Abstract
Background: Bipolar disorder (BD) is characterized by biased processing of emotional information. However, little research in this area has been conducted in youth with BD and at-risk individuals. The goal of this study was to determine whether children with BD displayed comparable or more severe manifestations of this bias relative to offspring of parents with BD.
Materials and methods: The sample (n = 57 children and adolescents) included 18 individuals with BD (age: 13.63 ± 2.99; 8 females), 16 offspring of parents with BD (age: 11.83 ± 2.96; 9 females) and 23 healthy controls (HC) (age: 12.789 ± 3.087; 8 females). All participants performed the Affective Go/No-Go (AGN) and the Rapid Visual Processing (RVP) tasks of the Cambridge Neuropsychological Test Automated Battery (CANTAB).
Results: Relative to HC, individuals with BD responded faster to correct trials and committed an elevated number of commission errors across all affective conditions of the AGN task. By contrast, BD offspring showed intact performance accuracy but quicker response times than HC. Post-hoc analyses revealed that this behavioral pattern was observed in BD offspring with mental health problems but not in healthy BD offspring. Overall, mean reaction times and total number of errors in the RVP task were comparable across groups.
Conclusions: In line with previous findings, subjects with BD encountered difficulties in processing affective information. The tendency toward faster but accurate responses to affective stimuli observed in BD offspring may be a marker of attentional bias toward affective information and constitute a vulnerability marker for mood disorder.
Introduction
Bipolar disorder (BD) is a serious illness characterized by mood fluctuations, brain abnormalities, poor affective processing, and cognitive deficits that, in the majority of cases, persist across mood phases (Bora et al. 2009). The onset of the disease typically occurs between the late teens and early 30s (Akiskal 1996; Merikangas et al. 2007) and a diagnosis before the age of 13 is associated with high rates of psychiatric comorbidities and poor long-term outcome (Perlis et al. 2004). Additionally, BD has a substantial genetic component (Akiskal 1996) with heritability estimates ranging from 70% to 80%, and the prevalence of mood disorders in offspring of parents with BD in the range of 5–67% (DelBello and Geller 2001; Chang et al. 2003; Duffy et al. 2013; Rasic et al. 2014).
The biases in affective information processing observed in BD have been linked with altered information processing speed and deficits in verbal memory and response inhibition (Schenkel et al. 2008; Passarotti et al. 2010; Singh et al. 2010; Jacobs et al. 2011; Deveney et al. 2012; Passarotti et al. 2013). In particular, previous studies in BD have consistently shown a robust effect of negative stimuli on cognitive processing (Pavuluri et al. 2008; Passarotti et al. 2011; Pavuluri et al. 2012). Although current literature does not view such biases as primary endophenotypic markers of BD, both healthy pediatric BD offspring (Gotlib et al. 2005) and adult siblings of BD patients exhibit affective processing biases toward negative stimuli in tasks of impulse control (Clark et al. 2005; Klimes-Dougan et al. 2006; Maziade et al. 2009; Brand et al. 2012). Similar to patients with BD, at-risk individuals display deficits in sustained attention and executive functioning (Zalla et al. 2004; Frangou et al. 2005; Klimes-Dougan et al. 2006; Trivedi et al. 2008; Kulkarni et al. 2010; Diwadkar et al. 2011) which suggests that cognitive deficits and affective processing biases could be interrelated, and may constitute markers of vulnerability to BD.
In agreement with the behavioral findings, functional neuroimaging studies have detected altered patterns of neural activity in the ventrolateral and dorsolateral prefrontal, cingulate, and limbic regions during the performance of tasks of affective processing and response inhibition (Malhi et al. 2005; Wessa et al. 2007; Pavuluri et al. 2008; Morris et al. 2012). In particular, adults with BD who were instructed to downregulate their emotional response to threatening stimuli exhibited greater activation in the frontal and amygdalar regions than did healthy controls (Houenou et al. 2011). The emotional processing bias observed in BD may, therefore, be associated with dysregulation in the frontolimbic network.
In summary, existing evidence suggests that both individuals with BD and at-risk individuals display affective processing biases. However, to the authors' knowledge, no published study has focused on whether children with BD display a comparable or more severe manifestation of such bias than do offspring of parents with BD. To address these research questions, the current study compared the performance of children and adolescents with BD, BD offspring with and without psychiatric disorders, and healthy controls (HC) on two attentional measures of affective and nonaffective processing – the Affective Go/No-Go (AGN) and the Rapid Visual Processing (RVP) tasks of the CANTAB battery. Based on previous findings, we predicted that subjects with BD would display a stronger affective processing bias compared to HC. Their performance on the RVP task was expected to be comparable to that of HC, thus showing that the affective processing bias, if any, is not the result of attentional deficits. Given the lack of studies focusing on affective processing and cognition in BD offspring compared with BD patients and HC, no a priori hypothesis regarding group differences was made.
Materials and Methods
Subjects
The sample (n = 57 children and adolescents) included 18 individuals with BD (age: 13.63 ± 2.99; 8 females; 5 BD type I; 4 BD type II; 10 BD not otherwise specified [NOS]), 16 offspring of parents with BD (age: 11.83 ± 2.96; 9 females) and 23 HC (age: 12.789 ± 3.087; 8 females). Participants were recruited at the University of North Carolina at Chapel Hill (UNC) and at the University of Texas Health Science Center at Houston. The study protocol was approved by the local institutional review boards and informed consent was obtained from all the participants. Participants included in this study had no current medical disorder, including neurological disorders and traumatic brain injury. Children and adolescents with BD and offspring of parents with BD had at least one parent who met criteria for BD as determined via a detailed family history assessment. The group of BD offspring included healthy individuals (n = 7), individuals with attention-deficit/hyperactivity disorder (ADHD) (n = 3), major depressive disorder (MDD) NOS (n = 1), oppositional defiant disorder (n = 2), and generalized anxiety disorder (n = 1). Diagnostic data were missing for two BD offspring. Eight individuals with BD and five offspring of parents with BD were on psychiatric medication at the time of assessment. HC with a history of any Axis I disorder in first-degree relatives or who had taken any prescribed psychotropic medication at any point in their lives were excluded. Across all groups, children and adolescents with history of substance abuse in the 6 months prior to enrollment, schizophrenia, developmental disorders, eating disorders, and intellectual disability were excluded. Female participants of reproductive age underwent a urine pregnancy test. All participants underwent a urine drug screen to exclude illegal drug use.
Clinical assessment
Psychiatric diagnosis was established using the Kiddie Schedule of Affective Disorders and Schizophrenia-Present and Lifetime Version (K-SADS-PL) interview (Kaufman et al. 1996) based on Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV) criteria, and confirmed subsequently in a clinical evaluation with a research psychiatrist (American Psychiatric Association 1994). All parents (of individuals with BD and BD offspring) who reported previous BD diagnosis had their diagnosis ascertained by the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders Axis I (SCID I) (First et al. 2012). All interviews were administered to participants by trained evaluators, and were later reviewed by a board-certified psychiatrist. The affective state was assessed with the Young Mania Rating Scale (YMRS) (Young et al. 1978) and the Children's Depression Rating Scale (CDRS) (Poznanski et al. 1984). Both instruments have satisfactory psychometric properties (YMRS: Cronbach α = 0.80; convergent validity: r = 0.83 [Fristad et al. 1995]; CDRS: Cronbach α = 0.85, item-total correlations ranged from 0.28 to 0.78, convergent validity: r = 0.92 [Poznanski and Mokros 1996]).
Cognitive assessment
Premorbid cognitive ability was estimated by the reading test of the Wide Range Achievement Test-4 (WRAT-4) (Wilkinson and Robertson 2006). Participants were then administered the Affective Go/No-Go paradigm (AGN) and the Rapid Visual Processing (RVP) of the computerized Cambridge Neurocognitive Test Automated Battery (CANTAB) (http://www.cantab.com). This cognitive battery was based on its well-established sensitivity to cognitive impairment in psychiatric disorders (Sweeney et al. 2000). The AGN and RVP tasks are briefly described subsequently, and a detailed description is given elsewhere (Robbins et al. 1994).
The AGN task evaluates the effect of the emotional valence of words on the participant's ability to identify the target valence (positive or negative) and to inhibit a response to the nontarget valence with the target and nontarget valences switching across trials. Participants are presented with positive (e.g., joyful, warm, courageous) and negative (e.g., mistake, hopelessness, burden) words in a counterbalanced manner, and instructed to respond to either happy or sad stimuli depending upon the task condition. The primary outcome measures of this study are the mean latencies to correct trials and the number of commission errors (false positive) across affective connotations.
The RVP task is a nonemotional analogue of the AGN task selected to assess information processing capacities under conditions of low working memory load. Participants are presented with sequences of digits from 2 to 9 and instructed to press on a response pad when they see the target sequence of numbers (e.g., 2-4-6). The main outcome measures were the mean response time to correct target sequences (mean latency) and the total number of commission errors.
Statistical analysis
Statistical analyses were performed using IBM SPSS statistics (Version 21.0). Normality assumptions were examined. Where appropriate, outliers were Winsorized and log, square root, or reciprocal transformations applied to achieve normality. One way ANOVAs and χ2 analyses were used to compare demographic, clinical and cognitive differences between groups. G*Power (Faul et al. 2007) was used for power calculations. Group differences in latencies and number of errors on the AGN and RVP tasks were estimated using multivariate analyses of variance (MANOVA). The threshold of statistical significance was set at p ≤ 0.05, and a Bonferroni correction was applied to post-hoc comparisons between HC and each clinical group, and between individuals with BD and offspring of BD parents to adjust the significance level. Subsequent exploratory post-hoc analyses compared HC with individuals with BD, healthy offspring of parents with BD (healthy at-risk: n = 7), and offspring of parents with BD with a psychiatric diagnosis (at-risk with diagnosis: n = 9) (three contrasts).
Results
Group characteristics
Demographics and clinical features of the participants included in this study are reported in Table 1. There were no age, gender, or education differences across groups. The WRAT reading test varied across groups (F[2, 37] = 8.98, p = 0.001, partial η2 = 0.33), with both children with BD and BD offspring displaying lower scores than HC (p ≤ 0.01). There were significant differences among the three groups on the CDRS (F[2, 48] = 10.91, p < 0.001, partial η2 = 0.31) and the YMRS (F[2, 53] = 10.91, p < 0.001, partial η2 = 0.29). As expected, both subjects with BD and offspring of BD parents had higher CDRS and YMRS scores than HC (p values ≤0.05).
Table 1.
Demographic and Clinical Characteristic of the Sample (Mean ± Standard Deviation)
| HC n = 23 | BD n = 18 | BD offspring n = 16 | F test or X | p value | |
|---|---|---|---|---|---|
| Age (years) | 12.79 ± 3.09 | 13.63 ± 2.99 | 11.83 ± 2.96 | 1.5 | 0.23 |
| Gender (F) | 8 | 8 | 9 | 1.77 | 0.41 |
| BD subtype | N/A | 5 BDI/4 BDII/10 BD-NOS | N/A | ||
| Education (years) | 6.43 ± 3.01 | 7.18 ± 3.36 | 5.94 ± 2.82 | 0.69 | 0.51 |
| WRAT | 118.56 ± 17.08 | 92.56 ± 9.38 | 105.07 ± 15.30 | 8.98 | ≤.001a,b |
| n | (n = 16) | (n = 9) | (n = 15) | ||
| CDRS | 17.63 ± 1.29 | 29.15 ± 12.86 | 21.69 ± 4.94 | 10.91 | ≤.001a,b |
| (n = 22) | (n = 13) | (n = 16) | |||
| YMRS | 0.35 ± 0.57 | 6.59 ± 4.16 | 7.13 ± 8.57 | 10.91 | ≤0.001a,b |
| (n = 23) | (n = 17) | (n = 16) |
BD, bipolar disorder; HC, healthy controls; WRAT, Wide Range Achievement Test; CDRS, Childhood Depression Rating Scale; YMRS, Young Mania Rating Scale.
a = BD vs. HC; b = BD offspring vs. HC.
AGN
All stimuli
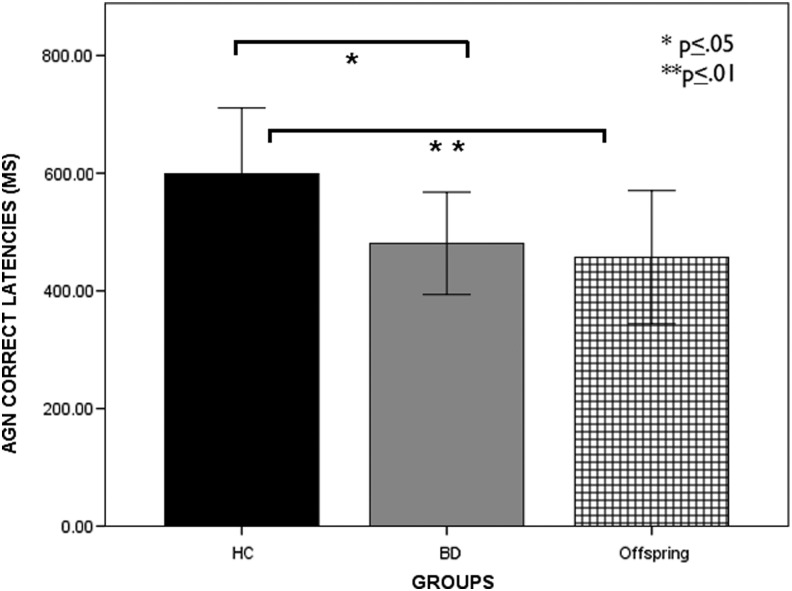
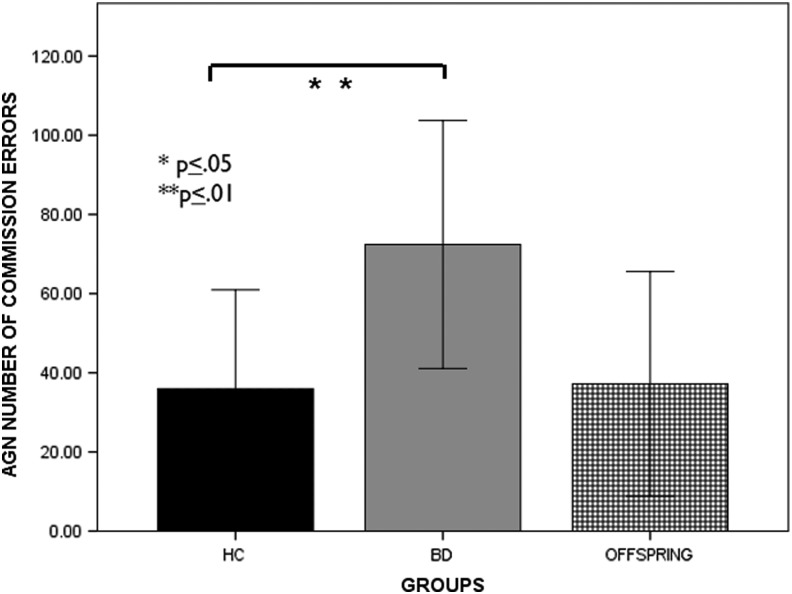
Statistical analyses across affective conditions of the AGN task revealed a significant group effect on mean response latencies (F[2, 54] = 10.61, p = 0.000, partial η2 = 0.28) and number of commission errors (F[2, 54] = 10.11, p < 0.001, partial η2 = 0.27). Both BD and BD offspring responded to stimuli faster than HC (latency: BD, p = 0.002; offspring, p = 0.000). Whereas BD committed more errors than HC (p = 0.002), BD offspring performed more accurately than BD (p < 0.001) (Figs. 1 and 2).
FIG. 1.
Group performance (means ± SE) on affective Go/No-Go. Mean correct latencies in youth with bipolar disorder (BD), healthy controls (HC), affected BD offspring (AOffspring), and unaffected BD offspring (UOffspring). The results indicate significantly reduced RTs during the correct trials in subjects with BD and Aoffspring compared with HC.
FIG. 2.
Group performance (means ± SE) on affective Go/No-Go. Total number of commission errors to positive and negative stimuli in youth with bipolar disorder (BD), healthy controls (HC), and offspring of BD parents. The results indicate a significantly higher number of errors in youth with BD compared to HC (BD > HC).
Positive versus negative stimuli
When comparing positive and negative stimuli, a significant group effect on response latencies to correctly identified positive (F[2, 53] = 7.37, p = 0.002, partial η2 = 0.22) and negative stimuli (F[2, 53] = 9.44, p = 0.000, partial η2 = 0.26) was observed. BD and BD offspring displayed faster latencies than HC (BD: p = 0.025, BD offspring: p = 0.002). There was also a group effect on the number of commissions in both the positive (F[2, 53] = 6.79, p = 0.002, partial η2 = 0.20) and negative conditions (F[2, 53] = 10.35, p < 0.001, partial η2 = 0.28). BD patients made more mistakes in response to both positive (p = 0.002) and negative stimuli (p = 0.000) than HC. There was no difference in accuracy in response to positive and negative stimuli in BD offspring when compared with HC and BD (p > 0.05) (Table 2).
Table 2.
Mean Reaction Times (RT) (in ms) and Number of Commission Errors (CE) Across Groups (Mean ± Standard Deviation)
| HC n = 23 | BD n = 18 | BD offspring n = 16 | F | p value | |
|---|---|---|---|---|---|
| AGN mean RT | 598.98 ± 111.81 | 480.77 ± 86.99 | 457.09 ± 113.26 | 10.61 | <0.001a,b |
| AGN mean number of CE | 35.96 ± 25 | 72.39 ± 31.31 | 37.19 ± 28.37 | 10.11 | <0.001a |
| AGN RT positive | 571.81 ± 109.49 | 483.950 ± 81.02 | 450.35 ± 112.22 | 7.37 | 0.002a,b |
| AGN CE positive | 5.87 ± 4.26 | 11.527 ± 5.66 | 8.20 ± 4.77 | 6.79 | 0.002a |
| AGN RT negative | 596.26 ± 113.91 | 471.04 ± 97.43 | 461.93 ± 117.04 | 9.44 | 0.000a,b |
| AGN CE negative | 4.87 ± 4.77 | 12.08 ± 5.12 | 8.16 ± 5.33 | 10.35 | <0.001a |
| RVP RT | 382.16 ± 93.37 | 433.69 ± 122.65 | 451.17 ± 144.51 | 1.73 | 0.19 |
| (n = 22) | (n = 18) | (n = 14) | |||
| RVP CE | 1.31 ± 1.29 | 3.56 ± 4.09 | 2.43 ± 4.55 | 2.15 | 0.13 |
| (n = 22) | (n = 18) | (n = 14) |
HC, healthy controls; BD, bipolar disorder; AGN, Affective Go/No-Go; RVP: Rapid Visual Processing.
a = BD vs. HC; b = offspring vs. HC.
RVP
There was no significant difference in reaction times (F[2,51] = 1.725, p = 0.19, partial η2 = 0.06) and the number of commission errors across groups (F[2,51] = 2.15, p = 0.13, partial η2 = 0.09).
Exploratory analyses comparing HC, BD, healthy at-risk, and at-risk with diagnosis
AGN: All stimuli
To explore the data further, we compared AGN and RVP findings among HC, BD, healthy at-risk (n = 7) and at-risk with diagnosis (n = 9). Groups differed in terms of both latencies (F[3, 53] = 8.49, p < 0.001, partial η2 = 0.33) and total number of commissions (F[3, 53] = 8.27, p < 0.001, partial η2 = 0.32). Results showed that BD patients and at-risk with diagnosis worked faster than HC (BD: p = 0.004, at-risk with diagnosis: p = 0.000). In terms of number of commissions, BD made more mistakes than HC (p = 0.000). Remaining group comparisons did not yield statistical significance.
AGN: Negative versus positive stimuli
Group differences were found in latencies to positive (F[3,52] = 6.03, p = 0.001, partial η2 = 0.26) and negative stimuli (F[3,52] = 8.69, p < 001, partial η2 = 0.33). BD and at-risk with diagnosis showed faster reaction times than HC (positive BD: p = 0.05, at-risk with diagnosis BD: p = 0.001; negative BD: p = 0.002, at-risk with diagnosis: p ≤ 0.001). In relation to the number of commissions, there was a group effect in response to positive (F[3,52] = 5.24, p = 0.003, partial η2 = 0.23) and negative stimuli (F[3,52] = 8.4, p < 0.001, partial η2 = 0.33). BD made more mistakes than HC in both conditions (positive: p = 0.003, negative: p < 0.001). The number of commission errors in response to negative stimuli in the at-risk with diagnosis group was elevated overall compared with HC, but did not reach statistical significance (p = 0.06).
RVP
Latencies and number of commission errors of all clinical groups in response to the RVP task was comparable with those of HC.
Discussion
Based on previous evidence of affective processing biases in adults and youth with BD, this study compared the performance of children and adolescents with BD, BD offspring (with and without psychiatric disorders), and HC on measures of affective and nonaffective cognitive information processing. The most compelling finding of this study was that offspring of parents with BD were as accurate as HC and displayed faster response to affective stimuli. In line with previous findings, youth with BD responded to correct trials at a faster speed and committed more commission errors in the AGN task than HC.
Because our operational definition of BD offspring included individuals with ADHD, BD NOS and MDD NOS, the tendency to respond more quickly could be associated with these psychiatric conditions rather than with genetic vulnerability to BD per se. Findings in this research field are mixed, as a recent study showed that children with BD performed as accurately as children with BD and/or comorbid ADHD symptoms (including children with ADHD only) on measures of sustained attention and response inhibition (Narvaez et al. 2014). Because the latter study did not include a healthy control group it is, however, unknown whether the cognitive performance of these children was within or below standard average values. Further, some consideration should be given to the fact that BD offspring displayed faster latencies in response to stimuli of the AGN but not the RVP task, which is a nonaffective analogue of the AGN. Notably, this pattern of faster responses was observed in the at-risk individuals with diagnoses but not in healthy individuals at risk for BD. Along the same line, a recent article showed that individuals with high scores on the Hypomanic Personality Scale (HPS) (Eckblad and Chapman 1986), a measure widely used to assess proneness to hypomanic symptoms and mood lability, responded faster to visual stimuli than individuals with low HPS scores (Bauer et al. 2015). Accuracy was, however, comparable between the two groups. Therefore, although our current findings suggest that BD offspring have sustained attentional skills comparable with those of HC, the tendency to respond more quickly to affective stimuli may correlate with increased reactivity or impulsivity, and be a vulnerability marker.
In line with this hypothesis, Surguladze et al. showed that patients with BD and their relatives displayed a robust increase in neural activation in the amygdala when exposed to happy faces, compared with healthy individuals (Surguladze et al. 2010). Another functional MRI (fMRI) study on affective processing in bipolar disorder showed that patients with BD and high-risk individuals displayed a stronger amygdala response than did healthy controls in response to fearful stimuli. Notably, the three groups did not differ in their ability to label face emotion (Olsavsky et al. 2012). Thereore, the pattern of response to affective stimuli observed in our BD offspring may be the result of limbic hyperactivation. It is, however, unclear whether this confers risk or protection against the development of mood disorders. Future longitudinal studies in this research area will assist in determining whether healthy pediatric BD offspring who present with the cognitive profile observed in our offspring sample are diagnosed with mood disorder at a later age. Further, the use of neuroimaging measures will be useful in detecting the differences in neural activation in response to affective stimuli between BD and BD offspring with and without diagnosis.
The lack of a specific bias toward negative information in the BD population is in contrast to studies in remitted and symptomatic adults with BD showing abnormal processing only to negative stimuli (Leppänen and Hietanen 2004; Atchley et al. 2007; Joormann and Gotlib 2007; LeMoult et al. 2009). However, findings in this research area are mixed, as a study found that remitted adults and children with BD did not differ from HC in terms of their ability to inhibit negative stimuli (Joormann and Gotlib 2010). Similarly, in another study, BD patients did not display negative emotional biases during the performance of a memory task (Timbremont and Braet 2004). A potential explanation for the divergence in findings could be that in previous studies the participants' mood was manipulated via negative or positive induction (Scher et al. 2005; Ramel et al., 2007). Mood induction is a valid and effective technique used to trigger latent vulnerability features and may help detect endophenotypic markers of BD to a greater extent than computerized cognitive measures. It is noteworthy that at-risk individuals with a diagnosis committed a higher number of commission errors to negative stimuli than did HC. This finding did not, however, reach statistical significance. Replication of the current study with a larger sample may help to determine whether a negative bias is a marker of vulnerability to mood disorder in a pediatric at-risk population.
Current mood state is known to affect the participants' response to affective stimuli. Previous literature showed that depressed patients respond more slowly to positive stimuli than to negative stimuli when compared with HC (Murphy et al. 1999). Depressed patients also show a stronger reaction to words associated with depression (Rinck and Becker 2005) and faces showing negative emotions (Joormann and Gotlib 2007). The lack of a pronounced attentional bias toward negative stimuli in the BD sample may be explained by the absence of clinically relevant depressive features in our BD sample, as shown by the low CDRS scores (BD: 29.15 ± 12.86; BD offspring: 21.69 ± 4. Additional empirical evidence is, however, needed to support this claim.
A limitation of the current study is the small number of participants included in each group. This weakness was accounted for by planning a limited number of comparisons between groups (Howell 2012). However, this approach did not allow us to estimate potential differences between healthy offspring and offspring with a diagnosis. Given that the present study was a novel investigation of offspring of parents with BD and we did not have expectations in terms of effect size, we decided to undertake retrospective post-hoc analyses with the view to informing future research design. These analyses showed that, assuming a large effect size (f = 0.40, d = 0.80, α = 0.05), the current study was sufficiently powered (0.74) to detect differences in cognitive performance across all groups. Further, in terms of a priori contrasts comparing healthy controls with other groups, power estimates were equal to 0.88 for detecting a large effect size (f = 0.40, d = 0.80, α = 0.05). The rationale for assuming a large effect size was based on a previous meta-analysis of neurocognitive findings in a sample of pediatric BD, which identified medium to large effects deficits across relevant domains (Joseph et al. 2008). Further, we wished to ensure that any identified effects were meaningful.
Conclusions
In conclusion, BD offspring displayed faster response times to affective stimuli than did HC, but were equally accurate in their performance. Post-hoc comparisons showed that this result was observed in at-risk offspring with diagnoses and not in healthy offspring. In line with previous findings, youth with BD responded faster but less accurately to affective stimuli.
Clinical Significance
The current results yield potential implications for the development of early prevention and intervention strategies addressing affective processing in youth with BD and at-risk individuals, and warrant further investigation of the impact of reduced affective processing on the long-term outcome of BD.
Disclosures
Drs Bauer, Meyer, and Zunta-Soares have no competing financial interests to disclose. Dr. Frazier has received federal funding or research support from, acted as a consultant to, received travel support from, and/or received a speaker's honorarium from Brain and Behavior Research Foundation, Bristol-Myers Squibb (BMS), Ecoeos, Forest Laboratories, Ingalls Foundation, IntegraGen, Kugona LLC, National Institutes of Health, Shire Development, and the Simons Foundation. Dr. Youngstrom has consulted with Lundbeck, Otsuka, and Pearson about assessment. Dr J. C. Soares has received grants/research support from BMS, Forrest, Merck, NIH 69774, and Stanley Medical Research Institute, and has been a speaker for Abbott and Pfizer.
References
- Akiskal HS: The prevalent clinical spectrum of bipolar disorders: Beyond DSM-IV. J Clin Psychopharmacol 16:4S–14S, 1996 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- Atchley RA, Stringer R, Mathias E, Ilardi SS, Diane Minatrea A: The right hemisphere's contribution to emotional word processing in currently depressed, remitted depressed, and never-depressed individuals. J Neurolinguistics 20:145–160, 2007 [Google Scholar]
- Bauer IE, Jordan G, Soares JC, Meyer TD: The role of negative mood induction on working memory capacity in individuals putatively at risk for bipolar disorder: A pilot study. J Affect Disord 185:60–66, 2015 [DOI] [PubMed] [Google Scholar]
- Bora E, Yucel M, Pantelis C: Cognitive endophenotypes of bipolar disorder: A meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. J Affect Disord 113:1–20, 2009 [DOI] [PubMed] [Google Scholar]
- Brand JG., Goldberg TE, Gunawardane N, Gopin CB, Powers RL, Malhotra A, Burdick KE: Emotional bias in unaffected siblings of patients with bipolar I disorder. J Affect Disord 136:1053–1058, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K, Steiner H, Dienes K, Adleman N, Ketter T: Bipolar offspring: A window into bipolar disorder evolution. Biol Psychiatry 53:945–951, 2003 [DOI] [PubMed] [Google Scholar]
- Clark L, Sarna A, Goodwin GM: Impairment of executive function but not memory in first-degree relatives of patients with bipolar I disorder and in euthymic patients with unipolar depression. Am J Psychiatry 162:1980–1982, 2005 [DOI] [PubMed] [Google Scholar]
- Delbello MP, Geller B. Review of studies of child and adolescent offspring of bipolar parents. Bipolar Disord 3:325–334, 2001 [DOI] [PubMed] [Google Scholar]
- Deveney CM, Connolly ME, Jenkins SE, Kim P, Fromm SJ, Brotman MA, Pine DS, Leibenluft E. Striatal dysfunction during failed motor inhibition in children at risk for bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry 38:127–133, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar VA, Goradia D, Hosanagar A, Mermon D, Montrose DM, Birmaher B, Axelson D, Rajarathinem R, Haddad L, Amirsadri A: Working memory and attention deficits in adolescent offspring of schizophrenia or bipolar patients: comparing vulnerability markers. Prog Neuropsychopharmacol Biol Psychiatry 35:1349–1354, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy A, Horrocks J, Doucette S, Keown–Stoneman C, Mccloskey S, Grof P: The developmental trajectory of bipolar disorder. Br J Psychiatry 113:126706, 2013 [DOI] [PubMed] [Google Scholar]
- Eckblad M, Chapman LJ: Development and validation of a scale for hypomanic personality. J Abnorm Psychol 95:214, 1986 [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang A.-G, Buchner A: G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39:175–191, 2007 [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB: Structured Clinical Interview for DSM-IV Axis I Disorders Clinician Version (SCID-CV). Washington, D.C.: American Psychiatric Press, Inc., 1996 [Google Scholar]
- Frangou S, Haldane M, Roddy D, Kumari V: Evidence for deficit in tasks of ventral, but not dorsal, prefrontal executive function as an endophenotypic marker for bipolar disorder. Biol Psychiatry 58:838–839, 2005 [DOI] [PubMed] [Google Scholar]
- Fristad MA, Weller RA, Weller EB. The Mania Rating Scale (MRS): Further reliability and validity studies with children. Ann Clin Psychiatry 7:127–132, 1995 [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Traill SK, Montoya RL, Joormann J, Chang K: Attention and memory biases in the offspring of parents with bipolar disorder: Indications from a pilot study. J Child Psychol Psychiatry 46:84–93, 2005 [DOI] [PubMed] [Google Scholar]
- Houenou J, Frommberger J, Carde S, Glasbrenner M, Diener C, Leboyer M, Wessa M: Neuroimaging-based markers of bipolar disorder: Evidence from two meta-analyses. J Affect Disord 132:344–355, 2011 [DOI] [PubMed] [Google Scholar]
- Howell D: Statistical Methods for Psychology (8th Edition). Belmont, CA: Wadsworth, Cengage Learning, 2012 [Google Scholar]
- Jacobs RH, Pavuluri MN, Schenkel LS, Palmer A, Shah K, Vemuri D, Whited S, Little DM: Negative emotion impacts memory for verbal discourse in pediatric bipolar disorder. Bipolar Disord 13:287–293, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH: Emotion regulation in depression: Relation to cognitive inhibition. Cogn Emot 24:281–298, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH: Selective attention to emotional faces following recovery from depression. J Abnorm Psychol 116:80, 2007 [DOI] [PubMed] [Google Scholar]
- Joseph MF, Frazier TW, Youngstrom EA, Soares JC: A quantitative and qualitative review of neurocognitive performance in pediatric bipolar disorder. J Child Adolesc Psychopharmacol 18:595–605, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Ryan N. Kiddie-SADS-Present and Lifetime Version. Pittsburgh:University of Pittsburgh, School of Medicine; 1996 [Google Scholar]
- Klimes–Dougan B, Ronsaville D, Wiggs EA, Martinez PE: Neuropsychological functioning in adolescent children of mothers with a history of bipolar or major depressive disorders. Biol Psychiatry 60:957–965, 2006 [DOI] [PubMed] [Google Scholar]
- Kulkarni S, Jain S, Janardhan RY, Kumar KJ, Kandavel T: Impairment of verbal learning and memory and executive function in unaffected siblings of probands with bipolar disorder. Bipolar Disord 12:647–656, 2010 [DOI] [PubMed] [Google Scholar]
- Lemoult J, Joormann J, Sherdell L, Wright Y, Gotlib IH: Identification of emotional facial expressions following recovery from depression. J Abnorm Psychol 118:828, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppänen JM, Hietanen JK. Positive facial expressions are recognized faster than negative facial expressions, but why? Psychol Res 69:22–29, 2004 [DOI] [PubMed] [Google Scholar]
- Malhi GS, Lagopoulos J, Sachdev PS, Ivanovski B, Shnier R. An emotional Stroop functional MRI study of euthymic bipolar disorder. Bipolar Disord 7:58–69, 2005 [DOI] [PubMed] [Google Scholar]
- Maziade M, Rouleau N, Gingras N, Boutin P, Paradis M-E, Jomphe V, Boutin J, Létourneau K, Gilbert E, Lefebvre AA. Shared neurocognitive dysfunctions in young offspring at extreme risk for schizophrenia or bipolar disorder in Eastern Quebec multigenerational families. Schizophr Bull 35:919–930, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RM, Petukhova M, Kessler RC: Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry 64:543–552, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RW, Sparks A, Mitchell PB, Weickert CS, Green MJ: Lack of cortico-limbic coupling in bipolar disorder and schizophrenia during emotion regulation. Transl Psychiatry 2:e90, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy F, Sahakian B, Rubinsztein J, Michael A, Rogers R, Robbins T, Paykel E: Emotional bias and inhibitory control processes in mania and depression. Psychol Med 29:1307–1321, 1999 [DOI] [PubMed] [Google Scholar]
- Narvaez JC, Zeni CP, Coelho RP, Wagner F, Pheula GF, Ketzer CR, Trentini CM, Tramontina S, Rohde LA: Does comorbid bipolar disorder increase neuropsychological impairment in children and adolescents with ADHD? Rev Bras Psiquiatr 36:53–59, 2014 [DOI] [PubMed] [Google Scholar]
- Olsavsky AK, Brotman MA, Rutenberg JG, Muhrer EJ, Deveney CM, Fromm SJ, Towbin K, Pine DS, Leibenluft E: Amygdala hyperactivation during face emotion processing in unaffected youth at risk for bipolar disorder. J Am Acad Child Adolesc Psychiatry 51:294–303, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti AM, Fitzgerald JM, Sweeney JA, Pavuluri MN: Negative emotion interference during a synonym matching task in pediatric bipolar disorder with and without attention deficit hyperactivity disorder. J Int Neuropsychol Soc 19:601–612, 2013 [DOI] [PubMed] [Google Scholar]
- Passarotti AM, Sweeney JA, Pavuluri MN: Fronto-limbic dysfunction in mania pre-treatment and persistent amygdala over-activity post-treatment in pediatric bipolar disorder. Psychopharmacology (Berl) 216:485–499, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti AM, Sweeney JA, Pavuluri MN. Neural correlates of response inhibition in pediatric bipolar disorder and attention deficit hyperactivity disorder. Psychiatry Res Neuroimaging 181:36–43, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, O'Connor MM, Harral EM, Sweeney JA: An fMRI study of the interface between affective and cognitive neural circuitry in pediatric bipolar disorder. Psychiatry Res 162:244–255, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, Passarotti AM, Fitzgerald JM, Wegbreit E, Sweeney JA: Risperidone and divalproex differentially engage the fronto-striato-temporal circuitry in pediatric mania: a pharmacological functional magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry 51:157–170 e155, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis RH, Miyahara S, Marangell LB, Wisniewski SR, Ostacher M, Delbello MP, Bowden CL, Sachs GS, Nierenberg AA: Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the systematic treatment enhancement program for bipolar disorder (STEP-BD). Biol Psychiatry 55:875–881, 2004 [DOI] [PubMed] [Google Scholar]
- Poznanski EO, Grossman JA, Buchsbaum Y, Banegas M, Freeman L, Gibbons R: Preliminary studies of the reliability and validity of the Children's Depression Rating Scale. J Am Acad Child Psychiatry 23:191–197, 1984 [DOI] [PubMed] [Google Scholar]
- Poznanski EO, Mokros HB: Children's Depression Rating Scale, Revised (CDRS-R). Los Angeles: Western Psychological Services; 1996 [Google Scholar]
- Ramel W, Goldin PR, Eyler LT, Brown GG, Gotlib IH, McQuaid JR: Amygdala reactivity and mood-congruent memory in individuals at risk for depressive relapse. Biol Psychiatry 61:231–239, 2007 [DOI] [PubMed] [Google Scholar]
- Rasic D, Hajek T, Alda M, Uher R: Risk of mental illness in offspring of parents with schizophrenia, bipolar disorder, and major depressive disorder: A meta-analysis of family high-risk studies. Schizophr Bull 40:28–38, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinck M, Becker ES. A comparison of attentional biases and memory biases in women with social phobia and major depression. J Abnorm Psychol 114:62, 2005 [DOI] [PubMed] [Google Scholar]
- Robbins T, James M, Owen A, Sahakian B, Mcinnes L, Rabbitt P: Cambridge Neuropsychological Test Automated Battery (CANTAB): A factor analytic study of a large sample of normal elderly volunteers. Dement Geriatr Cogn Dis 5:266–281, 1994 [DOI] [PubMed] [Google Scholar]
- Schenkel L, Marlow–O'Connor M, Moss M, Sweeney J, Pavuluri M. Theory of mind and social inference in children and adolescents with bipolar disorder. Psychol Med 38:791–800, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher CD, Ingram RE, Segal ZV. Cognitive reactivity and vulnerability: Empirical evaluation of construct activation and cognitive diatheses in unipolar depression. Clin Psychology Rev 25:487–510, 2005 [DOI] [PubMed] [Google Scholar]
- Singh MK, Chang KD, Mazaika P, Garrett A, Adleman N, Kelley R, Howe M, Reiss A: Neural correlates of response inhibition in pediatric bipolar disorder. J Child Adolesc Psychopharmacol 20:15–24, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surguladze SA, Marshall N, Schulze K, Hall MH, Walshe M, Bramon E, Phillips ML, Murray RM, McDonald C: Exaggerated neural response to emotional faces in patients with bipolar disorder and their first-degree relatives. Neuroimage 53:58–64, 2010 [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Kmiec JA, Kupfer DJ: Neuropsychologic impairments in bipolar and unipolar mood disorders on the CANTAB neurocognitive battery. Biol Psychiatry 48:674–684, 2000 [DOI] [PubMed] [Google Scholar]
- Timbremont B, Braet C. Cognitive vulnerability in remitted depressed children and adolescents. Behavior Res Ther 42:423–437, 2004 [DOI] [PubMed] [Google Scholar]
- Trivedi JK, Goel D, Dhyani M, Sharma S, Singh AP, Sinha PK, Tandon R: Neurocognition in first‐degree healthy relatives (siblings) of bipolar affective disorder patients. Psychiatry Clin Neurosci 62:190–196, 2008 [DOI] [PubMed] [Google Scholar]
- Wessa M, Houenou J, Paillère–Martinot ML, Berthoz S, Artiges E, Leboyer M, Martinot JL. Fronto-striatal overactivation in euthymic bipolar patients during an emotional go/nogo task. Am J Psychiatry 164:638–646, 2007 [DOI] [PubMed] [Google Scholar]
- Wilkinson GS, Robertson G: Wide Range Achievement Test (WRAT4). Lutz: Psychological Assessment Resources; 2006 [Google Scholar]
- Young R, Biggs J, Ziegler V, Meyer D. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 133:429–435, 1978 [DOI] [PubMed] [Google Scholar]
- Zalla T, Joyce C, Szöke A, Schürhoff F, Pillon B, Komano O, Perez–Diaz F, Bellivier F, Alter C, Dubois B. Executive dysfunctions as potential markers of familial vulnerability to bipolar disorder and schizophrenia. Psychiatry Res 121:207–217, 2004 [DOI] [PubMed] [Google Scholar]