Abstract
Objective: Offspring of depressed parents are at risk for developing depression at rates higher than the general population. One potential mechanism linking parent and offspring depression involves attenuated reward function. Despite the importance of social incentives for adolescents, no previous studies have relied on active social incentive reward paradigms in youth at risk for depression. The present study examined differences in youth self- and parent-report measures of and neural response to social reward between youth of mothers with and those of mothers without a history of depression.
Methods: Imaging data were collected on 10 youth with a depressed parent and 23 youth without depressed parent, which included a task examining neural response to social rewards. Youth and parents also completed self-report measures of social reward.
Results: Offspring of depressed parents had lower levels of parent-reported affiliation and reduced neural response to social reward in the ventral striatum and anterior cingulate cortex than offspring of parents without a history of depression. Higher parent-reported affiliation was associated with greater ventral striatal response to social reward. Data suggest that risk status differences in ventral striatal response to social acceptance may be accounted for by affiliation. No differences were found in youth self-reports of behavior.
Conclusions: The results suggest that attenuated response to social reward, assessed through neurobiology and behavior, may be mechanistically linked to the etiology and pathophysiology of depression. Targeting social interest and engagement may be a new direction in preventing the onset of depressive disorders in youth.
Introduction
Diminished interest and/or pleasure is a critical feature of major depressive disorder (MDD) (American Psychiatric Association 2013). Historically, this has been operationalized through self-report measures of personality, temperament, and anhedonia (Watson et al. 1994; Watson and Clark, 1997). More recently, neuroscientific research has conceptualized diminished interest and/or pleasure as attenuated reward responsiveness using neural indices (Hasler et al. 2004; Hasler and Northoff 2011). Beyond identifying cross-sectional differences between individuals with and without depression, a number of more recent studies have examined differences in reward responsiveness between individuals at high and those at low familial risk for depressive disorders (e.g., Monk et al. 2008; Gotlib et al. 2010). However, to our knowledge, no previous studies have examined attenuated reward responsiveness in individuals at high familial risk using social feedback.
Reward responsiveness has been linked to risk for onset, recurrence, and likelihood of remission of MDD (Pine et al. 1999; Kasch et al. 2002; McMakin et al. 2012). Reward responsiveness can be assessed across multiple units of behavior including self-reports and neural functioning (Pizzagalli et al. 2009; Wacker et al. 2009; Hasler and Northoff, 2011). Reward-related brain function involves a number of processes across multiple brain regions, including the ventral striatum (VS) (including the nucleus accumbens; subjective experience of pleasure), orbitofrontal cortex (reward evaluation), dorsal anterior cingulate cortex (dACC) (effort evaluation to gain a reward), and the ventromedial and dorsolateral prefrontal cortex (decision making concerning effort and value assessments) (Montague et al. 2006; Der-Avakian and Markou 2012). A recent meta-analysis (Zhang et al. 2013) quantitatively summarizing these studies reported that individuals with, relative to individuals without, depression demonstrated reduced caudate and nucleus accumbens and increased middle frontal gyrus and dACC response to an array of positively valenced stimuli (Zhang et al. 2013). However, these data did not speak to whether differences in reward response were present before the disorder onset.
As parental depression is a strong and consistent predictor of MDD in offspring (Lieb et al. 2002; Klein et al. 2005; Goodman et al. 2011; Klein et al. 2013), high-risk (HR) offspring designs can provide important information about whether reward responsiveness is implicated in risk for depression. Recent work has examined reward responsiveness in individuals at high risk for MDD. Gotlib et al. (2010) found that girls at high risk for MDD had attenuated response to monetary rewards in the putamen and left insula (but augmented response in the right insula) relative to girls at low risk for depression. However, in this study, there was a significant group difference in the levels of depressive symptoms between the girls at high and those at low risk for MDD. Therefore, it was unclear whether the observed differences were the result of their familial risk status or their own subthreshold depressive symptoms. Olino et al. (2014) also examined differences between youth at high and those at low risk for MDD using monetary incentives in a guessing task. The authors found diminished responsiveness to monetary incentives in the HR youth in the VS, and those differences were not attributable to the youth's self-reported levels of depression. McCabe et al. (2012) examined responses to gustatory reward (i.e., chocolate) in older adolescents at high risk for depression. The authors reported that HR youth demonstrated reduced responding in the orbitofrontal cortex and ACC relative to the low-risk (LR) youth. This collection of studies provides converging results about impaired reward responsiveness in youth at risk for depression. This is particularly impressive, as the studies span early through late adolescence, examine multiple reward modalities, and operationalize family risk status in different ways.
However, further work examining these issues is essential. An additional class of reward that is crucial to examine is social reward (Davey et al. 2008; Guyer et al. 2008; Forbes 2009; Silk et al. 2012, 2014). Depression is conceptualized as a disorder of social functioning (Badcock and Allen 2003), and social rewards could be especially salient for eliciting depression-related differences. Developmentally, the value and role of social incentives increases dramatically during adolescence (Crone and Dahl 2012) and may be more centrally related to the development of depression than general classes of reward. One previous study (Monk et al. 2008) compared youth at high and those at low risk for depression on responsiveness using social stimuli, specifically passive viewing of faces. In this study, happy faces were described as potential rewarding stimuli. The authors reported that HR youth demonstrated attenuated response in the VS, specifically the nucleus accumbens, when viewing happy faces relative to LR youth. However, critical tests of these differences are possible that rely on more active social incentive paradigms (Silk et al. 2012, 2014).
The present study examines differences in social reward responses in youth at high and low familial risk for MDD. In addition to neural response to rewards, we also consider both parent- and self-reports of social reward behaviors, specifically affiliation, which has been argued to be a measure of social anhedonia (Reise et al. 2011). Consistent with previous findings, we hypothesize that HR youth will demonstrate reduced ventral striatal response during social rewards than LR youth. We will also explore differences between HR and LR youth in other regions using whole-brain analyses (e.g., middle frontal gyrus and dACC).
Methods
The final sample for the present work included 47 youth, which consisted of 17 youth at familial high risk (HR) and 30 youth at low risk (LR) for depression. Of these, adequate imaging data was acquired from 10 HR and 23 LR youth. Data were unavailable for other participants because of not completing the imaging assessment (n = 5), task administration problems (n = 5), and poor coverage (i.e., <75% of voxel coverage) in either the ventral striatal or medial prefrontal cortex (n = 4). Only one youth per family was included in the study. Across all youth, the mean age was 12.98 years (SD = 2.14; range = 10.10–16.89); 57.4% were female; and 59.6% were white/Caucasian, 29.8% were African American, 8.5% were biracial, and 2.1% were another race. HR and LR youth did not differ on age or gender (ps > 0.20). Approval for the research was provided by the local Institutional Review Board. All parent participants provided written consent, and youth provided written assent for study participation before any study procedures began.
Participants were recruited from several sources from the general community in a metropolitan region of the northeast region of the United States. Recruitment methods included posting flyers in the community, placing advertisements on electronic bulletin boards and in print magazines, and identifying individuals through a university-based participant research registry. A total of 213 inquiries about the study were received by the research staff. Of these, 137 were fully screened (27 declined the phone screening, contact information for 37 families ceased to be effective, and 12 individuals were unable to ultimately be scheduled for screening). Of the 137 potential families, 66 parents (64 mothers and 2 fathers) met initial criteria for the study for being either an HR or a LR family. HR families included at least one parent with a history of chronic and/or recurrent depression, including both major depression and/or dysthymic disorder. Comorbidity was permitted within this group, except for parental history of any bipolar or psychotic disorder. Youth in HR families were excluded if they could not participate in a functional MRI assessment (because of metal implants, medical devices, or metal braces), had a previous or current unipolar or bipolar mood disorder, had a current or previous diagnosis of posttraumatic stress disorder or obsessive compulsive disorder, or were currently prescribed an antidepressant medication. Youth in HR families were eligible for study participation if they had anxiety or attention-deficit/hyperactivity disorder (ADHD) diagnoses. LR families included parents with no history of psychopathology. Following the phone screen, 70 potential families were found to be ineligible for the study (e.g., lacking multiple depressive episodes, lacking chronic depression, youth already had mood disorders, or potential LR families had a history of other disorders). Therefore, 67 parents were invited to participate in an in-person diagnostic interview to confirm eligibility. Of the 67 potential families invited to participate in the in-person assessment, 62 parents completed the diagnostic session. Following these interviews, 52 parent-child dyads continued to be eligible for and participate in the study. After youth diagnostic assessments, a past or current history of a mood disorder was discovered in five cases (across both HR and LR youth).
Interview assessments
Youth diagnostic assessments were conducted using the Schedule for Affective Disorders and Schizophrenia in School-Age Children-Present and Lifetime version (KSADS-PL) (Kaufman et al. 1997). Interviews were conducted with youth and parents individually by trained interviewers who integrated information from both sources to determine whether symptoms and diagnoses were present. Interviewers came from a pool of interviewers across a number of studies. Of the youth included in the present report, several HR youth had a lifetime history of specific phobia (n = 3), ADHD (n = 3), and oppositional defiant disorder (ODD) (n = 1). The single case of ODD had comorbid ADHD. As described, LR youth were not included if they met diagnostic criteria for any lifetime diagnosis.
The Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV) (SCID) (American Psychiatric Association 1994; First et al. 1996) was used to assess parental history of psychiatric diagnoses. The SCID was administered by the same pool of trained interviewers. To be included in the present study, a parent needed to have a history of chronic and/or recurrent depression. Chronic depression was operationalized as chronic major depression and/or dysthymic disorder, requiring the presence of symptoms for a 2 year period without remission of symptoms for at least 2 months. Recurrent depression was operationalized as the experience of at least two major depressive episodes with at least 2 months of full interepisode recovery between episodes.
Self-report assessments
Youth completed self-reports and parents completed informant-reports of the Early Adolescent Temperament Questionnaire (EATQ) (Ellis and Rothbart 2001), which assesses multiple dimensions of individual differences in youth behavior. In the present report, we focus on dimensions of behavior that are conceptually linked to approach motivation/reward-seeking and social affiliative behaviors that are assessed in both the youth self- and parent-report forms. The affiliation scale assesses pursuit of warmth and closeness with others (αs = 0.80 and 0.79, for youth and mothers, respectively). The high intensity pleasure/surgency scale assesses pleasure from activities involving high sensation seeking or novelty seeking (αs = 0.77 and 0.70, for youth and mothers, respectively). The shyness scale assesses inhibition to novelty and challenge, particularly in social contexts (αs = 0.77 and 0.85, for youth and mothers, respectively). Although the depressed mood scale is also common to youth- and parent-reports from the EATQ, the reliabilities of each scale were determined too poor to be meaningful (αs = 0.65 and 0.30, for youth and mothers, respectively), and were not considered further. Youth provided self-reports of developmental status using the Petersen Development Scale (Petersen et al. 1988) and depressive symptomatology using the Mood and Feelings Questionnaire (Angold et al. 1995). Each of these measures has strong psychometric functioning.
Parental current depressive symptomatology was assessed using the Patient Reported Outcomes Measurement Information Systems Depression scale (PROMIS-D) (Pilkonis et al. 2011). In the present study, parents completed the full 28 item bank. Reliability of this measure is strong (α = 0 .91).
Chatroom Interact Task
The Chatroom Interact Task was designed to investigate reactions to social acceptance and rejection from virtual peers in an online setting (Silk et al. 2012, 2014). On Day 1, participants were shown photographs and fictitious biographical profiles for potential virtual peers. Participants were asked to choose the top five same-sex peers that they would be interested in interacting with online at their next visit. Peer selections were made from within sets of 30 photographs for each age group (9–11, 12–14, or 15–17). Participants also provided their own biographical profile and photograph. We relied solely on same-sex peers based on previous findings of few substantive differences in responses to same- and opposite-sex peers (Silk et al. 2014). This decision also reduced the duration of the task.
On Day 2 (∼4 weeks later), participants returned to the laboratory and were told that they had been matched with two same-sex peers selected from the first visit and that these youth were ready to participate in a “chat game” online. They reviewed biographical profiles for selected peers prior to the task. The task takes the form of a structured online interaction, rather than a free-form “chat,” in order to give the impression that subjects and virtual peers are interacting in real time while maintaining sufficient standardization across subjects and sufficient repetition across trials to conduct analyses. During neuroimaging, pictures of the peers and participant were projected on the screen two at a time, as the subject and virtual peers took turns selecting whom they would rather talk to about a series of teen interests (e.g., music, friends, school, books). The task proceeded in three experimental blocks, each containing 15 trials in which a person was chosen or not chosen to discuss each topic and a fourth control block (total run time 13 minutes, 30 seconds). Stimuli were presented using E-prime 1.0 software (Psychology Software Tools, Pittsburgh, PA). Each block began with an instruction about who would be making choices for that block (agent). The photograph of the agent (i.e., the “chooser”) was shown at the bottom left corner of the screen and the photographs of the other two players were shown next to each other in the middle of the screen. At the beginning of each trial, the question “Whom would you rather talk to about. … .” with the selected topic for that trial (i.e. … “music?”) appeared on the screen for 3.34 seconds (task component durations were chosen to be multiples of the 1.67 second TR). Feedback was then provided about which person was chosen (the subject or the virtual peer) for 10.02 seconds (i.e., 6 TR). The photograph of the person who was not chosen was superimposed with an “X,” and the photograph of the person who was chosen was highlighted around the border. To maintain engagement in the task, in all trials in which the participant was not the agent, he or she was asked to press a button to indicate whether the person on the left or the right was chosen. Trials were arranged in blocks so that participants experienced one “accept” block in which they were chosen two thirds of the time and one “reject” block in which they were rejected two thirds of the time. Topics were presented randomly and repeated in each block, but with a different “agent” for each block. In block 1, the subject was the “agent” and made choices between the two same-gender virtual peers. Analyses focused on blocks two and three, during which the subject was chosen/not chosen by the virtual peers. The order of accept and reject blocks and trials were randomized within gender grouping. Participants were not led to believe that they would have additional interaction with the virtual peers beyond the structured “chat game” (i.e., although they chose which participants they would be more interested in discussing topics with, they were not led to expect to engage in an open discussion on these topics with the virtual peers). The fourth block was a motor and perceptual control task designed to control for viewing faces (self and others) and pressing a button to identify a stimulus appearing on one of the faces. In these trials, pictures of the participant and one peer were displayed on the screen and a small gray dot was presented on one of the faces. The participant was asked to indicate on which face the gray dot appeared.
Ratings were made after completing the task to determine whether HR and control subjects differed in mood following completion of the task; therefore, mood ratings were not specific to accept or reject trials. Participants were debriefed at a later date and informed that they had been playing with a preset computer program.
Blood-oxygen-level dependent (BOLD) fMRI acquisition, processing, and analysis
Each participant was scanned using a Siemens 3T Allegra scanner. BOLD functional images were acquired with a gradient echo planar imaging sequence and covered 32 axial slices (3.2 mm thick) beginning at the cerebral vertex and encompassing the entire cerebrum and the majority of the cerebellum (TR/TE = 1670/29 msec, field of view = 20 cm, matrix = 64 × 64). Scanning parameters were selected to optimize BOLD signal quality while maintaining a sufficient number of slices to acquire whole-brain data. Before the collection of fMRI data for each participant, a reference echoplanar imaging scan was acquired and visually inspected for artifacts (e.g., ghosting) and good signal across the entire volume. The data from all 33 included participants were clear of such problems.
Whole-brain image analysis was conducted with SPM8 (http://www.fil.ion.ucl.ac.uk/spm). For each scan, images for each participant were realigned to the mean volume in the time series, to correct for head motion. Realigned images were spatially normalized into Montreal Neurological Institute stereotactic space using a 12 parameter affine model, then smoothed to minimize noise and residual difference in gyral anatomy with a Gaussian filter set at 6 mm full width at half maximum. Voxel-wise signal intensities were ratio normalized to the whole-brain global mean.
Preprocessed data were analyzed using second-level random effects models that account for both scan-to-scan and participant-to-participant variability, to determine task-specific regional responses. These group level analyses were conducted in SPM8. Because a priori hypotheses concerned the role of social reward, analyses focused on the acceptance outcome trials. For each participant and scan, predetermined condition effects at each voxel were calculated using a t statistic, producing a statistical image for the contrast of activation during acceptance > activation during the motor control task. For describing overall patterns of task-related activation, we examined whole-brain analyses with thresholds for tests of significance at p < 0.01, uncorrected, with 50 contiguous voxels. For comparisons of HR and LR youth, we examined whole-brain analyses with thresholds for tests of significance at p < 0.05, uncorrected, with 50 contiguous voxels. Whole-brain analyses were based on a total volume of 112,309 voxels.
Results
Subjective report differences
Table 1 displays comparisons between HR and LR youth on youth self- and parent-reports of individual difference characteristics. These analyses revealed that HR and LR youth were not distinguished on any youth self-report measures, including current self-reported depressive symptoms. However, comparisons of parent-reports revealed that HR youth demonstrated significantly lower levels of affiliation than LR youth. As group differences revealed via maternal reports could be the result of the influence of mood state effects, we further examined this issue by examining HR versus LR differences in maternal ratings of youth affiliation after controlling for maternal current depressive symptoms. These analyses revealed that HR youth continued to be rated as lower on affiliation (F[1,46] = 5.29, p < 0.05) than LR youth after controlling for maternal current depression. Groups did not differ in pleasure/surgency and shyness. We also present interinformant associations among these individual difference characteristics, and found convergence for parent and youth reports of youth surgency, but not other dimensions, and also found that parent reports of surgency were associated with youth reports of depressive symptoms.
Table 1.
Comparisons of High-Risk (HR) and Low-Risk (LR) Youth on Self and Parent Reports
| Youth self-reports | Parental reports | |||||
|---|---|---|---|---|---|---|
| HR | LR | t | HR | LR | t | |
| Affiliation | 19.92 (6.36) | 20.68 (4.31) | 0.47 | 16.24 (4.1) | 18.74 (3.6) | 2.17* |
| Surgency | 26.13 (8.93) | 28.35 (7.04) | 0.92 | 21.12 (5.18) | 23.21 (5.36) | 1.29 |
| Shyness | 11.64 (4.44) | 12.46 (4.93) | 0.55 | 6.59 (4.73) | 6.31 (4.24) | 0.21 |
| MFQ | 9.77 (2.49) | 6.21 (1.37) | −1.36 | |||
| Youth self-reports | ||||
|---|---|---|---|---|
| Affiliation | Surgency | Shyness | MFQ | |
| Parent reports | 0.13 | 0.05 | 0.08 | −0.20 |
| Affiliation | 0.05 | 0.44** | −0.18 | −0.34* |
| Surgency | −0.25 | 0.01 | 0.20 | 0.02 |
| Shyness | ||||
Affiliation, Depressed Mood, Surgency, and Shyness were all assessed using the Early Adolescent Temperament Questionnaire with reports provided by youth and parents. Table entries for the top panel are means (standard deviations). Comparisons are based on all available data regardless of the presence of imaging data. Youth self-reports were available for 14 high-risk and, depending upon the measure, 25–26 low-risk cases. Parental reports were available for 15 high-risk and 27 low-risk cases.
p<.05; **p<.01.
MFQ, Mood and Feelings Questionnaire (continuous measure of depressive symptomatology).
Chatroom behavior differences
We examined differences in reaction times to button presses for being accepted versus being rejected. A paired t test revealed that youth pressed buttons to indicate that they were accepted faster than to indicate that they were rejected (mean = 947.79 ms, SD =290.57 ms for acceptance and mean = 1089.44 ms, SD = 474.29 ms for rejection, t = 2.44, p < 0.05). We further tested whether HR and LR youth differed in reaction times for either acceptance or rejection. However, no group differences were found (t = 0.67 for acceptance and t = 0.47 for rejection). Similarly, no significant HR–LR differences were found on any posttask ratings (all ps > 0.10).
Whole-brain chatroom BOLD response
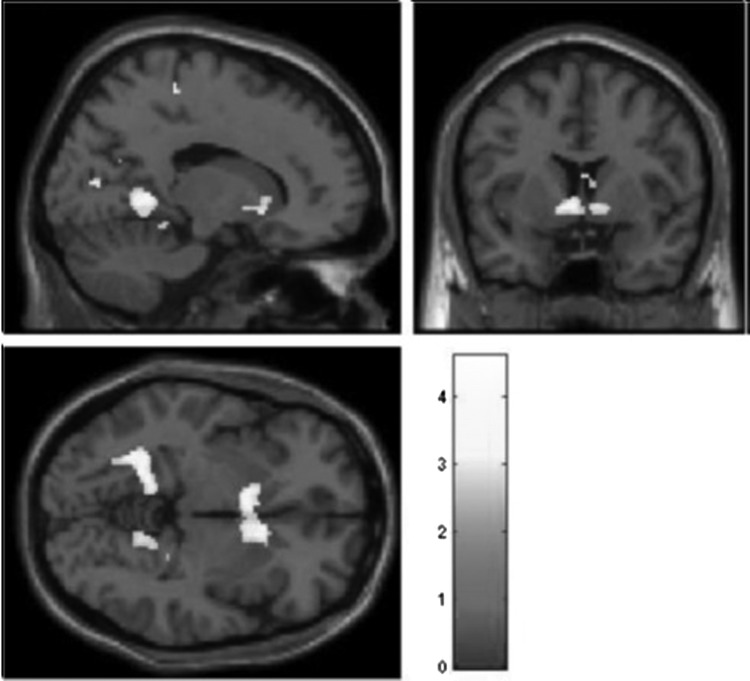
First, we estimated a one sample t test to determine whether the chatroom task elicited response in reward-related regions. As displayed in Table 2 and Figure 1, task-related response was found in the VS (including the caudate head), cuneus, insula, and superior temporal gyrus.
Table 2.
Task-Related Activation for Social Reward (> Control Task)
| Coordinates | |||||
|---|---|---|---|---|---|
| Anatomical region | Cluster sizekE | x | y | z | Statistic t |
| Cuneus | 211 | −20 | −84 | 26 | 4.61 |
| Insula | 444 | 40 | −18 | 19 | 4.11 |
| Parahippocampal gyrus | 413 | −22 | −50 | 4 | 4.06 |
| Parahippocampal gyrus | 460 | 16 | −48 | 4 | 3.74 |
| Precuneus and paracentral lobule | 79 | −8 | −32 | 59 | 3.73 |
| Precentral and postcentral gyrus | 218 | 18 | −29 | 56 | 3.61 |
| Caudate body | 65 | 2 | 10 | 12 | 3.57 |
| Caudate head | 380 | 12 | 13 | −4 | 3.45 |
| Pre-/postcentral gyrus | 120 | −49 | −8 | 32 | 3.28 |
| Calcarine/posterior cingulate | 99 | 9 | −65 | 14 | 3.22 |
| Superior temporal gyrus | 64 | −50 | −15 | 6 | 3.09 |
| Superior temporal gyrus/insula | 65 | −34 | −36 | 16 | 2.91 |
| Precentral gyrus | 65 | 45 | −16 | 32 | 2.91 |
Results are for whole-brain analyses with puncorrected < 0.01 with a minimum threshold of 50 contiguous voxels. t statistics refer to the peak t for the cluster.
FIG. 1.
Task related activation for acceptance > control. Threshold is set at p < 0.01, uncorrected, with a contiguous threshold of 50 clusters. The image is centered on Talairach coordinates 16 6 −4. The full set of clusters exceeding this threshold is displayed in Table 2.
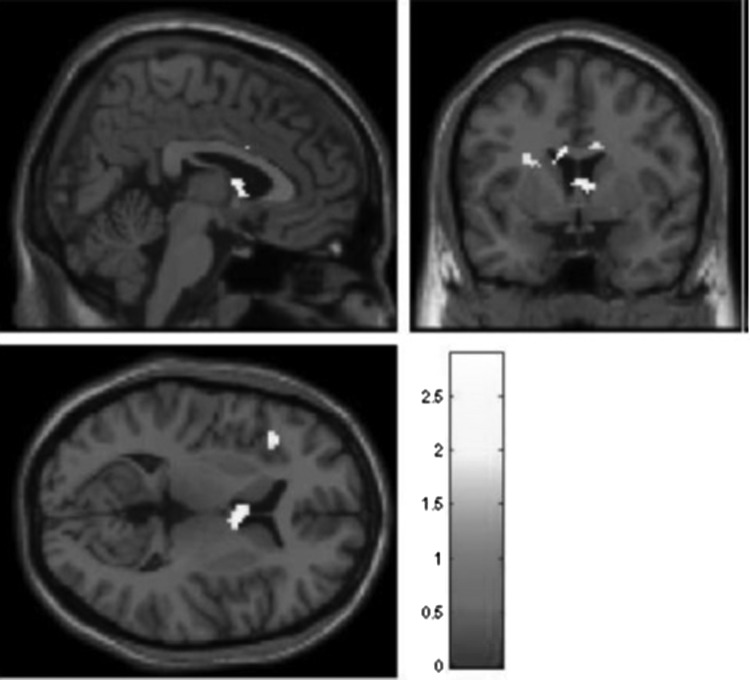
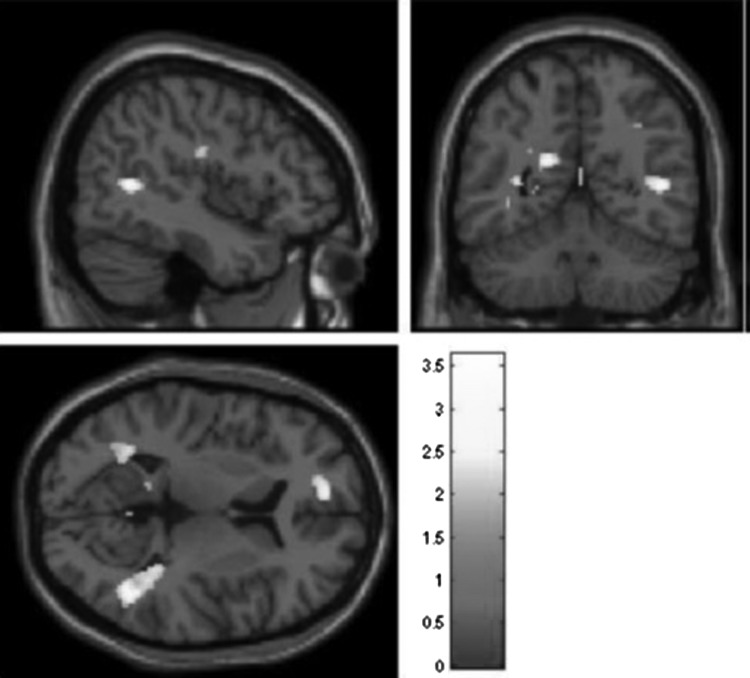
Next, we compared HR and LR youth on neural response to social acceptance. We estimated directional t tests focusing on regions where HR youth demonstrated lower response than LR youth. Whole-brain analyses revealed that HR youth demonstrated reduced response in nine independent clusters, spanning the VS, inferior frontal gyrus, and ACC (Table 3, top panel and Fig. 2). We then estimated directional t tests focusing on regions where HR youth demonstrated a higher response than LR youth. These analyses revealed that HR youth demonstrated increased response in 12 clusters, spanning the precuneus, cuneus, superior temporal gyrus, middle temporal gyrus, middle frontal gyrus, and precentral gyrus (Table 3, bottom panel and Fig. 3). Analyses were repeated controlling for 1) developmental status and 2) youth self-reported depressive symptoms, and results were substantively identical.
Table 3.
Regions Demonstrating Differences Between High-Risk (HR) and Low-Risk (LR) Youth for Social Reward
| Coordinates | |||||
|---|---|---|---|---|---|
| Anatomical region | Cluster sizekE | x | y | z | Statistic t |
| HR < LR | |||||
| Inferior frontal gyrus/insula/caudate | 304 | −26 | 18 | 12 | 2.89 |
| ACC | 62 | 8 | 11 | 25 | 2.88 |
| ACC | 60 | 20 | 28 | 21 | 2.72 |
| ACC/middle frontal gyrus/BA32 | 107 | −12 | 32 | 22 | 2.51 |
| Caudate head | 86 | 4 | 4 | 7 | 2.36 |
| HR > LR | |||||
|---|---|---|---|---|---|
| Precuneus | 555 | 20 | −60 | 38 | 3.63 |
| Lateral globus pallidus | 67 | 20 | −8 | −3 | 3.54 |
| Superior and middle temporal Gyrus (/temperoparietal junction) | 340 | 45 | −52 | 10 | 3.16 |
| Precuneus/cuneus | 417 | −16 | −50 | 21 | 2.79 |
| Middle frontal gyrus/BA10 | 82 | −16 | 47 | 7 | 2.76 |
| Postcentral gyrus | 230 | 55 | −14 | 21 | 2.63 |
| Precuneus/calcarine | 144 | 8 | −61 | 18 | 2.62 |
| Precentral and postcentral gyrus | 81 | −32 | −15 | 43 | 2.42 |
| Inferior parietal lobule | 80 | −26 | −45 | 41 | 2.40 |
| Precuneus | 58 | −20 | −62 | 33 | 2.26 |
| Postcentral gyrus | 74 | 28 | −39 | 44 | 2.26 |
| Postcentral gyrus | 83 | 38 | −27 | 47 | 2.18 |
Contrast is social reward (i.e., acceptance) > activation during the control task. Results are for whole-brain analyses with puncorrected < 0.05 with a minimum threshold of 50 contiguous voxels. t statistics refer to the peak t for the cluster.
BA, Brodmann Area; ACC, anterior cingulate cortex.
FIG. 2.
Group differences in acceptance > control for high-risk < low-risk youth. Threshold is set at p < 0.05, uncorrected, with a contiguous threshold of 50 clusters. The image is centered on Talairach coordinates 4 4 7. The full set of clusters exceeding this threshold is in Table 3, Top Panel.
FIG. 3.
Group differences in acceptance > control for high-risk > low-risk youth. Threshold is set at p < 0.05, uncorrected, with a contiguous threshold of 50 clusters. The image is centered on Talairach coordinates 45 −52 10. The full set of clusters exceeding this threshold is displayed in Table 3, Bottom Panel.
Parent-reported youth affiliation and chatroom response
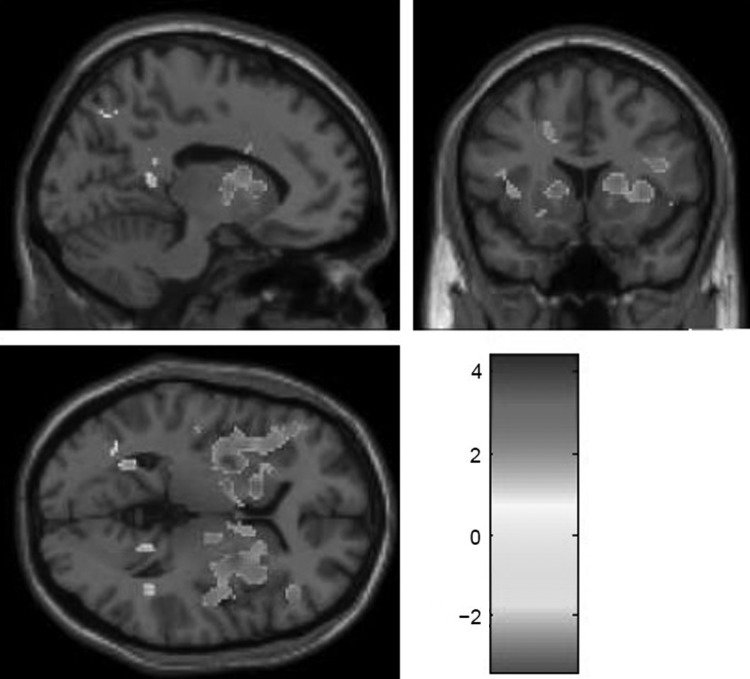
As parental reports of youth affiliation differed between youth at high and those at low familial risk for depression, we examined whether parental report of affiliation was similarly positively associated with response to acceptance during the chatroom task. We found that higher levels of affiliation were associated with greater response in the striatum (including both caudate and putamen), insula, inferior and middle temporal gyri, and BA 13, and reduced response in the precuneus, superior temporal gyrus, and middle temporal gyrus (Fig. 4 and Table 4 for full results). When analyses were conducted that simultaneously included both risk status and affiliation, whole-brain analyses found that, after controlling for parental report of youth affiliation, HR youth continued to demonstrate reduced activation in the insula and ACC/middle frontal gyrus and increased activation in the lateral globus pallidus, precuneus, inferior parietal lobule, postcentral gyrus, and temperoparietal junction relative to LR youth. Also, controlling for risk status, affiliation was positively associated with activation in the striatum, anterior cingulate, inferior frontal gyrus, middle frontal gyrus, and insula, and negatively associated with activation in the middle temporal gyrus and precuneus. These results suggested that risk status differences in the VS were accounted for youth individual differences in affiliation.
FIG. 4.
Associations between parent-reported youth affiliation and activation for acceptance > control. Threshold is set at p < 0.05, uncorrected, with a contiguous threshold of 50 clusters. Orange-red shadings indicate positive associations and blue shadings indicate negative associations. The image is centered on Talairach coordinates −12 14 3. The full set of clusters exceeding this threshold is displayed in Table 4.
Table 4.
Regions Demonstrating Associations Between Parent-Reported Youth Affiliation and Response to Social Reward (> Control Task)
| Coordinates | |||||
|---|---|---|---|---|---|
| Anatomical region | Cluster sizekE | x | y | z | Statistic t |
| Positive associations | |||||
| Insula/putamen/caudate/inferior frontal Gyrus/BA 13 | 1961 | −26 | 0 | 4 | 4.39 |
| Putamen/insula/caudate/anterior cingulate | 1897 | 26 | 2 | 4 | 3.72 |
| Superior/middle frontal gyrus | 125 | 26 | 55 | 16 | 3.32 |
| BA 13 | 96 | 40 | 14 | 18 | 2.76 |
| Inferior frontal gyrus | 127 | 45 | 31 | 4 | 2.66 |
| Middle frontal gyrus | 64 | 16 | −13 | 52 | 2.59 |
| Putamen | 56 | −28 | 7 | −5 | 2.30 |
| Insula/BA 13 | 77 | 38 | −3 | 15 | 2.22 |
| Negative associations | |||||
| Precuneus/BA7 | 321 | 24 | −66 | 40 | 3.28 |
| Precuneus/calcarine | 83 | 18 | −46 | 13 | 2.92 |
| Superior and middle temporal gyrus (Temperoparietal junction) | 155 | 38 | −52 | 12 | 2.89 |
| Posterior cingulate/precuneus | 199 | 4 | −42 | 11 | 2.89 |
| Precuneus/calcarine | 115 | −26 | −56 | 8 | 2.76 |
| Middle temporal gyrus | 60 | −51 | −47 | 1 | 2.75 |
| Middle occipital gyrus | 73 | −32 | −64 | 3 | 2.51 |
| Precuneus/BA7 | 58 | −20 | −62 | 51 | 2.06 |
Results are for whole-brain analyses with puncorrected < 0.05 with a minimum threshold of 50 contiguous voxels. t statistics refer to the peak t for the cluster.
BA, Brodmann Area; ACC, anterior cingulate cortex.
Exploratory analysis
We estimated risk group differences for the specific contrast of acceptance > rejection to determine whether regional activation differences were specific to acceptance or social feedback in general. In these analyses, we found a number of regions that were less strongly responsive for HR youth (relative to LR youth) for the contrast of acceptance – reject that were consonant with the findings for the acceptance – control contrast, particularly the ACC. However, a number of additional regions demonstrated differences in activation, including the precuneus, thalamus, superior temporal gyrus, middle temporal gyrus, and middle frontal gyrus. HR (relative to LR) youth showed greater activation for the contrast of acceptance versus rejection in the parahippocampal gyrus and middle frontal gyrus. Full results are available from T.M.O.
Region of interest (ROI) analysis
A major motivation of this work was to examine whether VS activation differed among HR and LR youth on social acceptance. Therefore, we conducted an ROI analysis using a sphere with a 20 mm radius, centered on Talairach coordinates x = 0, y = 10, z = −10, encompassing the entire bilateral VS and adjacent regions of the caudate. Using AlphaSim, we estimated a minimum contiguity threshold of 49 voxels for a corrected α of 0.05. In this analysis, we found the same exact VS cluster that differed between youth at high and low familial risk for depression (kE = 86, 4 4 7, t = 2.36, p < 0.05). These group differences persisted when controlling for youth self-reported depressive symptoms. Parallel ROI analyses were conducted including parent-reported youth affiliation as a predictor of VS activation. Two clusters within the VS mask (kE = 295, −14 −2 6, t = 3.52, p < 0.01 and kE = 182, −14 −2 6, t = 3.52, p < 0.01) were associated with parent-report affiliation. When both risk status and affiliation were included in the model simultaneously focusing on activation in the ROI, we found no significant differences in VS activation between groups; however, parent-reported youth affiliation continued to predict VS activation.
Discussion
Reward responsiveness is altered in depression, and a small number of studies have examined whether differences in reward responsiveness are present in individuals at risk for, but who do not have a history of depressive disorders. Previous studies primarily relied on monetary rewards. However, social incentives may be particularly important during adolescence and in the context of depression (Badcock and Allen, 2003; Crone and Dahl, 2012). Therefore, we examined differences between youth at high and those at low risk for depression using an active social reward paradigm. We also examined whether HR and LR youth differed on subjective reports of reward-seeking behavior. We found that HR and LR youth differed on neural response to social rewards and parental reports of affiliation; however, HR and LR youth did not differ on youth self-reports of affiliation. We also found that neural response to social reward was associated with parental reports of affiliation. Finally, we found that group differences in activation in the VS were better accounted for by parent reports of affiliation.
Our finding that HR and LR youth differed on neural response to social rewards extends the previous findings of differences in neural response to other types of rewards, including money and food (Gotlib et al. 2010; McCabe et al. 2012; Olino et al. 2014). Monk et al. (2008) found that HR youth demonstrated reduced striatal response compared with LR youth when passively viewing happy faces, which are often conceptualized as rewarding stimuli. However, the present study further extends this work by making the task more active and socially engaging. Attenuated striatal response is thought to be associated with diminished subjective reports of positive affect. In addition to attenuated striatal response during social rewards, we also found that HR youth demonstrated attenuated ACC and middle frontal gyrus response. This is consistent with recent meta-analytic findings of attenuated response in each of these areas in depressed, relative to nondepressed participants (Zhang et al. 2013). Taken together, these findings contribute to the emerging data that reduced reward function is present in individuals at risk for depression before they have developed a disorder.
In concert with the data on neural response, we also found that some parental reports, but not youth self-reports, of reward-relevant behaviors differed between HR and LR youth. Differences in parental report were found on the affiliation scales. The affiliation scale contains content highly relevant to the social reward task, such as having preferences for participating in social activities and being gregarious. Therefore, there is convergence in findings between neural response and parental reports of affiliation. However, we did not find significant differences in reports of high-intensity pleasure, which may reflect a more global approach motivational tendency. If a global attenuation to rewards had been present, differences between HR and LR youth should have been found on affiliation and high-intensity pleasure. The pattern of our results may suggest that attenuated interest in or seeking out of social rewards might be key in distinguishing between HR and LR youth. However, this may be limited to parental reports of social rewards because, as previously noted, youth neural response to an assortment of rewards differs between HR and LR youth. No differences were found on parent report or youth self-reports of shyness. Shyness is also characterized by low social engagement; however, shyness has been more linked to fear-based systems than to approach motivation systems (Coplan et al .2004; Fox et al. 2005). The pattern of findings is suggestive that youth at risk for depression have attenuated motivation for (vs. heightened fear of) social rewards. However, our initial examinations of risk group differences did not suggest that VS differences were more strongly present for acceptance versus rejection (see Exploratory Analysis section). Nonetheless, this is an important area of future work.
In addition to the finding that HR youth demonstrated attenuated reward function relative to LR youth, we found that HR youth demonstrated heightened response in regions associated with social information processing (or theory of mind) than LR youth, including the superior and middle temporal gyrus, middle frontal gyrus, and precuneus (Northoff and Bermpohl 2004; Northoff et al. 2006). These regions are of central importance in a number of self-relevant networks, including the default mode network (Qin and Northoff, 2011; Whitfield-Gabrieli and Ford 2012) and social cognition (Schilbach et al. 2012). Therefore, our findings suggest that HR youth are engaging in more effort to understand the selections made by their “peers.” Further work is necessary to understand this difference and which personality (e.g., neuroticism) or cognitive factors (e.g., rumination) may best account for these associations.
Finally, as we found differences between HR and LR youth on neural response to acceptance and parent reports of youth affiliation, we also examined the associations between these outcomes. Parent report of youth affiliation was associated with response to neural response to acceptance, and this included several regions that are critical for reward function, in particular the striatum, and for social information processing, including the temporoparietal junction. Therefore, parent reports demonstrate predictive value for youth neural response to social reward. Youth anticipation of, interest in, and reactions to social activities might be quite observable to parents. Therefore, parents may be able to recognize the strength of social reinforcers for their children. This may be manifest by parents being needed to facilitate social engagement during adolescence (e.g., providing transportation) or by having discussions about social relationships with their children. Therefore, parents may have good evidence to make their ratings of youth behavior, whereas youth reports may be influenced by ideals or self-presentation biases.
Interestingly, our observed group differences in VS response in particular were nonsignificant in both liberal whole-brain and more conservative ROI analyses after controlling for parent-report affiliation. This suggests that youth behavior may be a more proximal marker of risk than family history of depressive disorder. Again, as this study was modest in size, additional future work is needed to replicate these results.
The study had a number of strengths. We assessed social rewards in multiple ways from multiple informants, we focused on offspring of parents with chronic and/or recurrent depression as a means of reducing heterogeneity in risk, and we studied youth without a personal history of affective disorders. We also examined associations between conceptually similar indicators of risk across multiple units of analysis. However, there were a number of limitations. First, the sample was small. Because of limited power, we relied on liberal thresholds for our comparisons. Despite the small sample, the results were consistent with previously published findings. However, replication of these findings with larger samples and more stringent thresholds is necessary. Second, the sample spanned youth 10–16 years of age. Therefore, we could not conduct fine-grained analyses of developmental changes in social reward responsiveness. Future work in this area is necessary. Third, although we restricted heterogeneity in presentation of maternal depression, we included mothers who had comorbid diagnoses. Therefore, it is not clear if our findings are specific to parental history of depression or if additional anxiety and/or substance use disorders are implicated in attenuated response to social rewards. Fourth, along similar lines, we focused primarily on maternal psychopathology. The influence of paternal psychopathology may also be influential on youth brain function. However, we had data on too few fathers to empirically examine this issue. Fifth, whereas similar differences were found for parental reports and neural response, youth self-reports did not demonstrate similar patterns of results. It is possible that parental biases may have influenced their ratings of youth behavior, as opposed to reflecting true differences in offspring. However, we repeated analyses after controlling for maternal state depression scores and found that the differences in affiliation persisted. Therefore, we have some evidence against this possible confound. Further, in light of concerns in the developmental literature on the validity of parent reports for many domains of youth behavior and experience (e.g., Najman et al. 2001), it is quite impressive that maternal reports were associated with youth brain function. However, with a small sample, we cannot rule out that we did not have enough power to detect differences. Finally, there may be important differences between anticipation and receipt of rewards or acceptance. However, our task included a general anticipation period that could not discriminate between anticipation of acceptance or anticipation of rejection.
Conclusions
Overall, the present study found support for attenuated VS response to social rewards and augmented social information processing response in youth at risk for depression using fMRI methods. However, these group differences in neural function were better accounted for by individual differences in affiliation. Future work is needed to examine whether these indices are associated with the development of depressive disorders or symptoms. Similarly, it is also crucial to compare utility of neural measures to traditional self-report and family history measures to determine which predictor or which combination of predictors is best able to predict adverse outcomes.
Clinical Significance
Offspring of depressed parents demonstrated reduced reward-related brain function when receiving acceptance feedback, and were reported to be less affiliative than offspring of parents without depression. These results suggest that attenuated response to social reward may be an etiological factor in the development of depression, and may represent a novel target for prevention.
Disclosures
No competing financial interests exist.
References
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013 [Google Scholar]
- Angold A, Costello EJ, Messer SC, Pickles A: Development of a short questionnaire for use in epidemiological studies of depression in children and adolescents. Int J Methods Psychiatr Res 5:237–249, 1995 [Google Scholar]
- Badcock PB, Allen NB: Adaptive social reasoning in depressed mood and depressive vulnerability. Cogn Emot 17:647–670, 2003 [DOI] [PubMed] [Google Scholar]
- Coplan RJ, Prakash K, O'Neil K, Armer M: Do you “want” to play? Distinguishing between conflicted shyness and social disinterest in early childhood. Dev Psychol 40:244–258, 2004 [DOI] [PubMed] [Google Scholar]
- Crone EA, Dahl RE: Understanding adolescence as a period of social–affective engagement and goal flexibility. Nat Rev Neurosci 13:636–650, 2012 [DOI] [PubMed] [Google Scholar]
- Davey CG, Yücel M, Allen NB: The emergence of depression in adolescence: Development of the prefrontal cortex and the representation of reward. Neurosci Biobehav Rev 32:1–19, 2008 [DOI] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A: The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci 35:68–77, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis LK, Rothbart MK: Revision of the Early Adolescent Temperament Questionnaire. Paper presented at the Biennial Meeting of the Society for Research in Child Development, Minneapolis, Minnesota, 2001 [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW: The Structured Clinical Interview for DSM-IV Axis I Disorders – Non-patient Edition. New York: Biometrics Research Department, New York State Psychiatric Institute; 1996 [Google Scholar]
- Forbes EE: Where's the fun in that? Broadening the focus on reward function in depression. Biol Psychiatry 66:199–200, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM: Behavioral inhibition: Linking biology and behavior within a developmental framework. Ann Rev Psychol 56:235–262, 2005 [DOI] [PubMed] [Google Scholar]
- Goodman SH, Rouse MH, Connell AM, Broth MR, Hall CM, Heyward D: Maternal depression and child psychopathology: A meta-analytic review. Clin Child Fam Psychol Rev 14:1–27, 2011 [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Hamilton JP, Cooney RE, Singh MK, Henry M L, Joormann J: Neural processing of reward and loss in girls at risk for major depression. Arch Gen Psychiatry 67:380–387, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Lau JY, McClure–Tone EB, Parrish J, Shiffrin ND, Reynolds RC, Chen G, Blair RJ, Leibenluft E, Fox NA, Ernst M, Pine DS, Nelson EE: Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Arch Gen Psychiatry 65:1303–1312, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS: Discovering endophenotypes for major depression. Neuropsychopharmacology 29:1765–1781, 2004 [DOI] [PubMed] [Google Scholar]
- Hasler G, Northoff G: Discovering imaging endophenotypes for major depression. Mol Psychiatry 16:604–619, 2011 [DOI] [PubMed] [Google Scholar]
- Kasch KL, Rottenberg J, Arnow BA, Gotlib IH: Behavioral activation and inhibition systems and the severity and course of depression. J Abnorm Psychol 111:589–597, 2002 [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N: Schedule for Affective Disorders and Schizophrenia for School-Age Children Present and Lifetime version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36:980–988, 1997 [DOI] [PubMed] [Google Scholar]
- Klein DN, Glenn CR, Kosty DB., Seeley JR, Rohde P, Lewinsohn PM: Predictors of first lifetime onset of major depressive disorder in young adulthood. J Abnorm Psychol 122:1–6, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DN, Lewinsohn PM, Rohde P, Seeley JR, Olino TM: Psychopathology in the adolescent and young adult offspring of a community sample of mothers and fathers with major depression. Psychol Med 35:353–365, 2005 [DOI] [PubMed] [Google Scholar]
- Lieb R, Isensee B, Hofler M, Pfister H, Wittchen HU: Parental major depression and the risk of depression and other mental disorders in offspring: A prospective-longitudinal community study. Arch Gen Psychiatry 59:365–374, 2002 [DOI] [PubMed] [Google Scholar]
- McCabe C, Woffindale C, Harmer CJ, Cowen PJ: Neural processing of reward and punishment in young people at increased familial risk of depression. Biol Psychiatry 72:588–594, 2012 [DOI] [PubMed] [Google Scholar]
- McMakin DL, Olino TM, Porta G, Dietz LJ, Emslie G, Clarke G., Wagner KD, Asarnow JR, Ryan ND, Birmaher B, Shamseddeen W, Mayes T, Kennard B,Spirito A, Keller M, Lynch FL, Dickerson JF, Brent DA: Anhedonia predicts poorer recovery among youth with selective serotonin reuptake inhibitor treatment–resistant depression. J Am Acad Child Adolesc Psychiatry 51:404–411, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Klein RG, Telzer EH, Schroth EA, Mannuzza S, Moulton JL, Guardino M, Masten CL, McClure-Tone EB, Fromm S, Blair RJ, Pine DS, Ernst M: Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am J Psychiatry 165:90, 2008 [DOI] [PubMed] [Google Scholar]
- Montague PR, King–Casas B, Cohen JD: Imaging valuation models in human choice. Annu Rev Neurosci 29:417–448, 2006 [DOI] [PubMed] [Google Scholar]
- Najman JM, Williams GM, Nikles J, Spence S, Bor W, O'Callaghan M, Le Brocque R, Andersen MJ, Shuttlewood G: Bias influencing maternal reports of child behaviour and emotional state. Soc Psychiatry Psychiatr Epidemiol 36:186–194, 2001 [DOI] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F: Cortical midline structures and the self. Trends Cogn Sci 8:102–107, 2004 [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J: Self-referential processing in our brain—a meta-analysis of imaging studies on the self. Neuroimage 31:440–457, 2006 [DOI] [PubMed] [Google Scholar]
- Olino TM, McMakin DL, Morgan JK, Silk JS, Birmaher B, Axelson DA, Williamson DE, Dahl RE, Ryan ND, Forbes EE: Reduced reward anticipation in youth at high-risk for unipolar depression: A preliminary study. Dev Cogn Neurosci 8:55–64, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A: A self-report measure of pubertal status: Reliability, validity, and initial norms. J Youth Adolesc 17:117–133, 1988 [DOI] [PubMed] [Google Scholar]
- Pilkonis PA, Choi SW, Reise SP, Stover AM, Riley WT, Cella D. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS®): Depression, Anxiety, and Anger. Assessment 18:263–283, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS, Cohen E, Cohen P, Brook J: Adolescent depressive symptoms as predictors of adult depression: moodiness or mood disorder? Am J Psychiatry 156:133, 1999 [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Dougherty DD, Iosifescu DV, Rauch SL, Fava M. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry 166:702–710, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P, Northoff G: How is our self related to midline regions and the default-mode network? Neuroimage 57:1221–1233, 2011 [DOI] [PubMed] [Google Scholar]
- Reise SP, Horan WP, Blanchard JJ: The challenges of fitting an item response theory model to the Social Anhedonia Scale. J Pers Assess 93:213–224, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach L, Bzdok D, Timmermans B, Fox PT, Laird AR, Vogeley K, Eickhoff S: Introspective minds: using ALE meta-analyses to study commonalities in the neural correlates of emotional processing, social & unconstrained cognition. PloS one 7:e30920, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Siegle GJ, Lee KH, Nelson EE, Stroud LR, Dahl RE: Increased neural response to peer rejection associated with adolescent depression and pubertal development. Soc Cogn Affect Neurosci 9:1798–1807, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Stroud LR, Siegle GJ, Dahl RE, Lee KH, Nelson E E: Peer acceptance and rejection through the eyes of youth: Pupillary, eyetracking and ecological data from the Chatroom Interact task. Soc Cogn Affect Neurosci 7:93–105, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker J, Dillon DG, Pizzagalli DA: The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: Integration of resting EEG, fMRI, and volumetric techniques. NeuroImage 46:327–337, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA: Extraversion and its positive emotional core. In: Handbook of Personality Psychology. San Diego: Academic Press; 1997; pp. 767–793 [Google Scholar]
- Watson D, Clark LA, Harkness AR: Structures of personality and their relevance to psychopathology. J Abnorm Psychol 103:18–31, 1994 [PubMed] [Google Scholar]
- Whitfield–Gabrieli S, Ford JM: Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol 8:49–76, 2012 [DOI] [PubMed] [Google Scholar]
- Zhang WN, Chang SH, Guo LY, Zhang KL, Wang J: The neural correlates of reward-related processing in major depressive disorder: A meta-analysis of functional magnetic resonance imaging studies. J Affect Disord 151:531–539, 2013 [DOI] [PubMed] [Google Scholar]