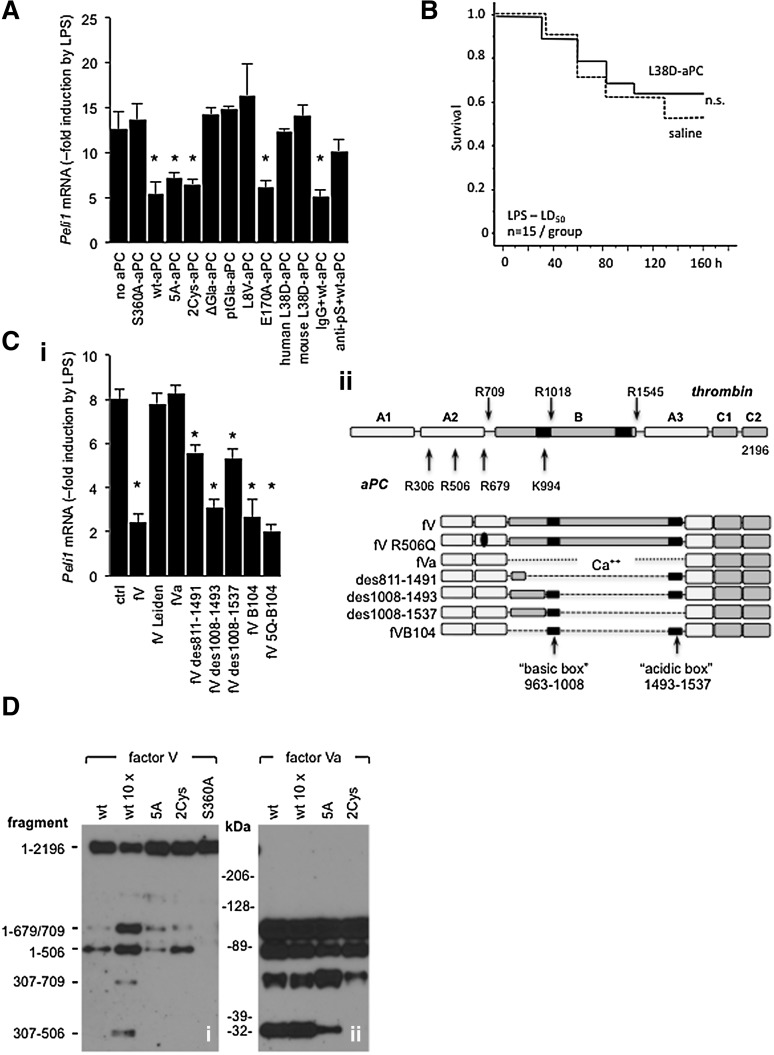
To gain further mechanistic insight into fV-dependent anti-inflammatory effects of aPC, we tested several function-selective aPC variants: the signaling-selective aPC variants human 2Cys-aPC,40 human and mouse 5A-aPC, human and mouse L38D-aPC (deficient in pS-dependent anticoagulant function41), the human E170A-aPC with normal anticoagulant function but defective PAR1 interaction,42 the proteolytically inactive mouse S360A-aPC variant,43 and 3 variants with defective EPCR binding due to either complete absence of the Gla-domain (ΔGla-aPC),9 replacement of the entire protein C Gla-domain with that of prothrombin (ptGla-aPC),24 or a point mutation disrupting EPCR-aPC/PC interaction (L8V-aPC).44 2Cys-aPC and E170A-aPC inhibited LPS-induced Peli1 mRNA expression to the same extent as wild-type aPC and 5A-aPC, whereas S360A-aPC, ptGla-aPC, L8V-aPC, and ΔGla-aPC all failed to downmodulate Peli1 mRNA (Figure 5A). Surprisingly, the signaling-selective human and mouse L38D-aPC variants also failed to uncouple inflammatory TF-EPCR-PAR2 signaling. pS function–blocking antibodies45 likewise inhibited suppression of Peli1 mRNA induction by wild-type aPC (Figure 5A). Consistent with its lack of in vitro activity, mouse L38D-aPC did not enhance survival of wild-type mice challenged with LPS at an LD50 (Figure 5B). Taken together, this analysis demonstrated that fV-dependent anti-inflammatory effects of aPC required its interaction with EPCR and pS plus its proteolytic activity.
Figure 5.
Inflammatory gene regulation by aPC requires procofactor V and pS. (A) RAW cells were incubated for 3 hours in normal human plasma (10% vol/vol) with 100 ng/mL of the indicated aPC variants, function-blocking anti-human pS antibody (anti-pS; 2 μg/mL), or nonimmune immunoglobulin G (IgG; 2 μg/mL), and Peli1 mRNA levels relative to LPS-treated RAW cells cultured without aPC were measured by quantitative PCR. Data are the mean ± standard deviation from 4 independent experiments. *P < .01 by Student t test relative to “no aPC” controls. (B) Survival of LPS-challenged (40 mg/kg; intraperitoneal) wild-type mice infused with mouse L38D-aPC (300 μg/kg; IV) within 30 minutes after LPS administration. (Ci) Plasma-derived fV, fVa, fV Leiden, and recombinant variants with partial B-domain deletions were tested for their ability to restore Peli1 downregulation by wild-type human aPC in RAW cells cultured with 10% human fV-depleted plasma. All variants were tested at 700 ng/mL. Data represent the average of 6 independent experiments ± standard deviation. *P < .01 by Student t test. (ii) Domain structure of intact fV with positions of cleavage sites recognized by aPC or thrombin indicated by arrows. Structures of fV-variants are shown below. (D) Signaling-selective aPC variants cleave procofactor V at R506: plasma-derived fV (i) or thrombin-activated fVa (ii) were added to a final concentration of 700 ng/mL to RAW cell culture medium supplemented with 10% (vol/vol) fV-depleted human plasma, phospholipid vesicles, and 2 U/mL of hirudin. The mixtures were incubated for 15 minutes at 22°C with 10 ng/mL of wt-aPC (wt), 100 ng of wt-aPC (wt 10 ×), or 10 ng/mL of 5A-aPC (5A), 2Cys-aPC (2Cys), or proteolytically inactive S360A-aPC (S360A). Cleavage products were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis under reducing conditions, and detected by western blot analysis with anti-human fV antibody AHV-5146 recognizing the 307-506 fragment of fV. Residue numbers of fV fragments are indicated on the left (fragment); molecular weight standards are shown in the center (kDa).
Although the signaling-selective aPC variants 5A-aPC and 2Cys-aPC exhibit extensive loss of anticoagulant activity due to reduced ability to cleave fVa at R506, both variants, like wild-type aPC, cleaved R506 in intact fV procofactor in RAW cell culture medium supplemented with phospholipids (Figure 5D). This finding indicates that such aPC variants lacking most but not all anticoagulant activity can convert fV to forms that enhance aPC signaling functions.
We next assessed the structural requirements for fV’s ability to support suppression of LPS-induced Peli1 expression by aPC. Supplementation of fV-depleted human plasma with various human fV molecules (Figure 5C) showed that thrombin-activated, plasma-derived human fV (fVa) lacking the entire B domain did not support this aPC function, showing a requirement for B-domain elements. Unactivated recombinant fV variants with defined deletions of the B domain either partially (fVdes811-1491 and fVdes1008-1537) or fully (fVdes1008-1493 and fVdes716-963/1008-1491; alias fVB104) restored Peli1 regulation by aPC (Figure 5C). A fV B104 variant in which all thrombin and aPC cleavage sites except for R506 were inactivated (designated fVB104-5Q with mutations R306Q, R679Q, R709Q, K994Q, and R1545Q) retained cofactor activity (Figure 5C), indicating that an intact R506 cleavage site of procofactor fV was necessary and sufficient to support this anti-inflammatory aPC function.
In summary, the inhibition of LPS-induced Peli1 expression required interactions among aPC, EPCR, pS, and fV, which could be cleaved at R506 while retaining at least parts of its B domain. Because fVdes811-1491 and fVdes1008-1537 were each partially active, it appears that both the basic (residues 963-1008) and the acidic box (residues 1493-1537) not only are necessary for full anticoagulant cofactor activity of fV46 and maintenance of the inactive procofactor conformation,47 but also for the maximal anti-inflammatory cofactor activity of fV.
Discussion
The current work showed, first, a remarkable reversal from overall detrimental to beneficial outcomes of therapy with wild-type aPC, depending on the disease stage at which aPC infusion was initiated. The comparison of function-selective aPC variants indicated that these disease stage–specific negative and positive effects may be attributed to aPC’s anticoagulant and cell signaling activity, respectively. Although we did not attempt to document internal hemorrhage in mice receiving early infusions of wild-type aPC, the detrimental effects exerted by aPC’s anticoagulant activity are conceivably a correlate of the increased risk for severe bleeding events in septic patients treated with aPC. Deleterious effects of early aPC infusion after infection are also evident in mouse models of focal polymicrobial peritonitis triggered by cecal ligation and puncture24 but are much less pronounced in sterile endotoxemia.10 These model-specific differences, together with the initial phase of unresponsiveness to 5A-aPC therapy in bacterial sepsis, point to yet-to-be-fully characterized differences in the role of coagulation activation between sterile inflammation and infection with live bacteria. In part, such differences likely reflect a more critical role of coagulation activation in sepsis models, as compared to sterile endotoxemia, in which fibrin-dependent host defense mechanisms play little, if any, role. Of importance, the prototypic signaling-selective 5A-aPC variant not only lacked adverse effects on the outcome of S aureus infection but also exhibited a significantly expanded window of therapeutic responsiveness, reflected in a striking gain of efficacy in intermediate disease stages, as compared to wild-type or hyperantithrombotic aPC. These findings extend earlier observations in mice using a 3-hour plus 10-hour dosing regimen that showed efficacy of 5A-aPC in gram-positive and gram-negative infection and in polymicrobial peritonitis.8 Results from coinfusion of 5A-aPC and the 14E11 anti-fXI antibody further suggest that it should be possible to combine signaling-selective aPC therapy with novel anticoagulants that target prothrombotic pathways without causing bleeding, thereby providing the plausible benefits of antithrombotic therapy in septic patients with severe microvascular coagulopathies. Human recombinant 3K3A-aPC is the equivalent of mouse 5A-aPC and has been administered to healthy volunteers at exceedingly high doses without causing significant anticoagulant or other adverse effects,48 providing an opportunity to determine whether high-dose infusion of signaling-selective aPC variants might exhibit efficacy in septic patients.
Second, we identified a novel mechanism by which aPC reduces in an fV- and pS-dependent manner the mortality of bacterial infection and sterile endotoxemia. This mechanism is distinct from previously reported cytoprotective aPC mechanisms, such as the EPCR- or Mac1-dependent activation of PAR1.4,5,9,13 Instead, it involves the inhibition of a recently described inflammatory signaling process that is mediated by the EPCR-dependent activation of PAR2 by the ternary TF-fVIIa-fXa blood coagulation initiation complex and that augments in innate immune cells a specific component of the inflammatory gene expression response to stimulation with LPS or endogenously released danger signals such as histones.27 Because EPCR-dependent PAR2 activation by the ternary TF complex involves direct interactions of fVIIa and/or fXa Gla-domain(s) with EPCR,37,38 and because the L8V-aPC variant with defective EPCR interaction lacks inhibitory activity, we hypothesize that inhibition of TF signaling by aPC involves the displacement of fVIIa or fXa from EPCR by aPC, resulting in the uncoupling of inflammatory PAR2 signaling. Surprisingly, this anti-inflammatory effect of signaling-selective 5A-aPC required pS and fV, 2 nonenzymatic cofactors typically associated with aPC’s anticoagulant activity. The molecular mechanism by which these cofactors might facilitate EPCR engagement by aPC remains unclear at the present time. Analyses of aPC and fV variants indicated that this mechanism involved direct aPC-pS interaction and required the same or similar structural features of fV that govern its anticoagulant cofactor activity (reviewed in Cramer and Gale12). Potential candidate mechanisms that do not involve the degradation of fVa or fVIIIa but are consistent with known functions of pS and fV include (1) the destabilization of the signaling-competent conformation of the TF complex secondary to physical interactions of B domain–containing fVa with fXa49 and of pS with TF and fXa50, and/or (2) the recruitment of TF pathway inhibitor α (TFPIα) to the TF-fVIIa-fXa complex via interaction of pS with the TFPIα K3 domain or of the fV B domain with the carboxyterminal fragment of TFPIα (reviewed in Wood et al51). It also remains to be clarified whether other naturally occurring fV variants such as fV Liverpool (I359T),52 fV Nara (W1920R),53 fV released from platelets,54 or fV present in homozygous carriers of the fV R2 haplotype11,55-57 would exhibit similarly defective cofactor function for the inhibition of TF signaling by aPC as does fV Leiden.
In summary, we provided evidence for a coagulation-independent function of fV and pS as essential cofactors for the aPC-mediated inhibition of TF signaling, and showed that aPC resistance of fV Leiden not only disrupts the anticoagulant activity of the protein C pathway but, in addition, abrogates the ability of aPC to inhibit inflammatory TF signaling and prevent death from infection with S aureus. However, hematopoietic EPCR gene ablation in mice abrogates aPC efficacy and does not improve sepsis survival in the absence of therapeutic intervention,7,8 implying that the fV-dependent inhibition of TF-EPCR-PAR2 signaling, although necessary, is not sufficient for sepsis mortality reduction by aPC. Thus, aPC resistance of fV Leiden not only modifies the endogenous host response to infection with highly virulent bacterial pathogens such as S aureus and Y pestis14 but also modulates the responsiveness to therapeutically administered aPC. The current findings could potentially explain why the beneficial effects of aPC resistance on infection and endotoxemia in mice (in the absence of aPC therapy) are limited to heterozygous fV Leiden mice,13,14 because the anti-inflammatory cofactor function of fV for endogenously formed aPC cannot be expressed in homozygous carriers. Of note, fV Leiden carriership had no effect on polymicrobial focal peritonitis or infection with E coli and attenuated strains of S aureus and Y pestis that lack virulence factors interacting with the host fibrinolytic system.14 It thus remains to be investigated whether the contribution of fV- and pS-dependent mechanisms to the efficacy of therapeutically administered aPC is similarly disease context- and pathogen-specific. The interchangeable use of human and mouse plasma, fV preparations, and aPC variants (Figure 4B-C) in in vitro assays of TF signaling is consistent with a cross-species conservation of the relevant interactions among fV, pS, and aPC, but the extent to which the described fV-dependent anti-inflammatory aPC effects can be translated from mice and the murine immune system response to human biology is currently unknown.
Acknowledgments
The authors thank Dr James Morrissey for providing guidance and the reagents for analyzing in vitro fV processing, and Dr David Gailani (Vanderbilt University School of Medicine, Nashville, TN) and Dr Andras Gruber (Oregon Health and Science University, Portland, OR) for providing copious amounts of 14E11 antibody.
This work was supported by National Institutes of Health National Institute of Allergy and Infectious Diseases grant AI080557 (H.W.) and National Heart, Lung, and Blood Institute grants HL44612-14 and HL093388 (H.W.), HL31950 and HL60742 (W.R.), HL31950 and HL52246 (J.H.G.), HL101917 (A.R.R.), and HL74124 and HL88010 (R.M.C.); Bridge Funding from the American Society of Hematology (H.W.); and the Ziegler Family Research Chair Foundation (H.W.).
Footnotes
The data reported in this article have been deposited in the Gene Expression Omnibus database (accession number GSE8328; raw data are found in data sets GSM1407132 through GSM1407141).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: H.P.H.L., E.J.K., S.B., I.H., and M.Z. conducted the experiments, analyzed the data, prepared the figures, and wrote the manuscript; S.J. and M.J.H. conducted and analyzed the gene expression experiments; A.R.R., R.T., and J.A.F. prepared and provided the critical reagents; R.M.C., W.R., and J.H.G. contributed to the experimental design and data interpretation, provided reagents, and wrote the manuscript; H.W. conducted the experiments and analyzed the data, wrote the manuscript, and was responsible for overseeing and coordinating this study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for H.P.H.L. is Sutton Arthritis Research Laboratory, Royal North Shore Hospital, St Leonards NSW, Australia.
Correspondence: Hartmut Weiler, Blood Research Institute, Blood Center of Wisconsin, 8727 Watertown Plank Rd, Milwaukee, WI 53226; e-mail: hartmut.weiler@bcw.edu.
References
- 1.Esmon CT. Inflammation and the activated protein C anticoagulant pathway. Semin Thromb Hemost. 2006;32(Suppl 1):49–60. doi: 10.1055/s-2006-939554. [DOI] [PubMed] [Google Scholar]
- 2.Mosnier LO, Griffin JH. Protein C anticoagulant activity in relation to anti-inflammatory and anti-apoptotic activities. Front Biosci. 2006;11:2381–2399. doi: 10.2741/1977. [DOI] [PubMed] [Google Scholar]
- 3.Dahlbäck B, Villoutreix BO. Regulation of blood coagulation by the protein C anticoagulant pathway: novel insights into structure-function relationships and molecular recognition. Arterioscler Thromb Vasc Biol. 2005;25(7):1311–1320. doi: 10.1161/01.ATV.0000168421.13467.82. [DOI] [PubMed] [Google Scholar]
- 4.Weiler H. Multiple receptor-mediated functions of activated protein C. Hamostaseologie. 2011;31(3):185–195. doi: 10.5482/ha-1166. [DOI] [PubMed] [Google Scholar]
- 5.Mosnier LO, Zlokovic BV, Griffin JH. The cytoprotective protein C pathway. Blood. 2007;109(8):3161–3172. doi: 10.1182/blood-2006-09-003004. [DOI] [PubMed] [Google Scholar]
- 6.Dahlbäck B, Villoutreix BO. The anticoagulant protein C pathway. FEBS Lett. 2005;579(15):3310–3316. doi: 10.1016/j.febslet.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Kerschen E, Hernandez I, Zogg M, et al. Activated protein C targets CD8+ dendritic cells to reduce the mortality of endotoxemia in mice. J Clin Invest. 2010;120(9):3167–3178. doi: 10.1172/JCI42629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kerschen EJ, Fernandez JA, Cooley BC, et al. Endotoxemia and sepsis mortality reduction by non-anticoagulant activated protein C. J Exp Med. 2007;204(10):2439–2448. doi: 10.1084/jem.20070404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao C, Gao Y, Li Y, Antalis TM, Castellino FJ, Zhang L. The efficacy of activated protein C in murine endotoxemia is dependent on integrin CD11b. J Clin Invest. 2010;120(6):1971–1980. doi: 10.1172/JCI40380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mosnier LO, Zampolli A, Kerschen EJ, et al. Hyperantithrombotic, noncytoprotective Glu149Ala-activated protein C mutant. Blood. 2009;113(23):5970–5978. doi: 10.1182/blood-2008-10-183327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castoldi E, Rosing J. APC resistance: biological basis and acquired influences. J Thromb Haemost. 2010;8(3):445–453. doi: 10.1111/j.1538-7836.2009.03711.x. [DOI] [PubMed] [Google Scholar]
- 12.Cramer TJ, Gale AJ. The anticoagulant function of coagulation factor V. Thromb Haemost. 2012;107(1):15–21. doi: 10.1160/TH11-06-0431. [DOI] [PubMed] [Google Scholar]
- 13.Kerlin BA, Yan SB, Isermann BH, et al. Survival advantage associated with heterozygous factor V Leiden mutation in patients with severe sepsis and in mouse endotoxemia. Blood. 2003;102(9):3085–3092. doi: 10.1182/blood-2003-06-1789. [DOI] [PubMed] [Google Scholar]
- 14.Kerschen E, Hernandez I, Zogg M, Maas M, Weiler H. Survival advantage of heterozygous factor V Leiden carriers in murine sepsis. J Thromb Haemost. 2015;13(6):1073–1080. doi: 10.1111/jth.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui J, Eitzman DT, Westrick RJ, et al. Spontaneous thrombosis in mice carrying the factor V Leiden mutation. Blood. 2000;96(13):4222–4226. [PubMed] [Google Scholar]
- 16.Bi L, Lawler AM, Antonarakis SE, High KA, Gearhart JD, Kazazian HH., Jr Targeted disruption of the mouse factor VIII gene produces a model of haemophilia A. Nat Genet. 1995;10(1):119–121. doi: 10.1038/ng0595-119. [DOI] [PubMed] [Google Scholar]
- 17.Sambrano GR, Weiss EJ, Zheng YW, Huang W, Coughlin SR. Role of thrombin signalling in platelets in haemostasis and thrombosis. Nature. 2001;413(6851):74–78. doi: 10.1038/35092573. [DOI] [PubMed] [Google Scholar]
- 18.Elphick GF, Sarangi PP, Hyun YM, et al. Recombinant human activated protein C inhibits integrin-mediated neutrophil migration. Blood. 2009;113(17):4078–4085. doi: 10.1182/blood-2008-09-180968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mosnier LO, Gale AJ, Yegneswaran S, Griffin JH. Activated protein C variants with normal cytoprotective but reduced anticoagulant activity. Blood. 2004;104(6):1740–1744. doi: 10.1182/blood-2004-01-0110. [DOI] [PubMed] [Google Scholar]
- 20.Qureshi SH, Yang L, Manithody C, Bae JS, Rezaie AR. Functional properties and active-site topographies of factor X Gla- and prothrombin Gla-domain chimeras of activated protein C. Biochim Biophys Acta. 2008;1780(9):1080–1086. doi: 10.1016/j.bbagen.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heeb MJ, Kojima Y, Greengard JS, Griffin JH. Activated protein C resistance: molecular mechanisms based on studies using purified Gln506-factor V. Blood. 1995;85(12):3405–3411. [PubMed] [Google Scholar]
- 22.Zhu H, Toso R, Camire RM. Inhibitory sequences within the B-domain stabilize circulating factor V in an inactive state. J Biol Chem. 2007;282(20):15033–15039. doi: 10.1074/jbc.M701315200. [DOI] [PubMed] [Google Scholar]
- 23.Furlan-Freguia C, Marchese P, Gruber A, Ruggeri ZM, Ruf W. P2X7 receptor signaling contributes to tissue factor-dependent thrombosis in mice. J Clin Invest. 2011;121(7):2932–2944. doi: 10.1172/JCI46129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tucker EI, Verbout NG, Leung PY, et al. Inhibition of factor XI activation attenuates inflammation and coagulopathy while improving the survival of mouse polymicrobial sepsis. Blood. 2012;119(20):4762–4768. doi: 10.1182/blood-2011-10-386185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Neut Kolfschoten M, Dirven RJ, Vos HL, Tans G, Rosing J, Bertina RM. Factor Va is inactivated by activated protein C in the absence of cleavage sites at Arg-306, Arg-506, and Arg-679. J Biol Chem. 2004;279(8):6567–6575. doi: 10.1074/jbc.M308574200. [DOI] [PubMed] [Google Scholar]
- 26.Lutz MB, Kukutsch N, Ogilvie AL, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223(1):77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 27.Liang HP, Kerschen EJ, Hernandez I, et al. EPCR-dependent PAR2 activation by the blood coagulation initiation complex regulates LPS-triggered interferon responses in mice. Blood. 2015;125(18):2845–2854. doi: 10.1182/blood-2014-11-610717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhattacharjee G, Ahamed J, Pedersen B, et al. Regulation of tissue factor--mediated initiation of the coagulation cascade by cell surface grp78. Arterioscler Thromb Vasc Biol. 2005;25(8):1737–1743. doi: 10.1161/01.ATV.0000173419.31242.56. [DOI] [PubMed] [Google Scholar]
- 29.Schoenmakers SH, Brüggemann LW, Groot AP, Maijs S, Reitsma PH, Spek CA. Role of coagulation FVIII in septic peritonitis assessed in hemophilic mice. J Thromb Haemost. 2005;3(12):2738–2744. doi: 10.1111/j.1538-7836.2005.01649.x. [DOI] [PubMed] [Google Scholar]
- 30.Franchini M, Lippi G. Factor V Leiden and hemophilia. Thromb Res. 2010;125(2):119–123. doi: 10.1016/j.thromres.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Hamilton JR, Cornelissen I, Coughlin SR. Impaired hemostasis and protection against thrombosis in protease-activated receptor 4-deficient mice is due to lack of thrombin signaling in platelets. J Thromb Haemost. 2004;2(8):1429–1435. doi: 10.1111/j.1538-7836.2004.00783.x. [DOI] [PubMed] [Google Scholar]
- 32.Xu J, Zhang X, Pelayo R, et al. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15(11):1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toltl LJ, Austin RC, Liaw PC. Activated protein C modulates inflammation, apoptosis and tissue factor procoagulant activity by regulating endoplasmic reticulum calcium depletion in blood monocytes. J Thromb Haemost. 2011;9(3):582–592. doi: 10.1111/j.1538-7836.2010.04177.x. [DOI] [PubMed] [Google Scholar]
- 34.Larsen KS, Ostergaard H, Olsen OH, Bjelke JR, Ruf W, Petersen LC. Engineering of substrate selectivity for tissue factor.factor VIIa complex signaling through protease-activated receptor 2. J Biol Chem. 2010;285(26):19959–19966. doi: 10.1074/jbc.M110.101030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahamed J, Ruf W. Protease-activated receptor 2-dependent phosphorylation of the tissue factor cytoplasmic domain. J Biol Chem. 2004;279(22):23038–23044. doi: 10.1074/jbc.M401376200. [DOI] [PubMed] [Google Scholar]
- 36.Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002;296(5574):1880–1882. doi: 10.1126/science.1071699. [DOI] [PubMed] [Google Scholar]
- 37.Disse J, Petersen HH, Larsen KS, et al. The endothelial protein C receptor supports tissue factor ternary coagulation initiation complex signaling through protease-activated receptors. J Biol Chem. 2011;286(7):5756–5767. doi: 10.1074/jbc.M110.201228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Disse J, Ruf W. Endothelial protein C receptor is required for tissue factor ternary complex signaling in the mouse. J Thromb Haemost. 2011;9(12):2516–2518. doi: 10.1111/j.1538-7836.2011.04521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rios-Steiner JL, Murakami MT, Tulinsky A, Arni RK. Active and exo-site inhibition of human factor Xa: structure of des-Gla factor Xa inhibited by NAP5, a potent nematode anticoagulant protein from Ancylostoma caninum. J Mol Biol. 2007;371(3):774–786. doi: 10.1016/j.jmb.2007.05.042. [DOI] [PubMed] [Google Scholar]
- 40.Bae JS, Yang L, Manithody C, Rezaie AR. Engineering a disulfide bond to stabilize the calcium-binding loop of activated protein C eliminates its anticoagulant but not its protective signaling properties. J Biol Chem. 2007;282(12):9251–9259. doi: 10.1074/jbc.M610547200. [DOI] [PubMed] [Google Scholar]
- 41.Harmon S, Preston RJ, Ni Ainle F, et al. Dissociation of activated protein C functions by elimination of protein S cofactor enhancement. J Biol Chem. 2008;283(45):30531–30539. doi: 10.1074/jbc.M802338200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang L, Bae JS, Manithody C, Rezaie AR. Identification of a specific exosite on activated protein C for interaction with protease-activated receptor 1. J Biol Chem. 2007;282(35):25493–25500. doi: 10.1074/jbc.M702131200. [DOI] [PubMed] [Google Scholar]
- 43.Gale AJ, Sun X, Heeb MJ, Griffin JH. Nonenzymatic anticoagulant activity of the mutant serine protease Ser360Ala-activated protein C mediated by factor Va. Protein Sci. 1997;6(1):132–140. doi: 10.1002/pro.5560060115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Preston RJ, Ajzner E, Razzari C, et al. Multifunctional specificity of the protein C/activated protein C Gla domain. J Biol Chem. 2006;281(39):28850–28857. doi: 10.1074/jbc.M604966200. [DOI] [PubMed] [Google Scholar]
- 45.Deng T, Zhang Y, Chen Q, Yan K, Han D. Toll-like receptor-mediated inhibition of Gas6 and ProS expression facilitates inflammatory cytokine production in mouse macrophages. Immunology. 2012;135(1):40–50. doi: 10.1111/j.1365-2567.2011.03511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thorelli E, Kaufman RJ, Dahlbäck B. The C-terminal region of the factor V B-domain is crucial for the anticoagulant activity of factor V. J Biol Chem. 1998;273(26):16140–16145. doi: 10.1074/jbc.273.26.16140. [DOI] [PubMed] [Google Scholar]
- 47.Camire RM, Bos MH. The molecular basis of factor V and VIII procofactor activation. J Thromb Haemost. 2009;7(12):1951–1961. doi: 10.1111/j.1538-7836.2009.03622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lyden P, Levy H, Weymer S, et al. Phase 1 safety, tolerability and pharmacokinetics of 3K3A-APC in healthy adult volunteers. Curr Pharm Des. 2013;19(42):7479–7485. doi: 10.2174/1381612819666131230131454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schuijt TJ, Bakhtiari K, Daffre S, et al. Factor Xa activation of factor V is of paramount importance in initiating the coagulation system: lessons from a tick salivary protein. Circulation. 2013;128(3):254–266. doi: 10.1161/CIRCULATIONAHA.113.003191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fernandes N, Mosnier LO, Tonnu L, Heeb MJ. Zn²(+) -containing protein S inhibits extrinsic factor X-activating complex independently of tissue factor pathway inhibitor. J Thromb Haemost. 2010;8(9):1976–1985. doi: 10.1111/j.1538-7836.2010.03919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wood JP, Ellery PE, Maroney SA, Mast AE. Biology of tissue factor pathway inhibitor. Blood. 2014;123(19):2934–2943. doi: 10.1182/blood-2013-11-512764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steen M, Norstrøm EA, Tholander AL, et al. Functional characterization of factor V-Ile359Thr: a novel mutation associated with thrombosis. Blood. 2004;103(9):3381–3387. doi: 10.1182/blood-2003-06-2092. [DOI] [PubMed] [Google Scholar]
- 53.Nogami K, Shinozawa K, Ogiwara K, et al. Novel FV mutation (W1920R, FVNara) associated with serious deep vein thrombosis and more potent APC resistance relative to FVLeiden. Blood. 2014;123(15):2420–2428. doi: 10.1182/blood-2013-10-530089. [DOI] [PubMed] [Google Scholar]
- 54.Gould WR, Silveira JR, Tracy PB. Unique in vivo modifications of coagulation factor V produce a physically and functionally distinct platelet-derived cofactor: characterization of purified platelet-derived factor V/Va. J Biol Chem. 2004;279(4):2383–2393. doi: 10.1074/jbc.M308600200. [DOI] [PubMed] [Google Scholar]
- 55.Castoldi E, Brugge JM, Nicolaes GA, Girelli D, Tans G, Rosing J. Impaired APC cofactor activity of factor V plays a major role in the APC resistance associated with the factor V Leiden (R506Q) and R2 (H1299R) mutations. Blood. 2004;103(11):4173–4179. doi: 10.1182/blood-2003-10-3578. [DOI] [PubMed] [Google Scholar]
- 56.Rosing J, Bakker HM, Thomassen MC, Hemker HC, Tans G. Characterization of two forms of human factor Va with different cofactor activities. J Biol Chem. 1993;268(28):21130–21136. [PubMed] [Google Scholar]
- 57.Hoekema L, Nicolaes GA, Hemker HC, Tans G, Rosing J. Human factor Va1 and factor Va2: properties in the procoagulant and anticoagulant pathways. Biochemistry. 1997;36(11):3331–3335. doi: 10.1021/bi9623284. [DOI] [PubMed] [Google Scholar]