Abstract
Background
Campylobacter jejuni is a leading cause of bacterial enteritis worldwide. This microaerophilic bacterium can survive in aerobic environments, suggesting it has protective mechanisms against oxidative stress. The clinical C. jejuni Bf strain is characterized by an increased resistance to oxygen. This study aimed to characterize the behavior of the clinical C. jejuni Bf strain under an aerobic atmosphere and in response to ROS-promoter agents.
Methods
Growth was studied in both aerobic and microaerobic conditions using classic cultivable methods. Electronic microscopy and mreB gene expression were used to evaluate the morphology of this strain under aerobic conditions. The survival under oxidative stress was tested in the presence of different concentrations of hydrogen peroxide (H2O2) and paraquat (PQ).
Results
The results showed that C. jejuni Bf strain can grow aerobically, unlike other strains of C. jejuni tested. Cells of C. jejuni Bf exposed to oxidative stress presented changes in morphology and the gene mreB, responsible for maintaining the bacillary cell morphology, was down-expressed. In aerobically acclimated conditions, C. jejuni Bf exhibited a higher survival rate of 52 % in the presence of H2O2 (1 mM) compared to the reference strain NCTC 11168. Concentrations above 1 mM PQ were lethal for the reference strain but not for C. jejuni Bf.
Conclusions
Taken together, these data highlight the resistance to oxidative stress conditions of C. jejuni Bf, indicating that this microorganism seems more adapted to survival in hostile environmental conditions.
Electronic supplementary material
The online version of this article (doi:10.1186/s13099-015-0077-x) contains supplementary material, which is available to authorized users.
Keywords: Campylobacter jejuni, Oxygen tolerance, Hydrogen peroxide, Paraquat, Cell shape
Background
Campylobacter jejuni was the most commonly reported bacteria associated with human intestinal infections in the European Union (EU) in 2013 [1]. In 2013, 64.8 confirmed campylobacteriosis cases per 100,000 inhabitants were reported in the EU, exceeding the number of salmonellosis cases with a notification rate of 20.4 per 100,000 population [1]. C. jejuni was isolated in 80.6 % of confirmed cases of campylobacteriosis [1]. In the United States, this pathogen was the second most commonly reported bacterial pathogen (behind Salmonella) with 6621 infections in 2013 and an incidence of 13.82 cases per 100,000 population [2]. C. jejuni is described as a non-spore-forming Gram-negative bacterium, highly motile, with a spiral shape [3, 4].
In most cases, people infected by C. jejuni often have watery diarrhea, cramp, abdominal pain and fever, which may be accompanied by nausea and vomiting. Campylobacteriosis may also result in post-infection complications such as Guillain–Barré Syndrome (GBS) and its variant form, Miller Fisher Syndrome (MFS), a chronic and potentially fatal form of paralysis [5, 6].
Unlike most pathogens, Campylobacter species show fastidious growth requirements and an unusual sensitivity to environmental stress. Campylobacter is considered as an obligate microaerobic bacterium. According to Kaakoush et al. [7], Campylobacter requires low oxygen concentration, between 2–10 %, for optimal growth. Moreover, Campylobacter seems to be sensitive to high oxygen tensions therefore, theoretically, it cannot grow under an ambient atmosphere, during food processing for instance.
As a microaerophilic bacterium, the main stress encountered by C. jejuni is the oxygen concentration in the ambient atmosphere. Under conditions of oxidative stress, oxygen can be reduced to a variety of toxic intermediate products called reactive oxygen species (ROS) including the superoxide anion radical (O2−), hydrogen peroxide (H2O2) and the hydroxyl radical (−OH) [8]. Oxidative stress is produced when ROS accumulates in the bacteria to a concentration level that exceeds the defense capacity of the cell. However, despite this fastidious requirement, the increasing incidence of human enteric campylobacteriosis indicates that Campylobacter has developed mechanisms to survive oxidative stress throughout the whole food chain.
In our laboratory, the clinical C. jejuni Bf strain was isolated from a French patient in 1994. No link was established between the illness and the food ingested, but the patient was working in a wastewater treatment plant, suggesting that this strain may be present in the environment. Some studies have evaluated the ability of this strain to enter a viable but non-culturable (VBNC) state [9–12], its increased resistance to oxygen and its ability to grow under an ambient atmosphere (unpublished data). However, no study has been performed to evaluate the resistance to oxygen of C. jejuni Bf.
In order to provide data about the oxidative stress resistance of C. jejuni, we proposed to characterize the behavior of the clinical C. jejuni Bf strain under an aerobic atmosphere and in response to ROS-promoter agents. We evaluated the atypical ability of C. jejuni Bf to multiply in an ambient atmosphere, in contrast to several other strains of C. jejuni tested. We also studied the morphological changes of this strain under aerobic conditions.
Results
Multilocus sequence typing of C. jejuni Bf
Using 16S rRNA PCR (Table 1), this isolate was shown to belong to the Campylobacter genus (data not shown). Moreover, the MLST analysis revealed that this isolate is a C. jejuni strain, which is a member of the clonal complex ST-403.
Table 1.
Primers used in this study
| Primer | Sequence 5′ → 3′ | Product size (bp) |
|---|---|---|
| mreB F | TTCGTACGGCTGGAGATAAG | 234 |
| mreB R | GTGAAAGAACAGGCGAAGAG | |
| rrs F | AAGGGCCATGATGACTTGACG | 207 |
| rrs R | AGCGCAACCCACGTATTTAG | |
| 341 F | CCTACGGGAGGCACGAG | 439 |
| 758 R | CTACCAGGGTATCTAATCC | |
| MD16S F | ATCTAATGGCTTAACCATTAAAC | 856 |
| MD16S R | GGACGGTAACTAGTTTAGTATT | |
| aspA F | CCAACTGCAAGATGCTGTACC | 625 |
| aspA R | GATGGTATTACCGCAAATGAA | |
| glnA F | GCTCAATTCATGGATGGC | 722 |
| glnA R | GTTCATATGAGCTTATGGAA | |
| gltA F | GTGGCTATCCTATAGAGTGGC | 575 |
| gltA R | CAGGTATTGGTGCGCTTTGG | |
| glyA F | AGCCTAATTCAGGTTCTCAA | 667 |
| glyA R | GCGATGGAACGGATAATCACCT | |
| pgm F | TTGGAACTGATGGAGTTCG | 1298 |
| pgm R | GAAAAAGAAATGAAAAATGTTGTG | |
| tkt F | TGCACCTTTGGGCTTAGC | 702 |
| tkt R | GCACCTTTGGGTGAAGAAGT | |
| uncA F | AAAGTACAGTGGCACAAGTGG | 611 |
| uncA R | GCTAGTGATTTAGATGAGGCA |
Growth/survival of C. jejuni strains in microaerobic (MAC) and aerobic conditions (AC)
Cultivation of C. jejuni Bf in aerobic conditions in BHI broth using shaking flasks or closed tubes showed large variations in cell numbers in AC (see Additional files 1, 2). These results demonstrate the low reproducibility of C. jejuni Bf cultivation under aerobic conditions for suspended cells. A better reproducibility in AC was obtained with cells growing on solid surfaces. Consequently, the growth rate in MAC and AC of the 10 strains was compared using solid surfaces.
To compare the growth and/or survival of C. jejuni Bf on solid surfaces according to the gas composition, ten strains of C. jejuni were cultured on Karmali agar in AC or MAC for 48 h at 42 °C (Table 2).
Table 2.
Strains used in this study
| Strain of C. jejuni | Origin | Clonal complex | RefSeq | References |
|---|---|---|---|---|
| Bf | Human | ST-403 | ND | This study |
| subsp. jejuni NCTC 11168 = ATCC 700819 | Human | ST-21 | NC_002163.1 | Gundogdu et al. [53] Parkhill [54] |
| RM1221 | Chicken | ST-354 | NC_003912.7 | Fouts et al. [55] |
| subsp. jejuni 81-176 | Human | ST-42 |
NC_008787.1 NC_008790.1 NC_008770.1 |
Hofreuter et al. [56] |
| subsp. doylei 269.97, RM 4099 | Chicken | ND | NC_009707.1 | Fouts et al. [57] |
| subsp. jejuni 81116; NCTC 11828 | Human | ST-283 | NC_009839.1 | Pearson et al. [58] |
| subsp. jejuni 00-2538 | Human | ST-21 | NC_022351.2 | Clark et al. [59] |
| subsp. jejuni 00-2544 | Human | ST-21 |
NC_022353.2 NC_022354.1 |
Clark et al. [59] |
| subsp. jejuni 00-2426 | Human | ST-21 | NC_022352.2 | Clark et al. [59] |
| subsp. jejuni 00-2425 | Human | ST-21 | NC_022362.2 | Clark et al. [59] |
ND not determined
In MAC, the number of cells increased gradually from approximately 106 CFU mL−1 to more than 1010 CFU mL−1 after 24 h of incubation (Fig. 1a). The same trend was observed for C. jejuni Bf strain, with a maximum number of cells of approximately 109 CFU mL−1 after 24 h of incubation.
Fig. 1.
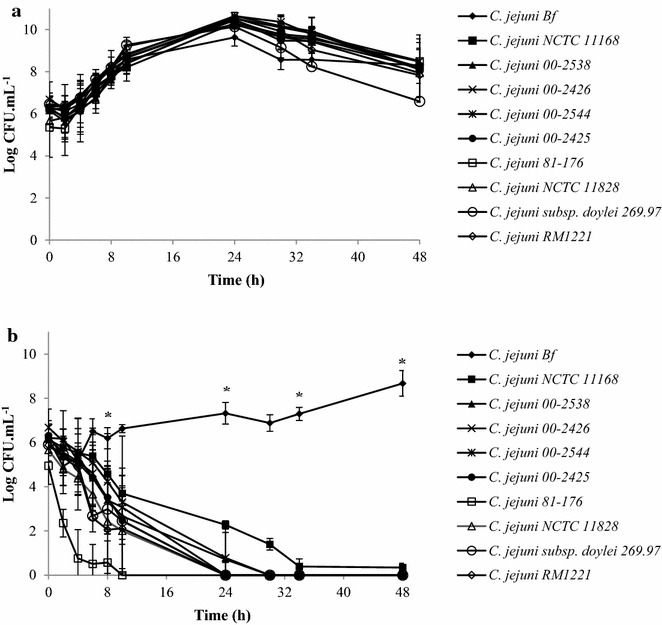
Growth and survival of C. jejuni strains under MAC (a) and AC (b) at 42 °C. The number of colony-forming units was determined by plating serial dilutions on Karmali agar using the microdroplet technique followed by incubation at 42 °C for 24 h in MAC. Plotted points are the mean measurements from three independent experiments. Error bars represent standard deviations, although they are too small to be visible in most cases. *Statistically significant difference between the strains in each condition tested (P < 0.05)
The decrease in the number of cells in MAC was heterogeneous among strains. After 48 h of incubation, the strains showed a decrease in cells to reach approximately 108 CFU mL−1, except for C. jejuni subsp. doylei 269.97, which showed a cell number of 107 CFU mL−1 (Fig. 1a).
All strains tested in this study presented a statistically higher specific growth rate under MAC than that of C. jejuni Bf strain (Table 3).
Table 3.
Growth rate after 24 h of incubation (µmax) and death times (D-values) in C. jejuni strains cultured in MAC and AC
| Strain of C. jejuni | Rate of growth (µmax) in microaerobic conditions | Death times (D-values) in aerobic conditions |
|---|---|---|
| Bf | 0.5763 | \** |
| subsp. jejuni NCTC 11168 | 0.7714* | 0.9737 |
| subsp. jejuni 00-2538 | 0.9198* | 1.1039 |
| subsp. jejuni 00-2426 | 0.8964* | 1.5442 |
| subsp. jejuni 00-2544 | 0.8303* | 1.4554 |
| subsp. jejuni 00-2425 | 0.9085* | 1.3785 |
| subsp. jejuni 81-176 | 0.9477* | 0.4765 |
| subsp. jejuni NCTC 11828 | 0.7899* | 1.3417 |
| subsp. doylei 269.97 | 0.7765* | 2.2691 |
| subsp. jejuni RM 1221 | 0.6793* | 1.9276 |
* Statistically significant difference between the strains (P < 0.05)
** Not determined because Bf is able to grow under AC
The incubation of the strains under AC led to a decrease in the number of cells of the strains tested, with complete loss of cultivability after approximately 24 h of incubation (Fig. 1b). Only C. jejuni NCTC 11168 strain showed a total loss of cultivability after approximately 34 h of incubation under AC. In contrast to the other strains, C. jejuni Bf strain in an ambient atmosphere showed a lag phase of around 10 h, which could be identified as an adaptation phase to the stress condition submitted, followed by a 2.5 log cell proliferation (Fig. 1b). Under AC, this strain showed a decrease in cell number after 24 h of incubation and subsequently remained in cell multiplication until the time of analysis (48 h) (Fig. 1b).
Moreover, no significant difference was observed in the number of cells of C. jejuni Bf strain cultured in AC and in aerobically acclimated conditions, AAC (Fig. 2). In the two conditions tested, C. jejuni Bf showed, on average, an increase of 2.8 log after 48 h of culture in AC at 42 °C (Fig. 2).
Fig. 2.
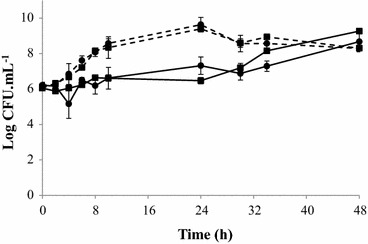
Effect of acclimation to aerobiosis on C. jejuni Bf growth under MAC (dotted line) and AC (continuous line) after aerobiosis acclimation (square) or without acclimation (circle) at 42 °C. The number of colony-forming units was determined by plating serial dilutions on Karmali agar using the microdroplet technique followed by incubation at 42 °C for 24 h in MAC. Plotted points are the mean measurements from three independent experiments. Error bars represent standard deviations, although they are too small to be visible in most cases
The great majority of strains showed death times (D-values) below 1.5 h, with a maximum value of 1.54 h for C. jejuni 00-2426 strain and a minimum value of 0.48 h for C. jejuni 81-176 strain. However, strains isolated from chicken, C. jejuni subsp. doylei and RM 1221, showed higher values of death times (2.27 and 1.93, respectively) (Table 3). These results suggest that the strain origin probably influenced the survival capacity under AC, even though only two strains of animal origin were tested.
According to the results obtained in Fig. 1b, and the similar overall response to AC of the different C. jejuni strains tested, we selected C. jejuni NCTC 11168 for further comparative analyses with C. jejuni Bf.
Cell morphology of C. jejuni NCTC 11168 and C. jejuni Bf in aerobic conditions
To characterize the effect of oxidative stress on the modification of the cell format of C. jejuni, its cell morphology was assessed.
After 12 h of incubation in MAC, the cellular morphology of C. jejuni NCTC 11168 and C. jejuni Bf exhibited an elongated spiral shape as observed using SEM (Fig. 3) with a size of approximately 2.5 and 1.5 µm, respectively (Fig. 3a, b), while after 12 h of incubation under AC, C. jejuni Bf showed a reduced cell size (approximately 0.5 µm), with a predominance of coccoid cells (Fig. 3d). Despite this oxidative stress condition, cells of C. jejuni NCTC 11168 kept their spiral shape, with a reduction in cell size to 2.0 µm (Fig. 3c).
Fig. 3.
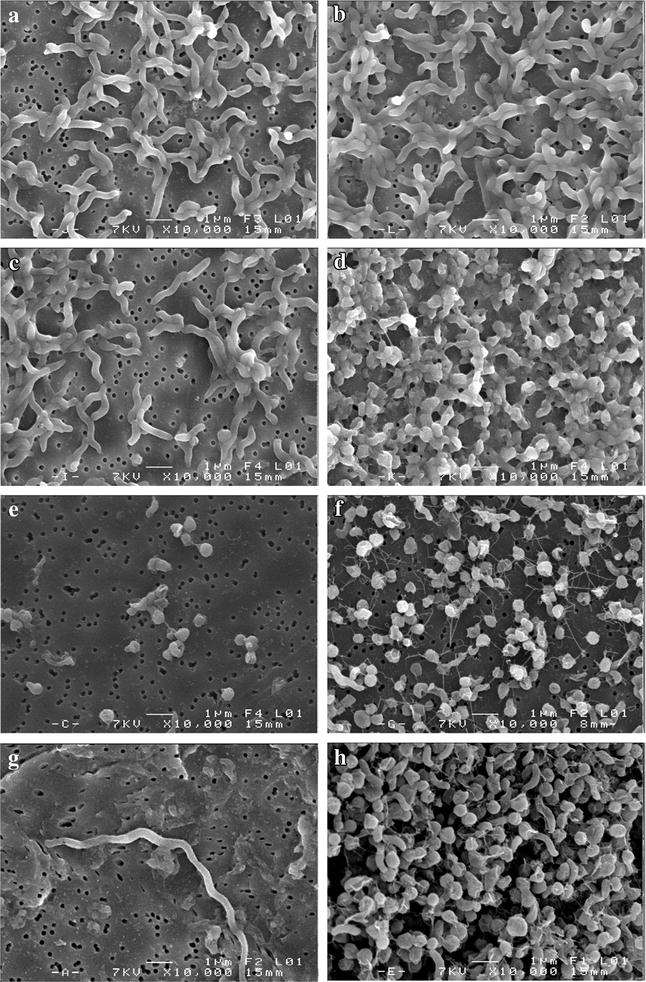
Micrographs of C. jejuni NCTC 11168 and C. jejuni Bf in MAC (a, b, respectively), and AC (c, d, respectively) after 12 h of incubation, and C. jejuni NCTC 11168 and C. jejuni Bf in MAC (e, f, respectively), and AC (g, h, respectively) after 24 h of incubation using SEM on polycarbonate membranes. Magnification: ×10,000
An incubation of 24 h under MAC led to a coccoid shape (approximately 0.5 µm) of the C. jejuni NCTC 11168 and Bf strains (Fig. 3e, f). C. jejuni Bf maintained this shape when it was exposed to an ambient atmosphere, unlike C. jejuni NCTC 11168, which exhibited elongated filaments, approximately 20.0 µm in size (Fig. 3g, h). Moreover, under both atmosphere conditions with an incubation of 24 h, cells of C. jejuni Bf seemed to interact by close contact and fiber-like structures, like a cobweb (Fig. 3f, h).
Effect of oxidative stress on the expression of the mreB gene in C. jejuni NCTC 11168 and C. jejuni Bf
The role of oxidative stress in the regulation of the mreB gene in C. jejuni was assessed at the transcript level, using qRT-PCR.
After 24 h of incubation under MAC, the mreB gene expression in the strain C. jejuni NCTC 11168 fell by 94.1 % compared with 12 h of incubation while mreB expression in C. jejuni Bf was reduced by 90.0 %. The expression of mreB under AC after 24 h of incubation in C. jejuni NCTC 11168 and C. jejuni Bf showed an increase in the relative expression of 13.9 and 0.8 times, respectively, when compared with 12 h of incubation.
After 12 h of incubation under AC, mreB gene expression significantly decreased in both strains tested in comparison with MAC. In fact, C. jejuni NCTC 11168 and C. jejuni Bf cultured aerobically had a reduced mreB gene expression of 86.3 and 91.7 %, respectively (Fig. 4a).
Fig. 4.
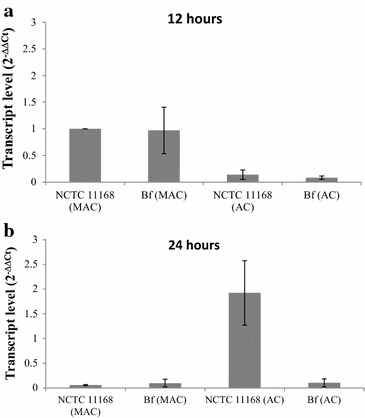
Transcript levels of the mreB gene in C. jejuni NCTC 11168 and C. jejuni Bf in AC and MAC at 12 h (a) and 24 h (b). Gene expression was estimated using RT-qPCR and the comparative critical threshold (ΔΔCT) method. The rrs gene was used as the internal control. The level of transcription of the C. jejuni NCTC 11168 strain in MAC was used to standardize the analysis
However, after 24 h of incubation, the C. jejuni NCTC 11168 strain grown aerobically showed a 1.9-fold increase in the relative mreB gene expression when compared to MAC for 12 h. On the other hand, C. jejuni NCTC 11168 and C. jejuni Bf grown in MAC and C. jejuni Bf grown in AC presented a reduced expression of the gene of 94.1, 90.3 and 89.7 %, respectively (Fig. 4b).
Effects of oxidative stress reagents on the growth of C. jejuni
To evaluate the resistance to oxidative stress and the aerotolerance of C. jejuni, NCTC 11168 and Bf strains were exposed to oxidative stress reagents (hydrogen peroxide—H2O2 and paraquat—PQ) in different concentrations.
Campylobacter jejuni Bf strain cultivated in MAC or AAC showed a higher survival rate in the presence of H2O2 and PQ than C. jejuni NCTC 11168 strain (Fig. 5).
Fig. 5.
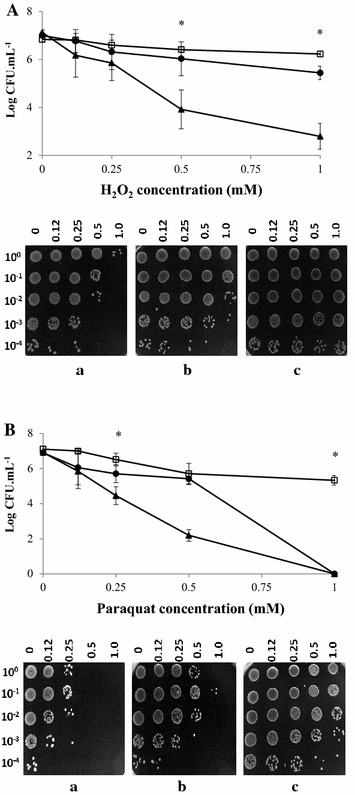
Effect of oxidative stress on the survival of C. jejuni NCTC 11168 (triangle a) and C. jejuni Bf (circle b) strains cultured in MAC, and C. jejuni Bf in AAC (square c) after exposure to different concentrations of H2O2 (A), and paraquat (B) for 1 h at 42 °C. The number of colony-forming units was determined by plating serial dilutions on Karmali agar using the microdroplet technique followed by incubation at 42 °C for 24 h in MAC. The viability of oxidative stress was determined by inoculating 10 µL of bacterial suspension on Karmali agar plates after exposure to different concentrations of H2O2 and paraquat followed by incubation at 42 °C for 24 h in MAC. Plotted points are the mean measurements from three independent experiments. Error bars represent standard deviations, although they are too small to be visible in most cases. *Statistically significant difference between the strains in each condition tested (P < 0.05)
Indeed, C. jejuni Bf strain cultivated in MAC and AAC exhibited a higher survival rate of 38.6 and 52 %, respectively, in the presence of H2O2 (1 mM) compared to the reference strain C. jejuni NCTC 11168 (Fig. 5A). The two C. jejuni strains evaluated showed no significant difference in survival at concentrations below 0.5 mM H2O2.
In the presence of 0.5 mM paraquat, C. jejuni NCTC 11168 strain showed a decrease of 4.7 log, while the C. jejuni Bf strain cultivated in MAC or AAC showed a limited reduction of 1.5 and 1.4 log, respectively. Concentrations above 1 mM PQ were lethal for the reference strain but not for aerobically-acclimated C. jejuni Bf (Fig. 5B).
Discussion
In this study, some phenotypic and genotypic characteristics of the atypical C. jejuni Bf strain were investigated, in order to provide data about the aerotolerance of this strain. It was shown that this strain can grow under aerobic conditions (AC), unlike other strains of C. jejuni. Oxidative stress changes the morphology of this strain and the transcription of the mreB gene, responsible for maintaining the bacillary cell morphology, is down-expressed. Moreover, C. jejuni Bf strain is less susceptible to oxidative stress mediated by ROS-promoter agents, in comparison with the other C. jejuni strains tested. Taken together, these data highlight the resistance to oxidative stress conditions of C. jejuni Bf.
The clonal complex ST-403, to which the C. jejuni Bf strain belongs, has been isolated from humans [13–15], but the origins of this complex are unclear, although it has been found in porcine isolates and occasionally in cattle [16–18]. This clonal complex is associated with Guillain–Barré Syndrome or enteritis as indicated by ST-403 C. jejuni HS:23 isolated from patients with these diseases [19].
As a microaerophilic microorganism, C. jejuni requires 2–10 % O2 for optimal growth [7]. However, we report here that the C. jejuni Bf strain can survive and multiply under certain AC, unlike several other strains of C. jejuni. Although some studies have already reported the ability of C. jejuni strains to survive oxygen [7, 19, 20], this is the first report demonstrating the capacity of a C. jejuni strain to multiply under atmospheric conditions, with a significant and continuous growth. Further studies are required to define whether this aero-tolerant feature observed in the current study is specific to C. jejuni Bf or could be generalized. Furthermore, this property could explain the persistence and spread of this organism in the food chain.
Many studies have investigated the survival of C. jejuni under oxidative stress. Chynoweth et al. [20] observed that strains of C. jejuni isolated from different sources showed the ability to adapt in atmospheric oxygen. Growth was limited to a few colonies on nutrient agar in atmospheric oxygen. Kaakoush et al. [7] evaluated the growth of C. jejuni strain NCTC 11168 at several cell densities in BHI broth cultures. As no growth under AC using initial cell densities lower than 104 CFU mL−1 and growth in a 19 % O2 atmosphere with Campylobacter densities higher than 105 CFU mL−1 were observed, they concluded that oxygen plays a growth-limiting role in high-density bacterial cultures under MAC and is toxic in low-density cultures under AC in C. jejuni NCTC 11168 [7]. However, the experiments conducted by Kaakoush et al. [7] were performed in liquid medium. Notably, oxygen transfer in liquid medium is low (kH = 9.28 × 10−4 mol L−1 atm−1 in water at 42 °C) and the oxygen demand by the microorganism with cell densities around 105–106 CFU mL−1 is higher than for lower densities (lower than 104 CFU mL−1). These two factors lead to a decrease in the dissolved oxygen concentration in liquid medium, which can induce the growth of Campylobacter in AC. In addition, we observed a low reproducibility of C. jejuni Bf cultivation under AC in liquid medium (BHI). Due to the absence of a robust biological reproducibility of these results, the growth curve in AC and MAC was performed on a solid surface using Karmali agar. Growth on the agar plate was reproducible for all strains used in the conditions tested, demonstrating the validity of the method. Although not a routine technique, the microbial surface growth of different strains has previously been carried out by many researchers [21–25]. Furthermore, Fujikawa and Morozumi [26] observed that the growth curves of Escherichia coli on a nutrient agar plate at constant temperatures were very similar to those in a liquid.
Jones et al. [19] reported that the C. jejuni 13997 strain grown under MAC on blood agar plates and subsequently stored under AC survived better than on plates stored under MAC. These results suggest the ability of this C. jejuni strain to adapt to an ambient gaseous atmosphere. However, these authors observed only a better survival of the C. jejuni strain tested, but no cellular growth under AC [19].
The viability of the reference strains of C. jejuni (NCTC 11168, NCTC 11828 and 81-176) under an atmospheric level of oxygen has also been evaluated in the literature [27–29]. After 9 h of exposure under atmospheric conditions, the viability of C. jejuni NCTC 11828 and C. jejuni 81-176 decreased to 10 and 50 %, respectively [28, 29] while C. jejuni NCTC 11168 exposed to ambient oxygen at 37 °C showed a reduction of approximately 2 log after 48 h of culture [27]. In the present study, C. jejuni NCTC 11828 and 81-176 strains presented a reduction of 54.4 and 88.6 % viability after 8 h of exposure to atmospheric oxygen, respectively, and C. jejuni NCTC 11168 showed a greater reduction (5.8 log) after 48 h of culture in AC at 42 °C.
In order to verify if the ability to grow in certain AC is due to an increase in oxidative stress resistance, we tested the behavior of C. jejuni Bf in the presence of H2O2 and paraquat. C. jejuni Bf was less susceptible to stress conditions induced by H2O2 and paraquat than the reference strain C. jejuni NCTC 11168, for which the lethal effect of paraquat was concentration-dependent. Unlike the results observed with C. jejuni Bf, previous studies have shown the sensitivity of C. jejuni referent strains to oxidative stress induced by H2O2 and paraquat. Garénaux et al. [30] observed that 1 h of exposure to 500 μM paraquat led to a 0.5 log10 reduction in the C. jejuni NCTC 11168 population. This same strain exposed to the final concentration of 1 mM H2O2 and paraquat for 1 h showed reduced viability (0.5 and 0.1 %, respectively) at 42 °C under MAC [31]. This resistance to oxidative stress induced by ROS-promoter agents has also been observed in other strains of C. jejuni [13, 29].
In the presence of oxygen, the microorganism undergoes genetic and physiological changes in response to oxidative stress. Some studies have reported a transition from the spiral to the coccoid form in cells of C. jejuni submitted to oxidative stress conditions [32, 33]. In addition, C. jejuni cells may be seen as filaments, doughnuts and straight rods [34–36]. According to Griffiths [35], the rod form of C. jejuni is considered to be the usual viable form in the exponential growth phase, whilst filaments and coccoid forms are associated with the stationary growth phase. We have confirmed this phenotype in C. jejuni Bf and C. jejuni NCTC 11168 submitted to oxidative stress conditions. According to our results, the stationary growth phase (after 24 h of incubation) and particularly oxidative stress conditions are the stimuli for cell transition from the spiral to the coccoid form. The presence of fiber-like structures in C. jejuni Bf cells under AC and MAC after 24 h of incubation probably plays a role in protecting them against oxidative stress [37]. Other studies have obtained similar results of cell morphology modification from spiral to coccoid forms after exposure to atmospheric oxygen [38, 39].
Cell size reduction and transition to coccoid forms might be associated with a modification of mreB gene expression, responsible for maintaining the bacillary cell morphology by forming helical filaments underneath the cell membrane [40–44]. It was suggested that the mreB-like gene present in the genome of C. jejuni NCTC 11168 plays a similar role in the maintenance of the bacterial cell shape [33]. The reduction in mreB expression in C. jejuni Bf and NCTC 11168 shown in this work during oxidative stress and stationary growth phase most likely leads to the morphological change in the cells under stress conditions. In E. coli, Bacillus subtilis and Caulobacter crescentus, the inactivation of mreB genes resulted in coccoid form formation [42, 45, 46]. Transcriptional down-regulation of Helicobacter hepaticus mreB and mreC genes also induced the formation of spherical cells [47].
This study clearly demonstrates that exposure to oxidative stress plays an important role in the morphological changes of C. jejuni under AC. These changes could contribute to the adaptation and survival of these strains in stressful conditions. Although the Bf strain exposed to AC presents a large amount of coccoid cells, this strain is able to multiply. This could be due to the presence of shorter spiral forms, which have previously been observed after exposure to atmosphere oxygen concentration [38].
Conclusions
In conclusion, C. jejuni Bf can grow and multiply under AC, unlike other strains of C. jejuni and showed a great resistance to compounds that induce oxidative stress, such as hydrogen peroxide and paraquat. This strain is therefore more adapted to survive oxidative stress conditions. During the transmission of C. jejuni to food preparation surfaces, the resistance of this strain to oxidative stress may be a key factor in explaining its spread and survival in the environment. The more this pathogen can survive in its environment, the greater is the probability of being exposed to this hazard.
However, it is important to note that we cannot say whether this property of oxidative stress resistance is intrinsic to or recently gained by a sub-type of C. jejuni, considering that only a total of ten strains of C. jejuni were tested. In addition, further studies are required to determine the cellular mechanisms that contribute to the resistance of this strain to oxidative stress. Moreover, the impact on different biological processes of C. jejuni Bf growth capacity under AC, such as its persistence in the environment, its virulence, or biofilm formation, could be investigated.
Methods
Bacterial strains and growth conditions
Strains used in this study are presented in Table 2. The genomes of the selected strains are entirely sequenced and available on NCBI. The cultures were stored at −80 °C in microtubes containing Brain–Heart Infusion medium (BHI) containing 20 % sterile glycerol. Before each experiment, the strains were grown at 42 °C on Karmali agar plates (Oxoid, Dardilly, France) for 48 h under microaerobic conditions, namely MAC (10 % CO2, 5 % O2 and 85 % N2) obtained using gas replacement jars (GRJ) 4× flushed/filled with a MACS MICS system (Whitley jar gassing system) at −50 kPa vacuum.
To prepare cells cultured in aerobic conditions (ambient atmosphere), namely AC, the strains were grown first in MAC at 42 °C on Karmali agar for 48 h. They were then harvested and resuspended in peptone saline solution and standardized to an optical density at 600 nm (OD600nm) of 0.03 (≈106 CFU mL−1). Each bacterial suspension, containing approximately 106 CFU mL−1, was then inoculated onto Karmali agar plates and incubated at 42 °C under AC for 24 h.
To prepare cells in aerobically acclimated conditions, namely AAC, C. jejuni Bf was first grown in MAC on Karmali agar for 48 h. Then, colonies were recovered and subcultured three times successively on Karmali agar plates followed by incubation at 42 °C for 24 h in AC.
DNA extraction, 16S rDNA and MLST typing of C. jejuni Bf
DNA of C. jejuni Bf was extracted using the Wizard® Genomic DNA Purification Kit (Promega, Charbonnières, France) according to the manufacturer’s instructions. DNA integrity and quantity were determined using an Experion™ Automated Electrophoresis System (BioRad, Marnes la Coquette, France) and NanoDrop 2000c (Fisher Scientific, Illkirch, France), respectively. A DNA fragment of 16S rDNA was amplified using primers specific to C. jejuni MD16S1 and MD16S2 [48] and sequenced using the Sanger protocol by Biofidal (Vaux-en-Velin, France). MLST was achieved according to Dingle et al. [49]. Briefly, the seven housekeeping genes for MLST (aspA, glnA, gltA, glyA, pgm, tkt and uncA) were amplified by PCR using the primers mentioned in Table 1. The PCR products were sequenced by Biofidal (Vaux-en-Velin, France). All allelic sequences were queried against the C. jejuni MLST database (http://pubmlst.org/campylobacter) to retrieve sequence types (STs) and determine the clonal complex of C. jejuni Bf. The nucleotide sequence data were submitted to the Campylobacter PubMLST database for allele assignments.
Growth curve of C. jejuni strains
Growth curves of C. jejuni NCTC 11168 (reference strain) and Bf were obtained from liquid cultures (using BHI) and the growth curve of C. jejuni strains shown in Table 2 were obtained from solid cultures. Bacteria were cultivated in MAC at 42 °C for 48 h. Then, colonies were transferred into a flask containing 10 mL of BHI broth grown in MAC at 42 °C with shaking (80 rpm) for 24 h. After incubation, aliquots corresponding to 2 % of the cultures (≈106 CFU mL−1) of C. jejuni NCTC 11168 and C. jejuni Bf were transferred to a flask containing 100 mL of BHI broth and incubated at 37 or 42 °C under shaking for 9 h in MAC and AC.
Alternatively, C. jejuni NCTC 11168 and Bf were cultivated in MAC at 42 °C for 48 h. Then, colonies were transferred to 50 mL of BHI in a hermetic closed tube in order to prevent a large exchange of oxygen between the culture medium and the environment. The tube was incubated under AC at 42 °C with shaking (80 rpm) for 24 h. After incubation, aliquots containing 106 cells mL−1 were inoculated in a flask containing 25 mL of BHI broth and incubated at 42 °C with shaking (80 rpm) for 18 days under AC.
Campylobacter jejuni NCTC 11168 and Bf were enumerated on Karmali agar plates using the microdroplet technique [50]. Briefly, 20 µL of serial dilutions were deposited on Karmali agar plates and incubated at 42 °C for 48 h. C. jejuni plate counts were expressed in CFU mL−1.
To determine the growth curve of C. jejuni strains in solid cultures, bacteria were cultivated in MAC at 42 °C for 48 h. However, C. jejuni Bf was cultured in AC and AAC as well. The cells obtained in these different conditions were resuspended in peptone saline solution and standardized to an OD600nm of 0.03. Then, cells were plated on Karmali agar plates and incubated at 42 °C in AC and MAC for 48 h. After incubation, cells were recovered in peptone saline solution. C. jejuni was enumerated on Karmali agar plates using the microdroplet technique followed by incubation at 42 °C for 48 h in MAC. The experiment was performed in triplicate using three biological replicates. The D-value was calculated according to the formula:
where N0 is the initial number of cells, N is the number of cells after time t of exposure to oxygen at 42 °C and D is the rate constant for inactivation of cells. Statistically significant differences were calculated using the Student t test. Values <0.05 were considered statistically significant.
Scanning electron microscopy
Campylobacter jejuni NCTC 11168 and C. jejuni Bf cells were cultivated in MAC at 42 °C for 48 h. Then, cells were resuspended in peptone saline solution, and standardized to an OD600nm of 0.01 (≈104 CFU mL−1). The cells were plated onto Karmali agar and incubated at 42 °C in AC and MAC for 12 and 24 h. After incubation, cells were recovered in peptone saline solution and aliquots of the cell suspensions were filtered on a white polycarbonate membrane (Millipore) with a 0.22 µm pore size. The membrane containing the specimens was fixed with 2.5 % (v/v) glutaraldehyde solution for 48 h at 4 °C. Then, the samples were washed in 0.2 mol L−1 sodium cacodylate (pH 7.2), and dehydrated in serial concentrations of ethanol. The membranes were transferred to a critical point dryer, and the specimens were subsequently sputter-coated and observed under a scanning electron microscope (Jeol JSM 6301F).
Bacterial RNA isolation and purification
Expression of the mreB gene, responsible for maintaining the bacillary cell morphology of the bacteria [40, 41, 51], was compared between MAC and AC. Bacterial RNA was obtained from C. jejuni NCTC 11168 and C. jejuni Bf strains cultured for 12 and 24 h in AC and MAC, at 42 °C.
After the incubation time, RNA Protect Reagent (Qiagen, Hilden) was added to the cells. Cells were centrifuged at 3300×g for 6 min at 4 °C, and then resuspended in 1 mL of Extract-All (Eurobio, France) and 0.2 mL of chloroform. After centrifugation at 12,000×g for 15 min at 4 °C, the aqueous phase was removed. Total RNA was precipitated in isopropanol, rinsed in cold ethanol 75 %, then solubilized in 50 μL of Rnase-free water (Qiagen, Hilden). DNA was removed by digestion using RQ1 RNase-free DNase (Promega, USA), and then total DNA removal was verified by PCR using 341F/758R primers. The quality and quantity of RNA were checked using a NanoDrop spectrophotometer (NanoDrop® 2000, Thermo Scientific). The integrity of RNA samples was verified on a 1 % agarose gel; electrophoresis was carried out in Tris–acetate–EDTA buffer for 50 min at 90 V. RNA concentrations were then standardized to 100 ng for each sample prior to reverse transcription.
Quantitative real-time reverse transcription PCR
The cDNA synthesis was performed using the RevertAid H Minus First-Strand cDNA synthesis kit (Euromedex) with the random hexamer primer according to the manufacturer’s instructions. The quantitative real-time PCR assay was performed with SYBR Green I (Applied Biosystems, USA) using an MJ Research PTC-200 Thermal Cycler and mreB and rrs primers (Table 1). The rrs gene was used as the internal control [52]. The composition of the PCR mix was as follows: 5.0 μL of sample, reverse primer (1 μM), forward primer (1 μM), and 12.5 μL of SYBR Green I Master Mix. The amplification program included an initial denaturing step at 95 °C (10 min), followed by 40 cycles at 95 °C (15 s) and 60 °C (1 min). A negative control (without cDNA) was included in each run. Relative quantification of mreB expression was calculated according to the 2−ΔΔCt method. The experiments were performed in triplicate from three independent cultures.
Survival of C. jejuni NCTC 11168 and C. jejuni Bf in oxidative stress conditions
Campylobacter jejuni NCTC 11168 and C. jejuni Bf grown in MAC and C. jejuni Bf in AAC were resuspended in peptone saline solution, and standardized to an OD600nm of 0.03. Subsequently, hydrogen peroxide (H2O2) (Sigma-Aldrich, Saint-Quentin Fallavier, France) or paraquat (PQ) (MP Biomedicals, Illkirch, France) solutions were used to induce a superoxidative and a peroxide stress, respectively. Concentrations of 0.12, 0.25, 0.50 and 1 mM of H2O2 or PQ were added to the bacterial suspension, which were incubated at 42 °C for 1 h under MAC. The ability of the strains to resist oxidative stress was assessed by plating serial dilutions on Karmali agar plates using the microdroplet technique followed by incubation at 42 °C for 48 h under MAC. The experiment was performed in triplicate from three independent cultures. Statistically significant differences were calculated using the Student t test. P values <0.05 were considered statistically significant.
Authors’ contributions
RCR carried out the growth/survival studies, the cell morphology analysis, the expression of genes studies, the analysis of the effects of oxidative stress reagents on the growth and drafted the manuscript. ALP participated in the growth/survival studies. MH carried out the multilocus sequence typing. NH, OT and JMC conceived of the study, participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We kindly acknowledge the financial support of one author (Ramila Cristiane Rodrigues) by the Brazilian government through the program “Ciências sem Fronteiras” and CNPq (Brazil). We thank Dr. Clifford Clark (National Laboratory for Enteric Pathogens, Winnipeg, Manitoba, Canada) for providing C. jejuni human isolates and the “Centre de Microscopie Electronique à Balayage et microAnalyse” (Rennes, France) for the electronic scanning microscopy images.
Competing interests
The authors declare that they have no competing interests.
Additional files
10.1186/s13099-015-0077-x Growth and survival of C. jejuni NCTC 11168 (●) and Bf (■) under MAC (continuous lines) and AC (dashed lines) at 37°C (A) and 42°C (B) in BHI broth. The number of colony-forming units was determined by plating serial dilutions on Karmali agar using the microdroplet technique followed by incubation at 42°C for 24 h in MAC. Plotted points are the mean measurements from three independent experiments. Error bars represent standard deviations, although they are too small to be visible in some cases.
10.1186/s13099-015-0077-x Growth and survival of C. jejuni NCTC and Bf in AC at 42°C in BHI broth.
Contributor Information
Ramila Cristiane Rodrigues, Email: ramilarodrigues@yahoo.com.br.
Anne-Lise Pocheron, Email: pocheron.annelise@gmail.com.
Mathieu Hernould, Email: mat_her33@yahoo.fr.
Nabila Haddad, Email: nabila.haddad@oniris-nantes.fr.
Odile Tresse, Email: odile.tresse@oniris-nantes.fr.
Jean-Michel Cappelier, Email: jean-michel.cappelier@oniris-nantes.fr.
References
- 1.European Food Safety Authority—EFSA The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2013. EFSA J. 2015;13:3991–4153. doi: 10.2903/j.efsa.2018.5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Center for Disease Control and Prevention—CDC. In: Incidence and trends of infection with pathogens transmitted commonly through food—Foodborne Diseases Active Surveillance Network, 10 U.S. 2014;63:328–332. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6315a3.htm?s_cid=mm6315a3w. Accessed 15 Jan 2015. [PMC free article] [PubMed]
- 3.Blaser MJ, Taylor DN, Feldman RA. Epidemiology of Campylobacter jejuni infections. Epidemiol Rev. 1983;5:157–176. doi: 10.1093/oxfordjournals.epirev.a036256. [DOI] [PubMed] [Google Scholar]
- 4.Tauxe RV. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations. In: Nachamkin I, Blaser MJ, Tompkins LS, editors. Campylobacter jejuni: current status and future trends. Washington, DC: American Society for Microbiology; 1992. pp. 9–19. [Google Scholar]
- 5.Altekruse SF, Stern NJ, Fields PI, Swerdlow DL. Campylobacter jejuni an emerging foodborne pathogen. Emerg Infect Dis. 1999;5:28–35. doi: 10.3201/eid0501.990104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuwabara S. Does Campylobacter jejuni infection elicit axonal or demyelinating Guillaine Barré syndrome, or both? J Neurol Neurosurg Psychiatry. 2011;82:238. doi: 10.1136/jnnp.2010.237040. [DOI] [PubMed] [Google Scholar]
- 7.Kaakoush NO, Miller WG, De Reuse H, Mendz GL. Oxygen requirement and tolerance of Campylobacter jejuni. Res Microbiol. 2007;158:644–650. doi: 10.1016/j.resmic.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Cabiscol E, Piulats E, Echave P, Herrero E, Ros J. Oxidative stress promotes specific protein damage in Saccharomyces cerevisiae. J Biol Chem. 2000;275:27393–27398. doi: 10.1074/jbc.M003140200. [DOI] [PubMed] [Google Scholar]
- 9.Federighi M, Tholozan JL, Cappelier JM, Tissier JP, Jouve JL. Evidence of non-coccoid viable but non-culturable Campylobacter jejuni cells in microcosm water by direct viable count, CTC-DAPI double staining, and scanning electron microscopy. Food Microbiol. 1998;15:539–550. doi: 10.1006/fmic.1998.0181. [DOI] [Google Scholar]
- 10.Cappelier JM, Minet J, Magras C, Colwell RR, Federighi M. Recovery in embryonated eggs of viable but nonculturable Campylobacter jejuni cells and maintenance of ability to adhere to HeLa cells after resuscitation. Appl Environ Microbiol. 1999;65:5154–5157. doi: 10.1128/aem.65.11.5154-5157.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tholozan JL, Cappelier JM, Tissier JP, Delattre G, Federighi M. Physiological characterization of viable-but-nonculturable Campylobacter jejuni cells. Appl Environ Microbiol. 1999;65:1110–1116. doi: 10.1128/aem.65.3.1110-1116.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cappelier JM, Rossero A, Federighi M. Demonstration of a protein synthesis in starved Campylobacter jejuni cells. Int J Food Microbiol. 2000;55:63–67. doi: 10.1016/S0168-1605(00)00195-1. [DOI] [PubMed] [Google Scholar]
- 13.Dingle KE, Colles FM, Ure R, Wagenaar JA, Duim B, Bolton FJ, et al. Molecular characterization of Campylobacter jejuni clones: a basis for epidemiologic investigation. Emerg Infect Dis. 2002;8:949–955. doi: 10.3201/eid0809.02-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duim B, Godschalk PC, van den Braak N, Dingle KE, Dijkstra JR, Leyde E, et al. Molecular evidence for dissemination of unique Campylobacter jejuni clones in Curacao, Netherlands Antilles. J Clin Microbiol. 2003;41:5593–5597. doi: 10.1128/JCM.41.12.5593-5597.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dingle KE, McCarthy ND, Cody AJ, Peto TE, Maiden MC. Extended sequence typing of Campylobacter spp., United Kingdom. Emerg Infect Dis. 2008;14:1620–1622. doi: 10.3201/eid1410.071109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.French N, Barrigas M, Brown P, Ribiero P, Williams N, Leatherbarrow H, et al. Spatial epidemiology and natural population structure of Campylobacter jejuni colonizing a farmland ecosystem. Environ Microbiol. 2005;7:1116–1126. doi: 10.1111/j.1462-2920.2005.00782.x. [DOI] [PubMed] [Google Scholar]
- 17.Kwan PS, Birtles A, Bolton FJ, French NP, Robinson SE, Newbold LS, et al. Longitudinal study of the molecular epidemiology of Campylobacter jejuni in cattle on dairy farms. Appl Environ Microbiol. 2008;74:3626–3633. doi: 10.1128/AEM.01669-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson DJ, Gabriel E, Leatherbarrow AJ, Cheesbrough J, Gee S, Bolton E, et al. Tracing the source of campylobacteriosis. PLoS Genet. 2008;4:e1000203. doi: 10.1371/journal.pgen.1000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones DM, Sutcliffe EM, Rios R, Fox AJ, Curry A. Campylobacter jejuni adapts to aerobic metabolism in the environment. J Med Microbiol. 1993;38:145–150. doi: 10.1099/00222615-38-2-145. [DOI] [PubMed] [Google Scholar]
- 20.Chynoweth RW, Hudson JA, Thom K. Aerobic growth and survival of Campylobacter jejuni in food and stream water. Lett Appl Microbiol. 1998;27:341–344. doi: 10.1046/j.1472-765X.1998.00453.x. [DOI] [PubMed] [Google Scholar]
- 21.McKay AL, Peters AC. The effect of sodium chloride concentration and pH on the growth of Salmonella typhimurium colonies on solid medium. J Appl Microbiol. 1995;79:353–359. doi: 10.1111/j.1365-2672.1995.tb03148.x. [DOI] [PubMed] [Google Scholar]
- 22.Amanatidou A, Smid EJ, Gorris LGM. Effect of elevated oxygen and carbon dioxide on the surface growth of vegetable-associated micro-organisms. J Appl Microbiol. 1999;86:429–438. doi: 10.1046/j.1365-2672.1999.00682.x. [DOI] [PubMed] [Google Scholar]
- 23.Ingham SC, Losinski JA, Dropp BK, Vivio LL, Buege DR. Evaluation of Staphylococcus aureus growth potential in ham during a slow-cooking process: use of predictions derived from the U.S. Department of Agriculture Pathogen Program Modeling Program 6.1 Predictive Model and an inoculation study. J Food Protect. 2004;67:1512–1516. doi: 10.4315/0362-028x-67.7.1512. [DOI] [PubMed] [Google Scholar]
- 24.Koutsoumanis KP, Kendall PA, Sofos JN. A comparative study on growth limits of Listeria monocytogenes as affected by temperature, pH and aw when grown in suspension or on a solid surface. Food Microbiol. 2004;21:415–422. doi: 10.1016/j.fm.2003.11.003. [DOI] [Google Scholar]
- 25.Meldrum RJ, Brocklehurst TF, Wilson DR, Wilson PDG. The effects of cell immobilization, pH and sucrose on the growth of Listeria monocytogenes Scott A at 10 °C. Food Microbiol. 2003;20:97–103. doi: 10.1016/S0740-0020(02)00083-7. [DOI] [Google Scholar]
- 26.Fujikawa H, Morozumi S. Modeling surface growth of Escherichia coli on agar plates. Appl Environ Microbiol. 2005;71:7920–7926. doi: 10.1128/AEM.71.12.7920-7926.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kassem II, Khatri M, Sanad YM, Wolboldt M, Saif YM, Olson JW, et al. The impairment of methylmenaquinol:fumarate reductase affects hydrogen peroxide susceptibility and accumulation in Campylobacter jejuni. Microbiologyopen. 2014;3:168–181. doi: 10.1002/mbo3.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baillon ML, van Vliet AH, Ketley JM, Constantinidou C, Penn CW. An iron-regulated alkyl hydroperoxide reductase (AhpC) confers aerotolerance and oxidative stress resistance to the microaerophilic pathogen Campylobacter jejuni. J Bacteriol. 1999;181:4798–4804. doi: 10.1128/jb.181.16.4798-4804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fields JA, Thompson SA. Campylobacter jejuni CsrA mediates oxidative stress responses, biofilm formation, and host cell invasion. J Bacteriol. 2008;190:3411–3416. doi: 10.1128/JB.01928-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garénaux A, Guillou S, Ermel G, Wren B, Federighi M, Ritz M. Role of the Cj1371 periplasmic protein and the Cj0355c two-component regulator in the Campylobacter jejuni NCTC 11168 response to oxidative stress caused by paraquat. Res Microbiol. 2008;159:718–726. doi: 10.1016/j.resmic.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Hwang S, Kim M, Ryu S, Jeon B. Regulation of oxidative stress response by CosR, an essential response regulator in Campylobacter jejuni. PLoS One. 2011;6:e22300. doi: 10.1371/journal.pone.0022300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moran AP, Upton ME. Factors affecting production of coccoid forms by Campylobacter jejuni on solid media during incubation. J Appl Bacteriol. 1987;62:527–537. doi: 10.1111/j.1365-2672.1987.tb02685.x. [DOI] [PubMed] [Google Scholar]
- 33.Ikeda N, Karlyshev AV. Putative mechanisms and biological role of coccoid form formation in Campylobacter jejuni. Eur J Microbiol Immunol. 2012;2:41–49. doi: 10.1556/EuJMI.2.2012.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ng LK, Sherburne R, Taylor DE, Stiles ME. Morphological forms and viability of Campylobacter species studied by electron microscopy. J Bacteriol. 1985;164:338–343. doi: 10.1128/jb.164.1.338-343.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Griffiths PL. Morphological changes of Campylobacter jejuni growing in liquid culture. Lett Appl Microbiol. 1993;17:152–155. doi: 10.1111/j.1472-765X.1993.tb00382.x. [DOI] [PubMed] [Google Scholar]
- 36.Cameron A, Frirdich E, Huynh S, Parker CT, Gaynor EC. Hyperosmotic stress response of Campylobacter jejuni. J Bacteriol. 2012;194:6116–6130. doi: 10.1128/JB.01409-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hilbert F, Scherwitzel M, Paulsen P, Szostak MP. Survival of Campylobacter jejuni under conditions of atmospheric oxygen tension with the support of Pseudomonas spp. Appl Environ Microbiol. 2010;76:5911–5917. doi: 10.1128/AEM.01532-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klancnik A, Zorman T, Mozina SS. Effects of low temperature, starvation and oxidative stress on the physiology of Campylobacter jejuni cells. Croat Chem Acta. 2008;81:41–46. [Google Scholar]
- 39.Lee YD, Choi JP, Mok CK, Ji GE, Kim HY, Noh BS, et al. Expression of flagellin proteins of Campylobacter jejuni within microaerobic and aerobic exposures. Microbiol Biotechnol. 2004;14:1227–1231. [Google Scholar]
- 40.Daniel RA, Errington J. Control of cell morphogenesis in bacteria: two distinct ways to make a rod-shaped cell. Cell. 2003;113:767–776. doi: 10.1016/S0092-8674(03)00421-5. [DOI] [PubMed] [Google Scholar]
- 41.Graumann PL. Cytoskeletal elements in bacteria. Annu Rev Microbiol. 2007;61:589–618. doi: 10.1146/annurev.micro.61.080706.093236. [DOI] [PubMed] [Google Scholar]
- 42.Figge RM, Divakaruni AV, Gober JW. MreB, the cell shape-determining bacterial actin homologue, co-ordinates cell wall morphogenesis in Caulobacter crescentus. Mol Microbiol. 2004;51:1321–1332. doi: 10.1111/j.1365-2958.2003.03936.x. [DOI] [PubMed] [Google Scholar]
- 43.Divakaruni AV, Baida C, White CL, Gober JW. The cell shape proteins MreB and MreC control cell morphogenesis by positioning cell wall synthetic complexes. Mol Microbiol. 2007;66:174–188. doi: 10.1111/j.1365-2958.2007.05910.x. [DOI] [PubMed] [Google Scholar]
- 44.Wang S, Arellano-Santoyo H, Combs PA, Shaevitz JW. An actin-like cytoskeleton contributes to cell mechanics in bacteria. Proc Natl Acad Sci. 2010;107:9182–9185. doi: 10.1073/pnas.0911517107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bendezú FO, Hale CA, Bernhardt TG, de Boer PA. RodZ (YfgA) is required for proper assembly of the MreB actin cytoskeleton and cell shape in E. coli. EMBO J. 2008;28:193–204. doi: 10.1038/emboj.2008.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawai Y, Daniel RA, Errington J. Regulation of cell wall morphogenesis in Bacillus subtilis by recruitment of PBP1 to the MreB helix. Mol Microbiol. 2009;71:1131–1144. doi: 10.1111/j.1365-2958.2009.06601.x. [DOI] [PubMed] [Google Scholar]
- 47.Okoli AS, Wilkins MR, Raftery MJ, Mendz GL. Response of Helicobacter hepaticus to bovine bile. J Proteome Res. 2010;9:1374–1384. doi: 10.1021/pr900915f. [DOI] [PubMed] [Google Scholar]
- 48.Denis M, Soumet C, Rivoal K, Ermel G, Blivet D, Salvat G, et al. Development of a m-PCR for simultaneous identification of Campylobacter jejuni and Campylobacter coli. Lett Appl Microbiol. 1999;29:406–410. doi: 10.1046/j.1472-765X.1999.00658.x. [DOI] [PubMed] [Google Scholar]
- 49.Dingle KE, Colles FM, Wareing DRA, Ure R, Fox AJ, Bolton FE, et al. Multilocus sequence typing system for Campylobacter jejuni. J Clin Microbiol. 2001;39:14–23. doi: 10.1128/JCM.39.1.14-23.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morton RD. Aerobic plate count. In: Downes FP, Ito K, editors. Compendium of methods for the microbiological examination of foods. Washington: American Public Health Association; 2001. pp. 63–67. [Google Scholar]
- 51.van den Ent F, Johnson CM, Persons L, de Boer P, Löwe J. Bacterial actin MreB assembles in complex with cell shape protein RodZ. EMBO J. 2010;29:1081–1090. doi: 10.1038/emboj.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hyytiaïnen H, Juntunen P, Akrenius N, Hanninen ML. Importance of RNA stabilization: evaluation of ansB, ggt, and rpoA transcripts in microaerophilic Campylobacter jejuni 81-176. Arch Microbiol. 2012;194:803–808. doi: 10.1007/s00203-012-0820-3. [DOI] [PubMed] [Google Scholar]
- 53.Gundogdu O, Bentley SD, Holden MT, Parkhill J, Dorrell N, Wren BW. Re-annotation and re-analysis of the Campylobacter jejuni NCTC 11168 genome sequence. BMC Genomics. 2007;8:162. doi: 10.1186/1471-2164-8-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parkhill J, Wren BW, Mungall K, Ketley JM, Churcher C, Basham D, et al. The genome se ll BG quence of the foodborne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature. 2000;403:665–668. doi: 10.1038/35001088. [DOI] [PubMed] [Google Scholar]
- 55.Fouts DE, Mongodin EF, Mandrell RE, Miller WG, Rasko DA, Ravel J, et al. Major structural differences and novel potential virulence mechanisms from the genomes of multiple campylobacter species. PLoS Biol. 2005;3:e15. doi: 10.1371/journal.pbio.0030015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hofreuter D, Tsai J, Watson RO, Novik V, Altman B, Benitez M, et al. Unique features of a highly pathogenic Campylobacter jejuni strain. Infect Immun. 2006;74:4694–4707. doi: 10.1128/IAI.00210-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fouts DE, Mongodin EF, Puiu D, Sebastian Y, Miller WG, Mandrell RE, et al. Complete genome sequence of Campylobacter jejuni subsp doylei 269.97 isolated from human blood. Submitted (JUL-2007) to the EMBL/GenBank/DDBJ databases. 2007.
- 58.Pearson BM, Gaskin DJ, Segers RP, Wells JM, Nuijten PJ, van Vliet AH. The complete genome sequence of Campylobacter jejuni strain 81116 (NCTC11828) J Bacteriol. 2007;189:8402–8403. doi: 10.1128/JB.01404-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clark CG, Chong PM, McCorrister SJ, Simon P, Walker M, Lee DM, et al. The CJIE1 prophage of Campylobacter jejuni affects protein expression in growth media with and without bile salts. BMC Microbiol. 2014;14:70–92. doi: 10.1186/1471-2180-14-70. [DOI] [PMC free article] [PubMed] [Google Scholar]


