Abstract
Computational biology and ‘omics’ systems sciences are greatly impacting research on common diseases such as cancer. By contrast, dermatology covering an array of skin diseases with high prevalence in society, has received relatively less attention from ‘omics’ and computational biosciences. We are focusing on psoriasis, a common and debilitating autoimmune disease involving skin and joints. Using computational systems biology and reconstruction, topological, modular, and a novel correlational analyses (based on fold changes) of biological and transcriptional regulatory networks, we analyzed and integrated data from a total of twelve studies from the Gene Expression Omnibus (sample size = 534). Samples represented a comprehensive continuum from lesional and nonlesional skin, as well as bone marrow and dermal mesenchymal stem cells. We identified and propose here a JAK/STAT signaling pathway significant for psoriasis. Importantly, cytokines, interferon-stimulated genes, antimicrobial peptides, among other proteins, were involved in intrinsic parts of the proposed pathway. Several biomarker and therapeutic candidates such as SUB1 are discussed for future experimental studies. The integrative systems biology approach presented here illustrates a comprehensive perspective on the molecular basis of psoriasis. This also attests to the promise of systems biology research in skin diseases, with psoriasis as a systemic component. The present study reports, to the best of our knowledge, the largest set of microarray datasets on psoriasis, to offer new insights into the disease mechanisms with a proposal of a disease pathway. We call for greater computational systems biology research and analyses in dermatology and skin diseases in general.
Introduction
Understanding the dynamics of a complex biological system requires the construction of systems based models. By using these models, researchers can identify problems and also interpret differences between disease and healthy states. Systems biomedicine integrates computational analysis with various data sources such as genomic and proteomic data to decipher complex human diseases and hopefully create a solution for ongoing health issues regarding human diseases to improve a patient's quality of life. Hence, computational biology and ‘omics’ systems sciences are increasingly impacting research on common diseases such as cancer. By contrast, dermatology covering an array of skin diseases, with high prevalence in society, has received relatively less attention from the tools of ‘omics’ and computational biosciences.
High-throughput gene expression profiling (i.e., transcriptomics) technologies permit the identification of disease-related genes by exposing the difference between healthy and disease states. Integration of transcriptomics and protein interaction data helps understanding the disease mechanisms (Pache et al., 2008) as well as evaluating therapeutic intervention (i.e., drug targets) (Bartfai et al., 2012). Moreover, disease genes tend to be highly expressed, have tissue-specific expression patterns, and a higher mutation rate over evolutionary time (Oti and Brunner, 2007). Using these characteristics, several studies have been performed on ‘omics’ platforms to identify, predict, or prioritize disease genes, and were recently reviewed by Sevimoglu and Arga (2014). Additionally, multi-omics resources such as MOPED (Multi-Omics Profiling Expression Database) have provided preprocessed expression data and visualization tools (Montague et al., 2014).
Psoriasis is a complex, autoimmune, multifactorial systemic disease affecting the skin and joints. Genetic components of the immune system and the epidermis play a role, along with environmental factors that trigger or exacerbate the symptoms of the disease. Psoriasis can have a negative impact on a patient's physical and mental well being and correlates with inconsistent response to therapy, resulting in unsuccessful clinical treatments (Perera et al., 2012).
Though various microarray studies, beginning with Oestreicher et al. (2001), have been employed in hopes of detecting disease genes for psoriasis, the gaps in the disease mechanism still exist. In particular, we have not yet understood the initiators, as well as the overall mechanism of the disease. Computational analysis is essential in analyzing the extensive data retrieved from these studies by incorporating the integration of various ‘omics’ platforms to fully represent the underlying mechanism of psoriasis.
Gudjonsson et al. (2009) characterized and compared gene expression in skin from psoriatic patients to identify patterns that are involved in lipid metabolism, innate immunity, and keratinocyte differentiation. Coda et al. (2012) investigated differential gene expression in samples of lesional and nonlesional skin to identify psoriatic disease-associated pathways at the tissue level. Lu et al. (2013) used microarray and transcriptiıonal regulatory data to identify transcription regulation relationships in psoriasis. Recently, Guo et al. (2014) employed microarray-based gene expression profiling, in which three widely used feature selection algorithms were applied to screen the psoriasis-associated features.
Despite the significant findings of psoriatic transcriptomics analyses, sufficient conclusions on the central molecular mechanisms responsible in triggering or inflaming the disease were not attained. A major problem in identifying psoriasis-related genes is figuring out which of these genes are initiators, triggers, or regulators, resulting in various proteins encoded by genes represented in the pool of differentially expressed genes (DEGs). Therefore, trying to determine the source of the problem itself becomes difficult, since there are more than two thousand DEGs that are identified with each experimental analysis.
In the present study, a comprehensive analysis was performed using twelve psoriasis datasets from three different microarray platforms with a holistic point of view. The integration of multiple ‘omics’ databases coupled with a novel correlation approach aids at identifying the disease topology. To this end, a psoriasis pathway and several biomarker candidates are proposed.
Materials and Methods
Gene expression datasets of psoriasis
The raw data of high throughput gene expression datasets associated with psoriasis (Table 1) from a total of 12 studies were obtained from Gene Expression Omnibus (GEO) (Barrett et al., 2013) and analyzed. These datasets were from three different microarray platforms: Affymetrix, Illumina, and Agilent. A total of 534 samples were analyzed. These samples were often taken from skin, in addition to samples from bone marrow (GSE40033) and dermal mesenchymal stem cells (GSE42632). Lesional versus nonlesional samples were analyzed from ten datasets and psoriasis versus normal samples were analyzed for the remaining two (GSE40033 and GSE42632).
Table 1.
Gene Expressions Datasets of Psoriasis Employed in the Present Study
| Dataset No | GEO ID | Sample size | Platform | # of probesets | Description | Reference |
|---|---|---|---|---|---|---|
| 1 | GSE14905 | 82 | Affymetrix Human Genome U133 Plus 2.0 Array | 54675 | Analysis of lesional and nonlesional skins from patients with psoriasis. | Yao et al., 2008 |
| 2 | GSE34248 | 28 | Affymetrix Human Genome U133 Plus 2.0 Array | 54675 | Analysis of lesional and nonlesional skins from patients with psoriasis. | Bigler et al., 2013 |
| 3 | GSE41662 | 48 | Affymetrix Human Genome U133 Plus 2.0 Array | 54675 | Analysis of lesional and nonlesional skins from patients with psoriasis. | Bigler et al., 2013 |
| 4 | GSE30999 | 170 | Affymetrix Human Genome U133 Plus 2.0 Array | 54675 | Analysis of lesional and nonlesional skins from patients with moderate-to-severe psoriasis. | Suárez-Fariñas et al., 2012 |
| 5 | GSE13355 | 180 | Affymetrix Human Genome U133 Plus 2.0 Array | 54675 | Analysis of lesional and nonlesional skins from patients with psoriasis as well as normal skin from control individuals. | Nair et al., 2009 |
| 6 | GSE26866 | 37 | Affymetrix Human Genome U133A 2.0 Array | 22277 | Analysis of paired lesional and nonlesional skins from patients with psoriasis. | Mitsui et al., 2012 |
| 7 | GSE6710 | 26 | Affymetrix Human Genome U133A Array | 22280 | Analysis of lesional and nonlesional skins from patients with plaque-type psoriasis. | Reischl et al., 2007 |
| 8 | GSE40263 | 10 | Affymetrix Human Gene 1.0 ST Array | 32321 | Analysis of skins from patients with psoriasis as well as normal skin from healthy control individuals. | Unpublished data |
| 9 | GSE2737 | 11 | Affymetrix Human Genome U95A Array | 12626 | Analysis of paired lesional and nonlesional skins from patients with psoriasis as well as normal skin of healthy control individuals. | Kulski et al., 2005 |
| 10 | GSE41745 | 6 | Illumina Genome Analyzer IIx (Homo Sapiens) | 33655 | Analysis of paired lesional and nonlesional skins from patients with psoriasis. | Jabbari et al., 2012 |
| 11 | GSE42632 | 12 | Agilent-026652 Whole Human Genome Microarray 4x44K v2 | 28908 | Analysis of dermal mesenchymal stem cells between psoriatic patients and normal adults. | Unpublished data |
| 12 | GSE40033 | 14 | Agilent-028004 SurePrint G3 Human GE 8x60K Microarray | 42405 | Analysis of bone marrow mesenchymal stem cells between psoriatic patients and normal adults. | Unpublished data |
Identification of differentially expressed genes
The previously designed methodology (Karagoz et al., 2015), employing RMA normalization (Irizarry et al., 2003) and linear models for microarray data (LIMMA) methods (Smyth et al., 2004) was followed in statistical analysis of each dataset in order to identify DEGs. DEGs were selected according to computed p values <0.05 and the same p value cut-off values were used for comparison across datasets of all microarray platforms. Up- and downregulation of genes was identified according to fold changes. The DEGs with fold change greater than 1.5 were accepted as upregulated and the DEGs with fold change of less than 0.5 were accepted as downregulated.
Gene–protein associations
To avoid possible ambiguity due to different identifiers employed in separate microarray platforms and for ease of comparison between gene sets, all the identifiers of different platforms and series were converted to ENTREZ identifiers. The gene–protein associations were obtained from UniProt database (Uniprot Consortium, 2014). Some of the genes had multiple probe sets (a total of 182 probesets for 145 DEGs in the present study) owing to splice variants or cross-hybridization. To avoid any confusion, these probesets are named accordingly (for instance, BUB1-1 and BUB1-2).
Gene set enrichment analyses
The gene set enrichment analyses were achieved using DAVID (Huang et al., 2007) with a p value cutoff <0.05 for statistical significance. DAVID tool has options to enrich gene sets according to Genetic Association Database (http://geneticassociationdb.nih.gov/) disease, disease class as well as Gene Ontology terms (molecular function, biological process, and cellular component) and signaling pathways. The Gene Ontology Annotations are obtained from Gene Ontology Consortium (GOC, http://www.geneontology.org). Pathways associated with psoriasis genes were collected from Kyoto Encyclopedia of Genes and Genomes (KEGG) (Kanehisa et al., 2012).
Transcriptional regulatory data (TF–gene associations)
The resources used to identify the regulatory associations between transcription factors (TFs) and their gene targets were: Transcriptional Regulatory Element Database (TRED) (Jiang et al., 2007), GENOMATIX (Genomatix Software Inc, Ann Arbor, MI, USA), and The Human Transcriptional Regulation Interactions database (HTRIdb) (Bovolenta et al., 2012).
Psoriasis associated protein–protein interaction (PPI) networks
The protein–protein interactions were obtained from iRefIndex Database (Razick et al., 2008) and PPI networks were reconstructed around DEGs. The visualization and topological analysis of the PPI network was performed via Cytoscape (Shannon et al., 2003). Hub proteins were identified using the dual-metric approach that simultaneously employs degree and betweenness centrality measures (Karagoz et al., 2015) and Cytohubba plugin (Chin et al., 2014).
Fold change correlation (FCC) analysis
FCC analysis employs fold change (FC) profiles of DEGs instead of expression profiles. In order to obtain a comprehensive list of DEGs for FCC analysis, annotation databases (KEGG, DAVID, and GO) were surveyed to gather genes associated with the core DEGs. Among these, the genes that were in at least five of the datasets as DEGs were also included to the DEG pool along with the core DEGs.
Fold change (FC) values of individual DEGs in each dataset were calculated using their gene expression profiles in order to present a FC profile for each DEG among datasets. These FC profiles were used to check for correlations between selected DEGs utilizing Pearson correlation coefficients (PCC). DEGs with PCC values greater than 0.7 were accepted as positively correlated and with values less than −0.7 were accepted as negatively correlated. A correlation network was formed between DEGs based on these cutoff values. Modules within the correlation network were identified using Cytoscape plugin Clust&See (Spinelli et al., 2013). Hubs of the correlation network as well as each module were identified using Cytohubba.
Results
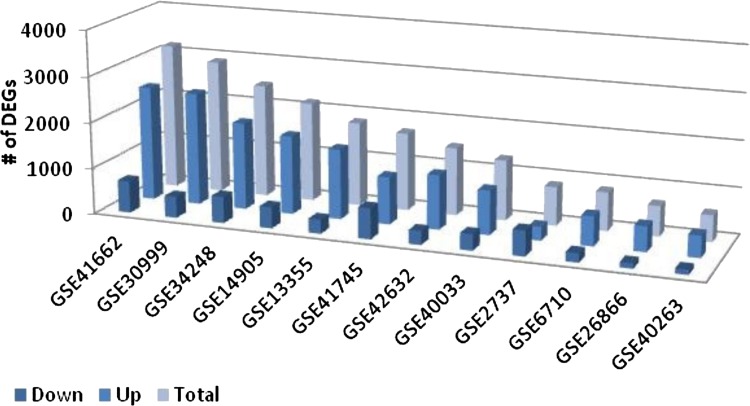
In this study, twelve transcriptome datasets of psoriasis were used with samples from lesional and nonlesional skin, as well as bone marrow and dermal mesenchymal stem cells (Table 1). The number of DEGs across datasets ranges between 572 (GSE40263) and 3184 (GSE41662). In all datasets (except GSE2737), the number of upregulated genes were higher than that of downregulated genes (Fig. 1).
FIG. 1.
The numbers of differentially expressed genes in each dataset employed.
Differentially expressed genes of psoriasis
IFI44, which is an interferon-stimulated gene (ISG) encoding an interferon-induced cytoplasmic protein with antiviral activity, was the sole common DEG among 11 of the 12 datasets. Though its function is still unknown, it is believed to play a role in host defense. Recombinant expression of IFI44 alone is sufficient to inhibit cell proliferation, indicating that it does not require the presence or activity of any additional ISGs (Hallen et al., 2007). It is upregulated with a FC as high as 4.8.
Ten DEGs (IFIT1, OAS2, PI3, STAT1, NMI, TRIM22, RSAD2, WIF1, SUB1, and MAD2L1) were found in ten of the twelve datasets. These DEGs along with IFI44 will be named as “core DEGs” for the rest of this publication since they are commonly identified in at least ten of the twelve datasets. Eight of the core DEGs are cytoplasmic proteins (GO:0005737) except for WIF1 and PI3, which are in the extracellular region, and SUB1, which is located in the nucleus. Six of the DEGs (OAS2, NMI, IFI44, RSAD2, STAT1, and TRIM22) share a common GO Biological Process Term (response to stimulus, GO:0050896), while NMI, SUB1, STAT1, and TRIM22 share the same GO Molecular Function Terms (transcription factor binding, GO:0003712 and transcription co-factor activity, GO:0008134). Six of these core DEGs are ISG's (IFI44, IFIT1, OAS2, STAT1, TRIM22, and RSAD2).
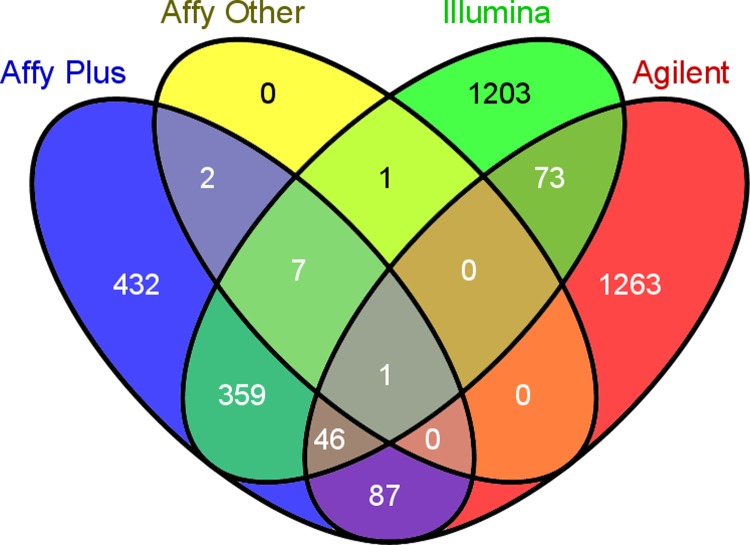
The mutual DEGs across datasets were also analyzed according to the microarray platform employed in the analyses (Fig. 2). The highest number of mutual DEGs (934) was in the five datasets of Affymetrix Human Genome U133 Plus 2.0 Array. This is possibly due to the fact that it is the latest series of Affymetrix platform and contains the highest number of probesets (54675) that covers approximately 20,000 genes.
FIG. 2.
Comparative analysis of DEGs according to microarray platforms employed in the analyses. (“Affy plus”: Affymetrix Human Genome U133 Plus 2.0 Array. “Affy other”: Affymetrix Human Genome arrays other than U133 Plus 2.0.)
Transcription factor–DEG relationship
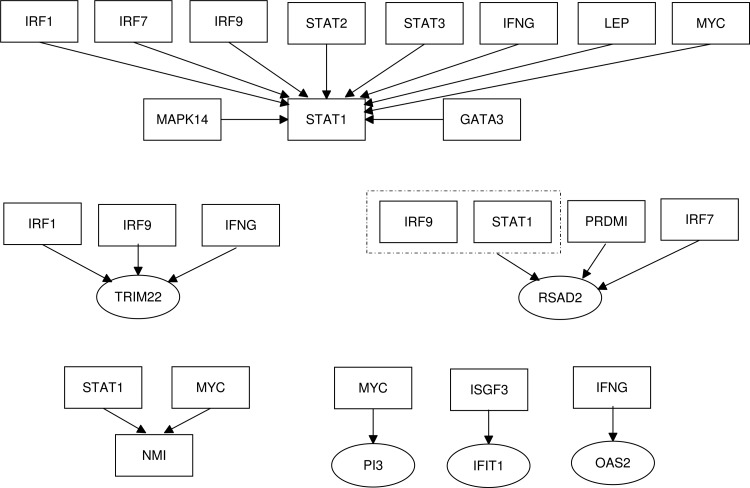
The regulatory relationship between some of the core DEGs and their TFs (which were also differentially expressed in psoriasis datasets) are depicted in Figure 3. Seven of the core DEGs have TFs associated with them. TRIM22, RSAD2, IFIT1, STAT1, and OAS2 are all ISGs, so it not a surprise that they are regulated by either IRFs (Interferon Regulated Factors) or IFNG. Three of the TFs involved in the regulatory relationships with the core DEGs (IRF1, IRF7, and IRF9) are members of the interferon regulatory transcription factor family. They are multifunctional transcription factors that are involved in the regulation of immune cells as well as cell cycle regulation and apoptosis in response to a variety of stimuli (Ning et al., 2011; Schwartz et al., 2011).
FIG. 3.
The transcriptional regulatory modules controlling the core DEGs.
STAT1 and STAT2 associate to form a heterodimer, which in turn recruits IRF9 to form the IFN-stimulated gene factor 3 (ISGF3) complex, which is a TF regulating the expression of IFIT1. These three TFs (STAT1, STAT2, and IRF9) can work together as a complex and also individually to regulate different processes (Fink and Grandvaux, 2013). STAT1 is a major TF by which cytokines induce transcription (Delgoffe and Vignali, 2013). MYC is a TF regulating the expression of STAT1 and NMI as well as expression of PI3 which activates the transcription of growth related genes.
Protein–protein interaction (PPI) networks associated with psoriasis
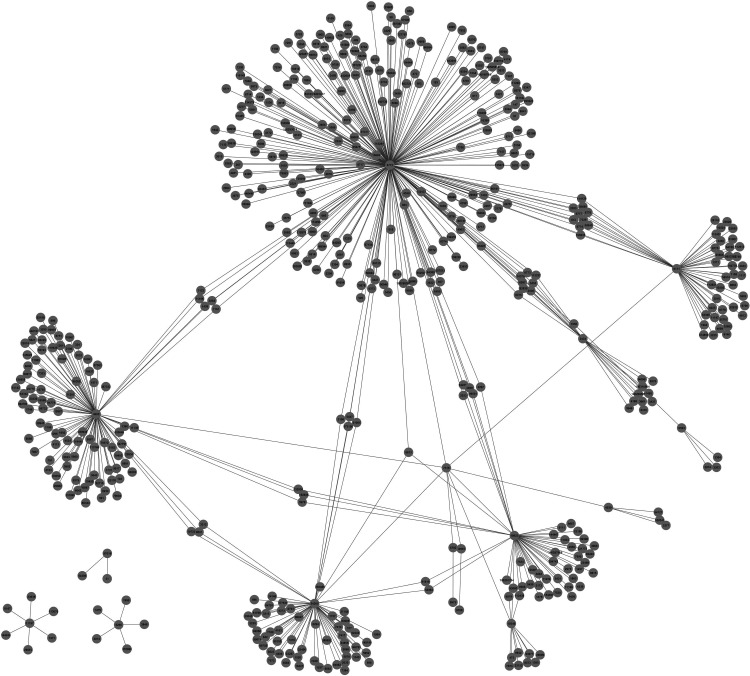
A PPI network associated with psoriasis was reconstructed. The reconstructed PPI network of psoriasis consisted of 576 binary interactions between 534 proteins, which were physically interacting with our core DEGs (Fig. 4). Analyses of the reconstructed psoriasis network identified five proteins as hubs (STAT1, MAD2L1, CYCS, NMI, and SUB1), which exhibit high topological centrality in the network (Table 2). These hub proteins should be considered in future experimental studies as candidate biomarkers or theurapautic targets.
FIG. 4.
PPI Network of core DEGs (with Entrez ID's).
Table 2.
Central Proteins (Hubs) of Reconstructed Psoriasis Network
| Protein symbol | Description | Degree | Betweenness |
|---|---|---|---|
| STAT1 | Signal transducer and activator of Transcription 1 | 266 | 101661.2 |
| MAD2L1 | Mitotic Arrest Deficient-Like 1 | 99 | 41626.4 |
| CYCS | Cytochrome C, somatic | 63 | 26730.1 |
| NMI | N-Myc (and STAT) interactor | 52 | 19275.5 |
| SUB1 (PC4) | SUB1-homolog, Positive Cofactor 4 | 50 | 19114.4 |
Four of the hub proteins of the reconstructed psoriasis network were also in our core DEG list. STAT1 is from the family of STAT (signal transducer and activator of transcription) proteins, which play important roles in cellular processes such as cell growth and differentiation, cell survival and apoptosis, and immune responses. These bind to receptors and function as transcription factors that trigger gene activation (Shuai, 2000). STAT1 is overexpressed with a FC ranging between 1.65 and 3.75.
MAD2L1 (mitotic arrest deficient-like 1) is required for the execution of spindle assembly checkpoint during mitosis to ensure the proper segregation of chromosomes under normal growth conditions (Xu et al., 1997). It is overexpressed with a FC range of 1.87 and 5.5. NMI (N-myc (and STAT) interactor) is a TF that can enhance STAT1-mediated transcription, implicating a broader role for NMI in cytokine signaling (Zhu et al., 1999). The FC for NMI is between 1.65 and 2.37.
SUB1 (also known as PC4: Positive Cofactor 4) plays a dual role in regulation of gene transcription as an activator or repressor and functions in distinct stages of the transcription process (Conesa and Acker, 2010). A possible role of SUB1 in the early response to DNA damage was also proposed by recognizing single-stranded DNA and facilitating the subsequent steps of DNA repair (Mortusewicz et al., 2008). It is upregulated with a FC range of 1.54–3.26. CYCS (Cytochrome c), which was not one of the core DEGs but a DEG in nine of the analyzed datasets, is a component of the electron transport chain of mitochondria (Gonzales and Neupert, 1990). It is involved in cell apoptosis (programmed cell death) (Liu et al., 1996) and in antioxidant defense system of mitochondria (Skulachev, 1998) and is upregulated in psoriasis (FC ranging between 1.54 and 2.85).
Fold change correlation (FCC) analysis of psoriasis
According to the FCC analysis of the core DEGs IFI44, IFIT1, OAS2, STAT1, NMI, TRIM22, and RSAD2 were correlated with each other (Table 3). PI3 was not correlated with any of the other core DEGs. WIF1 was negatively correlated with OAS2.
Table 3.
FCC Analysis Results for Core DEGs*
| IFI44 | IFIT1 | OAS2 | PI3 | STAT1 | NMI | TRIM22 | RSAD2 | WIF1 | SUB1 | MAD2L1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| IFI44 | 1.00 | 0.84 | 0.85 | 0.52 | 0.92 | 0.89 | 0.94 | 0.77 | −0.33 | 0.49 | 0.41 |
| IFIT1 | 0.84 | 1.00 | 0.83 | 0.20 | 0.74 | 0.96 | 0.92 | 0.98 | −0.56 | 0.72 | 0.78 |
| OAS2 | 0.85 | 0.83 | 1.00 | 0.65 | 0.69 | 0.83 | 0.80 | 0.77 | −0.87 | 0.24 | 0.35 |
| PI3 | 0.52 | 0.20 | 0.65 | 1.00 | 0.42 | 0.26 | 0.34 | 0.09 | −0.37 | −0.33 | −0.37 |
| STAT1 | 0.92 | 0.74 | 0.69 | 0.42 | 1.00 | 0.82 | 0.92 | 0.66 | −0.14 | 0.55 | 0.49 |
| NMI | 0.89 | 0.96 | 0.83 | 0.26 | 0.82 | 1.00 | 0.96 | 0.94 | −0.53 | 0.80 | 0.85 |
| TRIM22 | 0.94 | 0.92 | 0.80 | 0.34 | 0.92 | 0.96 | 1.00 | 0.88 | −0.42 | 0.68 | 0.64 |
| RSAD2 | 0.77 | 0.98 | 0.77 | 0.09 | 0.66 | 0.94 | 0.88 | 1.00 | −0.51 | 0.80 | 0.86 |
| WIF1 | −0.33 | −0.56 | −0.87 | −0.37 | −0.14 | −0.53 | −0.42 | −0.51 | 1.00 | −0.12 | 0.04 |
| SUB1 | 0.49 | 0.72 | 0.24 | −0.33 | 0.55 | 0.80 | 0.68 | 0.80 | −0.12 | 1.00 | 0.96 |
| MAD2L1 | 0.41 | 0.78 | 0.35 | −0.37 | 0.49 | 0.85 | 0.64 | 0.86 | 0.04 | 0.96 | 1.00 |
Significant correlations were marked with gray color.
In addition to our core DEGs, a set of 145 genes (182 probesets) was established through a comprehensive literature survey on signaling pathways and biological processes associated with mutual DEGs of psoriasis datasets and central proteins of the reconstructed psoriasis network. FCC analysis was done for this set of genes as well.
A correlation network was reconstructed based on the results of the FCC analysis, which consisted of 182 nodes (representing the probesets) and 4152 edges (representing a significant correlation with Pearson > 0.70). Topological analysis of the network indicated a highly dense, scale-free degree distribution with average connectivity of 45.6. SUB1, IL13RA1, and SOCS1 have the highest number of correlations (86 of the 182 probesets), while DEFB4A has the lowest number of correlations with only four of the DEGs (PI3, CCNB2, UBA6, and LEPR).
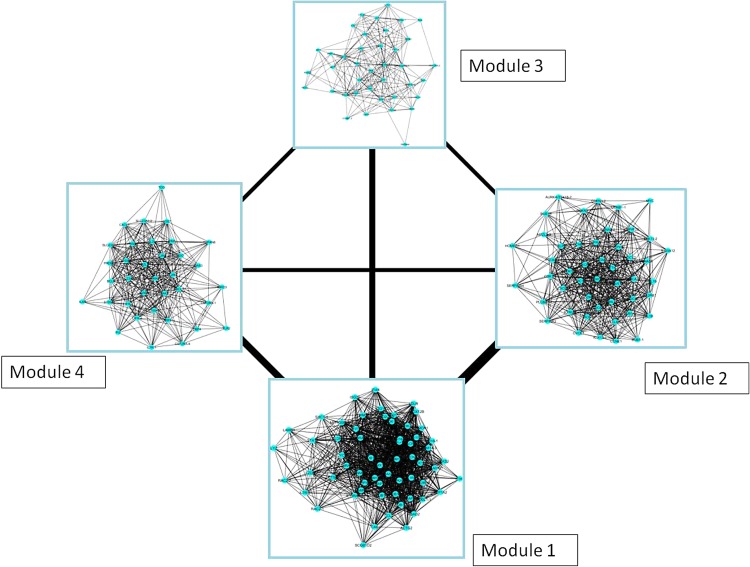
The modular topology of the FCC network was further investigated to better assess the psoriasis disease network. Four modules were identified as a result of the clustering analysis. Module 1 appears to be the central module of the FCC network of psoriasis with a high-level connection to Module 2 (Fig. 5).
FIG. 5.
Modules of the FCC network of psoriasis (the line widths are proportional to the correlations between the modules).
The hubs of Module 1 shares proteins with the global hub analysis of the FCC Network as well as PPI network of psoriasis (Table 4). Pathway enrichment analysis for the 145 DEGs and the modules are given in Table 5. The enrichment analysis indicated that JAK/STAT signaling pathway is significant in all the modules. Most of the DEGs in Module 1 are involved in biological processes such as response to stress, chemical stimulus, and biotic stimulus. Module 2 has 39 members, 15 of which have molecular activities in cell cycle and oocyte meiosis. The most significant biological pathways of this module are cell cycle (p = 1.34 × 10−10) and oocyte meiosis (p = 1.13 × 10−5). Module 3 has 35 members that are mostly involved in immune system processes and positive regulation of biological processes. Module 4 has 32 members involved in biological processes such as immune, defense, and inflammatory responses.
Table 4.
Central DEGs (Hubs) of the FCC Network of Psoriasis and Its Modules
| Protein symbol | Degree | Protein symbol | Betweenness |
|---|---|---|---|
| Overall network | |||
| SUB1-1 | 86 | CSF2RA-3 | 474.6 |
| SOCS1 | 86 | LIFR-1 | 394.4 |
| IL13RA1-3 | 86 | SHC1-2 | 288.1 |
| IL12RB2 | 85 | IL12RB1 | 266.7 |
| TRIM14-2 | 84 | LEPR-2 | 243.01 |
| MAPKAPK3 | 82 | CTSC | 228.6 |
| NMI | 82 | SUB1-1 | 223.9 |
| OAS1 | 81 | ITGA4 | 216.5 |
| CDK1-2 | 80 | IL13RA1-3 | 216.4 |
| Module 1 | |||
| SUB1-1 | 86 | CTSC | 753.4 |
| SOCS1 | 86 | SUB1-1 | 642.9 |
| IL12RB2 | 85 | TRIM14-2 | 542.3 |
| TRIM14-2 | 84 | SOCS1 | 471.8 |
| MAPKAPK3 | 82 | ATP1A2 | 469.9 |
| NMI | 82 | IL12RB2 | 461.3 |
| Module 2 | |||
| CDK1-1 | 77 | LIFR-1 | 2652.3 |
| CDC6-1 | 75 | SHC1-2 | 1225.1 |
| CXCR2 | 73 | CDK1-1 | 711.2 |
| CDK1-3 | 72 | SLPI | 666.3 |
| SHC1-2 | 70 | CDK1-3 | 627.5 |
| SLPI | 69 | CDC6-1 | 616.4 |
| LIFR-1 | 69 | CXCR2 | 545.9 |
| Module 3 | |||
| CSF2RA-3 | 76 | CSF2RA-3 | 2646.9 |
| ITGA4 | 56 | LEPR-2 | 2005.2 |
| STAT3 | 55 | STAT3 | 1471.8 |
| LEPR-2 | 45 | ITGA4 | 1463.9 |
| Module 4 | |||
| IRF9 | 62 | IRF9 | 1543.8 |
| S100A9 | 56 | CSF2RA-4 | 1190.2 |
| LIFR-2 | 49 | S100A9 | 1030.7 |
| CSF2RA-4 | 45 | LIFR-2 | 633.4 |
Table 5.
Pathway Enrichment Results of Psoriasis Network
| P value | |||||
|---|---|---|---|---|---|
| Pathway (KEGG ID) | Overall | Module 1 | Module 2 | Module 3 | Module 4 |
| Jak-STAT signaling pathway (hsa04630) | 1.16 × 10−12 | 8.43 × 10−3 | 3.91 × 10−2 | 4.68 × 10−3 | 3.34 × 10−9 |
| Cytokine-cytokine receptor interaction (hsa04060) | 1.67 × 10−8 | – | 7.94 × 10−3 | – | 4.01 × 10−6 |
| Cell cycle (hsa04110) | 2.64 × 10−8 | – | 1.34 × 10−10 | – | – |
| Chemokine signaling pathway (hsa04062) | 2.03 × 10−5 | – | 1.22 × 10−2 | – | – |
| NOD-like receptor signaling (hsa04621) | 9.59 × 10−5 | – | – | – | – |
| Oocyte meiosis (hsa04114) | 1.22 × 10−4 | – | 1.13 × 10−5 | – | – |
| Progesterone-mediated oocyte maturation (hsa04914) | 7.43 × 10−4 | – | 7.36 × 10−4 | – | – |
| RIG-I-like receptor signaling (hsa04622) | 8.08 × 10−3 | – | – | – | – |
| Toll-like receptor signaling (hsa04620) | 8.58 × 10−3 | – | – | – | – |
| Pathways in cancer (hsa05200) | 1.22 × 10−2 | – | – | – | – |
| Hematopoietic cell lineage (hsa04640) | 1.76 × 10−2 | – | – | – | 2.57 × 10−2 |
| p53 signaling pathway (hsa04115) | 3.16 × 10−2 | – | 4.34 × 10−2 | – | – |
| Adipocytokine signaling pathway (hsa04920) | – | – | – | 3.21 × 10−3 | – |
Discussion
While there are reports on transcriptomics studies of psoriasis, molecular pathophysiology of this disease still remains elusive. Several studies have examined the psoriasis-related transcriptome by comparing lists of differentially expressed genes with inconsistent results (Bigler et al., 2013; Gudjonsson et al., 2010; Suarez-Farinas et al., 2010). The absence of agreement between these studies might be due to threshold affects (e.g., p-value, fold change, false discovery rate) on selection of over- and underexpressed genes and also parameter differences in analyzing microarrays (Pan et al., 2005).
In the present study, we explored the largest set of microarray datasets to date to generate a comprehensive pool of DEGs, to provide deep insight into the disease mechanism, and to propose a disease pathway in psoriasis. To this end, in addition to the statistical analysis of transcriptomics datasets, the holistic approach comprising the reconstruction and topological analysis of biological networks around DEGs was enriched with a novel correlation analysis based on fold changes.
Each of the studied datasets has hundreds of DEGs when analyzed individually, however they lack a mutual gene when all datasets were compared. This might be due to the platform differences, naming issues, and heterogeneity of patient selection criteria. The numbers of DEGs that are common drop sharply to a small number when a comparison between platforms was performed (Fig. 2). Even within the Affymetrix platform the number of common DEGs shows a great decline between the newest and early generations.
The main difference between the three separate platforms is the number of probe sets employed. Arrays with the highest number of probe sets happen to be in the Affymetrix platform: Human Genome U133 Plus 2.0 array, which include 54675 probe sets that collectively target 20026 human genes. The comparative analysis of the five datasets (GSE 14905, GSE34248, GSE41662, GSE30999, and GSE13355) employing this array resulted in 934 mutual DEGs, suggesting a great agreement between them.
On the other hand, the lowest number of probe sets is also in an Affymetrix platform, Human Genome U95A Array, which is an early generation array with only 12626 probe sets. Some of the well-recognized genes involved in psoriasis such as IL17, IL22, and INOS were not detected as DEGs by most of the twelve studies. However, differential expressions of these genes in psoriasis have been confirmed by RT-PCR analysis (Suarez-Farinas et al., 2010). This observation indicates a major limitation of microarrays. Expressions of these genes are usually low on microarray platforms; hence their fold changes may not be accurately measured. (Suarez-Farinas et al., 2010).
An alternate to utilizing microarray data is employing expression level correlation to identify new functional modules and gene sets (Ye and Eskin, 2007). Reynier et al. (2011) suggest that a correlation between gene expression levels can allow us to identify the activated mechanisms at the cellular level. On the other hand, the initial set of gene expression data acquired from microarray experiments is broad, with as many as 54575 probes and the correlation analysis of these data may result in a high variance in the number of DEGs as well as substantial amount of false positives (Tamayo et al., 2012). Besides the results are mostly difficult to comprehend and analyze.
Therefore, an alternate approach, so-called FCC analysis, was employed in the present study. In the FCC analysis, fold change (FC) values of the DEGs are used instead of gene expression levels to determine pairwise correlations. Hence, possible uncertainties that may arise from high variance are eliminated, and the effect of false positives is possibly reduced. In addition, the employment of FCC analysis is uncomplicated and requires fewer amounts of data compared to traditional correlation analysis.
The statistical and comparative analysis of the individual gene expression datasets resulted in eleven core DEGs, around which PPI network was reconstructed. Central proteins (called hubs) were identified based on a local (i.e., degree) and a global topological metric (i.e., betweenness centrality). The hub proteins (STAT1, MAD2L1, CYCS, NMI, and SUB1) require special attention since they can be considered as candidates for biomarker studies and potential drug targets.
Three of these hub proteins (STAT1, NMI, and SUB1) were transcription factors. SUB1, which is upregulated in our datasets, plays a dual role (as an activator or repressor) in gene expression and has multiple effects in distinct steps of the transcription cycle, consisting of initiation, elongation, termination, and reinitiation (Conesa and Acker, 2010). NMI is a transcription cofactor that potentiates STAT-dependent transcription and also augments coactivator protein recruitment (Zhu et al., 1999). Another hub protein, STAT1 is also one of our upregulated core DEGs and is a member of STAT proteins that play central roles in Janus kinase/signal transducers and activators of transcription (JAK/STAT) pathway and cytokine signaling (Bromberg and Darnell, 2000).
The literature was further surveyed for signaling pathways associated with the hub proteins and proteins encoded by core DEGs. So, other protein encoding DEGs functionally associated with the core DEGs and hub proteins were extracted. As a result, a comprehensive pool of 145 DEGs consisting of transcription factors, cytokines, receptors, enzymes, and ISGs, was constructed (Table 6).
Table 6.
Proteins Associated with the Proposed Psoriasis Pathwaya
| Protein symbol | Protein name | Molecular function | Direction of regulation | Comments |
|---|---|---|---|---|
| Module 1 | ||||
| ID1 | Inhibitor of DNA binding 1 | Transcription Regulator | ↑ | |
| IFI16 | Interferon, gamma-inducible protein 16 | Transcription Regulator | ↑ | Interferon Stimulated Gene |
| NMI* | N-myc (and STAT) interactor | Transcription Regulator | ↑ | |
| STAT1* | signal transducer and activator of transcription 1 | Transcription Regulator | ↑ | JAK/STAT pathway and chemokine signaling pathway |
| SUB1* | SUB1 homolog | Transcription Regulator | ↑ | |
| TRIM22 | tripartite motif containing 22 | Transcription Regulator | ↑ | Interferon Stimulated Gene |
| CYCS | cytochrome c, somatic | Transporter | ↑ | Sulfur metabolism |
| SLC5A1 | solute carrier family 5 (sodium/glucose cotransporter), member 1 (SGLT1) | Transporter | ↑ | Carbohydrate digestion and absorption, and mineral absorption and bile secretion |
| CCL2 | chemokine (C-C motif) ligand 2 | Cytokine | ↑ | Chemokine signaling pathway |
| IL1RN | interleukin 1 receptor antagonist | Cytokine | ↑ | |
| IL12RB2 | interleukin 12 receptor, beta 2 | Receptor | ↑ | JAK/STAT pathway |
| IL13RA1 | interleukin 13 receptor, alpha 1 | Receptor | ↑ | JAK/STAT pathway |
| FZD5 | frizzled class receptor 5 | Receptor | ↑ | WNT signaling pathway |
| LDLR | low density lipoprotein receptor | Receptor | ↑ | Bile secretion |
| IFIH1 | interferon induced with helicase C domain 1 | Hydrolase (EC:3.6.4.13) | ↑ | RIGI like receptor signaling pathway; Interferon Stimulated Gene |
| ATP1A2 | ATPase, Na+/K+ transporting, alpha 2 polypeptide | Hydrolase (EC:3.6.3.9) | ↓ | Mineral absorption, carbohydrate digestion and absorption |
| CTSC | cathepsin C | Hydrolase (EC:3.4.14.1) | ↑ | Lysosome |
| LYZ | lysozyme | Hydrolase (EC:3.2.1.17) | ↑ | Salivary secretion |
| ISG20 | interferon stimulated exonuclease gene | Hydrolase (EC:3.1.13.1) | ↑ | Interferon Stimulated Gene |
| CA6 | carbonic anhydrase VI | Lyase (EC:4.2.1.1) | ↓ | Nitrogen metabolism |
| HMOX1 | heme oxygenase (decycling) 1 | Oxidoreductase (EC:1.14.99.3) | ↑ | Mineral absorption |
| ALOX12B | arachidonate 12-lipoxygenase, 12R type | Oxidoreductase (EC:1.13.11.-) | ↑ | Epidermal barrier function |
| OAS1 | 2′-5′-oligoadenylate synthetase 1 | Transferase (EC:2.7.7.84) | ↑ | Interferon Stimulated Gene |
| MAPK13 | mitogen-activated protein kinase 13 | Transferase (EC:2.7.11.24) | ↑ | Cell cycle, RIGI like receptor signaling pathway |
| CDK1 | cyclin-dependent kinase 1 | Transferase (EC:2.7.11.22) | ↑ | Cell cycle and oocyte meiosis |
| MAPKAPK3 | mitogen-activated protein kinase-activated protein kinase 3 | Transferase (EC:2.7.11.1) | ↑ | |
| HK2 | hexokinase 2 | Transferase (EC:2.7.1.1) | ↑ | Carbohydrate digestion and absorption |
| ACTG2 | actin, gamma 2, smooth muscle, enteric | Other | ↓ | |
| CKS2 | CDC28 protein kinase regulatory subunit 2 | Other | ↑ | Cell cycle |
| CLDN8 | claudin 8 | Other | ↓ | |
| HOMER1 | homer homolog 1 | Other | ↑ | |
| IFI44* | interferon-induced protein 44 | Other | ↑ | Interferon Stimulated Gene |
| IFIT1* | interferon-induced protein with tetratricopeptide repeats 1 | Other | ↑ | Interferon Stimulated Gene |
| ISG15 | ISG15 ubiquitin-like modifier | Other | ↑ | RIGI like receptor signaling pathway |
| KIAA0101 | KIAA0101 | Other | ↑ | Cell cycle |
| LAMB4 | laminin, beta 4 | Signaling protein | ↓ | |
| LAMP3 | lysosomal-associated membrane protein 3 | Signaling protein | ↑ | Autophagy |
| MAD2L1* | MAD2 mitotic arrest deficient-like 1 | Other | ↑ | Cell cycle and oocyte meiosis |
| MX1 | MX dynamin-like GTPase 1 | Other | ↑ | Interferon Stimulated Gene |
| PLSCR1 | phospholipid scramblase 1 | Other | ↑ | Interferon Stimulated Gene |
| RAC2 | ras-related C3 botulinum toxin substrate 2 (rho family, small GTP binding protein Rac2) | Other | ↑ | Chemokine signaling pathway |
| RSAD2* | radical S-adenosyl methionine domain containing 2 | Other | ↑ | Interferon Stimulated Gene |
| SCGB1D2 | secretoglobin, family 1D, member 2 | Signaling protein | ↓ | |
| SERPINB1 | serpin peptidase inhibitor, clade B (ovalbumin), member 1 | Other | ↑ | |
| SOCS1 | suppressor of cytokine signaling 1 | Other | ↑ | JAK/STAT pathway; Interferon Stimulated Gene |
| TRIM14 | tripartite motif containing 14 | Other | ↑ | Interferon Stimulated Gene |
| SHC1 | SHC (Src homology 2 domain containing) transforming protein 1 | Other | ↑ | Chemokine signaling pathway; also in module 2 |
| IL7R | interleukin 7 receptor | Receptor | ↑ | JAK/STAT pathway; also in module 4 |
| Module 2 | ||||
| CCNE1 | Cyclin E1 | Transcription Regulator | ↑ | Cell Cycle and oocyte meiosis |
| MYC | v-myc avian myelocytomatosis viral oncogene homolog | Transcription Regulator | ↑ | JAK/STAT pathway |
| OVOL1 | ovo-like zinc finger 1 | Transcription Regulator | ↑ | Cell cycle |
| ABCA12 | ATP-binding cassette, sub-family A (ABC1), member 12 | Transporter | ↑ | Epidermal barrier function |
| CXCL2 | chemokine (C-X-C motif) ligand 2 | Cytokine | ↑ | Chemokine signaling pathway |
| CXCL8 | chemokine (C-X-C motif) ligand 8 | Cytokine | ↑ | JAK/STAT pathway and Chemokine signaling pathway |
| IL19 | interleukin 19 | Cytokine | ↑ | JAK/STAT pathway and RIGI like receptor signaling pathway |
| IL1B | interleukin 1, beta | Cytokine | ↑ | |
| IL12RB1 | interleukin 12 receptor, beta 1 | Receptor | ↑ | JAK/STAT pathway |
| CXCR2 | chemokine (C-X-C motif) receptor 2 | Receptor | ↑ | JAK/STAT pathway and chemokine signaling pathway |
| KLK13 | kallikrein-related peptidase 13 | Hydrolase (EC:3.4.21.-) | ↑ | |
| PLCB4 | phospholipase C, beta 4 | Hydrolase (EC:3.1.4.11) | ↓ | Chemokine signaling pathway and WNT signaling pathway |
| CDKN3 | cyclin-dependent kinase inhibitor 3 | Hydrolase (EC:3.1.3.16) | ↑ | Cell cycle |
| ADCY2 | adenylate cyclase 2 | Lyase (EC 4.6.1.1) | ↓ | Oocyte meiosis and chemokine signaling pathway |
| TTK | TTK protein kinase | Transferase (EC:2.7.12.1) | ↑ | Cell cycle |
| AURKA | aurora kinase A | Transferase (EC:2.7.11.1) | ↑ | Cell cycle and oocyte meiosis |
| BUB1 | BUB1 mitotic checkpoint serine/threonine kinase | Transferase (EC:2.7.11.1) | ↑ | Cell cycle and oocyte meiosis |
| BUB1B | BUB1 mitotic checkpoint serine/threonine kinase B | Transferase (EC:2.7.11.1) | ↑ | Cell cycle |
| TGM1 | transglutaminase 1 | Transferase (EC:2.3.2.13) | ↑ | Epidermal barrier function |
| CCNB2 | cyclin B2 | Other | ↑ | Cell cycle and oocyte meiosis |
| CDC45 | cell division cycle 45 | Other | ↑ | Cell cycle |
| CDC6 | cell division cycle 6 | Other | ↑ | Cell cycle |
| CYP4F22 | cytochrome P450, family 4, subfamily F, polypeptide 22 | Other | ↑ | Epidermal barrier function |
| FBXO5 | F-box protein 5 | Other | ↑ | Cell cycle and oocyte meiosis |
| GNA15 | guanine nucleotide binding protein (G protein), alpha 15 (Gq class) | Other | ↑ | |
| MALL | mal, T-cell differentiation protein-like | Other | ↑ | |
| PCNA | proliferating cell nuclear antigen | Other | ↑ | Cell cycle |
| S100A12 | S100 calcium binding protein A12 | Other | ↑ | |
| SERPINB3 | serpin peptidase inhibitor, clade B (ovalbumin), member 3 | Other | ↑ | |
| SERPINB4 | serpin peptidase inhibitor, clade B (ovalbumin), member 4 | Other | ↑ | |
| SLPI | secretory leukocyte peptidase inhibitor | Signaling protein | ↑ | |
| TPX2 | TPX2, microtubule-associated | Other | ↑ | |
| GZMA | granzyme A (granzyme 1, cytotoxic T-lymphocyte-associated serine esterase 3) | Hydrolase (EC:3.4.21.78) | ↑ | |
| KYNU | Kynureninase | Hydrolase (EC:3.7.1.3) | ↑ | Tryptophan metabolism; also in module 3 |
| CCNA2 | cyclin A2 | Other | ↑ | Cell cycle; also in module 3 |
| CCNB1 | cyclin B1 | Other | ↑ | Cell cycle and oocyte meiosis; also in module 3 |
| MPZL2 | myelin protein zero-like 2 | Signaling protein | ↑ | Also in module 4 |
| LIFR | leukemia inhibitory factor receptor alpha | Receptor | ↓ | JAK/STAT pathway; also in module 4 |
| Module 3 | ||||
| ID4 | Inhibitor of DNA binding 4 | Transcription Regulator | ↓ | |
| IRF1 | Interferon regulatory factor 1 | Transcription Regulator | ↑ | Interferon Stimulated Gene |
| IRF7 | Interferon regulatory factor 7 | Transcription Regulator | ↑ | RIGI like receptor signaling pathway, Interferon Stimulated Gene |
| STAT3 | signal transducer and activator of transcription 3 | Transcription Regulator | ↑ | JAK/STAT pathway and chemokine signaling pathway |
| TCN1 | transcobalamin I (vitamin B12 binding protein, R binder family) | Transporter | ↑ | |
| CCL20 | chemokine (C-C motif) ligand 20 | Cytokine | ↑ | Chemokine signaling pathway |
| WNT5A | wingless-type MMTV integration site family, member 5A | Cytokine | ↑ | WNT signaling pathway |
| GPC4 | glypican 4 | Receptor | ↓ | WNT signaling pathway |
| DDX58 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 58 (RIGI) | Hydrolase (EC 3.6.3.14) | ↑ | RIGI like receptor signaling pathway |
| ENO1 | enolase 1, (alpha) | Lyase (EC:4.2.1.11) | ↑ | |
| SOD2 | superoxide dismutase 2, mitochondrial | Oxidoreductase (EC:1.15.1.1) | ↑ | |
| AKR1B10 | aldo-keto reductase family 1, member B10 (aldose reductase) | Oxidoreductase (EC 1.1.1.21) | ↑ | |
| OAS2* | 2′-5′-oligoadenylate synthetase 2 | Transferase (EC:2.7.7.84) | ↑ | Interferon Stimulated Gene |
| CDC20 | cell division cycle 20 | Other | ↑ | Cell cycle and oocyte meiosis |
| DEFB4A | defensin, beta 4A | Signaling protein | ↑ | |
| KRT16 | keratin 16 | Other | ↑ | |
| MGB2 | secretoglobin, family 2A, member 1 (SCGB2A1) | Signaling protein | ↓ | |
| MPHOSPH6 | M-phase phosphoprotein 6 | Other | ↑ | Cell cycle |
| NOD2 | nucleotide-binding oligomerization domain containing 2 | Other | ↑ | |
| RGS1 | regulator of G-protein signaling 1 | Other | ↑ | |
| RGS20 | regulator of G-protein signaling 20 | Other | ↑ | |
| SOCS3 | suppressor of cytokine signaling 3 | Other | ↑ | JAK/STAT pathway |
| WIF1* | WNT inhibitory factor 1 | Signaling protein | ↓ | WNT signaling pathway |
| ITGA4 | integrin, alpha 4 (antigen CD49D, alpha 4 subunit of VLA-4 receptor) | Receptor | ↑ | |
| LEPR | leptin receptor | Receptor | ↓ | JAK/STAT pathway |
| LEP | Leptin | Growth Factor | ↓ | JAK/STAT pathway |
| MMP12 | matrix metallopeptidase 12 (macrophage elastase) | Hydrolase (EC:3.4.24.65) | ↑ | |
| MMP9 | matrix metallopeptidase 9 | Hydrolase (EC:3.4.24.35) | ↑ | |
| CFB | complement factor B | Hydrolase (EC:3.4.21.47) | ↑ | |
| PNP | purine nucleoside phosphorylase | Hydrolase (EC:2.4.2.1) | ↑ | |
| CSF2RA | colony stimulating factor 2 receptor, alpha, low-affinity (granulocyte-macrophage) | Receptor | ↑ | JAK/STAT pathway; also in module 4 |
| Module 4 | ||||
| BCL3 | B-cell CLL/lymphoma 3 | Transcription Regulator | ↑ | |
| IRF9 | Interferon regulatory factor 9 | Transcription Regulator | ↑ | JAK/STAT pathway |
| PRDM1 | PR domain containing 1, with ZNF domain | Transcription Regulator | ↑ | |
| SLC23A2 | solute carrier family 23 (ascorbic acid transporter), member 2 | Transporter | ↑ | |
| CXCL1 | chemokine (C-X-C motif) ligand 1 | Cytokine | ↑ | Chemokine signaling pathway |
| CXCL9 | chemokine (C-X-C motif) ligand 9 | Cytokine | ↑ | Chemokine signaling pathway |
| IL20 | interleukin 20 | Cytokine | ↑ | JAK/STAT pathway |
| IL2RA | interleukin 2 receptor, alpha | Receptor | ↑ | JAK/STAT pathway |
| IL2RG | interleukin 2 receptor, gamma | Receptor | ↑ | JAK/STAT pathway |
| IL4R | interleukin 4 receptor | Receptor | ↑ | JAK/STAT pathway |
| TLR2 | toll-like receptor 2 | Receptor | ↑ | |
| GZMB | granzyme B (granzyme 2, cytotoxic T-lymphocyte-associated serine esterase 1) | Hydrolase (EC:3.4.21.79) | ↑ | |
| PRSS8 | protease, serine, 8 | Hydrolase (EC:3.4.21.-) | ↑ | Epidermal barrier function |
| UBA6 | ubiquitin-like modifier activating enzyme 6 | Ligase (EC 6.3.2.19) | ↑ | |
| IDO | indoleamine 2,3-dioxygenase 1 | Oxidoreductase (EC:1.13.11.52) | ↑ | Tryptophan metabolism |
| TDO | tryptophan 2,3-dioxygenase | Oxidoreductase (EC:1.13.11.11) | ↑ | Tryptophan metabolism |
| OAS3 | 2′-5′-oligoadenylate synthetase 3 | Transferase (EC:2.7.7.84) | ↑ | Interferon Stimulated Gene |
| PIM1 | Pim-1 proto-oncogene, serine/threonine kinase | Transferase (EC:2.7.11.1) | ↑ | JAK/STAT pathway and Cell cycle |
| JAK3 | Janus kinase 3 | Transferase (EC:2.7.10.2) | ↑ | JAK/STAT pathway and chemokine signaling pathway |
| BIRC3 | baculoviral IAP repeat containing 3 | Other | ↑ | |
| DBF4 | DBF4 zinc finger | Other | ↑ | Cell cycle |
| PI3* | peptidase inhibitor 3, skin-derived (SKALP) | Signaling protein | ↑ | |
| S100A7 | S100 calcium binding protein A7 (psoriasin) | Other | ↑ | |
| S100A8 | S100 calcium binding protein A8 | Cytokine | ↑ | |
| S100A9 | S100 calcium binding protein A9 | Other | ↑ | |
| SOST | sclerostin | Signaling protein | ↑ | WNT signaling pathway |
| SPRR2C | small proline-rich protein 2C | Other | ↑ | Epidermal barrier function |
| TSPAN8 | tetraspanin 8 | Other | ↓ | |
The core DEGs are listed in boldface and marked with *.
Among the 145 DEGs, 32 of them were involved in chemokine signaling pathways, 21 of which are particularly in JAK/STAT pathway. The activation of this pathway stimulates cell proliferation, differentiation, cell migration, and apoptosis (Rawlings et al., 2004). The JAKs and STATs are essential intracellular mediators of immune cytokine action, and lack of these proteins causes immunological defects (Ivashkiv, 2000). The chemokines found in the pool were CXCL1, CXCL2, CXCL8 (IL8), CXCL9, CCL2, and CCL20, along with their G-protein coupled receptor (GPCR) CXCR2.
In addition, two RGS (regulator of G protein signaling) proteins (RGS1 and RGS20) regulating the GPCRs were included. These DEGs were all significantly upregulated in psoriasis with fold changes up to 27. Chemokines coordinate immune cell trafficking both during the development of the immune system and during responses to exogenous or infectious agents by signaling through their receptors (Moratz et al., 2004; Schneider et al., 2014). RGS proteins are key regulators of leukocyte trafficking and RGS1 is critical in downregulating the response to sustained chemokine signaling (Patel et al., 2013).
The cytokines, IL19 and IL20, which were involved in the JAK/STAT pathway, were also upregulated in psoriasis. Among skin cells, keratinocytes are important targets of IL19. They also increase the production of three S100 family proteins S100A7, S100A8, S100A9, and to a moderate extent IL1B, IL20, CXCL8, and MMP1. The psoriasin protein encoded by the S100A7 gene is overexpressed in hyperproliferative skin diseases, and exhibits antimicrobial activities against bacteria, and induces immunomodulatory activities (Celis et al., 1990).
IL19 also activates the transcription factor STAT3 (Witte et al., 2014). IL-20 has a distinct role in promoting hyperproliferation of keratinocytes, hence modulating inflammation in the skin. The ability of keratinocytes to release pro-inflammatory factors when stimulated by cytokines or physical distress allows them to recruit inflammatory cells and regulate their behavior (Rich and Kupper, 2001). Although TEN cytokine receptors (IL4R, IL7R, IL2RA, IL12RB1 IL12RB2, IL13RA1, IL2RG, LIFR, LEPR, and CSF2RA) involved in JAK/STAT pathway were represented in the pool, the receptors for IL19 and IL20 were surprisingly not differentially expressed in the transcriptomics datasets examined here.
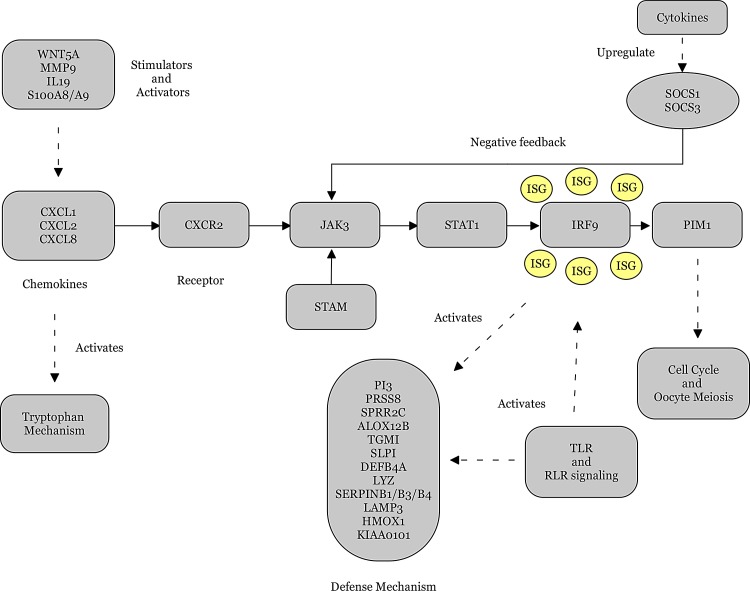
The cytokine receptors in the DEG pool were all upregulated except for LIFR and LEPR. As part of a feedback loop, cytokines upregulate the suppressors of cytokine signaling (SOCS) that inhibit the activity of JAKs and STATs (Slattery et al., 2013). SOCS3 inhibits the JAK catalytic activity via binding to receptor rather than a direct interaction with JAKs (Alexander, 2002). SOCS1 and SOCS3 were upregulated in psoriasis, proving that the JAK/STAT pathway is activated with its key elements CXCL1, CXCL2, CXCL8, CXCR2, STAT1, JAK3, SOCS1, and SOCS3, in psoriasis (Fig. 6).
FIG. 6.
Proposed pathway for pathophysiology of psoriasis.
The production of inflammatory cytokines is induced by the activation of neutrophils (Sadik and Luster, 2012), which play an important role in the regulation of the innate immune response by recruiting chemokine gradients to the area of injury or infection (Kobayashi and DeLeo, 2009). CXCL8 is the main chemokine produced by neutrophils, activating them through CXCR2 in an autocrine loop (Soehnlein and Lindbom, 2010). Two matrix metallopeptidases, MMP9 and MMP12, were overexpressed in psoriatic skin. MMP9 increases the chemotactic activity of chemokines, CXCL1 and CXCL8, hence increasing their potency to attract neutrophils. The activities of MMP proteins are regulated by tissue inhibitors of metalloproteinases (TIMPs) (Nagase et al., 2006). TIMP3, which is an inhibitor of MMP12, was downregulated in psoriatic skin.
WNT5A, FZD5, WIF1, GPC4, SOST, and PLCB4 are the members of the WNT signaling pathway, an ancient and evolutionarily conserved pathway that regulates crucial aspects of cell fate determination, cell migration, cell polarity, neural patterning, and organogenesis during embryonic development (Komiya and Habas, 2008). WNT5A stimulates the production of several inflammatory cytokines and chemokines, such as IL6, CXCL1, and CXCL8. WNT5A also binds to several members of the Frizzled receptor family, including FZD5 (Jung, 2013), and is inhibited by WIF1 (Surmann-Schmitt et al., 2009). FZD5 was upregulated and WIF1 was downregulated in our analysis. Our results proposed that the non-canonical Wnt/Ca2+ branch of the Wnt signaling pathway was activated in psoriasis.
Studies of ISGs have increased our knowledge in areas of translational control, regulation of RNA stability and editing, protein transport and turnover. The ISGs in the DEG pool included IRF1, IRF7, IRF9, IFIT1, IFI16, STAT1, OAS1, OAS2, OAS3, MX1, ISG15, ISG20, IFIH1, IFI44, PLSCR1, and RSAD2, some of which were also defined as core DEGs. RSAD2 (Viperin) and ISG15 are associated with antiviral response (Borden and Williams, 2011). Viperin is an antiviral enzyme induced by JAK/STAT signaling (Zhou et al., 2007) or direct activation by IRF1 (Stimweiss et al., 2010) and its expression was significantly upregulated in psoriasis datasets (up to 16-fold). STATs and the IRFs amplify the effects of ISGs (Tamura et al., 2008). IFIT proteins may also be induced through toll-like receptor (TLR) signaling (Fensterl and Sen, 2010).
On the other hand, numerous ISGs (such as STAT1 and IRF7) were activated in the absence of IFN signaling (Schoggins and Rice, 2011), indicating that IRF-mediated transcriptional regulatory cascades may be natural antiviral mechanisms that allow rapid ISG expression before IFN itself can be produced. This might explain the lack of IFNs in our DEG pool, while IRF1 and IRF7 along with IRF9 were overexpressed and a number of ISGs were present. In addition, the silencing of ISGs may cause increased infection (Li et al., 2013). We propose that the ISGs are needed in psoriasis to activate the antimicrobial peptides (AMPs).
Of the 145 DEGs, 25 play a role in cell cycle and oocyte meiosis. All the DEGs in this group were upregulated, proposing that cell cycle and oocyte meiosis processes are activated during the disease progress. Among those, cyclins (CCNA2, CCNB1, CCNB2), cyclin-dependent kinases (CDK1, CDK2) and cyclin-dependent kinase inhibitors (CDKN3), which are required for progression of cell cycle, were prominent. Additionally, AURKA is required for proper mitosis of cells (Marumoto et al., 2005) and its overexpression induces checkpoint disruption, possibly leading to aneuploidy (Katayama et al., 2004).
MAD2L1, BUB1B, and BUB1 are involved in the mitotic checkpoint, serving as a surveillance mechanism (Manning and Dyson, 2012). The alterations in these mitotic arrest genes may play a role in psoriasis by disrupting control mechanism for the normal mitotic checkpoint (Percy et al., 2000).
Kynurenine (KYNU), indoleamine 2,3-dioxygenase (IDO), and tryptophan 2,3-dioxygenase (TDO) of the tryptophan metabolism were also represented in the DEG pool, all of which were overexpressed in psoriasis. Previously, Nomura and co-workers (2003) reported KYNU as a marker gene for psoriasis as compared with atopic dermatitis. The present study incorporating all the published datasets verified the upregulation of KYNU in psoriasis (i.e., its expression level was significantly elevated (up to 16 fold) in psoriatic skin when compared to healthy skin).
Tryptophan metabolism is known to mediate both genetic and environmental mechanisms of depression, and depression has been documented as a significant disability in many patients of psoriasis (Krueger et al., 2001). Simultaneous presence of high producer alleles of pro-inflammatory cytokine genes determines the genetic predisposition to depression via upregulation of IDO, while impact of environmental stresses is mediated via hormonal activation of TDO (Oxenkrug, 2010).
Aberrant RIGI-like receptor (RLR) signaling or dysregulation of RLR expression have been implicated in the development of autoimmune diseases (Loo and Gale, 2011). In the present study, six proteins that play central roles in RLR signaling were differentially overexpressed: RIGI (DDX58), ISG15, IFIH1, IRF7, CXCL8, and MAPK13. In addition, seven proteins (TLR2, CXCL8, CXCL9, IRF7, IL1B, MAPK13, and STAT1) that play central roles in TLR signaling were differentially overexpressed in psoriasis. The innate immune system can sense the invasion of pathogenic microorganisms by TLR signaling, which recognizes specific molecular patterns that are present in microbial components (Akira and Takeda, 2004). It was reported that stimulation of different TLRs activates signals that are involved in the initiation of adaptive immune responses (Iwasaki and Medzhitov, 2004). The overexpressions of TLR2 as well as CXCL8 and STAT1 have previously been reported in psoriatic skin (Baker et al., 2003).
The outermost layer of the skin, the stratum corneum, functions as the body's main protective barrier against physical and chemical damage, dehydration, and microbial pathogens. Stratum corneum desquamation, which is premature in psoriasis, is a tightly regulated process, orchestrated by the combined function of serine proteases and their inhibitors within the intercorneal matrix. KLK13, a serine protease that has a role in stratum corneum desquamation, was highly overexpressed in psoriasis datasets (up to 7-fold).
PI3 (Elafin), an AMP that is cross-linked into the cornified cell envelopes from the inside of psoriatic keratinocytes (Nakane et al., 2009), was among the core DEGs and significantly upregulated in psoriasis (up to 100-fold). SLPI also exhibits antimicrobial properties and immunomodulatory activity (Doumas et al., 2005) and is implicated in the regulation of desquamation (Borgono et al., 2007; Zhu et al., 2002).
Patients with psoriasis have fewer skin infections than expected, leading to the hypothesis that lesional psoriatic skin has a chemical shield in the form of AMPs (Harder and Schröder, 2005). Defensins are AMPs secreted by various cells as a component of the innate host defence (Wehkamp et al., 2007). DEFB4A (defensin, beta 4A) was highly overexpressed in psoriasis (up to 135-fold). The cytokines IL1B and IL1RN are regulators of DEFB4A (Liu et al., 2002). S100A7 (psoriasin), LYZ and calprotectin (S100A8/S100A9 protein complex) are other AMPs that function as signaling molecules associated with cytokines (Lee et al., 2012; Nukui et al., 2008) and were upregulated in psoriasis.
The highly overexpressed S100A12 (Calgranulin C), a calcium-binding pro-inflammatory protein predominantly secreted by granulocytes, was proposed as a marker of inflammation (Pietzsch and Hoppmann, 2009). Our analyses indicated that the AMPs (DEFB4A, PI3, S100A8, S100A9 and S100A12) were overexpressed in psoriatic skin than any other genes and we propose that these peptides may play significant roles as downstream effectors in the defense mechanism of the biological system in response to psoriasis.
SERPINB1, SERPINB3, and SERPINB4 are members of the serpin family of proteinase inhibitors that are also overexpressed in psoriatic skin. This group of proteins protects tissues from damage at inflammatory sites. Among them, SERPINB4 had the highest overexpression with 126-fold difference from nonlesional skin. Other DEGs such as ID1, ID4, KRT16 and HMOX1, which have been previously indicated in psoriasis disease (Hanselmann et al., 2001; Leigh et al., 1995; Ronpirin et al., 2010; Ruchusatsawat et al., 2011; Zebedee et al., 2001) were also identified in the present study.
The DEGs in the presented pool have been separated into four highly-connected and correlated modules (Fig. 5) and function in an integrated manner as a defense mechanism of the cell in response to the biological processes that have been affected by psoriasis. Overall, the JAK/STAT signaling pathway constititues the central part of the proposed pathway of psoriasis (Fig. 6), with possible crosstalks with several signaling pathways (such as TLR, WNT, and RLR signaling) and biological processes (including cell cycle, oocyte meiosis, and tryptophan mechanism). Cytokines are influential in the presentation of the disease since they play major roles in the activation and regulation of the JAK/STAT pathway. ISGs, AMPs, and several other proteins are involved in intrinsic parts of the proposed pathway.
Conclusions
The disease mechanisms of psoriasis remain poorly understood. In this study, we have analyzed expression patterns from twelve microarray studies with the largest cohort of patients to date (a total of 534 patients) to identify DEGs associated with psoriasis. Eleven core DEGs were identified and TF–core DEG relationships were displayed. The PPI network of these core DEGs was reconstructed. A DEG pool was formed to elucidate the psoriasis mechanism as a whole, including our core DEGs and DEGs that are found in related signaling pathways as a result of literature survey.
Instead of utilization of a gene co-expression network analysis to describe the correlation patterns among gene expression levels across microarray samples, FC values were recruited to analyze the correlation between DEGs. Identification of the central molecules (i.e., hub DEGs) and highly interconnected modules of the reconstructed FCC network resulted in a summary of the gene profiles located centrally in the modules, which illuminate the disease mechanism of psoriasis.
In conclusion, we present a comprehensive pool of differentially expressed genes in psoriasis, which have the potential for providing deep insight into the disease mechanism. In addition, a psoriasis disease pathway is proposed.
The present study offers a comprehensive outlook on the molecular framework of psoriasis and proposes new hypotheses with a view to biomarkers and future experimental studies, and establishes a computational systems biomedicine framework that can be applied to other complex human diseases in dermatology. We also call for greater computational systems biology research and analyses in dermatology and skin diseases in general.
Acknowledgments
The financial support by the Marmara University Research Fund through project FEN-B-090414-0089 is greatly acknowledged. The authors thank the Editor, Reviewers, and Prof. Raghu Sinha for their significant contributions during the revision period.
Author Disclosure Statement
No conflicting financial interests exist.
References
- Akira S, and Takeda K. (2004). Toll-like receptor signalling. Nature Rev Immunol 4, 499–511 [DOI] [PubMed] [Google Scholar]
- Alexander WS. (2002). Suppressors of cytokine signalling (SOCS) in the immune system. Nat Rev Immunol 241, 410–416 [DOI] [PubMed] [Google Scholar]
- Baker BS, Ovigne JM, Powles AV, Corcoran S, and Fry L. (2003). Normal keratinocytes express Toll-like receptors (TLRs) 1, 2 and 5: Modulation of TLR expression in chronic plaque psoriasis. Br J Dermatol 148–167, 670–679 [DOI] [PubMed] [Google Scholar]
- Barrett T, Wilhite SE, Ledoux P, et al. (2013). NCBI GEO: Archive for functional genomics data sets—update. Nucleic Acids Res 41, D991–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartfai T, Buckley PT, and Eberwine J. (2012). Drug targets: Single-cell transcriptomics hastens unbiased discovery. Trends Pharmacol Sci 33, 9–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigler J, Rand HA, Kerkof K, Timour M, and Russell CB. (2013). Cross-study homogeneity of psoriasis gene expression in skin across a large expression range. PLoS One 8, e52242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borden EC, and Williams BR. (2011). Interferon-stimulated genes and their protein products: What and how? J Interferon Cytokine Res 31, 1–4 [DOI] [PubMed] [Google Scholar]
- Borgono CA, Michael IP, Komatsu N, et al. (2007). Enzyme catalysis and regulation: A potential role for multiple tissue kallikrein serine proteases in epidermal desquamation. J Biol Chem 282, 3640–3652 [DOI] [PubMed] [Google Scholar]
- Bovolenta LA, Acencio ML, and Lemke N. (2012). HTRIdb: An open-access database for experimentally verified human transcriptional regulation interactions. BMC Genomics 13, 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg J, and Darnell JE., Jr. (2000). The role of STATs in transcriptional control and their impact on cellular function. Oncogene 19, 2468–2473 [DOI] [PubMed] [Google Scholar]
- Celis JE, Cruger D, Kiil J, et al. (1990). A two-dimensional gel protein database of noncultured total normal human epidermal keratinocytes: Identification of proteins strongly up-regulated in psoriatic epidermis. Electrophoresis 11, 242–254 [DOI] [PubMed] [Google Scholar]
- Chin CH, Chen SH, Wu HH, Ho CW, Ko MT, and Lin CY. (2014). CytoHubba: Identifying hub objects and subnetworks from complex interactome. BMC Systems Biol 8, S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coda AB, Icen M, Smith JR, and Sinha AA. (2012). Global transcriptional analysis of psoriatic skin and blood confirms known disease-associated pathways and highlights novel genomic “hot spots” for differentially expressed genes. Genomics 100, 18–26 [DOI] [PubMed] [Google Scholar]
- Conesa C, and Acker J. (2010). Sub1/PC4 a chromatin associated protein with multiple functions in transcription. RNA Biol 7, 287–290 [DOI] [PubMed] [Google Scholar]
- Delgoffe GM, and Vignali DAA. (2013). STAT heterodimers in immunity: A mixed message or a unique signal? JAKSTAT 2, e23060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doumas S, Kolokotronis A, and Stefanopoulos P. (2005). Anti-inflammatory and antimicrobial roles of secretory leukocyte protease inhibitor. Infect Immun 73, 1271–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fensterl V, and Sen GC. (2010). The ISG56/IFIT1 gene family. J Interferon Cytokine Res 31, 71–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink K, and Grandvaux N. (2013). STAT2 and IRF9: Beyond ISGF3. JAKSTAT 2, e27521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick D, Barth S, and Macleod KF. (2010). Autophagy: Cellular and molecular mechanisms. J Pathol 21, 3–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales DH, and Neupert W. (1990). Biogenesis of mitochondrial c-type cytochromes. J Bioenerg Biomembr 22, 753–768 [DOI] [PubMed] [Google Scholar]
- Gudjonsson JE, Ding J, Johnston A, et al. (2010). Assessment of the psoriatic transcriptome in a large sample: Additional regulated genes and comparisons with in vitro models. J Invest Dermatol 130, 1829–1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudjonsson JE, Ding J, Li X, et al. (2009). Global gene expression analysis reveals evidence for decreased lipid biosynthesis and increased innate immunity in uninvolved psoriatic skin. J Invest Dermatol 129, 2795–2804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo P, Luo Y, Mai G, et al. (2014). Gene expression profile based classification models of psoriasis. Genomics 103, 48–55 [DOI] [PubMed] [Google Scholar]
- Hallen LC, Burki Y, Ebeling M, et al. (2007). Antiproliferative activity of the human IFN-alpha-inducible protein IFI44. J Interferon Cytokine Res 27, 675–680 [DOI] [PubMed] [Google Scholar]
- Hanselmann C, Mauch C, and Werner S. (2001). Haem oxygenase-1: A novel player in cutaneous wound repair and psoriasis? Biochem J 353, 459–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder J, and Schröder JM. (2005). Psoriatic scales: A promising source for the isolation of human skin-derived antimicrobial proteins. J Leukoc Biol 77, 476–486 [DOI] [PubMed] [Google Scholar]
- Huang DW, Sherman BT, Tan Q, et al. (2007). DAVID Bioinformatics Resources: Expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res 35, W169–W175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry RA, Hobbs B, Collin F, et al. (2003). Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4, 249–264 [DOI] [PubMed] [Google Scholar]
- Ivashkiv LB. (2000). Jak-STAT signaling pathways in cells of the immune system. Rev Immunogenet 2, 220–230 [PubMed] [Google Scholar]
- Iwasaki A, and Medzhitov R. (2004). Toll-like receptor control of the adaptive immune responses. Nat Immunol 5, 987–995 [DOI] [PubMed] [Google Scholar]
- Jabbari A, Suárez-Fariñas M, Dewell S, and Krueger JG. (2012). Transcriptional profiling of psoriasis using RNA-seq reveals previously unidentified differentially expressed genes. J Invest Dermatol 132, 246–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Xuan Z, Zhao F, and Zhang MQ. (2007). TRED: A transcriptional regulatory element database, new entries and other development. Nucleic Acids Res 35, D137–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung YS, Lee HY, Kim SD, et al. (2013). Wnt5a stimulates chemotactic migration and chemokine production in human neutrophils. Exper Mol Med 45, e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M, Goto S, Sato Y, Furumichi M, and Tanabe M. (2012). KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res 40, D109–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagoz K, Sinha R, and Arga KY. (2015). Triple negative breast cancer: A multi-omics network discovery strategy for candidate targets and driving pathways. OMİCS 19, 115–130 [DOI] [PubMed] [Google Scholar]
- Katayama H, Sasai K, Kawai H, et al. (2004). Phosphorylation by aurora kinase A induces Mdm2-mediated destabilization and inhibition of p53. Nat Genet 36, 55–62 [DOI] [PubMed] [Google Scholar]
- Kobayashi SD, and DeLeo FR. (2009). Review: Role of neutrophils in innate immunity: A systems biology-level approach. Wiley Interdiscip Rev Syst Biol Med 1, 309–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiya Y, and Habas R. (2008). Wnt signal transduction pathways. Organogenesis 4, 68–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger G, Koo J, Lebwohl M, Menter A, Stern RS, and Rolstad T. (2001). The impact of psoriasis on quality of life: Results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol 137, 280–284 [PubMed] [Google Scholar]
- Kulski JK, Kenworthy W, Bellgard M, et al. (2005). Gene expression profiling of Japanese psoriatic skin reveals an increased activity in molecular stress and immune response signals. J Mol Med (Berl) 83, 964–975 [DOI] [PubMed] [Google Scholar]
- Lee Y, Jang S, Min JK, et al. (2012). S100A8 and S100A9 are messengers in the crosstalk between epidermis and dermis modulating a psoriatic milieu in human skin. Biochem Biophys Res Commun 423, 647–653 [DOI] [PubMed] [Google Scholar]
- Leigh IM, Navsaria H, Purkis PE. McKay IA, Bowden PE, and Riddle PN. (1995). Keratins (K16 and K17) as markers of keratinocyte hyperproliferation in psoriasis in vivo and in vitro. Br J Dermatol 133, 501–511 [DOI] [PubMed] [Google Scholar]
- Li J, Ding SC, Cho H, Chung BC, et al. (2013). A short hairpin RNA screen of interferon-stimulated genes identifies a novel negative regulator of the cellular antiviral response. M Bio 4, e00385-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu AY, Destoumieux D, Wong AV, et al. (2002). Human beta-defensin-2 production in keratinocytes is regulated by interleukin-1, bacteria, and the state of differentiation. J Investig Dermatol 118, 275–281 [DOI] [PubMed] [Google Scholar]
- Liu X, Kim CK, Yang J, Jemmerson R, and Wang X. (1996). Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome c. Cell 86, 147–157 [DOI] [PubMed] [Google Scholar]
- Loo YM, and Gale M., Jr. (2011). Immune signaling by RIG-I-like receptors. Immunity 34, 680–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Du J, Liang J, Zhu X, Yang Y, and Xu J. (2013). Transcriptional regulatory network for psoriasis. J Dermatol 40, 48–53 [DOI] [PubMed] [Google Scholar]
- Manning AL, and Dyson NJ. (2012). RB: Mitotic implications of a tumour suppressor. Nature Rev Cancer 12, 220–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marumoto T, Zhang D, and Saya H. (2005). Aurora-A–A guardian of poles. Nature Rev Cancer 5, 42–50 [DOI] [PubMed] [Google Scholar]
- Mitsui H, Suárez-Fariñas M, Belkin DA, et al. (2012). Combined use of laser capture microdissection and cDNA microarray analysis identifies locally expressed disease-related genes in focal regions of psoriasis vulgaris skin lesions. J Invest Dermatol 132, 1615–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague E, Stanberry L, Higdon R, et al. (2014) MOPED 2.5—An integrated multi-omics resource: Multi-omics profiling expression database now includes transcriptomics data. OMICS 18: 335–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moratz C, Harrison K, and Kehrl JH. (2004). Regulation of chemokine-induced lymphocyte migration by RGS proteins. Methods Enzymol 389, 15–32 [DOI] [PubMed] [Google Scholar]
- Mortusewicz O, Roth W, Li N, Cardoso MC, Meisterernst M, and Leonhardt H. (2008). Recruitment of RNA polymerase II cofactor PC4 to DNA damage sites. J Cell Biol 183, 769–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase H, Visse R, and Murphy G. (2006). Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res 15, 562–573 [DOI] [PubMed] [Google Scholar]
- Nair RP, Duffin KC, Helms C, Ding J, Stuart PE, and Goldgar D. (2009). Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat Genet 41, 199–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane H, Ishida-Yamamoto A, Takahashi H, and Iizuka H. (2002). Elafin, a secretory protein, is cross-linked into the cornified cell envelopes from the inside of psoriatic keratinocytes. J Invest Dermatol 119, 50–55 [DOI] [PubMed] [Google Scholar]
- Ning S, Pagano JS, and Barber GN. (2011). IRF7: Activation, regulation, modification and function. Genes Immun 12, 399–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura I, Gao B, Boguniewicz M, Darst MA, Travers JB, and Leung DY. (2003). Distinct patterns of gene expression in the skin lesions of atopic dermatitis and psoriasis: A gene microarray analysis. J Allergy Clin Immunol 112, 1195–1202 [DOI] [PubMed] [Google Scholar]
- Nukui T, Ehama R, Sakaguchi M, et al. (2001). S100A8/A9, a key mediator for positive feedback growth stimulation of normal human keratinocytes. J Cell Biochem 104, 453–464 [DOI] [PubMed] [Google Scholar]
- Oestreicher JL, Walters IB, Kikuchi T, et al. (2001). Molecular classification of psoriasis disease-associated genes through pharmacogenomic expression profiling. Pharmacogenomics J 1, 272–287 [DOI] [PubMed] [Google Scholar]
- Oti M, and Brunner HG. (2007). The modular nature of genetic diseases. Clin Genet 71, 1–11 [DOI] [PubMed] [Google Scholar]
- Oxenkrug GF. (2010). Tryptophan–kynurenine metabolism as a common mediator of genetic and environmental impacts in major depressive disorder: The serotonin hypothesis revisited 40 years later. Isr J Psychiatry Relat Sci 47, 56–63 [PMC free article] [PubMed] [Google Scholar]
- Pache RA, Zanzoni A, Naval J, Mas JM, and Aloy P. (2008). Towards a molecular characterization of pathological pathways. FEBS Lett 582, 1259–1265 [DOI] [PubMed] [Google Scholar]
- Pan KH, Lih CJ, and Cohen SN. (2005). Effects of threshold choice on biological conclusions reached during analysis of gene expression by DNA microarrays. Proc Natl Acad Sci USA 21;102, 8961–8965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel J, Channon KM, and McNeill E. (2013). The downstream regulation of chemokine receptor signalling: Implications for atherosclerosis. Mediators of Inflammation Article ID 459520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy MJ, Myrie KA, Neeley CK, Azim JN, Ethier SP, and Petty EM. (2000). Expression and mutational analyses of the human MAD2L1 gene in breast cancer cells. Genes Chromosomes Cancer 29, 356–362 [DOI] [PubMed] [Google Scholar]
- Perera GK, Di Meglio P, and Nestle FO. (2012). Psoriasis. Annu Rev Pathol Mech Dis 7, 385–422 [DOI] [PubMed] [Google Scholar]
- Pietzsch J, and Hoppmann S. (2009). Human S100A12: A novel key player in inflammation? Amino Acids 36, 381–389 [DOI] [PubMed] [Google Scholar]
- Rawlings JS, Rosler KM, and Harrison DA. (2004). The JAK/STAT signaling pathway. J Cell Sci 117, 1281–1283 [DOI] [PubMed] [Google Scholar]
- Razick S, Magklaras G, and Donaldson IM. (2008). iRefIndex: A consolidated protein interaction database with provenance. BMC Bioinformat 9, 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reischl J, Schwenke S, Beekman JM, Mrowietz U, Stürzebecher S, and Heubach JF. (2007). Increased expression of Wnt5a in psoriatic plaques. J Invest Dermatol 127, 163–169 [DOI] [PubMed] [Google Scholar]
- Reynier F, Petit F, Paye M, Turrel-Davin F, Imbert P-E, et al. (2011). Importance of correlation between gene expression levels: Application to the type I interferon signature in rheumatoid arthritis. PLoS ONE 6, e24828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich BE, and Kupper TS. (2001). Cytokines: IL-20—A new effector in skin inflammation. Curr Biol 11, R531–R534 [DOI] [PubMed] [Google Scholar]
- Ronpirin C, Achariyakul M, Tencomnao T, Wongpiyabovorn J, and Chaicumpa W. (2010). Up-regulation of Id1 in peripheral blood of psoriatic patients. Genetics Mol Res 9, 2239–2247 [DOI] [PubMed] [Google Scholar]
- Ruchusatsawat K, Wongpiyabovorn J, Protjaroen P, et al. (2011). Parakeratosis in skin is associated with loss of inhibitor of differentiation 4 via promoter methylation. Hum Pathol 42, 1878–1887 [DOI] [PubMed] [Google Scholar]
- Sadik CD, and Luster AD. (2012). Lipid-cytokine-chemokine cascades orchestrate leukocyte recruitment in inflammation. J Leukoc Biol 91, 207–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider WM, Chevillotte MD, and Rice CM. (2014). Interferon-stimulated genes: A complex web of host defenses. Annu Rev Immunol 32, 513–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoggins JW, and Rice CM. (2011). Interferon-stimulated genes and their antiviral effector functions. Curr Opin Virol 1, 519–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz JL, Shajahan AN, and Clarke R. (2011). The role of interferon regulatory factor-1 (IRF1) in overcoming antiestrogen resistance in the treatment of breast cancer. Intl J Breast Cancer 2011, 912102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevimoglu T, and Arga KY. (2014). The role of protein interaction networks in systems biomedicine. Computat Struct Biotechnol J 11, 22–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon P, Markiel A, Ozier O, et al. (2003). Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res 11, 2498–2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai K. (2000). Modulation of STAT signaling by STAT-interacting proteins. Oncogene 15: 19, 2638–2644 [DOI] [PubMed] [Google Scholar]
- Skulachev VP. (1998). Cytochrome c in the apoptotic and antioxidant cascades. FEBS Letters 423, 275–280 [DOI] [PubMed] [Google Scholar]
- Slattery ML, Lundgreen A, Kadlubar SA, Bondurant KL, and Wolff RK. (2013). JAK/STAT/SOCS-signaling pathway and colon and rectal cancer. Mol Carcinogen 52, 155–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth GK. (2004). Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3, Article3. [DOI] [PubMed] [Google Scholar]
- Soehnlein O, and Lindbom L. (2010). Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol 10, 427–439 [DOI] [PubMed] [Google Scholar]
- Spinelli L, Gambette P, Chapple CE, et al. (2013). Clust&See: A Cytoscape plugin for the identification, visualization and manipulation of network clusters. Biosystems 113, 91–95 [DOI] [PubMed] [Google Scholar]
- Stimweiss A, Ksienzyk A, Klages K, et al. (2010). IFN regulatory factor-1 bypasses IFN-mediated antiviral effects through viperin gene induction. J Immunol 184, 5179–5185 [DOI] [PubMed] [Google Scholar]
- Suárez-Fariñas M, Li K, Fuentes-Duculan J, Hayden K, Brodmerkel C, and Krueger JG. (2012). Expanding the psoriasis disease profile: Interrogation of the skin and serum of patients with moderate-to-severe psoriasis. J Invest Dermatol 132, 2552–2564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Farinas M, Lowes MA, Zaba LC, and Krueger JG. (2010). Evaluation of the psoriasis transcriptome across different studies by gene set enrichment analysis (GSEA). PLoS ONE 5, e10247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmann-Schmitt C, Widmann N, Dietz U, et al. (2009). Wif-1 is expressed at cartilage-mesenchyme interfaces and impedes Wnt3a-mediated inhibition of chondrogenesis. J Cell Sci 122, 3627–3637 [DOI] [PubMed] [Google Scholar]
- Tamayo P, Steinhardt G, Liberzon A, and Mesirov JP. (2012). The limitations of simple gene set enrichment analysis assuming gene independence. Stat Methods Med Res doi: 10.1177/0962280212460441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T, Yanai H, Savitsky D, and Taniguchi T. (2008). The IRF family transcription factors in immunity and oncogenesis. Annu Rev Immunol 26, 535–584 [DOI] [PubMed] [Google Scholar]
- Wehkamp J, Schmid M, and Stange EF. (2007). Defensins and other antimicrobial peptides in inflammatory bowel disease. Curr Opin Gastroenterol 23, 370–378 [DOI] [PubMed] [Google Scholar]
- Witte E, Kokolakis G, Witte K, Philipp S, Doecke WD, and Babel N. (2014). IL-19 is a component of the pathogenetic IL-23/IL-17 cascade in psoriasis. J Invest Dermatol 134, 2757–2767 [DOI] [PubMed] [Google Scholar]
- Xu L, Deng H, Yang Y, Xia J, Hung W, and Siddque T. (1997). Assignment of mitotic arrest deficient protein 2 (MAD2L1) to human chromosome band 5q23.3 by in situ hybridization. Cytogenet Cell Genet 78, 63–64 [DOI] [PubMed] [Google Scholar]
- Yao Y, Richman L, Morehouse C, et al. (2008). Type I interferon: Potential therapeutic target for psoriasis? PLoS One 16, e2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye C, and Eskin E. (2007). Discovering tightly regulated and differentially expressed gene sets in whole genome expression data. Bioinformatics 23, e84–e90 [DOI] [PubMed] [Google Scholar]
- Zebedee Z, and Hara E. (2001). Id proteins in cell cycle control and cellular senescence. Oncogene 20, 8317. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Hamming OJ, Ank N, Paludan SR, Nielsen AL, and Hartmann R. (2007). Type III interferon (IFN) induces a type I IFN-like response in a restricted subset of cells through signaling pathways involving both the Jak-STAT pathway and the mitogen-activated protein kinases. J Virol 81, 7749–7758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Nathan C, Jin W, et al. (2002). Conversion of proepithelin to epithelins: Roles of SLPI and elastase in host defense and wound repair. Cell 111, 867–878 [DOI] [PubMed] [Google Scholar]
- Zhu M, John S, Berg M, and Leonard WJ. (1999). Functional association of Nmi with Stat5 and Stat1 in IL-2- and IFNgamma-mediated signaling. Cell 96, 121–130 [DOI] [PubMed] [Google Scholar]