Abstract
Background
The aim of our study is to compare the effects of thoracic epidural analgesia combined with general anesthesia (GA) vs. general anesthesia on oxygenation and pulmonary shunt fraction during one-lung ventilation (OLV).
Methods
Literature research was firstly conducted for studies related to comparison of epidural anesthesia combined with GA vs. GA with reporting of hemodynamic and oxygenation variables and published from Jan 1990 to Jan 2014 in EMBAS, MEDLINE and Cochrane Central Register of Controlled Trials databases. The studies were reviewed and data were extracted and analyzed using fixed-effect and random-effect models.
Results
There are 14 trials with 60 separate comparisons enrolling 653 patients for analysis. Regarding systemic hemodynamics, thoracic epidural analgesia decreased the mean arterial pressure and mean pulmonary arterial pressure with weighted mean difference 95 % confidence interval (−6.64 [−9.57 to −3.71] vs. -6.33 [−9.25 to −3.41] and −3.18 [−5.07 to −1.28] vs. -2.05 [−3.35 to −0.75]) respectively at the two measurements time, however, only decreasing heart rate and systemic vascular resistance (−3.28 [−5.98 to −0.67] and −319.99 [−447.05 to −192.94]) over the first 30 min after OLV. For oxygenation variables, thoracic epidural analgesia is associated with significant reduction in partial arterial oxygen pressure, mixed arterial saturation of oxygenation and increased pulmonary venous admixture fraction compared to general anesthesia with weighted mean difference 95 % confidence interval (−16.52 [−21.98 to − 11.05] vs. − 14.23 [−20.81 to − 7.65]), (0.74 [0.33 to 1.15] vs. − 0.63 [−1.23 to −0.04]) and (2.53 [1.35 to 3.72] vs. 2.77 [1.81 to 3.74]) respectively before and after 30 min of one-lung ventilation. A decrease in mixed venous saturation of oxygenation occurred after 30 min of OLV (−2.39 [−3.73 to −0.99]). Besides, a higher mean value of airway pressure was found in the thoracic epidural analgesia with weighted mean difference 95 % confidence interval (1.95 [1.61 to 2.28] vs. 0.87 [0.54 to 1.20]) at the measurements.
Conclusion
Based on the existing limited data puts forward recommendations for cautious usage of thoracic epidural analgesia in case of underlying risks in lower systemic hemodynamics, decreased partial arterial oxygen pressure but increases pulmonary shunt during one-lung ventilation.
Electronic supplementary material
The online version of this article (doi:10.1186/s12871-015-0142-5) contains supplementary material, which is available to authorized users.
Keywords: One-lung ventilation, Oxygenation, Pulmonary shunt fraction, Thoracic epidural analgesia
Background
One-lung ventilation (OLV) in the lateral position is the unique character of the thoracic surgery. During the procedure, with a potential risk of increased pulmonary shunt and occurrence of hypoxemia, the physiologic defense mechanism, termed hypoxic pulmonary vasoconstriction (HPV), starts with a rapid onset [1]. HPV is generally considered to be a factor of governing the redistribution of blood flow to prevent partial arterial oxygen pressure (PaO2) from excessively decreasing and to optimize pulmonary gas exchange during OLV. This physiologic response may be altered by many factors and the effects on oxygenation or HPV should be taken into consideration when choosing the anesthetic regimen. It was suggested that the usage of thoracic epidural anesthesia (TEA) may provide adequate analgesia, reduce postoperative mortality, improve pulmonary outcomes and facilitate fast-track approach for patients undergoing thoracic surgery [2–4]. Currently, continuous TEA combined with general anesthesia has been recommended widely in patients undergoing thoracic surgery. Due to the fact that pulmonary vasculature is dominant in sympathetic tone, it may be influenced by segmental blocking the activity of the sympathetic system over the vascular pulmonary responses. However, experimental and clinical studies on controversial effects of TEA with local anesthetics or opioids on HPV response during OLV are rare [5–7]. The optimal anesthetic management during OLV has not been yet clearly determined. Therefore, our meta-analysis is aiming to address this issue based on studies published over 15 years.
Methods
All experimental procedures listed below were approved by the Ethics Committee of China Medical University and were performed in accordance with the Helsinki Declaration for Medical Research Involving Human Subjects (the World Medical Association, Finland, created in 1964 and revised 2013).
Literature review
This meta-analysis was performed with a prospective protocol (outline below) using recommended literature search strategies incorporating multiple search terms. The literature search was performed in EMBAS, MEDLINE and the Cochrane central register of Controlled Trials databases published from Jan 1990 to Jan 2014 for the trials related to thoracic epidural anesthesia. Thoracic epidural analgesia, thoracic epidural block or ‘epidural-general’ were combined with procedure specific search terms (one-lung ventilation, intrapulmonary shunt) and limited by Human and Clinical trials. For the TEA portion, MESH term thorax, epidural, anesthesia AND text word thoracic epidural anesthesia were used and combined with OR (n = 1603). MESH term thorax, analgesia, epidural and the word thoracic epidural analgesia were used and combined with OR (n = 1292). MESH term lung, ventilation, respiration and text word one lung ventilation were used to search the database and combined with the term OR (7902). The primary investigations of 14 trials were acquired when all search terms were combined with the term AND. Each was then further checked by another two authors for any additional studies, as were the author’s personal files for additional references that met all inclusion criteria.
Study inclusion criteria
Thoracic epidural anesthesia (TEA) is defined as medicine delivered into the thoracic epidural space by injection or repeated bolus dosing local anesthetics (LA) or opioids. Studies given only a single epidural dose at the beginning or end of surgery (single shot) were not included.
Among all trials included in our meta-analysis, adult patients (aged > =18 years) were randomly assigned to one of the two groups: general intravenous or inhalation anesthesia (GA group); general anesthesia combined with TEA (TEA group). The observed variables are the hemodynamic changes, arterial and mixed venous blood gas analysis and the effects of on intrapulmonary shunt fraction during OLV. Non-English language reports were excluded.
Methodological qualities of included studies are graded using Cochrane scoring systems with a 5-point scale, in which a score of 1 is given for each of the following: 1) the description of the study as randomized, 2) the description of an appropriate method of randomization, 3) the description of the study as double-blinded, 4) the description of an appropriate method of double-blinding and 5) a statement of withdraws. Since nonrandomized studies are excluded, the minimum score is one and the maximum is five.
However, there are very few studies completely satisfied with all the criteria above. Hence, good-quality studies (prospective, randomized, and controlled) are included without weight by sample size. Any disputes were resolved by agreement of at least two reviewers
Data extraction and statistical analysis
Study information was summarized and listed in Table 1. The comparisons of intraoperatively hemodynamic parameters, blood gas analysis and secondary outcomes calculated by the standard formulas, such as mixed venous blood gas analysis and pulmonary shunt fraction during OLV are included. The comparisons in trials were then analyzed independently. Patients were subgrouped by different medications in epidural catheter of TEA group or different anesthetic regimens in GA group.
Table 1.
Included randomized controlled trials for effects of thoracic epidural analgesia on oxygenation and pulmonary shunt fraction during One-lung ventilation
| Study | Participants | Interventions | Abstracted outcomes |
|---|---|---|---|
| Ozcan et al [5] | 25 G-TIVA | G-TIVA/ISO: induced: fentanyl (3 μg.kg−1), propofol (BIS < 45) , vecuronium (0.1 mg.kg−1) | hemodynamic variables: HR, MAP; Intrapulmonary shunt: PvO2, PaO2,Qs/Qt |
| 25G-TIVA-TEA | Maintained: propofol or isoflurane respectively (according to BIS value) | ||
| 25 G-ISO | G-TIVA/ISO-TEA: T7-T8 epidural with initial 2 % lidocaine 2 mL ,0.1 % bupivacaine +0.1 mg.kg−1 morphine 10 mL, then induced the same as G-TIVA/ISO group | ||
| 25 G-ISO-TEA | Maintained: 0.1 % bupivacaine +0.1 mg.kg−1 morphine 7 ml.h-1, and maintained with propofol or isoflurane respectively (according to BIS value) | ||
| Garutti et al. [6] | 30 G-TIVA | G-TIVA: induced: fentanyl (3 μg.kg−1), midazolam (2-3 mg) , propofol (2 mg/kg), rocuronium (0.6 mg/kg) | hemodynamic variables: HR, MAP; Intrapulmonary shunt: PvO2,PaO2,Qs/Qt,PaCO2, PH,SvO2,SaO2,CaO2,CvO2; Other: Hb, Paw |
| 30 G-TIVA-TEA | Maintained: fentanyl (3 μg.kg−1), propfol (6–7 mg.kg−1.h−1); rocuronium (0.5 mg.kg−1.h−1) | ||
| G-TIVA-TEA: T6-T7 or T7-T8 epidural with initial 6–8 ml bupivacaine, then induced: same as G-TIVA group | |||
| Maintained: 0.375 % bupivacaine (6–7 mL.h−1), propofol (6–7 mg.kg−1.h−1), rocuronium (0.6 mg/kg) | |||
| Jung et al. [8] | 13G-TIVA | G-TIVA: induced: fentanyl (50–100 μg), propofol (4–5 μg/ml), vecuronium (0.1 mg.kg−1) | hemodynamic variables: HR, MAP, CVP. MPAP, PAOP, CO, SVR, PVR; Intrapulmonary shunt: PvO2,PaO2,Qs/Qt,PaCO2, PH, SvO2,SaO2; Other: Hb, Paw |
| 13G-TIVA-TEA-B | Maintained: 20 μg/ml remifentanil, 0.2 ml.kg−1.h−1; vecuronium (2–2.5 mg..h−1), propofol (according to BIS) | ||
| 13G-TIVA-TEA-S | G-TIVA-TEA-B: T5-T6 or T6-T7 epidural with initial 10 ml 5 % bupivacaine, then induced: same as G-TIVA group. | ||
| Maintained: 0.25 % bupivacaine 0.1 ml.kg−1.h−1, propofol (6–7 mg.kg−1.h−1), vecuronium (2–2.5 mg..h−1), propofol (according to BIS) | |||
| G-TIVA-TEA-S: T5-T6 or T6-T7 epidural with initial 10 ml 50 μg sufentanil , then induced :same as G-TIVA group | |||
| Maintained: 1 μg/ml sufentanil 0.1 ml.kg−1.h−1), propofol (6–7 mg.kg−1.h−1), vecuronium (2–2.5 mg..h−1), propofol (according to BIS) | |||
| Garutti et al. [9] | 37 G-TIVA | G-TIVA: induced: fentanyl (3 μg.kg−1), midazolam (0.04 mg/kg), propofol (2 mg/kg), rocuronium (0.6 mg.kg−1) | hemodynamic variables: HR, MAP; Intrapulmonary shunt: PvO2,PaO2,Qs/Qt,PaCO2, PH,SvO2,SaO2,CaO2,CvO2; Other: Hb, Paw |
| Maintained: fentanyl (2–3 μg.kg−1), propfol (6–7 mg.kg−1.h−1); rocuronium (0.5 mg.kg−1.h−1) | |||
| 35 G-TIVA-TEA | G-TIVA-TEA: T6-T7 or T7-T8 epidural with initial meperidine 2 mg.kg−1 diluted in avolume of 10–12 mL, then induced :same as G-TIVA group | ||
| Maintained: propofol (6–7 mg.kg−1.h−1), rocuronium(0.5 mg.kg−1.h−1) | |||
| Dossow et al. [10] | 25 G-TIVA | G-TIVA: induced: fentanyl (5–10 μg.kg−1), thiopental (3–5 mg.kg−1), panccuronium (0.1 mg.kg−1) | hemodynamic variables; HR, MAP, PAOP, MPAP, CVP; Intrapulmonary shunt: PvO2,SVR,PVR |
| 25 G-TIVA-TEA | Maintained: fentanyl (5–10 μg.kg−1.h−1), propfol (6–10 mg.kg−1.h−1), panccuronium(0.05–0.15 mg.kg−1) | ||
| G-TIVA-TEA: T6–7 or T7–8 epidural with initial 0.5 % bupivacaine 15 mg, then induced the same as G-TIVA group | |||
| Maintained: 0.5 % bupivacaine (range 15–25 mg), propfol (6–10 mg.kg−1.h−1), panccuronium (0.05–0.15 mg.kg−1) | |||
| Feng Y et al. [11] | 12G-TIVA | G-TIVA: induced :fentanyl (3 μg.kg−1), propfol (1 mg.kg−1) , Vecuronim (0.1 mg.kg−1) | hemodynamic variables; HR, MAP, MPAP, CVP, CO; Intrapulmonary shunt: PaO2, PaCO2, Qs/Qt |
| Maintained: propfol (9–12 mg.kg−1.h−1), Vecuronim (0.1 mg.kg−1), T7–8 or T8–9 epidural with initial 1 % lidocaine 5 ml ,then maintained with normal saline 5 ml/h | |||
| 12G-TIVA-TEA | G-TIVA-TEA: T7–8 or T8–9 epidural with initial 0.5 % ropivacaine 7–9 ml, then induced the same as G-TIVA group. | ||
| Maintaine with epidural ropivacaine 3–5 ml.h−1 combined with propfol (4.8–7.2 mg.kg−1.h−1), Vecuronim (0.1 mg.kg−1) | |||
| Lu JH et al. [12] | 10 G-SEV | G-SEV: induced: fentanyl (100 μg ), propfol (2 mg.kg−1), vecuronim (0.1 mg.kg−1) | Intrapulmonary shunt: PaO2, PaCO2, PETCO2; Other: Hb, Paw |
| 10 G-SEV-TEA | Maintained: 0.5–1.3 MAC sevofrane, panccuronium (not mentioned) | ||
| G-SEV-TEA: T5–6 or T6–7 epidural with initial 1.0 % lidocaine 5 ml then induced as G-SEV. | |||
| Maintained: epidural with 1.0 % lidocaine 5 ml.45mins−1, combined with sevofrane(0.5–1.3MAC), panccuronium (not mentioned) | |||
| Wang et al. [13] | 30 G-TIVA | G-TIVA: induced: fentanyl (2 μg.kg−1), midazolam (0.1 mg.kg-1), propfol (1.2–2.0 mg.kg−1) , vecuronim (0.1 mg.kg−1) | Intrapulmonary shunt: PaO2, PaCO2, Qs/Qt |
| 30G-TIVA-TEA | Maintained: fentanyl (0.2 μg.kg−1.h−1), propfol (3–6 mg.kg−1.h−1), vecuronium(0.05 mg.kg−1) | ||
| G-TIVA-TEA: T6–7 epidural with initial 1.0 % lidocaine 5 ml then induced as G- TIVA. | |||
| Maintained: epidural with 1.0 % lidocaine mixed with 0.25 % bupivacaine 5 ml.h−1, combined with propfol (3–6 mg.kg−1.h−1), vecuronium(0.05 mg.kg−1) | |||
| Wang et al. [14] | 16 G-ISO | G-ISO: induced: fentanyl (2 μg.kg−1), midazolam (0.1 mg.kg-1), propfol (1.0–2.0 mg.kg−1), vecuronim (0.16 mg.kg−1) | hemodynamic variables: HR, MAP; Intrapulmonary shunt: PaO2, PaCO2, PvO2, Qs/Qt |
| 14 G-ISO-TEA | Maintained: isoflurane (2.0–4.0MAC), fentanyl (50 μg) | ||
| G-ISO-TEA: T6–7 or T7-8epidural with initial 2.0 % lidocaine 3 ml then induced as G- ISO. | |||
| Maintained: epidural with 0.5 % bupivacaine 5 ml.h−1, combined with propfol (4–6 mg.kg−1.h−1) | |||
| Wang et al. [15] | 15 G-ISO | G-ISO: induced: fentanyl (2 μg.kg−1), midazolam (0.05 mg.kg-1), propfol (1.5–2.0 mg.kg−1), vecuronim (0.1 mg.kg−1) | hemodynamic variables: HR, MAP; Intrapulmonary shunt: PaO2/Fio2, Qs/Qt Other: Paw |
| 15 G-ISO-TEA | Maintained: isoflurane (0.5–1.3MAC), vecuronim (not mentioned) | ||
| G-ISO-TEA: T10–11 epidural with initial 0.5 % lidocaine then induced as G- ISO. | |||
| Maintained: epidural with 0.5 % lidocaine 5 ml.h−1, combined with isoflurane (0.5–1.3MAC), vecuronim (not mentioned) | |||
| Chen et al. [16] | 13 G-TIVA | G-TIVA: induced: fentanyl (3 μg.kg−1), midazolam (0.1 mg.kg-1), propfol (1.0–2.0 mg.kg−1), vecuronim (0.1 mg.kg−1) | hemodynamic variables; HR, MAP; Intrapulmonary shunt: PaO2,PaCO2,PvO2,Qs/Qt, SvO2,SaO2,CaO2,CvO2, PETCO2, pH; Other: Paw |
| 13G-TIVA-TEA | Maintained: fentanyl (0.2 μg.kg−1.h−1), propfol (3–6 mg.kg−1.h−1), vecuronium(0.05 mg.kg−1) | ||
| G-TIVA-TEA: T6–7 epidural with initial 1.0 % lidocaine mixed with 0.375 % bupivacaine 8–10 ml then induced as G- TIVA. | |||
| Maintained: epidural with mixture of lidocaine and bupivacaine 5 ml.h−1, combined with propfol (3–6 mg.kg−1.h−1), vecuronium(0.05 mg.kg−1) | |||
| Wu et al. [17] | 41G-TIVA | G-TIVA: induced: midazolam (0.05 mg.kg-1), fentanil, propfol and vecuronim (no details) | hemodynamic variables; HR, MAP; Intrapulmonary shunt: PaO2,PaCO2,Qs/Qt, pH |
| 41G-TIVA-TEA | Maintained: fentanil, propfol and vecuronium (no details, according to BIS). | ||
| G-TIVA-TEA: T5–6 or T6–7 epidural with initial 0.5 % ropivacaine 7–12 ml then induced as G- TIVA. | |||
| Maintained: epidural with 0.5 % ropivacaine 4–5 ml.h−1, combined with propfol and vecuronium (no details). | |||
| Zhang et al. [18] | 43G-TIVA | G-TIVA: induced: midazolam (0.05 mg.kg-1), fentanil, propfol and vecuronim (no details) | hemodynamic variables; HR, MAP; Intrapulmonary shunt: PaO2,PaCO2,Qs/Qt, |
| 43G-TIVA-TEA | Maintained: fentanil, propfol and vecuronium (no details, according to BIS). | ||
| G-TIVA-TEA: T5–6 or T6–7 epidural with initial 0.5 % ropivacaine 7–12 ml then induced as G- TIVA. | |||
| Maintained: epidural with 0.5 % ropivacaine 4–6 ml.h−1, combined with propfol and vecuronium (no details). | |||
| Sun et al. [19] | 12 G-ISO-TEA | G-ISO-TEA and G-TIVA-TEA: induced: T7–8 or T8–9 epidural with initial 0.5 % ropivacaine 7–9 ml then induced with fentanyl (3 μg.kg−1), midazolam (2-3 mg), propfol (1.5 mg.kg−1), vecuronim (0.1 mg.kg−1) | hemodynamic variables; HR, MAP,MPAP Intrapulmonary shunt: PaO2,PaCO2,Qs/Qt, pH |
| 12 G-TIVA-TEA | |||
| Maintained: isoflurane and propfol respectively(no details, according to BIS). |
TEA thoracic epidural anesthesia with local anesthetic, opioids or both, TIVA total-intravenous anesthesia, ISO isoflurane inhalation anesthesia
Quantitative analyses were performed using Review Manager Software (version 5.0; Cochrane Collaboration, Oxford shire, England). The level of significance for all tests is set at a α level of 0.05. For dichotomous data, Petro odds ratios (ORs) with 95 % confidence intervals (CIs) were computed. When possible, data were converted to means and standard deviations (SD) for continuous outcomes and calculated as weighted mean differences with 95 % CIs between active and control groups for each study. For heterogeneity analyses: data that were not significantly homogeneous (P > 0.1) were analyzed with a fixed-effect model, whereas heterogeneous data (P ≤ 0.1) were analyzed with a random effect model. Sensitivity analyses were performed to identify sources of heterogeneity. Studies which do not report mean and SD or standard error of the mean (SEM) are not included in the meta-analysis.
Results
Retrieved and included studies
Fourteen reports were retrieved while one trial with self-comparison was rejected (Fig. 1). A total of 653 patients with 60 separate comparisons met all inclusion criteria [5, 6, 8–19] (Table 1). These reports were published between 1999 and 2010 and reported data were from 653 patients. Forty one studies were excluded: 1) not thoracic surgery; 2) no available data on observed indexes; 3) the intervention used in studies not consistent with the criteria.
Fig. 1.
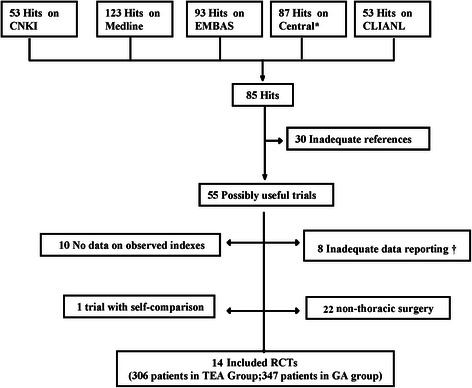
The flow diagram of retrieved, excluded, and analyzed trials. * Cochrane Central Register of Controlled Trials. †Continuous data presented as means but without standard deviation, no dichotomous data. RCT indicates randomized controlled trials
Among included trials, the epidural catheter was all placed at T6–7, T7–8 or T8–9 interspace in the TEA group and continuously injected with local anesthetics, opioids or both via the catheter during the whole OLV procedure more than 30 min. Controls received intravenous opioids with or without nonopioid analgesics (Table 1).
Risk of bias in included studies
Overall study quality was moderate. There was adequate randomization in 10/14 (71.4 %) of studies, double-blinding in 12/14 (85.7 %) of studies and statement of withdraw in 3/14 (21.4 %) of studies. Three studies were assessed all in three domains and five studies were assessed in two domains.
Thoracic Epidural analgesia on systemic hemodynamics variables
Overall, it was shown that TEA did not significantly affect the changes of hemodynamic variables during two lung ventilation, and it is associated with significantly reduction in heart rate (HR) and systemic vascular resistance (SVR) only during OLV within 30 min. However, there was a continuously significant decrease in mean arterial pressure (MAP) and mean pulmonary arterial pressure (MPAP) during the whole OLV period until re-two lung ventilation (re-TLV) (Table 2).
Table 2.
Effects of thoracic epidural analgesia on hemodynamics variables during OLV
| Median of Means (Range) | |||||
|---|---|---|---|---|---|
| All available data | No. of comparisons | TEA group | GA group | WMD (95 % CI) | P value |
| During two-lung ventilation | |||||
| HR(beat / min) | 7 | 72.98 (68.80 to 83.00) | 73.71 (69.00 to 78.00) | −1.33 [−5.16 to 2.50] | 0.50 |
| MAP(mmHg) | 5 | 83.62 (76.00 to 87.00) | 86.26. (75.00 to 93.00) | −2.28 [−5.51 to 0.95] | 0.17 |
| CO (L/min) | 2 | 5.50 (5.20 to 5.80) | 4.90 (4.90 to 4.90) | 0.55 [−0.21 to 1.30] | 0.16 |
| CVP (cmH2O) | 3 | 7.00 (6.00 to 8.00) | 8.00 (6.00 to 9.00) | −1.00 [−3.94 to 1.95] | 0.51 |
| SVR (dynes · sec · cm−5) | 3 | 1180.70(1022.00 to 1308.00) | 1401.67(1395.00 to 1405.00) | −246.16[−408.55 to -83.77] | 0.29 |
| MPAP (mmHg) | 3 | 20.67 (18.00 to 22.00) | 18.67 (18.00 to 19.00) | 2.09 [−0.94 to 5.13] | 0.18 |
| PAOP(mmHg) | 2 | 12.50 (12.00 to 13.00) | 13.00 (13.00 to 13.00) | −0.59 [−2.48 to 1.31] | 0.55 |
| During one-lung ventilation within 30 min | |||||
| HR(beat / min) | 7 | 74.14 (66.00 to 85.00) | 75.48 (73.20 to 80.00) | −3.28 [−5.89 to −0.67] | 0.01 |
| MAP(mmHg) | 7 | 85.11 (81.00 to 89.00) | 93.29 (87.60 to 100.00) | −6.64 [−9.57 to −3.71] | <0.01 |
| CO (L/min) | 2 | 5.40 (5.40 to 5.40) | 5.70 (5.70 to 5.70) | −0.30 [−1.15 to 0.55] | 0.49 |
| CVP (cmH2O) | 3 | 8.30 (7.00 to 10.00) | 9.00 (8.00 to 11.00) | −0.61 [−1.72, 0.49] | 0.28 |
| SVR (dynes · sec · cm−5) | 3 | 1003.33(890.00 to 1124.00) | 1349.67(1141.00 to 1454.00) | −319.99[−447.05 to -192.94] | <0.01 |
| MPAP (mmHg) | 3 | 20.00 (19.00 to 22.00) | 23.00 (23.00 to 23.00) | −3.18 [−5.07 to −1.28] | <0.01 |
| PAOP(mmHg) | 2 | 12.00 (12.00 to 12.00) | 13.00 (13.00 to 13.00) | −1.00 [−2.87 to 0.87] | 0.30 |
| One-lung ventilation more than 30 min | |||||
| HR(beat / min) | 6 | 76.00 (75.00 to 87.00) | 75.32 (71.50 to 80.00) | −0.94 [−3.81 to 1.92] | 0.52 |
| MAP(mmHg) | 6 | 84.12 (75.00 to 87.00) | 91.85 (85.80 to 98.00) | −6.33 [−9.25 to −3.41] | <0.01 |
| CO (L/min) | 4 | 5.55 (4.70 to 6.10) | 5.55 (5.50 to 5.60) | −0.07 [−0.64 to 0.51] | 0.82 |
| CVP (cmH2O) | 4 | 7.75 (7.00 to 9.00) | 8.00 (7.00 to 9.00) | −0.37 [−1.24 to 0.51] | 0.41 |
| SVR (dynes · sec · cm−5) | 4 | 1168.50(981.00 to 1356.00) | 1209.00(1209.00 to 1209.00) | −38.17[−201.75 to 125.42] | 0.07 |
| MPAP (mmHg) | 4 | 20.00 (18.00 to 21.00) | 22.50 (22.00 to 23.00) | −2.05 [−3.35 to −0.75] | <0.01 |
| PAOP(mmHg) | 4 | 12.50 (12.00 to 13.00) | 13.50 (13.00 to 14.00) | −1.11 [−2.40 to 0.18] | 0.09 |
| Re-two ventilation | |||||
| HR(beat / min) | 5 | 74.40 (65.00 to 87.00) | 71.60 (67.00 to 78.00) | −0.41 [−4.08 to 3.25] | 0.82 |
| MAP(mmHg) | 5 | 84.40 (81.00 to 87.00) | 95.80 (92.00 to 95.00) | −11.83 [−15.87 to −7.79] | <0.01 |
| CO (L/min) | 2 | 5.55 (5.50 to 5.60) | 5.20 (5.20 to 5.20) | 0.35 [−0.58 to 1.29] | 0.46 |
| CVP (cmH2O) | 3 | 7.67 (7.00 to 9.00) | 6.33 (6.00 to 7.00) | 1.20 [−0.15 to 2.55] | 0.08 |
| SVR (dynes · sec · cm−5) | 3 | 1157.00(1052.00 to 1316.00) | 1358.67(1226.00 to 1425.00) | −186.69[−312.37 to −61.01] | <0.01 |
| MPAP (mmHg) | 3 | 19.67 (18.00 to 21.00) | 21.33 (21.00 to 22.00) | −1.77 [−3.61 to 0.07] | 0.06 |
| PAOP(mmHg) | 2 | 11.00 (10.00 to 12.00) | 12.00 (12.00 to 12.00) | −1.03[−2.75 to 0.68] | 0.24 |
Data were presented as Mean and rang in bracket
Thoracic Epidural analgesia on oxygenation and pulmonary shunt fraction variables
It was shown that TEA did not significantly affect the pulmonary shunt fraction during TLV. From TLV to OLV, TEA modestly reduced arterial oxygen pressure (PaO2), mixed arterial saturation of oxygenation (SaO2) and increased the pulmonary venous admixture fraction (Qs/Qt%) and mean airway pressure (Paw) occurred during OLV. A decrease in mixed venous saturation of oxygenation (SvO2) occurred after 30 min of OLV (Fig. 2, Table 3).
Fig. 2.
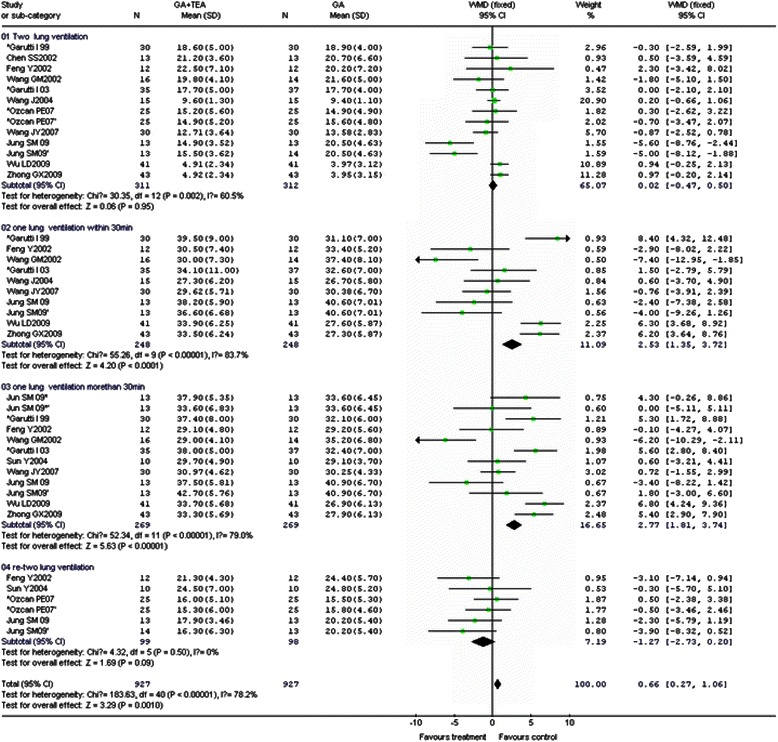
Effect of thoracic epidural analgesia (TEA) on pulmonary venous admixture fraction (Qs/Qt) in general anesthesia (GA) combined with TEA group and GA group during mechanical ventilation: Forest plot showing pooled analysis of the WMD of Qs/Qt in two groups during two-lung ventilation (01), one-lung ventilation within 30 min (02), one-lung ventilation more than 30 min (03) and re-two-lung ventilation (04) based on the fixed effects model. CI = confidence interval
Table 3.
Effects of thoracic epidural analgesia on oxygenation and pulmonary shunt fraction variables during OLV
| Median of Means (Range) | |||||
|---|---|---|---|---|---|
| All available data | No. of comparisons | TEA group | GA group | WMD (95 % CI) | P value |
| During two-lung ventilation | |||||
| PaO2 (mmHg) | 13 | 382.42(205.00 to 85.00) | 377.92(191.0 to 453.00) | 6.29 [0.04 to 12.17] | 0.35 |
| PaCO2 (mmHg) | 10 | 39.67 (32.50 to 37.6) | 40.23 (35.60 to 37.40) | −0.21 [−1.11 to 0.68] | 0.64 |
| PvO2 (mmHg) | 7 | 51.50(48.00 to 55.0) | 51.62(48.0 to 53.30) | 0.27 [−1.51 to 2.06] | 0.76 |
| Qs/Qt (%) | 13 | 14.80 (4.90 to 22.50) | 15.50 (3.95 to 21.60) | 0.02[−0.47 to 0.50] | 0.95 |
| SvO2 (%) | 4 | 85.37(83.30 to 87.10) | 86.68(84.90 to 88.20) | −1.22[−2.36 to −0.07] | 0.58 |
| SaO2 (%) | 4 | 99.75(99.70 to 99.90) | 99.45(99.00 to 99.90) | −0.06[−0.08 to −0.04] | 0.30 |
| PH | 4 | 7.43 (7.42 to 7.43) | 7.44 (7.43 to 7.44) | −0.01 [−0.02 to 0.00] | 0.21 |
| Paw (cmH2O) | 4 | 18.63 (17.00 to 20.00) | 19.03 (17.00 to 21.40) | −0.24 [−1.14 to 0.66] | 0.60 |
| During one-lung ventilation within 30 min | |||||
| PaO2 (mmHg) | 10 | 166.90(118.00 to 211.00) | 171.2(122.00 to 201.00) | −16.52[−21.98 to -11.05] | <0.01 |
| PaCO2 (mmHg) | 10 | 37.12 (36.10 to 44.20) | 36.85(34.20 to 42.20) | 0.29 [−0.53 to 1.10] | 0.49 |
| PvO2 (mmHg) | 7 | 47.78(44.90 to 54.00) | 46.14(44.50 to 51.00) | 1.13 [−0.50 to 2.76] | 0.17 |
| Qs/Qt (%) | 10 | 33.32 (27.30 to 39.50) | 32.77 (26.70 to 40.60) | 2.53 [1.35 to 3.72] | <0.01 |
| SvO2 (%) | 4 | 80.53(78.30 to 82.60) | 81.58(78.40 to 84.20) | −1.13[−2.74 to 0.48] | 0.17 |
| SaO2 (%) | 4 | 97.98(96.80 to 99.30) | 97.65(97.30 to 98.00) | 0.74[0.33 to 1.15] | <0.01 |
| PH | 4 | 7.43 (7.42 to 7.44) | 7.44 (7.42 to 7.46) | −0.01 [−0.02 to 0.001] | 0.41 |
| Paw (cmH2O) | 4 | 28.00 (23.00 to 32.50) | 27.00 (24.00 to 30.90) | 1.95 [1.61 to 2.28] | <0.01 |
| One-lung ventilation more than 30 min | |||||
| PaO2 (mmHg) | 11 | 162.27(117.00 to 203.00) | 168.72(148.00 to 221.00) | −14.23[−20.81 to -7.65] | <0.01 |
| PaCO2 (mmHg) | 11 | 36.61 (33.90 to 44.60) | 36.78 (35.00 to 44.20) | −0.22 [−0.96 to 0.53] | 0.57 |
| PvO2 (mmHg) | 6 | 44.70(42.60 to 46.10) | 44.98(43.40 to 46.60) | −0.61[−2.23 to 1.02] | 0.46 |
| Qs/Qt (%) | 12 | 34.40 (33.60 to 42.70) | 32.67 (32.10 to 40.90) | 2.77 [1.81 to 3.74] | <0.01 |
| SvO2 (%) | 6 | 79.33(77.40 to 81.10) | 81.97(78.50 to 83.90) | −2.39[−3.73 to −0.99] | <0.01 |
| SaO2 (%) | 6 | 97.68(96.60 to 98.10) | 98.20(97.20 to 99.00) | −0.63[−1.23 to −0.04] | 0.04 |
| PH | 6 | 7.43 (7.43 to 7.44) | 7.44 (7.41 to 7.46) | 0.00 [−0.01 to 0.01] | 0.50 |
| Paw (cmH2O) | 6 | 26.80 (24.00 to 32.50) | 26.72 (24.00 to 31.40) | 0.87 [0.54 to 1.20] | <0.01 |
| Re-two ventilation | |||||
| PaO2 (mmHg) | 7 | 322.28(173.00 to 482.00) | 307.28(168.00 to 407.00) | 11.54[−4.25 to 27.34] | 0.15 |
| PaCO2 (mmHg) | 3 | 37.8 (34.00 to 46.10) | 35.6 (34.90 to 36.90) | −0.60 [−3.03 to 1.83] | 0.63 |
| PvO2 (mmHg) | 5 | 47.82(43.00 to 50.00) | 48.68(46.20 to 51.00) | 0.06[−2.24 to 2.36] | 0.96 |
| Qs/Qt (%) | 6 | 18.55(15.30 to 24.50) | 20.15(15.50 to 24.80) | −1.27 [−2.73 to 0.20] | 0.09 |
| SvO2 (%) | 2 | 81.90(81.50 to 82.30) | 85.30(85.30 to 85.30) | −3.62[−6.28 to −0.95] | 0.80 |
| SaO2 (%) | 2 | 99.75(99.70 to 99.80) | 99.00 (99.00 to 99.00) | 0.75[0.52 to 0.98] | 0.67 |
| PH | 2 | 7.42 (7.41 to 7.42) | 7.42 (7.42 to 7.42) | 0.00 [−0.03 to 0.02] | 0.77 |
| Paw (cmH2O) | 2 | 19.00 (18.00 to 20.00) | 18.00 (18.00 to 18.00) | 0.95 [−0.81 to 2.71] | 0.29 |
Data were presented as Mean and rang in bracket
To overcome clinical heterogeneity, data were pooled for sensitivity analysis between different techniques of general anesthesia (e.g. total intravenous anesthesia vs. balanced anesthesia) and types of medications in epidural catheter of TEA group. There were no obviously differences in all observed variables. However, no pooling of data from epidural anesthesia with different local anesthetics was possible for small number of studies.
Discussion and conclusions
The anesthetic technique is one of several factors that can affect oxygenation and hemodynamics during one-lung ventilation (OLV), among which thoracic epidural analgesia (TEA) has demonstrated to provide statistically better acute pain relief after thoracotomy and now widely used in clinic [2, 3]. However, there were few and contradictory studies considering the effects of TEA on hemodynamics and oxygenation changes during the procedure of OLV [5, 6, 9]. This is the first meta-analysis comparing the effects of TEA on oxygenation and pulmonary shunt fraction during OLV. The most significant finding of our meta-analysis is an equivalent effect of combining TEA with any technique of general anaesthesia (GA) on hemodynamic and oxygenation variables in patients undergoing OLV after re-conversion to TLV in supine position, having positive effects on patient safety during surgery. However, the current study demonstrates that TEA inhibited hypoxic pulmonary vasoconstriction (HPV) as producing larger shunt fractions and lower PaO2, accompanied with decreased systemic hemodynamics compared with GA after undergoing OLV for more than 30 min. It is consistent with recent studies, in which the authors showed that, an increase in Qs/Qt was accompanied with a decrease in PaO2, but cardiac output (CO) and pulmonary artery pressure (PAP) were preserved between the groups [7, 20, 21]. At the same time, TEA was found to be associated with lower MPAP in line with decreased MAP and SVR, whereas CO was comparable. Ephedrine is a partial α-and ß-agonist. All trials included in our meta-analysis showed a tendency towards lower mean arterial blood pressure in the TEA group, which was treated by administering a dose of 5 or 10 mg ephedrine intravenously. Mechanism leading to less marked effects on CO was attributed to the higher incidence of ephedrine use in TEA groups. It had significant vasopressor activity in the pulmonary vascular bed that predominantly mediated by α-adrenergic receptor activation, although ephedrine dose less than 0.15 mg/kg did not increase the intrapulmonary shunt during OLV [8, 10]. Additionally, increasing in HR and ventricular contractility strengthen by ß-adrenergic subtype activation in left ventricular tissue could also explain the similarity of the compared values of CO [10]. Hence, the decrease in oxygenation was secondary to the effect of TEA on HPV and probably not on the changes of CO.
The pulmonary vasculature is dominant in sympathetic activity by the norepinephrine released from sympathetic nerve endings [6, 9, 22]. Potential mechanism of the influence of pulmonary shunt fraction during OLV was prone to cardiovascular and hemodynamic effects of TEA. Decrease in HR, MAP, stoke volume due to blockade of sympathetic activity over the vascular pulmonary responses was shown closely associated with decreased PaO2 [6, 9]. The decreased PaO2 may further have an opposite effects on HPV [5, 6, 23]. It may produce vasodilatation of the pulmonary vessels by blocking the activity of the thoracic sympathetic response [24] or stimulate precapillary vasoconstriction via a pathway involving NO and/or cyclooxygenase synthesis inhibition [17]. In an animal study by Ishibe, TEA was demonstrated to affect the ventilation/reperfusion relationship by stimulating precapillary vasoconstriction to redistribute pulmonary blood flow away from hypoxemic lung regions to the other well oxygenated areas of the lung [7], which was consistent with the study of Garutti I [6].
However, similar but significant differences in magnitude of changes in pulmonary hemodynamic circulation accorded with systemic in both groups may contribute to the analgesic effect of TEA or the systemic effects of the absorption of the local anesthetics for overall reduced sympathetic tone, and blockade of cardiac accelerator fibers [6]. The principal weakness of our meta-analysis is that we combined data for comparison even with some studies which were heterogeneous such as epidural treatments with different drugs (e.g. local anesthetics, opioids etc.) or different patient population. On the other hand, more significant differences might attain when better designed published trails with large sample size become available.
Another main reason for failing to show the beneficial effect of TEA during OLV was that in clinical circumstances, there were several other factors affecting HPV in different direction, finally resulting in clinically significant net effects. In our study, TEA was associated with higher mean airway pressure in the dependent lung compared with GA, which may counteract HPV in the non-dependent lung by diverting blood flow away from the ventilated lung, thereby increasing the pulmonary shunt. Besides, the decreased SvO2 in the TEA after 30 mins of OLV in addition to changes in shunt fraction may better explain the mechanism of our obverted oxygenation changes. Furthermore, there is essentially uncharged PaCO2 in both groups. Though, the efficacy of HPV in hypoxic lung regions is increased in the presence of respiratory acidosis and inhibited by respiratory alkalosis. There is no net benefit to exchange gas during OLV from hypoventilation for the hypercapnia. It seems to act as a vasoconstrictor by selectively increasing ventilated lung pulmonary vascular resistance (enhanced directly regional HPV) during OLV [25]. Additional, patients having right-sided thoracotomies tend to have a larger shunt and lower PaO2 during OLV. This is because the right lung is larger and normally better perfused than the left [26]. The unwarranted effects of higher FiO2 ratios have been shown to atelectasis even after very short periods of ventilation [27, 28]. There were no observed significant differences in the other oxygenation or hemodynamic variables in our study.
Some limitations are inherent to our meta-analysis. Firstly, it is possible we have missed trial that satisfied the inclusion criteria, and some data have to be excluded as the reports are incomplete. Secondly, the quality of the randomized trials in the systemic review is varied. So few alternative protocols with small sample sizes have been studied in effects of TEA on oxygenation and pulmonary shunt fraction during OLV, and quantitative analyses were limited as a result of heterogeneity and outcomes measures. Epidural thoracic anesthesia can be performed with LA, opioids or both, which limits the studies with homogeneous design from which data can be pooled. The sensitivity analyses were only chosen according to the different anesthetic regimens of GA group, although it is unlikely that different subgroups would have changed our findings, such as re-subgrouped by the different medication of TEA group, use of right arterial blood samples instead of pulmonary arterial blood [5, 29] or different sympathetic block level. Thirdly, the time intervals for outcome assessments were chosen with principle of the greatest degree of inclusion. Although the findings are reported as statistically significant, they are very discrete in clinical terms. It might be a possibility of different results because different measurements were evaluated after OLV, but it is still difficult to draw definite conclusions until further large, well conducted trials are performed.
Nevertheless, the analyses performed with limited studies allowed us to put forward recommendations for cautious usage of TEA in counteracting HPV undergoing OLV by producing a larger shunt and a decrease in oxygenation during the OLV as the vasodilatation caused by sympathetic blockade.
Acknowledgments
Funding for this project was provided by Doctoral Fund of Ministry of Education of China, No. 20092104110009 and Natural Science Foundation of China, No. 81271370.
Abbreviations
- CIs
Confidence intervals
- CO
Cardiac output
- GA
General anesthesia
- Hb
Hemoglobin
- HR
Heart rate
- HPV
Hypoxic pulmonary vasoconstriction
- MAP
Mean arterial pressure
- MPAP
Mean pulmonary arterial pressure
- OLV
One-lung ventilation
- Ors
Peto odds ratios
- Paw
Mean airway pressure
- PaO2
Partial arterial oxygen pressure
- PAOP
Pulmonary artery occlusion pressure
- Qs/Qt
Pulmonary venous admixture fraction
- SD
Standard deviations
- SEM
Standard error of the mean
- SaO2
Mixed arterial saturation of oxygenation
- SvO2
Mixed venous saturation of oxygenation
- TEA
Thoracic epidural analgesia
- TIVA
Total intravenous anesthesia
- TLV
Two lung ventilation
- WMD
Weighted mean difference
Additional file
PRISMA 2009 checklist. (DOC 68 kb)
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
XQL, WFT, JW and BF participated in the literature research and review. XQL, HM and WFT participated in data Extraction, and performed most statistical analysis; BF, HM and W-FT performed in making inclusion criteria and statistical analysis; JW and HM participated involved in the guide of model design and study design; JW gave important directions to data analysis and manuscript writing. All authors read and approved the final manuscript.
Contributor Information
Xiao-Qian Li, Email: shirley037305@hotmail.com.
Wen-Fei Tan, Email: winfieldtan@hotmail.com.
Jun Wang, Email: wjun2000@hotmail.com.
Bo Fang, Email: drunk0630@aliyun.com.
Hong Ma, Phone: +86-24-8328-3157, Email: mahong5466@yahoo.com.
References
- 1.Karzai W, Schwarzkopf K. Hypoxemia during one-lung ventilation. Curr Opin Anaesthesiol. 2009;22:1–3. doi: 10.1097/ACO.0b013e32831a43dc. [DOI] [PubMed] [Google Scholar]
- 2.Guay J. The benefits of adding epidural analgesia to general anesthesia: a meta analysis. J Anesth. 2006;20(4):335–40. doi: 10.1007/s00540-006-0423-8. [DOI] [PubMed] [Google Scholar]
- 3.Popping DM, Elia N, Marret E, Remy C, Tramer MR. Protective effects of epidural analgesia on pulmonary complications after abdominal and thoracic surgery. Arch Surg. 2008;143(10):990–9. doi: 10.1001/archsurg.143.10.990. [DOI] [PubMed] [Google Scholar]
- 4.Lawrence VA, Cornell JE, Smetana GW. Strategies to reduce postoperative pulmonary complications after noncardiothoracic surgery: systematic review of the American College of Physicians. Ann Intern Med. 2006;114(8):596–608. doi: 10.7326/0003-4819-144-8-200604180-00011. [DOI] [PubMed] [Google Scholar]
- 5.Ozcan PE, Senturk M, Sungur Ulke Z, Toker A, Ozden E, Camci E. Effects of thoracic epidural anaesthesia on pulmonary venous admixture and oxygenation during one-lung ventilation. Acta Anaesthesiol Scand. 2007;51:1117–22. doi: 10.1111/j.1399-6576.2007.01374.x. [DOI] [PubMed] [Google Scholar]
- 6.Garutti I, Quintana B, Olmedilla L, Cruz A, Barranco M, Garcia E. Arterial oxygenation during one-lung ventilation: Combined versus general anesthesia. Anesth Analg. 1999;88:494–9. doi: 10.1213/00000539-199903000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Ishibe Y, Shiokawa Y, Umeda T, Uno H, Nakamura M, Izumi T. The effect of thoracicepidural anesthesia on hypoxic pulmonary vasoconstriction in dog : Ananalysis of the pressure-flow curve. Anesth Analg. 1996;82:1049–55. doi: 10.1097/00000539-199605000-00030. [DOI] [PubMed] [Google Scholar]
- 8.Jung SM, Cho CK, Kim YJ, Cho HM, Kim CW, Kwon HU, et al. The effect of thoracic epidural anesthesia on pulmonary shunt fraction and arterial oxygenation during one-lung ventilation. J Cardiothorac Vasc Anesth. 2010;24(3):456–62. doi: 10.1053/j.jvca.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 9.Garutti I, Cruz P, Olmedilla L, Barrio JM, Cruz A, Fernandez C, et al. Effects of thoracic epidural meperidine on arterial oxygenation during one-lung ventilation in thoracic surgery. J Cardiothorac Vasc Anesth. 2003;17(3):302–5. doi: 10.1016/S1053-0770(03)00056-9. [DOI] [PubMed] [Google Scholar]
- 10.Von Dossow V, Welte M, Zaune U, Martin E, Walter M, Rückert J, et al. Thoracic epidural anesthesia combined with general anesthesia: the preferred anesthetic technique for thoracic surgery. Anesth Analg. 2001;92(4):848–54. doi: 10.1097/00000539-200104000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Feng Y, Sun Y, Yang BX. The effect of epidural ropicacaine on arterial oxygenation and intrapulmonary shunt during one-lung ventilation. Chin J Anesthesiol. 2002;22:143–7. [Google Scholar]
- 12.Lu JH, Chang YL, Niu L. The effect of epidural anesthesia combined with inhaled anesthesia on oxygenation during one-lung ventilation. Chin J Anesthesiol. 2001;21:371–2. [Google Scholar]
- 13.Wang ZY, Luo X, Wang DJ, Ma ZJ. Influence of general-thoracic epidural anaesthesia on intrapulmonary shunt and blood oxygenation during one-lung ventilation. Modem Medical Journal. 2007;35:390–2. [Google Scholar]
- 14.Wang GM, Xu H, Zeng YM. The effect of epidural anesthesia combined with propfol intravenous anesthesia on intrapulmonary shunt during one-lung ventilation. Journal of Practical Medicine. 2002;18:943–4. [Google Scholar]
- 15.Wang J, Li W, Li Y. The effect of lower thoracic epidural block on intrapulmonary shunt (Qs/Qt) during one-lung ventilation. J Emerg Med. 2004;9(4):277–9. [Google Scholar]
- 16.Chen SS, Fang NX, Zhao Q, Zhang J. The effect of thoracic epidural block on atrial oxygenation and intrapulmonary shunt during one-lung ventilation. J clin Anesthesiol. 2002;18:432–43. [Google Scholar]
- 17.Wu LD, Zhu XH, Zhou Y, Dong LP, Xu GH. Influence of general anesthesia combined with epidural block on the aged during one-lung ventilation in patients undergoing thoracic surgery. Chinese Journal of Clinical Medicine. 2009;16:443–5. [Google Scholar]
- 18.Zhang GX, Wu LD. Clinical observations of influence of general anesthesia combined with epidural block on the aged patients during one-lung ventilation. Acta Academiae Medicineae JiangXi. 2009;49:84–6. [Google Scholar]
- 19.Sun Y, Feng Y, Yang BX. Comparison of the effects of isoflurane and propofol in conjunction with thoracic epidural block on oxygenation and shunt fraction during one-lung anesthesia. Chin J Anesthesiol. 2002;22:403–5. [Google Scholar]
- 20.Pagel PS, John JL, Damask MC, Davis RF, Samuelson PN, Howie MB, et al. Desflurane and isoflurane produce similar alterations in systemic and pulmonary hemodynamics and arterial oxygenation in patients undergoing one-lung ventilation during thoracotomy. Anesth Analg. 1998;87:800–7. doi: 10.1097/00000539-199810000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Casati A, Mascotto G, Iemi K, Nzepa-Batonga J, de Luca M. Epidural block does not worsen oxygenation during one-lung ventilation for lung resections under isoflurane/nitrous oxide anesthesia. Euro J Anesth. 2005;22:363–8. doi: 10.1017/S0265021505000621. [DOI] [PubMed] [Google Scholar]
- 22.Freise H, Meissner A, Lauer S, Aellger B, Radke R, Bruewer M, et al. Thoracic epidural analgesia with low concentration of bupivacaine induces thoracic and lumbar sympathetic block. Anesthesiology. 2008;109:1107–12. doi: 10.1097/ALN.0b013e31818db16c. [DOI] [PubMed] [Google Scholar]
- 23.Xu YJ, Tan ZM, Wang S, Shao HJ, Zhu XQ. Effect of thoracic epidural anesthesia with different concentration of Ropivacaine on arterial oxygenation during one-lung ventilation. Anesthesiology. 2010;112:1146–54. doi: 10.1097/ALN.0b013e3181d40347. [DOI] [PubMed] [Google Scholar]
- 24.Moudgil R, Michelakis D, Archer SL. Hypoxic pulmonary vasoconstriction. J Appl Physiol. 2005;98:390. doi: 10.1152/japplphysiol.00733.2004. [DOI] [PubMed] [Google Scholar]
- 25.Loepply JA, Scotto P, Riedel CE, Roach RC, Chick TW. Effects of acid/base status on acute pulmonary vasoconstriction and gas exchange. J Appl Physiol. 1993;72:1787–97. doi: 10.1152/jappl.1992.72.5.1787. [DOI] [PubMed] [Google Scholar]
- 26.Kurilova OA, Vyzhigina MA, Titov VA, Kozlov SP, Zhukova SG, Parshin VD. High level thoracic epidural analgesia as a special component of anesthesia duringthoracic surgeries. Anesteziol Reanimatol. 2011;10:7. [PubMed] [Google Scholar]
- 27.Edmark L, Kostova-Aherdan K, Enlund M, Hedenstierna G. Optimal oxygen concentration during induction of general anesthesia. Anesthesiology. 2003;98:28–33. doi: 10.1097/00000542-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Turnaoglu S, Tugrul M, Camei E, Cakar N, Akinci O, Ergin P. Clinical applicability of the substitution of mixed venous oxygen saturation by central venous saturation. J Cardiothorac Vasc Anesth. 2001;15:574–9. doi: 10.1053/jcan.2001.26534. [DOI] [PubMed] [Google Scholar]
- 29.Senturk NM, Dilek A, Camci R, Senturk E, Orhan M, Tugrul M, et al. Effects of positive end-expiratory pressure on ventilatory and oxygenation parameters during pressure-controlled one-lung ventilation. J Cardiothorac Vasc Anesth. 2005;19:71–5. doi: 10.1053/j.jvca.2004.11.013. [DOI] [PubMed] [Google Scholar]


