Abstract
Background
Unhealthy alcohol use is a major contributor to the global burden of disease and injury. The US Preventive Services Task Force has recommended alcohol screening and intervention in general medical settings since 2004. Yet less than one in six US adults report health care professionals discussing alcohol with them. Little is known about methods for increasing implementation; different staffing models may be related to implementation effectiveness. This implementation trial compared delivery of alcohol screening, brief intervention and referral to specialty treatment (SBIRT) by physicians versus non-physician providers receiving training, technical assistance, and feedback reports.
Methods
The study was a cluster randomized implementation trial (ADVISe [Alcohol Drinking as a Vital Sign]). Within a private, integrated health care system, 54 adult primary care clinics were stratified by medical center and randomly assigned in blocked groups of three to SBIRT by physicians (PCP arm) versus non-physician providers and medical assistants (NPP and MA arm), versus usual care (Control arm). NIH-recommended screening questions were added to the electronic health record (EHR) to facilitate SBIRT. We examined screening and brief intervention and referral rates by arm. We also examined patient-, physician-, and system-level factors affecting screening rates and, among those who screened positive, rates of brief intervention and referral to treatment.
Results
Screening rates were highest in the NPP and MA arm (51 %); followed by the PCP arm (9 %) and the Control arm (3.5 %). Screening increased over the 12 months after training in the NPP and MA arm but remained stable in the PCP arm. The PCP arm had higher brief intervention and referral rates (44 %) among patients screening positive than either the NPP and MA arm (3.4 %) or the Control arm (2.7 %). Higher ratio of MAs to physicians was related to higher screening rates in the NPP and MA arm and longer appointment times to screening and intervention rates in the PCP arm.
Conclusion
Findings suggest that time frames longer than 12 months may be required for full SBIRT implementation. Screening by MAs with intervention and referral by physicians as needed can be a feasible model for increasing the implementation of this critical and under-utilized preventive health service within currently predominant primary care models.
Trial registration: Clinical Trials NCT01135654
Keywords: Alcohol screening, Brief intervention for alcohol misuse, Primary care, Unhealthy alcohol use, Cluster randomized trial, Implementation
Background
Unhealthy alcohol use is a major contributor to the global burden of disease and injury [1, 2] and accounts for one in ten deaths of US adults aged 20–64 [3]. Screening and brief intervention to address non-dependent unhealthy alcohol use within primary care is effective [4–6] and recommended in national practice guidelines (including the U.S. Preventive Services Task Force (USPSTF) [7], the U.S. National Institute on Alcohol Abuse and Alcoholism’s (NIAAA) Clinician’s Guide “Helping Patients Who Drink Too Much” [8], and Canada’s College of Family Physicians Guideline [9]), but is seldom delivered in health care settings. Fewer than one in six Americans report being asked about or discussing their drinking with a health professional [10], and screening is rarely conducted in US primary care settings [11] outside the Veteran’s Affairs Health System (VA) [12]. Since efforts to implement alcohol Screening and Brief Intervention (for non-dependent unhealthy use, such as binge drinking with no current adverse consequences [13]) and Referral to Treatment (for alcohol dependence) (SBIRT) have fallen short [14], the field has called for more SBIRT implementation research [15–17]. This is particularly timely given important new environmental factors, such as the alcohol screening and brief counseling performance measures developed and approved by American Medical Association’s Physician Consortium for Performance Improvement, the Affordable Care Act’s provisions for substance use services, the Mental Health Parity and Addiction Equity Act, and Medicare reimbursement codes for SBIRT [18].
Studies of SBIRT implementation within primary care settings have found implementation difficult [19–21] and have typically focused on delivery of SBIRT by physicians. A European study of a tailored, multi-faceted improvement program for physician-delivered SBIRT [20] found little change in screening and intervention behavior at 12 months. Implementation of SBIRT delivery by primary care physicians, including brief advice, has been difficult to achieve even with financial incentives provided [19].
Non-physician provision of SBIRT may also be considered as a viable approach. Babor and colleagues found higher implementation rates by non-physicians and similar effectiveness to physician-delivered screening and brief intervention [22, 23]. Moreover, use of non-physician providers to conduct behavioral and disease management interventions is increasingly being considered as an appropriate and cost-effective alternative to physician delivery, and has been found to be acceptable to patients [24–29]. There is evidence that primary care teams involving nurses or health educators for behavioral interventions have higher rates of preventive screenings, such as colorectal cancer screening [30].
The Alcohol Drinking as a Vital Sign (ADVISe) study is a cluster, randomized trial in 54 adult primary care clinics within 11 Kaiser Permanente Northern California (KPNC) medical centers. It compares implementation of primary care physician (PCP)-delivered to non-physician provider-delivered SBIRT, and to usual care. In the non-physician provider-delivered arm, medical assistants (MAs) screen and clinical health educators, behavioral medicine specialists or registered nurses deliver brief intervention and referral to treatment (NPP and MA arm). The outcomes examined are screening rates and rates of brief intervention and referral to treatment (BI/RT) among those who screened positive. Cluster randomization of clinics prevented contamination between clinicians, and also was necessary because training and quality improvement is generally conducted at the clinic level. Because there is some variation in practice and leadership across the organization by medical center [31] and consistent with prior research on physician versus non-physician SBIRT implementation [22], we present findings overall and by medical center. Based on prior research showing that non-physicians can increase implementation of preventive and behavioral interventions, and findings that the most common barriers to SBIRT delivery in primary care are limited physician time and competing priorities [28, 32–35], we hypothesized that the NPP & MA arm would have higher rates of screening and BI/RT than the PCP arm.
To inform implementation strategies, we also examine how, within each intervention arm (PCP and NPP and MA), patient, physician, and system-level factors may be associated with SBIRT implementation as the literature suggests that such factors influence implementation [36, 37]. Prior research suggests that critical factors within these domains include provider training, time constraints [28, 32, 35], lack of treatment referral resources [38], systems issues such as staff workloads, patient demographic and clinical characteristics, and integration of screening into the electronic health record (EHR) [36, 39–41]. Thus, we examined how implementation outcomes were related to patient factors including demographic characteristics and medical and psychiatric problems [42], physician factors including training, demographics and specialty [28, 43, 44], and system factors [22, 45] including office visit length, the ratio of support staff to physicians, and co-location of specialty alcohol and drug (AOD) services [46].
Due to the naturalistic design of the current study we did not measure provider attitudes. However, recent research from a large European implementation trial [47, 48] suggests that providers’ role security with and commitment to addressing alcohol misuse are cross-sectionally related to reports of addressing alcohol misuse [47]. Yet, neither role security nor therapeutic commitment were prospectively related to screening or intervention [48].
To better understand the system context, we surveyed physician leaders within the study sites to understand alcohol screening practices and policies within the internal setting prior to the training and implementation processes. We hold constant a key factor found to be a salient influence on provider performance of care services—an EHR screening tool [49] made available in all three study arms. EHR use is growing steadily, both domestically [50, 51] and in many developed [52] and developing [53, 54] countries, increasing opportunities to incorporate such tools broadly. The findings on physician versus non-physician implementation and factors related to implementation within each delivery model can inform delivery and implementation of alcohol SBIRT and similar USPSTF-recommended behavioral care services [46] in primary care settings.
Methods
Setting
KPNC is a private, integrated health care delivery system covering 15 counties and serving 3.8 million members, [55] and KP covers 40 % of the insured population in California [56] with AOD services provided internally. The membership reflects the population of the region [57, 58], with more than 40 % of adult members being non-white or Hispanic, nearly 30 % with only a high school or lower level of education, and 44 % with annual incomes less than $65,000. KPNC’s and the University of California San Francisco’s institutional review boards approved the study.
Randomization, sample and intervention
The unit of randomization was clinic. Eleven medical centers (of 47 in KPNC) were chosen to represent both urban and suburban locations and various clinic sizes. Fifty-four primary care clinics were in the 11 KPNC medical centers, located in Fairfield, Napa, Redwood City, San Francisco, San Jose, Santa Clara, Santa Rosa, South Sacramento, South San Francisco, Vacaville, and Vallejo. The 54 clinics were stratified by medical center and randomly assigned (parallel-group design) in blocked groups of three to SBIRT by physicians (PCP arm) versus non-physician providers and medical assistants (NPP and MA arm) versus usual care (Control arm), with 18 clinics in each of the three arms.
There were 204 physicians in the PCP clinics, 189 in the NPP and MA clinics, and 163 in the Control arm clinics. We excluded two physicians in the PCP arm who used an earlier version of the screening tool (see methods below for screening tool details). In the year following the date at which each clinic had started the study (ranging from September 22, 2010 to November 24, 2010), a total of 218,667 unique patients had visits to the PCP arm physicians, 223,147 to the NPP and MA arm physicians, and 197,799 to the Control arm physicians (Fig. 1). Consistent with other studies [41, 59, 60], and because screening and interventions were intended to be part of usual care, we used KPNC’s EHR and other administrative data to examine patient and provider data and did not recruit individual providers or patients.
Fig. 1.
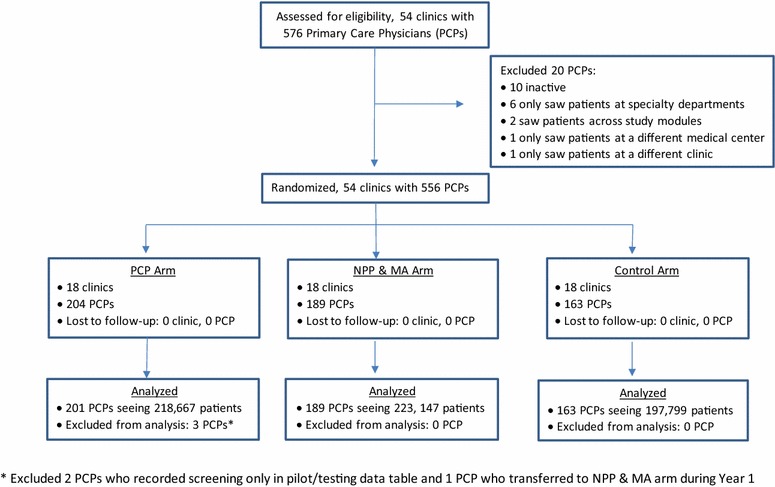
Flow diagram of participating clinics, physicians and patients through phases of the trial
Screening tools
The evidence-based [61] screening tool from the NIAAA Clinician’s Guide [8] was added to the adult primary care EHR with an automated clinical reminder at each adult (ages 18+) patient visit. It included the NIAAA single question screener “How many times in the past year have you had 5 or more drinks in a day” (for men aged 18–65, and “4 or more drinks” for women and individuals aged 66 and older) [8, 61]. Providers and MAs were instructed to use a laminated chart (distributed to exam rooms in the intervention arms) during screening to show patients various alcoholic beverage types and their standard drink sizes. Per the NIAAA Guide and prior studies, if a patient’s response was positive to the single question (i.e., >0 times exceeding the daily low-risk limit), the patient is considered an unhealthy drinker. Thus, for responses >0 times to this question, the tool prompted additional questions to assess use exceeding the NIAAA weekly low-risk limit (“On average, how many days a week do you have an alcoholic drink?” and “On a typical drinking day, how many drinks do you have?”), plus a validated two-question screener for alcohol use disorders (“In the past year, have you sometimes been under the influence of alcohol in situations where you could have caused an accident or gotten hurt?” and “Have there often been times when you had a lot more to drink than you intended to have?”; an affirmative response to either is considered a positive screening result) [62]. The EHR contained fields for recording whether a BI or RT was performed.
Intervention
In both PCP and NPP and MA arms, providers were trained to deliver the same intervention, drawn from the NIAAA Guide. Patients who screened positive on the alcohol use disorders risk questions would be referred to AOD treatment for further assessment and possible treatment. Patients who screened positive on the unhealthy drinking questions but negative for alcohol use disorder risk would receive brief intervention consisting of providers stating their concern and advising them to cut back to low risk limits or abstain, as outlined in the NIAAA Guide [8]. Further, providers were trained in brief motivational intervention, and to: (1) incorporate salient medical conditions if possible (e.g., “drinking above these limits can worsen your hypertension and insomnia”), and (2) ask patients how ready they were to make the recommended changes, and (3) assist in goal-setting to reduce or stop drinking if the patient was willing. Patients were given the NIAAA publication “Tips for Cutting Down on Drinking” [63].
PCP arm
Physicians were trained to conduct all aspects of SBIRT.
NPP and MA arm
MAs were trained to ask the screening question, and non-physician providers were trained to ask the questions about weekly drinking and the two alcohol disorder screening questions and to conduct BI/RT. Chiefs of medicine and administrative managers at each site determined which type of non-physician provider would be trained to provide BI/RT. In Medical Centers 1, 6, 10, and 11, clinical health educators were trained; in Medical Centers 2, 3, 4, 5, and 9, behavioral medicine specialists (clinical psychologists or licensed clinical social workers) were trained; and in Medical Centers 7 and 8, registered nurses were trained. MAs roles include escorting patients to exam rooms, collecting and recording (in the EHR) health screening data, including blood pressure and smoking. They are required to have completed a vocational medical assisting training program and are supervised by nurse managers.
Control arm
Patients received usual care. Providers and MAs had access to the same EHR resources as in the intervention arms and physicians were invited to a 30-min webinar information session on the EHR evidence-based screening tool. Prior to the study, alcohol screening was not part of the organizational workflow. There was no defined screening or intervention policy or standardized workflow for either physicians or non-physicians to address unhealthy alcohol use in the Control arm. In the Control arm webinar information session, the screening tool was described as evidence-based but additional details (such as sensitivity and specificity) were not provided. The NIAAA daily and weekly low-risk limits were given. No information or training were given on BI/RT in the Control condition.
Training
Training the trainers
Intervention training was adapted from the Alcohol Clinical Training (ACT) curriculum [64]. Trainers (internists, psychiatrists, psychologists, and social workers) attended one 4-h session on SBIRT and another 2-h session covering the KPNC-specific components including the EHR tool and referral information.
Training physicians and non-physician providers
PCP arm physicians and NPP and MA arm non-physician providers were given a 2-h training. The curriculum and duration of training were equivalent across arms; the trainings differed only in instruction about the workflow (with the NPP and MA arm including instruction about the handoff to the non-physician provider). In the NPP and MA arm, physicians were also included in the first hour of the training to learn about the importance and effectiveness of SBIRT and the workflow. The first hour included the NIAAA low-risk drinking limits [8], the USPSTF recommendations, the prevalence of unhealthy alcohol use in primary care in KPNC, evidence of the adverse health effects of unhealthy alcohol use, and effectiveness/cost-effectiveness of specialty treatment for alcohol, screening procedures and the screening tool (including sensitivity and specificity for identifying unhealthy alcohol use and a demonstration of the EHR screening tool); and an overview of the basics of the BI/RT and how to code for interventions/referrals. The second hour covered the BI/RT in more depth, including brief motivational interviewing, video case studies, and role-play practice. It also included a brief presentation by an AOD clinician about how to refer and assessment and treatment services offered at the local AOD clinic.
Each clinic also received in-person technical assistance 3 months after training. Trainers reviewed the clinics’ quality feedback reports on screening, intervention, and referral rates; and addressed questions and challenges. At 6 months, clinics received a 30-min “booster” training. All physicians in the PCP arm were included in the booster training. In the NPP and MA arm, physicians, NPPs and MAs were included.
MA training
In the NPP and MA arm, MAs were trained for one hour by registered nurses. The training included information about the health impact of unhealthy alcohol use, the effectiveness of SBIRT, the evidence-based screening questions and their ability to identify unhealthy alcohol use. MAs were provided with a demonstration of the EHR screening tool. They were given training on how to ask the screening questions in the EHR, which they practiced through role-play. The trainers also addressed “normalizing” alcohol screening and intervention (e.g., comparing with smoking and exercise) as integral to routine primary care services.
Training participation
In the PCP arm, 90 % of physicians attended training. In the NPP and MA arm, 100 % of non-physician providers, 85 % of MAs, and 80 % of physicians attended. For physicians who missed training, online, on-demand training was available. For MAs who missed training, their managers and senior peers provided training using the training materials. Brief videos on the EHR tool use were available to all on the EHR information and support intranet site. In the Control arm, a half-hour webinar session on use of the EHR screening tool was available on-demand via the KPNC intranet following the live session.
Quality feedback reports
In the PCP and NPP and MA arms, quarterly reports were emailed to each clinic presenting their average screening and BI/RT rates and the names of the top five performing clinics within each arm with their mean rates.
Leadership engagement
The CEO of The Permanente Medical Group (which employs the organization’s physicians) endorsed the project via a video introduction to the study training in which he discussed the links between unhealthy alcohol use and common health conditions and how SBIRT can benefit patients with unhealthy alcohol use. The Chair of the Chiefs of Adult Primary Care Medicine and the previous Chair (who retired a few months into the study) were both supportive and volunteered their clinics—“modeling” behavior for other Chiefs and physician colleagues. In the NPP and MA arm, clinic managers (to whom the MAs report) generally directed the MAs to use the screening tool, and it was generally seen as part of the MAs’ responsibilities. In each medical center, the physician chiefs of medicine (to whom the physicians report) agreed to having their sites used for the project and generally encouraged (but did not mandate) the use of SBIRT by physicians and non-physician providers.
Incentives
Incentives to conduct SBIRT were limited to clinic recognition in the quality feedback reports (see “quality feedback reports” above) for high performing clinics. There were no financial incentives for any staff or providers to conduct screening, brief intervention, or referral to treatment.
Data sources and measures
Primary care leader survey
We conducted an online survey of the Chiefs of Adult Primary Care in each medical center and the Physician Leader of each clinic, to understand practices and policies related to alcohol screening and intervention prior to the training. The response rate among the 62 physicians was 73 %. Participants were offered a $50 gift card. The survey also asked the allocated office visit length in the clinic which was used to calculate average visit time for each medical center.
EHR and administrative databases
The EHR was used to extract patient age, gender, and medical comorbidities. In the year prior to each patient’s index screening visit, we identified presence of a psychiatric diagnosis (i.e., psychoses, neurotic, personality and other psychiatric disorders, excluding AOD disorders) and presence of an other chronic disease diagnosis (yes/no) (i.e., Arthritis, Hypertension, Chronic Pain, Diabetes Mellitus, Asthma, Ischemic Heart Disease, Congestive Heart Failure, Stroke/Cerebrovascular Accident, Epilepsy, Parkinson’s Disease, End-Stage Renal Disease, HIV, Osteoporosis, Chronic Obstructive Pulmonary Disease (COPD) (ICD-9 codes available upon request). Physician race/ethnicity, gender, age, and MD specialty (Internal Medicine, Family Medicine or other) as well as the ratio of MAs to PCPs in each clinic were drawn from the administrative databases.
For each patient in the sample (i.e., who had one or more visits to a study physician in the study year), screening (1 if screened/else 0) was measured as having been asked the single-item screener as recorded in the EHR tool on any visit to the provider within the study year. If occurring in multiple visits within 1 year, the index screening visit was defined as the first visit. BI/RT was measured as having a visit in which an intervention or referral was recorded during or within 45 days of the screening visit for those who screened positive at the screening visit. We allowed a 45-day window because providers were trained to schedule a follow-up visit for the BI/RT within 6 weeks if unable to complete during the screening visit. We examined the EHR tool (containing fields for providers to record interventions and referrals) and also the diagnostic “V-code” for “Counseling, Alcohol Prevention” as indication of brief intervention or referral (as per the training). We also reported the BI and RT rates, separately, for each arm.
Other data collection
Co-location of primary care and specialty AOD clinics was measured by examining medical center maps and confirming with local staff. Anonymous questionnaires gathered at booster sessions asked providers and MAs the average amount of time spent on screening and asked providers the average total amount of time spent on BI/RT. To inform quantitative results, provider and staff feedback on the implementation and workflow challenges at the booster sessions was documented as part of data collection, and qualitative interviews with staff and providers at each study clinic were conducted.
Analyses
Analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA) and Stata version 10.1 (StataCorp, College Station, TX, USA). Physician survey results were examined descriptively. We calculated average monthly and annual screening rates for each medical center by study arm, and examined differences in screening rates between study arms using z statistics for comparisons of proportions. We also examined the number and percent screened for the best month over the 12 months after study start, which is the highest screening rate that each entity attained. Similarly, screening rates of BI/RT performed among those screened positive were calculated for each medical center and overall by study arm, and differences examined using Chi square tests. We also report a population-based BI/RT rate [65] by arm—this figure is the proportion of patients of all those with visits at the primary care clinics who received the intervention (regardless of being screened or not).
Within each intervention arm, multilevel logistic regression models were fitted to examine associations between patient-, physician- and system-level factors and screening and BI/RT implementation outcomes while accounting for clustering within physician and within clinic correlation of patient outcomes. Patient characteristics included age (18–24, 25–44, 45–59, and 60+), gender, and comorbid psychiatric and chronic conditions. Physician characteristics included age, gender, race/ethnicity, years serving the health plan, and specialty. System characteristics included co-location of primary care and AOD clinics, ratio of MAs to PCPs at the primary care clinic, average allocated office visit length, and number of physicians trained. For each outcome, we first fitted a series of multilevel univariate models to examine each predictor individually. All predictors associated with the outcome at p < 0.10 for either intervention arm were successively included in the multilevel models, with categories in the same direction being collapsed for some predictors to avoid extreme Odds Ratios (ORs) and 95 % Confidence Intervals (CIs) due to empty or small cells (for example, PCP race/ethnicity categories were collapsed to Non-White and White). Generalized Linear Latent and Mixed Models (GLLAMM) were used for multilevel analyses. We examined physician characteristics as predictors only in the PCP arm because SBIRT delivery in the NPP & MA arm was contingent on an entire team rather than an individual. No adjustments were made for multiple comparison [66].
Results
Screening practices prior to study implementation
In the survey of primary care leaders (N = 45) prior to the training, 88 % reported no policies or requirements to ask patients about alcohol. Those with policies indicated no consistent evidence-based screening methods.
Screening rates and proportion screened positive
Table 1 shows the average number of unique visits, screens per month, average percent of patients screened and the number and percent screened for the best month over the 12 months after study start in each medical center and overall. For average percent screened, the NPP and MA arm (50.9 %) was higher (p < 0.0001) than both the PCP arm (9.2 %) and the Control arm (3.5 %); and the PCP arm higher (p < 0.0001) than Control. Exceptions were three medical centers (1, 3, and 11) in which rates were higher in the Control than the PCP arm. A similar pattern was found for the best screening rate. The NPP and MA arm had higher rates (60.1 %) than either the PCP (10.7 %) or Control arm (6.0 %), and the PCP arm had higher rates than the Control arm (p < 0.0001), with higher rates in the Control than PCP arm for Medical Centers 1, 3, and 11. Some movement of MAs from the NPP and MA arm to other arms occurred in these medical centers; in some sites MAs covered duties for absent MAs in other clinics.
Table 1.
Screening rates by treatment arms in first year
| Average unique visits per month | Average screens per month | Average % screened | p value | Best month N screened | Best month % screened | p value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCP | NPP & MA | Control | PCP | NPP & MA | Control | PCP | NPP & MA | Control | PCP | NPP &MA | Control | PCP | NPP & MA | Control | |||
| Medical center | |||||||||||||||||
| 1 | 1464 | 2797 | 2928 | 41 | 1107 | 148 | 2.80 | 39.56 | 5.07 | a,b,c | 161 | 1505 | 391 | 11.47 | 46.88 | 13.81 | a,b’,c |
| 2 | 1088 | 1424 | 1392 | 51 | 824 | 24 | 4.67 | 57.84 | 1.73 | a,b,c | 102 | 1039 | 68 | 9.31 | 66.65 | 5.00 | a, b,c |
| 3 | 925 | 748 | 1779 | 58 | 197 | 403 | 6.28 | 26.33 | 22.64 | a,b,c | 126 | 467 | 697 | 13.15 | 55.12 | 35.49 | a,b,c |
| 4 | 4318 | 4536 | 1639 | 552 | 2962 | 4 | 12.77 | 65.29 | 0.26 | a,b,c | 861 | 3681 | 23 | 19.79 | 71.71 | 1.18 | a,b,c |
| 5 | 1569 | 884 | 1717 | 509 | 411 | 107 | 32.43 | 46.51 | 6.22 | a,b,c | 635 | 723 | 235 | 37.90 | 64.21 | 14.61 | a,b,c |
| 6 | 3124 | 7668 | 3372 | 177 | 3705 | 29 | 5.68 | 48.32 | 0.85 | a,b,c | 332 | 5644 | 63 | 10.15 | 74.68 | 2.02 | a,b,c |
| 7 | 3336 | 2452 | 1898 | 339 | 225 | 6 | 10.17 | 9.16 | 0.33 | a,b,c | 980 | 636 | 25 | 26.04 | 29.42 | 1.49 | a,b,c’ |
| 8 | 3866 | 4067 | 5686 | 565 | 2778 | 167 | 14.62 | 68.32 | 2.94 | a,b,c | 964 | 3355 | 350 | 28.41 | 75.48 | 5.81 | a,b,c |
| 9 | 5111 | 955 | 2531 | 405 | 142 | 8 | 7.92 | 14.86 | 0.33 | a,b,c | 608 | 254 | 41 | 12.36 | 31.59 | 1.76 | a,b,c |
| 10 | 4297 | 5034 | 6031 | 345 | 2827 | 79 | 8.03 | 56.17 | 1.32 | a,b,c | 637 | 3938 | 195 | 15.07 | 73.16 | 3.39 | a,b,c |
| 11 | 6636 | 3715 | 3125 | 239 | 2223 | 138 | 3.60 | 59.85 | 4.41 | a,b,c | 355 | 2586 | 443 | 5.04 | 70.78 | 15.08 | a,b,c |
| All | 35,519 | 34,167 | 31935 | 3280 | 17397 | 1113 | 9.23 | 50.92 | 3.49 | a,b,c | 3,729 | 20,445 | 1918 | 10.68 | 60.07 | 6.04 | a,b,c |
Differences in screening rates between each of the two intervention arms vs. Control arm as well as between the two intervention arms were examined by hand calculating z statistics for comparisons of two proportions
ap<0.0001, NPP & MA vs. Control; bp<0.0001, PCP vs. Control; b’p<0.05, PCP vs. Control; cp<0.0001, NPP & MA vs. PCP; c’p<0.01, NPP & MA vs. PCP
Figure 2 presents monthly average screening rates by arm across the 12 study months. NPP and MA arm rates increased from 38 % in month one to 60 % the final two months. PCP arm rates fluctuated between 8 and 10 % over time, and the Control arm had a small uptick from 2 to 6 %.
Fig. 2.
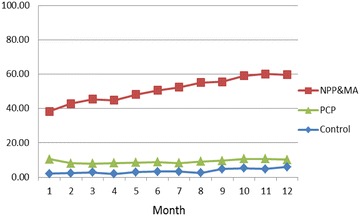
Average screening rates by month for NPP and MA arm, PCP arm and control arm
Of patients who were screened in the 12 months, the proportion screened positive for unhealthy drinking was 9.7 % in the PCP arm, 10.8 % in the NPP and MA arm, and 9.9 % in the Control arm.
Intervention and referral rates among those screened positive for unhealthy alcohol use
Table 2 presents numbers and percentages of patients with positive screens who received BI/RT. The PCP arm had higher (p < 0.0001) BI/RT rates (44.4 %) than either the NPP & MA arm (3.4 %) or the Control arm (2.7 %); this pattern differed only for medical center 5 (with no difference between PCP and NPP and MA arms) and medical center 9 (NPP and MA arm had higher rates than PCP arm, p < 0.0001). Generally, BI/RT rates did not differ between NPP and MA and Control arms with the exceptions of medical center 5 with a slightly higher NPP and MA arm rate (p < 0.01) and medical center 8 with a slightly higher Control arm rate (p < 0.05). The percent of patients who received RT (not shown) was 9.8, 0.6 and 0.2 % of those screening positive in the PCP, NPP and MA and Control arms, respectively. We also examined the proportion of all patients with primary care visits (regardless of screening) who received the BI/RT. We found that 0.63, 0.24 and 0.02 % of patients seen in the PCP, NPP and MA, and Control arms, respectively, received the BI/RT (not shown).
Table 2.
“Unhealthy drinkers” who received brief interventions or referral to treatment (BI/RT) by treatment arm
| PCP | NPP & MA | Control | p values2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N screened positive | N received BI/RT | % | N screened positive | N received BI/RT | % | N screened positive | N received BI/RT | % | ||
| Medical center | ||||||||||
| 1 | 90 | 51 | 56.67 | 1916 | 47 | 2.45 | 185 | 1 | 0.54 | b, c |
| 2 | 129 | 16 | 12.40 | 1849 | 24 | 1.30 | 41 | 1 | 2.44 | b’’’, c |
| 3 | 51 | 8 | 15.69 | 123 | 5 | 4.07 | 216 | 3 | 1.39 | b’, c’’ |
| 4 | 241 | 141 | 58.51 | 1829 | 96 | 5.25 | 6 | 0 | 0.00 | b’’, c |
| 5 | 145 | 17 | 11.72 | 229 | 23 | 10.04 | 142 | 3 | 2.11 | a’, b’’ |
| 6 | 246 | 120 | 48.78 | 2114 | 69 | 3.26 | 19 | 1 | 5.26 | b’, c |
| 7 | 394 | 200 | 50.76 | 172 | 9 | 5.23 | 26 | 0 | 0.00 | b, c |
| 8 | 503 | 153 | 30.42 | 1776 | 47 | 2.65 | 137 | 9 | 6.57 | a’’, b, c |
| 9 | 306 | 15 | 4.90 | 54 | 16 | 29.63 | 9 | 0 | 0.00 | a’’’, c |
| 10 | 697 | 545 | 78.19 | 4260 | 140 | 3.29 | 175 | 7 | 4.00 | b, c |
| 11 | 306 | 115 | 37.58 | 1259 | 55 | 4.37 | 176 | 5 | 2.84 | b, c |
| All | 3108 | 1381 | 44.43 | 15581 | 531 | 3.41 | 1132 | 30 | 2.65 | b, c |
1Chi-square tests were used to compare the proportions of patients screened between each of the two intervention arms vs. control as well as between the two intervention arms
2a: p<0.0001, NPP & MA vs. Control. a’ p<0.01, NPP & MA vs. Control. a’’: p<0.05, NPP & MA vs. Control. a’’’: p<0.10, NPP & MA vs. Control. b: p<0.0001, PCP vs. Control. b’: p<0.001, PCP vs. Control. b’’: p<0.01, PCP vs. Control. b’’’: p<0.10, PCP vs. Control. c: p<0.0001, NPP & MA vs. PCP. c’: p<0.01, NPP & MA vs. PCP. c’’: p<0.05, NPP & MA vs. PCP
Post-hoc analysis for medical center 9 found that a few physicians in the NPP and MA arm delivered BI/RT themselves to their few patients screening positive; these physicians were responsible for the vast majority of BI/RTs delivered in this clinic. Post-hoc analysis comparing subtype of non-physician provider found no clinically significant difference; rates of BI/RT for behavioral medicine specialists, clinical health educators, and nurses was 4.0, 3.3, and 2.9 %, respectively (not shown).
Predictors of screening
Table 3 presents results of the multilevel models predicting screening in each of the intervention arms. Younger patients were less likely to be screened than patients over 60 years old in both arms. In both arms, women were less likely, and patients with psychiatric diagnoses and chronic medical conditions were more likely, to be screened.
Table 3.
Patient-, physician- and system-level factors associated with delivery of screening by treatment arm
| PCP Arm | NPP & MA Arm | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient value | Std. err. | OR | 95% CI | p value | Coefficient value | Std. err. | OR | 95% CI | p value | |
| Patient level | ||||||||||
| Age | ||||||||||
| 18–24 vs. ≥60 | −0.1018 | 0.0430 | 0.90 | (0.83, 0.98) | 0.018 | −0.1726 | 0.0656 | 0.84 | (0.74 , 0.96) | 0.009 |
| 25–44 vs. ≥60 | −0.1582 | 0.0303 | 0.85 | (0.80, 0.91) | 0.000 | −0.1329 | 0.0426 | 0.88 | (0.81, 0.95) | 0.002 |
| 45–59 vs. ≥60 | −0.1074 | 0.0280 | 0.90 | (0.85, 0.95) | 0.000 | −0.1319 | 0.0408 | 0.88 | (0.81, 0.95) | 0.001 |
| Gender | ||||||||||
| Female vs. Male | −0.2447 | 0.0217 | 0.78 | (0.75, 0.82) | 0.000 | −0.2008 | 0.0305 | 0.82 | (0.77, 0.87) | 0.000 |
| Comorbid psychiatric conditions | ||||||||||
| Yes vs. no | 0.1231 | 0.0269 | 1.13 | (1.07, 1.19) | 0.000 | 0.2436 | 0.0383 | 1.28 | (1.18, 1.38) | 0.000 |
| Chronic diseases | ||||||||||
| Yes vs. no | 0.1934 | 0.0243 | 1.21 | (1.16, 1.27) | 0.000 | 0.3407 | 0.0345 | 1.41 | (1.31, 1.50) | 0.000 |
| Physician level | ||||||||||
| Age | ||||||||||
| (per 1 year increase) | −0.2183 | 0.0034 | 0.80 | (0.80, 0.81) | 0.000 | |||||
| Gender | ||||||||||
| Female vs. male | 1.1647 | 0.0247 | 3.21 | (3.05, 3.36) | 0.000 | |||||
| Race/ethnicity | ||||||||||
| Non-white vs. white | 1.4355 | 0.0263 | 4.20 | (3.99, 4.42) | 0.000 | |||||
| Years of services at the health plan | ||||||||||
| (per 1 year increase) | 0.2587 | 0.0032 | 1.30 | (1.29, 1.30) | 0.000 | |||||
| Specialty | ||||||||||
| Non-internal medicine vs. internal medicine | 3.0877 | 0.0366 | 21.93 | (20.41, 23.56) | 0.000 | |||||
| System level | ||||||||||
| Colocation of AOD and primary care departments | ||||||||||
| Yes vs. no | −2.3244 | 0.0689 | 0.10 | (0.09, 0.11) | 0.000 | −0.4432 | 0.2274 | 0.64 | (0.41, 1.00) | 0.051 |
| MA/PCP ratio at the clinic | −0.4393 | 0.0493 | 0.64 | (0.59, 0.71) | 0.000 | 3.0850 | 0.0748 | 21.87 | (18.89, 25.32) | 0.000 |
| Ave. visit time | 0.3606 | 0.0097 | 1.43 | (1.41, 1.46) | 0.000 | 0.1379 | 0.0104 | 1.15 | (1.12, 1.17) | 0.000 |
| Number of PCPs trained at the clinic | −0.0387 | 0.0047 | 0.96 | (0.95, 0.97) | 0.000 | |||||
A three-level multivariate logistic model adjusting for clustering effects within physician and clinic was run for each of the two intervention arms
PCP primary care physicians, NPP non-physician providers, MA medical assistants, AOD alcohol and other drug
In the PCP arm, we examined physician-level factors associated with screening. Older physicians were less likely, and female physicians more likely, to screen than their counterparts. Non-White physicians had four times higher odds of screening than White physicians (OR = 4.20, 95 % CI 3.99, 4.42). Each year of employment in the health plan was associated with a 30 % increase in odds of screening (OR = 1.30, 95 % CI 1.29, 1.30). Physicians with Family Practice or other specialties were far more likely to screen than Internal Medicine physicians.
Regarding system characteristics, lower odds of screening were associated with co-located primary care and AOD clinics in the PCP arm. A higher ratio of MAs vs. physicians was associated with lower odds of screening for the PCP arm, but higher odds for the NPP and MA arm. Longer visit time was related to a higher likelihood of screening in both arms. In the PCP arm, having more physicians trained was related to a lower odds of screening. In the NPP and MA arm, all non-physician providers were trained, so we excluded this variable from the NPP and MA analysis.
Predictors of brief intervention and referral among those screened positive for unhealthy alcohol use
Table 4 presents multilevel models of BI/RT among those screened positive in the intervention arms. Females were less likely to receive BI/RT in both. In the NPP and MA arm, patients with psychiatric or chronic conditions were more likely to receive BI/RT and those aged 45–59 years had higher odds than those aged 60 and older.
Table 4.
Patient-, physician- and system-level factors associated with delivery of BI/RT by treatment arm
| PCP Arm | NPP & MA Arm | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient value | Std. err. | OR | 95% CI | p value | Coefficient value | Std. err. | OR | 95% CI | p value | |
| Patient level | ||||||||||
| Age | ||||||||||
| 18–24 vs. ≥60 | 0.2127 | 0.2119 | 1.24 | (0.82, 1.87) | 0.315 | 0.3589 | 0.2019 | 1.43 | (0.96, 2.13) | 0.075 |
| 25–44 vs. ≥60 | −0.1290 | 0.1755 | 0.88 | (0.62, 1.24) | 0.462 | 0.2557 | 0.1632 | 1.29 | (0.94, 1.78) | 0.117 |
| 45–59 vs. ≥60 | −0.1571 | 0.1730 | 0.85 | (0.61, 1.20) | 0.364 | 0.5254 | 0.1615 | 1.69 | (1.23, 2.32) | 0.001 |
| Gender | ||||||||||
| Female vs. Male | −0.5081 | 0.1166 | 0.60 | (0.48, 0.76) | 0.000 | −0.4701 | 0.1067 | 0.62 | (0.51, 0.77) | 0.000 |
| Comorbid psychiatric conditions | ||||||||||
| Yes vs. no | 0.1489 | 0.1403 | 1.16 | (0.88, 1.53) | 0.289 | 0.2391 | 0.1121 | 1.27 | (1.02, 1.58) | 0.033 |
| Chronic diseases | ||||||||||
| Yes vs. no | −0.0359 | 0.1165 | 0.96 | (0.77, 1.21) | 0.758 | 0.2444 | 0.0995 | 1.28 | (1.05, 1.58) | 0.014 |
| Physician level | ||||||||||
| Age | ||||||||||
| (per 1 year increase) | −0.0608 | 0.0161 | 0.94 | (0.91, 0.97) | 0.000 | |||||
| Gender | ||||||||||
| Female vs. male | 0.2221 | 0.1932 | 1.25 | (0.86, 1.82) | 0.250 | |||||
| Race/ethnicity | ||||||||||
| Non-white vs. white | −0.5904 | 0.1599 | 0.55 | (0.41, 0.76) | 0.000 | |||||
| Years of services at the health plan | ||||||||||
| (per 1 year increase) | 0.0614 | 0.0152 | 1.06 | (1.03, 1.10) | 0.000 | |||||
| Specialty | ||||||||||
| Non-internal medicine vs. internal medicine | −0.2045 | 0.1932 | 0.82 | (0.56, 1.19) | 0.290 | |||||
| System level | ||||||||||
| Colocation of AOD and primary care departments | ||||||||||
| Yes vs. no | −0.1525 | 0.5258 | 0.86 | (0.31, 2.41) | 0.772 | 0.6106 | 0.4493 | 1.84 | (0.76, 4.44) | 0.174 |
| MA/PCP ratio at the clinic | −3.1177 | 0.3265 | 0.04 | (0.02, 0.08) | 0.000 | −0.4583 | 0.3325 | 0.63 | (0.33, 1.21) | 0.168 |
| Ave. visit time | 0.3111 | 0.0546 | 1.36 | (1.23, 1.52) | 0.000 | 0.0106 | 0.0529 | 1.01 | (0.91, 1.12) | 0.841 |
| Number of PCPs trained at the clinic | −0.1695 | 0.0360 | 0.84 | (0.79, 0.91) | 0.000 | |||||
A three-level multivariate logistic model adjusting for clustering effects within physician and clinic was run for each of the two intervention arms
BI/RT brief intervention or referral to treatment, PCP primary care physicians, NPP non-physician providers, MA medical assistants, AOD alcohol and other drug
Older physicians were less likely, while those who had worked at the organization longer were more likely, to provide BI/RT. On the other hand, Non-White physicians were less likely to provide BI/RT than were White physicians. Gender and specialty did not predict BI/RT delivery.
In the PCP arm, a higher ratio of MAs vs. physicians and having more physicians trained on SBIRT was associated with lower odds of BI/RT. Longer visit time was related to higher BI/RT rates. No significant associations between rates of BI/RT and system-level factors were found in the NPP and MA arm.
Discussion
Pre-implementation SBIRT practices
Consistent with research suggesting a widespread lack of SBIRT implementation [10], systematic alcohol screening was not conducted as part of usual care in this organization prior to the study. For the few physician leaders who reported any policies, the recommended practice was to use questions embedded in the EHR system for which there is no evidence, and which are not included in guidelines [7]. These findings underline the need for formalized SBIRT implementation programs, incorporating the use of evidence-based instruments, to ensure that patients obtain optimal primary care treatment, including addressing alcohol as a potential complicating factor in many prevalent health conditions.
Implementation by physicians versus non-physicians
In this cluster randomized controlled trial in adult PC, we compared implementation of alcohol SBIRT in three arms: physician-delivered, non-physician-delivered and Control. Consistent with prior research [22], screening rates were much higher in the NPP and MA arm than PCP arm, and both had higher rates than the Control arm. Generally, this pattern was true across all sites, with some exceptions, particularly one medical center in which MAs (who were trained as part of the NPP and MA arm) “floated” between the NPP and MA and Control arm clinics, resulting in similar screening rates. Two of the medical centers had slightly higher rates in the Control than PCP arm due to some MAs “floating” into the Control arm.
MAs are well-placed to provide alcohol screening and it is consistent with their role to collect and record vital signs in the EHR, including weight, height, blood pressure and (in KPNC) smoking screening and exercise [67]. The NPP and MA arm’s steadily improving screening rates from 38 % in month 1 to 60 % in month 12 suggests that barriers to screening that MAs may encounter are temporary and may be addressed through managers’ use of ongoing monitoring and performance feedback reports and practice. Improvements may also have been affected by booster sessions and technical assistance visits, which minimized discomfort with screening through reframing and normalizing it as a routine part of health care. Normalizing was accomplished by acknowledging that drinking is common in our culture and not problematic if within low-risk limits, and by comparing alcohol screening to asking about smoking and exercise—both are routinely addressed in KPNC’s primary care clinics.
Different approaches to screening may also be more efficient and increase rates in the NPP and MA delivery model. The two NPP and MA clinics with the highest rates attributed success to the use of paper forms provided to patients by the receptionist and collected by MAs, who simply recorded responses in the EHR screening form; an approach they already used for other screening activities. This “paper and pencil” screening may increase workflow efficiency and increase quality and reliability of screening [68]. It may be that PCPs were less likely to consistently record screening when it was performed because of their many competing priorities, whereas MAs were more likely to record screening in the EHR tool because they were more closely supervised and monitored.
The BI/RT results were counter to prior research which found good implementation rates among non-physicians [22]. The PCP arm rates were 44.4 % versus the NPP and MA arm’s 3.4 %. This was the pattern in most medical centers, with one exception where two physicians provided interventions themselves. When examining a population-based intervention rate (i.e., the proportion of all patients seen who received BI/RT), the PCP arm still had an advantage (0.63 versus 0.24 %).
The lower BI/RT rate in the NPP and MA arm was counter to our hypothesis that non-physician providers would have higher BI/RT rates. Several factors may have contributed to low BI/RT rates in the NPP and MA arm. First, because this was a naturalistic implementation study, we trained non-physician providers working in the health system rather than using research or externally or grant-funded clinicians, who may have different characteristics and motivations (because of being paid by a research study and invested in the success of the intervention) rather than having an existing operational role with competing duties. Non-physician providers were not always available immediately and thus the workflow often required an additional appointment either by phone or in person, or required phoning the provider when possible via a “consult phone”, which meant a delay. Second, exam room availability was often an issue, which translated into concerns about backlogs if interventions were provided there. Third, providers reported that patients were resistant to seeing the non-physician providers, regardless of whether they were told that the discussion would be about their alcohol screening results or whether a more vague script about “healthy lifestyle” was used.
Overall, the findings from the current study suggest an efficient, but less complex workflow of MA screening followed by BI/RT by physicians, consistent with how other preventive services are delivered in this health system [69]. It also parallels recent findings that alcohol screening by staff and follow-up by clinicians is associated with more frequent use of screening and intervention [70]. These findings are also consistent with aspects of the Consolidated Framework on Implementation Research (CFIR) model [45]. The CFIR suggests that evidence-based interventions with greater complexity (such as the NPP and MA delivery model examined here) are less likely to be implemented. Also consistent with the CFIR framework, the lack of resources in the form of sufficient available time of non-physician providers and exam rooms were barriers to implementation of interventions and referrals in the NPP and MA arm. Further, the CFIR suggests that high compatibility of new evidence-based interventions with existing roles and duties can increase implementation as is the case for adding alcohol screening for MAs who already ask about other health behaviors (smoking and exercise).
One potential disadvantage with the physician-delivered intervention is that physicians reported shorter interventions (5 min average) versus non-physicians (23 min average). Yet longer interventions may not significantly increase effectiveness [71]. Physicians reported that the single item screening question required less than 1 min. We did not gather more specific information on how long providers spent on brief interventions versus referrals to specialty treatment.
These results may raise questions about the ability of currently predominant primary care models to use non-physician clinical team members to perform SBIRT and other new evidence-based practices. Team-based care has potential for addressing behavioral health problems including unhealthy alcohol and tobacco use and depression and for improving health conditions affected by these problems such as hypertension and obesity [72, 73]. Yet, a recent study documented staffing models in primary care practices which participated in a nationwide US team-based care initiative and found that even among those designated as patient-centered medical homes, fewer than 23 % employed health educators, pharmacists, social workers, nutritionists or community service coordinators and fewer than half employed care coordinators [74]. Ideally, medical home models and integrated care systems would include adequate levels of behavioral health care staff, on-call and readily available to deliver behavioral health interventions such as multiple-session brief interventions for unhealthy alcohol use [75]. Further, sufficient exam room space is needed to prevent backlogs, and the purpose of exam rooms may need to be expanded so that care coordination and behavioral services can be provided. Alternatively, primary care office design may need to be reconsidered so that sufficient non-physician provider consult room spaces are interspersed next to exam rooms.
In addition to increased team staffing, performance measures linked to incentives could aid implementation. The CFIR model and the experience at the US VA Health System, where alcohol SBIRT is mandated by an internal performance measure [41, 76], suggest that policies such as performance measures may increase implementation. Yet to date there are no external performance measures applicable to private, capitated systems or to US Federally Qualified Health Centers. Recently released findings that fewer than one in six Americans reported being asked about or discussing their alcohol consumption with a health professional are striking, given that the USPSTF has recommended it in general medical settings since 2004 [7]. In the system studied here, MA screening and physician intervention were found to be a feasible solution for implementation of SBIRT. Without structural changes in primary care practices that would improve their ability to provide consistent team-based care, MA to physician workflow may be one viable approach, and may also be considered for other underutilized USPSTF recommended services [46] such as depression screening. Full implementation of SBIRT likely also requires a time frame of more than 1 year for both individual providers and organizations to progress through stages of readiness for change in order to achieve optimal results.
Predictors of implementation within each model
In both PCP and NPP and MA arms, younger patients were less likely to be screened; this was counter-intuitive given their higher prevalence of unhealthy drinking [77, 78] and also counter to prior research [79]. Similarly, in general, younger patients were not more likely to receive BI/RT, though in the NPP & MA arm, patients aged 45–59 who screened positive were more likely to receive BI/RT than those aged 60 or older. Physicians may assume that unhealthy drinking is “to be expected” in younger patients, and thus not be as concerned about identifying it. Also, the training emphasized the link between alcohol and chronic conditions, which may have made it easier for physicians to screen and discuss drinking among older patients at risk for such conditions.
Consistent with results suggesting that men are more likely to binge drink, male patients were more likely to be screened and receive BI/RT in both arms. This is consistent with prior research indicating that men are more likely to be screened [79–81] and raises concerns given that women are less likely to seek alcohol intervention and treatment and may be more vulnerable to adverse health effects of unhealthy use [82]. That more individuals with psychiatric and chronic disease diagnoses were screened than those without (in both arms) is also consistent with prior research [79] and with the emphasis in the provider training on the relationship of unhealthy alcohol use to adverse health effects and reduced medication adherence [83]. However, psychiatric and chronic conditions only predicted receiving BI/RT in the NPP and MA arm. Such patients are generally more likely to see non-physician providers for chronic conditions, e.g., for behavioral interventions related to diabetes, so the intervention may have been paired with conversations about their chronic condition.
As found in prior research, female physicians were more likely than male to screen [44, 84], though there was no difference by physician gender in providing BI/RT. Physicians from non-White and Latino ethnic/racial groups were more likely to screen, although White physicians were more likely to record providing BI/RT. Few studies have examined these characteristics as predictors of SBIRT; the large sample size and racial/ethnic diversity of the provider sample allowed us to examine this. More research is needed to understand these differences in screening and BI/RT rates between gender and racial/ethnic groups. For example, some national studies have found higher rates of alcohol abuse in whites than some other ethnic groups and in men than women [85, 86]. Perhaps these differences in screening may reflect that physicians with healthier habits themselves (which was unmeasured in this study) are more likely to provide counseling [87]. Alternatively, the findings may reflect gender and ethnic differences in communication styles in which case additional or different approaches to training may particularly be needed for these subgroups.
Consistent with a greater focus on preventive care, a specialty of Family Medicine or training other than Internal Medicine was related to screening, but this characteristic did not affect BI/RT rates. Physicians newer to the system were less likely to both screen and intervene. Perhaps longer experience in the system may help physicians to practice more efficiently, leaving time for preventive screening and intervention.
Consistent with studies suggesting lack of screening is due to time constraints [28, 88], longer visits were related to higher odds of screening in both arms and intervention in the PCP arm. Provision of BI/RT was not related to appointment time in the NPP and MA arm likely because the intervention usually required a separate visit. However, having a higher ratio of MAs per physician increased the likelihood of screening in the NPP and MA arm. Having more MAs did not seem to off-load the work of physicians enough in the PCP arm to result in higher rates of screening or intervention as we had expected, and in fact was related to a lower odds of screening and BI/RT. This may be because a higher MA ratio is a reflection of a busier clinic.
Some findings on system-level predictors were counter-intuitive. Having more physicians trained in SBIRT in a medical center was related to lower odds of screening. The findings that co-location between primary care and specialty AOD treatment clinics was associated with a lower likelihood of receiving screening or intervention are also counter to the ideas that proximity of specialty treatment would increase awareness and that easier patient access to specialty treatment would increase physician confidence in discussing alcohol with patients. More research is needed to understand how co-location affects primary care clinician behavior, and whether there is a confounding factor related to both variables.
Study strengths and limitations
Conducting this study in real-world busy clinics, and using existing staff are important characteristics of an implementation study, and at the same time enhance both limitations and strengths. We could not control the schedules or priorities of clinic managers or providers or the movement of staff across clinics. However, using existing staff is important for understanding whether a model is feasible and sustainable regardless of externally funded interventionists.
Provider factors not examined include outcome expectancies, concerns about intrusiveness, beliefs that SBIRT is ineffective, negative attitudes about patients with alcohol problems, and self-efficacy; these have been previously well-documented [65, 79, 89–97].
Similar to studies for other health conditions, use of EHR data means that our data on screening and BI/RT are limited to that documented in the EHR. Yet this approach allowed for studying the natural process of care, studying a large number of patients, and having a population-based sample of patients and providers, rather than limiting our sample to those willing to be recruited into a study.
This study is the first to examine implementation of the NIAAA Guide for SBIRT [8] in adult primary care in a private, non-profit US health care system. It also uses an established EHR, a mechanism recommended by the field [15, 46], and known to be salient in predicting better implementation of primary care practices [98–101]. EHR use is also more generalizable to the future of private US health care settings because of Phase III Meaningful Use requirements [102].
Because of the large sample size, some statistically significant differences may not be clinically meaningful. Variations in the pattern of results across medical centers highlight the importance of including multiple medical centers or clinics when studying factors that influence implementation.
A further limitation is that data on alcohol use disorder screening were not recorded for a considerable proportion of those who screened positive for unhealthy alcohol use. We thus were unable to examine the proportion of those screening positive on the alcohol use disorders screen who received RT.
Finally, it is not known how the study’s findings would generalize to systems with fewer exam room constraints or systems in which clinical health educators are dedicated to SBIRT (which would allow for same-time, warm hand-offs to exam room consultations). Moreover, currently predominant primary care models do not include health educators or behavioral specialists [74].
Conclusions
Our findings from this randomized trial of physician versus non-physician alcohol SBIRT implementation suggest that a model of MA screening and physician intervention may have the highest odds of implementation in currently predominant primary care models. Further, in large organizations such as this one, where policies and practices for systematic screening were not in place prior to training, implementation may take a longer time period than 1 year after an initial training and a higher “dosage” of quality improvement and feedback; longer time frames may be required for provider change [17] and full implementation, as has been found in the VA [68]. Many facilitators of implementation, such as organizational and provider stages of readiness for change [45] may increase over time after a new initiative begins. Informed by this study, a model using MA screening and physician intervention was adopted by Kaiser Permanente Northern California across all adult primary care clinics in June of 2013 for annual, evidence-based alcohol screening and intervention or referral to specialty treatment [103]. Future research is needed across systems that have implemented SBIRT to examine duration of time since initial implementation and duration and amount of related quality improvement activities as factors predicting SBIRT implementation outcomes. Research is also needed on whether implementation of BI and RT by non-physicians is more effectively implemented within fully realized and staffed medical home models.
Authors’ contributions
JM, FC, CW and SS obtained funding and devised the concept for the clinical trial design, and analysis. JM, CW, DP, TR, SA, DS and SS developed the training curriculum. FC and WL conducted data acquisition and performed the statistical analyses. JM, FC, CW, DS, SA, TB, DD, CC and SS designed the concept for the paper, interpreted the results and drafted the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We thank Agatha Hinman and Sabrina Wood for editorial assistance with the manuscript. We thank the KPNC Chemical Dependency Quality Improvement Committee and KPNC Chemical Dependency Program Directors and Addiction Medicine Physicians for their partnership, particularly Carol Havens, MD, Barrett Levine, MD, Michael Rubino, MD, Karen Spedowfski, PhD, and Matthew Tarran, PhD. We also thank the physicians, medical assistants, clinical health educators, behavioral medicine specialists, nurses, and managers of KPNC’s adult and family medicine departments for their participation in the quality improvement activities related to this study. We especially thank Don Mordecai, M.D. and Sheryl Sun, M.D, for their strong leadership support of this project and of implementation of alcohol screening, brief intervention and referral to treatment within KPNC adult primary care. Thanks are also due to Richard Saitz, MD who led a “Train the Trainers” workshop for this project.
Funding/Support and role of the sponsor
This study was funded by the National Institute on Alcohol Abuse and Alcoholism (R01 AA018660). The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication.
Competing interests
The authors all declare that they have no conflicting interests.
Abbreviations
- AOD
alcohol and other drug
- BI
brief intervention
- EHR
electronic health record
- MA
medical assistant
- NIAAA
US National Institute on Alcohol Abuse and Alcoholism
- NPP
non-physician provider
- PCP
primary care physician
- RT
referral to treatment
- SBIRT
screening, brief intervention and referral to treatment
- USPSTF
United States Preventive Services Task Force
Contributor Information
Jennifer R. Mertens, Email: MertensJenniferR@gmail.com
Felicia W. Chi, Email: Felicia.W.Chi@kp.org
Constance M. Weisner, Email: Constance.Weisner@kp.org
Derek D. Satre, Email: Derek.Satre@kp.org
Thekla B. Ross, Email: Thekla.B.Ross@kp.org
Steve Allen, Email: drsteveallen@gmail.com.
David Pating, Email: David.Pating@kp.org.
Cynthia I. Campbell, Email: Cynthia.I.Campbell@kp.org
Yun Wendy Lu, Email: Wendy.Y.Lu@kp.org.
Stacy A. Sterling, Email: Stacy.A.Sterling@kp.org
References
- 1.US Department of Health and Human Services, National Institute on Alcohol Abuse and Alcoholism. 10th special report to the US Congress on alcohol and health : highlights from current research from the Secretary of Health and Human Services. Rockville, MD: US Department of Health and Human Services. June 2000. http://pubs.niaaa.nih.gov/publications/10Report/10thSpecialReport.pdf. Accessed 8 Oct 2015.
- 2.Rehm J, Room R, Monteiro M, Gmel G, Graham K, Rehn N, et al. Alcohol as a risk factor for global burden of disease. Eur Addict Res. 2003;9(4):157–164. doi: 10.1159/000072222. [DOI] [PubMed] [Google Scholar]
- 3.Stahre M, Roeber J, Kanny D, Brewer RD, Zhang X. Contribution of excessive alcohol consumption to deaths and years of potential life lost in the United States. Prev Chronic Dis. 2014;11:E109. doi: 10.5888/pcd11.130293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaner E, Bland M, Cassidy P, Coulton S, Deluca P, Drummond C, et al. Screening and brief interventions for hazardous and harmful alcohol use in primary care: a cluster randomised controlled trial protocol. BMC Public Health. 2009;9:287. doi: 10.1186/1471-2458-9-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertholet N, Daeppen JB, Wietlisbach V, Fleming M, Burnand B. Reduction of alcohol consumption by brief alcohol intervention in primary care: systematic review and meta-analysis. Arch Intern Med. 2005;165(9):986–995. doi: 10.1001/archinte.165.9.986. [DOI] [PubMed] [Google Scholar]
- 6.O’Donnell A, Anderson P, Newbury-Birch D, Schulte B, Schmidt C, Reimer J, et al. The impact of brief alcohol interventions in primary healthcare: a systematic review of reviews. Alcohol Alcohol. 2014;49(1):66–78. doi: 10.1093/alcalc/agt170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moyer VA, Preventive Services Task Force Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: US preventive services task force recommendation statement. Ann Intern Med. 2013;159(3):210–218. doi: 10.7326/0003-4819-159-3-201308060-00652. [DOI] [PubMed] [Google Scholar]
- 8.National Institute on Alcohol Abuse and Alcoholism. Helping patients who drink too much: a clinician’s guide, updated 2005 edition. US Department of Health and Human Services [NIH Publication No. 07–3769], Rockville, MD. 2005. http://pubs.niaaa.nih.gov/publications/Practitioner/CliniciansGuide2005/clinicians_guide.htm. Accessed 8 Oct 2015.
- 9.Canadian Centre on Substance Abuse (CCSA) and College of Family Physicians of Canada (CFPC). Alcohol screening, brief intervention and referral: a clinical guide. In: Alcohol screening, brief intervention & referral helping patients reduce alcohol-related risks. College of Family Physicians of Canada and Canadian Centre on Substance Abuse. 2012. http://www.sbir-diba.ca/. Accessed 6 Jan 2015.
- 10.McKnight-Eily LR, Liu Y, Brewer RD, Kanny D, Lu H, Denny CH, et al. Vital signs: communication between health professionals and their patients about alcohol use–44 states and the District of Columbia, 2011. MMWR Morb Mortal Wkly Rep. 2014;63(1):16–22. [PMC free article] [PubMed] [Google Scholar]
- 11.Garnick DW, Horgan CM, Merrick EL, Hoyt A. Identification and treatment of mental and substance use conditions: health plans strategies. Med Care. 2007;45(11):1060–1067. doi: 10.1097/MLR.0b013e31812e01bb. [DOI] [PubMed] [Google Scholar]
- 12.Bradley KA, Williams EC, Achtmeyer CE, Volpp B, Collins BJ, Kivlahan DR. Implementation of evidence-based alcohol screening in the Veterans Health Administration. Am J Manag Care. 2006;12(10):597–606. [PubMed] [Google Scholar]
- 13.Saitz R. Unhealthy alcohol use. N Engl J Med. 2005;352(6):596–607. doi: 10.1056/NEJMcp042262. [DOI] [PubMed] [Google Scholar]
- 14.Kuehn BM. Despite benefit, physicians slow to offer brief advice on harmful alcohol use. JAMA. 2008;299(7):751–753. doi: 10.1001/jama.299.7.751. [DOI] [PubMed] [Google Scholar]
- 15.Saitz R, Svikis D, D’Onofrio G, Kraemer KL, Perl H. Challenges applying alcohol brief intervention in diverse practice settings: Populations, outcomes, and costs. Alcohol Clin Exp Res. 2006;30(2):332–338. doi: 10.1111/j.1530-0277.2006.00038.x. [DOI] [PubMed] [Google Scholar]
- 16.Stewart SH, Miller PM. Alcohol screening and intervention in medical and surgical settings. In: Miller PM, Kavanagh DJ, editors. Translation of addictions science into practice. New York: Elsevier Science; 2007. pp. 379–398. [Google Scholar]
- 17.Nilsen P. Brief alcohol intervention–where to from here? Challenges remain for research and practice. Addiction. 2010;105(6):954–959. doi: 10.1111/j.1360-0443.2009.02779.x. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Medicaid and Medicare Services. Screening, Brief Intervention, and Referral to Treatment (SBIRT) services. Medicare Learning Services. 2014, June. http://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/SBIRT_Factsheet_ICN904084.pdf. Accessed 7 July 2015.
- 19.Kaner E, Bland M, Cassidy P, Coulton S, Dale V, Deluca P, et al. Effectiveness of screening and brief alcohol intervention in primary care (SIPS trial): pragmatic cluster randomised controlled trial. BMJ. 2013;346:e8501. doi: 10.1136/bmj.e8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Beurden I, Anderson P, Akkermans RP, Grol RP, Wensing M, Laurant MG. Involvement of general practitioners in managing alcohol problems: a randomized controlled trial of a tailored improvement programme. Addiction. 2012;107(9):1601–1611. doi: 10.1111/j.1360-0443.2012.03868.x. [DOI] [PubMed] [Google Scholar]
- 21.Williams EC, Johnson ML, Lapham GT, Caldeiro RM, Chew L, Fletcher GS, et al. Strategies to implement alcohol screening and brief intervention in primary care settings: a structured literature review. Psychol Addict Behav. 2011;25(2):206–214. doi: 10.1037/a0022102. [DOI] [PubMed] [Google Scholar]
- 22.Babor TE, Higgins-Biddle J, Dauser D, Higgins P, Burleson JA. Alcohol screening and brief intervention in primary care settings: implementation models and predictors. J Stud Alcohol. 2005;66(3):361–368. doi: 10.15288/jsa.2005.66.361. [DOI] [PubMed] [Google Scholar]
- 23.Babor TF, Higgins-Biddle JC, Dauser D, Burleson JA, Zarkin GA, Bray J. Brief interventions for at-risk drinking: patient outcomes and cost-effectiveness in managed care organizations. Alcohol Alcohol. 2006;41(6):624–631. doi: 10.1093/alcalc/agl078. [DOI] [PubMed] [Google Scholar]
- 24.Cutrona SL, Choudhry NK, Stedman M, Servi A, Liberman JN, Brennan T, et al. Physician effectiveness in interventions to improve cardiovascular medication adherence: a systematic review. J Gen Intern Med. 2010;25(10):1090–1096. doi: 10.1007/s11606-010-1387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Georgeu D, Colvin CJ, Lewin S, Fairall L, Bachmann MO, Uebel K, et al. Implementing nurse-initiated and managed antiretroviral treatment (NIMART) in South Africa: a qualitative process evaluation of the STRETCH trial. Implement Sci. 2012;7:66. doi: 10.1186/1748-5908-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Denberg TD, Ross SE, Steiner JF. Patient acceptance of a novel preventive care delivery system. Prev Med. 2007;44(6):543–546. doi: 10.1016/j.ypmed.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Tsai AG, Wadden TA. Treatment of obesity in primary care practice in the United States: a systematic review. J Gen Intern Med. 2009;24(9):1073–1079. doi: 10.1007/s11606-009-1042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahm AK, Boggs JM, Martin C, Price DW, Beck A, Backer TE, et al. Facilitators and barriers to implementing Screening, Brief Intervention, and Referral to Treatment (SBIRT) in primary care in integrated health care settings. Subst Abus. 2015;36(3):281–288. doi: 10.1080/08897077.2014.951140. [DOI] [PubMed] [Google Scholar]
- 29.Substance Abuse and Mental Health Services Administration. Systems-level Implementation of Screening, Brief Intervention, and Referral to Treatment. Rockville, MD: Substance Abuse and Mental Health Services Administration. 2013. Report No.: Technical Assistance Publication (TAP) Series 33. HHS Publication No. (SMA) 13-4741. http://store.samhsa.gov/shin/content//SMA13-4741/TAP33.pdf. Accessed 31 Aug 2015.
- 30.Hudson SV, Ohman-Strickland P, Cunningham R, Ferrante JM, Hahn K, Crabtree BF. The effects of teamwork and system support on colorectal cancer screening in primary care practices. Cancer Detect Prev. 2007;31(5):417–423. doi: 10.1016/j.cdp.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selby JV, Schmittdiel JA, Lee J, Fung V, Thomas S, Smider N, et al. Meaningful variation in performance: what does variation in quality tell us about improving quality? Med Care. 2010;48(2):133–139. doi: 10.1097/MLR.0b013e3181c15a6e. [DOI] [PubMed] [Google Scholar]
- 32.Aira M, Kauhanen J, Larivaara P, Rautio P. Factors influencing inquiry about patients’ alcohol consumption by primary health care physicians: qualitative semi-structured interview study. Fam Pract. 2003;20(3):270–275. doi: 10.1093/fampra/cmg307. [DOI] [PubMed] [Google Scholar]
- 33.Keurhorst MN, Anderson P, Spak F, Bendtsen P, Segura L, Colom J, et al. Implementing training and support, financial reimbursement, and referral to an internet-based brief advice program to improve the early identification of hazardous and harmful alcohol consumption in primary care (ODHIN): study protocol for a cluster randomized factorial trial. Implement Sci. 2013;8:11. doi: 10.1186/1748-5908-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller NS, Sheppard LM, Colenda CC, Magen J. Why physicians are unprepared to treat patients who have alcohol- and drug-related disorders. Acad Med. 2001;76(5):410–418. doi: 10.1097/00001888-200105000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Yarnall KS, Pollak KI, Ostbye T, Krause KM, Michener JL. Primary care: is there enough time for prevention? Am J Public Health. 2003;93(4):635–641. doi: 10.2105/AJPH.93.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson M, Jackson R, Guillaume L, Meier P, Goyder E. Barriers and facilitators to implementing screening and brief intervention for alcohol misuse: a systematic review of qualitative evidence. J Public Health (Oxf) 2011;33(3):412–421. doi: 10.1093/pubmed/fdq095. [DOI] [PubMed] [Google Scholar]
- 37.Thomas CP, McCarty D. Appendix D: adoption of drug abuse treatment technology in specialty and primary care settings. In: Harwood HJ, Myers TG, editors. New treatments for addiction: behavioral, ethical, legal, and social questions. Washington, DC: National Academies Press; 2004. pp. 140–172. [PubMed] [Google Scholar]
- 38.Spandorfer JM, Israel Y, Turner BJ. Primary care physicians’ views on screening and management of alcohol abuse: inconsistencies with national guidelines. J Fam Pract. 1999;48(11):899–902. [PubMed] [Google Scholar]
- 39.Miller PM, Stockdell R, Nemeth L, Feifer C, Jenkins RG, Nietert PJ, et al. Initial steps taken by nine primary care practices to implement alcohol screening guidelines with hypertensive patients: the AA-TRIP project. Subst Abus. 2006;27(1–2):61–70. doi: 10.1300/J465v27n01_08. [DOI] [PubMed] [Google Scholar]
- 40.Rose HL, Miller PM, Nemeth LS, Jenkins RG, Nietert PJ, Wessell AM, et al. Alcohol screening and brief counseling in a primary care hypertensive population: a quality improvement intervention. Addiction. 2008;103(8):1271–1280. doi: 10.1111/j.1360-0443.2008.02199.x. [DOI] [PubMed] [Google Scholar]
- 41.Lapham GT, Achtmeyer CE, Williams EC, Hawkins EJ, Kivlahan DR, Bradley KA. Increased documented brief alcohol interventions with a performance measure and electronic decision support. Med Care. 2012;50(2):179–187. doi: 10.1097/MLR.0b013e3181e35743. [DOI] [PubMed] [Google Scholar]
- 42.Satre DD, Leibowitz A, Mertens JR, Weisner C. Advising depression patients to reduce alcohol and drug use: factors associated with provider intervention in outpatient psychiatry. Am J Addict. 2014;23(6):570–575. doi: 10.1111/j.1521-0391.2014.12140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ewing GB, Selassie AW, Lopez CH, McCutcheon EP. Self-report of delivery of clinical preventive services by US physicians. Comparing specialty, gender, age, setting of practice, and area of practice. Am J Prev Med. 1999;17(1):62–72. doi: 10.1016/S0749-3797(99)00032-X. [DOI] [PubMed] [Google Scholar]
- 44.Friedmann PD, McCullough D, Saitz R. Screening and intervention for illicit drug abuse: a national survey of primary care physicians and psychiatrists. Arch Intern Med. 2001;161(2):248–251. doi: 10.1001/archinte.161.2.248. [DOI] [PubMed] [Google Scholar]
- 45.Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. doi: 10.1186/1748-5908-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Institute of Medicine. Improving the quality of health care for mental and substance-use conditions. Quality Chasm Series. Washington, DC: National Academies Press; 2006. [PubMed]
- 47.Anderson P, Wojnar M, Jakubczyk A, Gual A, Segura L, Sovinova H, et al. Managing alcohol problems in general practice in Europe: results from the European ODHIN Survey of General Practitioners. Alcohol Alcohol. 2014;49(5):531–539. doi: 10.1093/alcalc/agu043. [DOI] [PubMed] [Google Scholar]
- 48.Bendtsen P, Anderson P, Wojnar M, Newbury-Birch D, Mussener U, Colom J, et al. Professional’s attitudes do not influence screening and brief interventions rates for hazardous and harmful drinkers: results from ODHIN study. Alcohol Alcohol. 2015;50(4):430–437. doi: 10.1093/alcalc/agv020. [DOI] [PubMed] [Google Scholar]
- 49.Garg AX, Adhikari NK, McDonald H, Rosas-Arellano MP, Devereaux PJ, Beyene J, et al. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. JAMA. 2005;293(10):1223–1238. doi: 10.1001/jama.293.10.1223. [DOI] [PubMed] [Google Scholar]
- 50.Ryan J, Doty MM, Abrams MK, Riley P. The adoption and use of health information technology by community health centers, 2009–2013. New York: The Commonwealth Fund. May 2014. http://www.commonwealthfund.org/publications/issue-briefs/2014/may/adoption-and-use-of-hit-by-chcs. Accessed 8 Oct 2015.
- 51.Abrams MK, Doty MM, Ryan J, Hall D, Riley P. Ready or not? How community health centers view their preparedness to care for newly insured patients. The Commonwealth Fund. May 2014. http://www.commonwealthfund.org/publications/issue-briefs/2014/may/ready-or-not-how-chcs-view-their-preparedness. Accessed 6 Oct 2015.
- 52.Schoen C, Osborn R, Squires DD, Doty MM, Rasmussen P, Pierson R, et al. A survey of primary care doctors in ten countries shows progress in use of health information technology, less in other areas. Health Aff (Millwood) 2012;31(2):2805–2816. doi: 10.1377/hlthaff.2012.0884. [DOI] [PubMed] [Google Scholar]
- 53.Hall SD. AHRQ: Open source EHR improves care in developing nations. Fierce EMR. March 19, 2013. http://www.fierceemr.com/story/ahrq-open-source-ehr-improves-care-developing-nations/2013-03-19. Accessed 8 Oct 2015.
- 54.World Health Organization, World Intellectual Property Organization, World Trade Organization. Promoting Access to Medical Technologies and Innovation: Intersections Between Public Health, Intellectual Property and Trade. Geneva, Switzerland: World Health Organization; 2013.
- 55.Kaiser Permanente. Fast facts about Kaiser Permanente. Kaiser Permanente Share. 2011. http://xnet.kp.org/newscenter/aboutkp/fastfacts.html. Accessed 8 Oct 2015.
- 56.Terhune C. Report: Kaiser tops state health insurance market with 40 % share. Los Angeles Times. 2013. http://articles.latimes.com/2013/jan/29/business/la-fi-mo-health-insure-market-20130129. Accessed 8 Oct 2015.
- 57.Ray GT, Collin F, Lieu T, Fireman B, Colby CJ, Quesenberry CP, Van den Eeden SK, Selby JV. The cost of health conditions in a health maintenance organization. Med Care Res Rev. 2000;57(1):92–109. doi: 10.1177/107755870005700106. [DOI] [PubMed] [Google Scholar]
- 58.Gordon NP. Similarity of the adult Kaiser Permanente membership in Northern California to the insured and general population in Northern California: statistics from the 2009 California Health Interview Survey. Kaiser Permanente Northern California Division of Research. 2012. http://www.dor.kaiser.org/external/chis_non_kp_2009/. Accessed 2015 Jul 29.
- 59.Harris AH, Bowe T, Finney JW, Humphreys K. HEDIS initiation and engagement quality measures of substance use disorder care: impact of setting and health care specialty. Popul Health Manag. 2009;12(4):191–196. doi: 10.1089/pop.2008.0028. [DOI] [PubMed] [Google Scholar]
- 60.Selby JV, Schmittdiel JA, Fireman B, Jaffe M, Ransom LJ, Dyer W, et al. Improving treatment intensification to reduce cardiovascular disease risk: a cluster randomized trial. BMC Health Serv Res. 2012;12:183. doi: 10.1186/1472-6963-12-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith PC, Schmidt SM, Allensworth-Davies D, Saitz R. Primary care validation of a single-question alcohol screening test. J Gen Intern Med. 2009;24(7):783–788. doi: 10.1007/s11606-009-0928-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vinson DC, Kruse RL, Seale JP. Simplifying alcohol assessment: two questions to identify alcohol use disorders. Alcohol Clin Exp Res. 2007;31(8):1392–1398. doi: 10.1111/j.1530-0277.2007.00440.x. [DOI] [PubMed] [Google Scholar]
- 63.National Institute on Alcohol Abuse and Alcoholism. Tips for cutting down on drinking. 2008. http://pubs.niaaa.nih.gov/publications/Tips/tips.pdf. Accessed 15 Oct 2014.
- 64.Boston University School of Medicine/Boston Medical Center. Alcohol screening and brief intervention curriculum. http://www.bu.edu/act/mdalcoholtraining/index.html. Accessed 7 July 2015.
- 65.Bradley KA, Chavez LJ, Lapham GT, Williams EC, Achtmeyer CE, Rubinsky AD, Hawkins EJ, Saitz R, Kivlahan DR. When quality indicators undermine quality: bias in a quality indicator of follow-up for alcohol misuse. Psychiatr Serv. 2013;64(10):1018–1025. doi: 10.1176/appi.ps.201200449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–46. doi: 10.1097/00001648-199001000-00010. [DOI] [PubMed] [Google Scholar]
- 67.Coleman KJ, Ngor E, Reynolds K, Quinn VP, Koebnick C, Young DR, et al. Initial validation of an exercise “vital sign” in electronic medical records. Med Sci Sports Exerc. 2012;44(11):2071–2076. doi: 10.1249/MSS.0b013e3182630ec1. [DOI] [PubMed] [Google Scholar]
- 68.Williams EC, Achtmeyer CE, Thomas RM, Grossbard JR, Lapham GT, Chavez LJ, et al. Factors underlying quality problems with alcohol screening prompted by a clinical reminder in primary care: a multi-site qualitative study. J Gen Intern Med. 2015;30(8):1125–1132. doi: 10.1007/s11606-015-3248-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grant RW, Schmittdiel JA, Neugebauer RS, Uratsu CS, Sternfeld B. Exercise as a vital sign: a quasi-experimental analysis of a health system intervention to collect patient-reported exercise levels. J Gen Intern Med. 2014;29(2):341–348. doi: 10.1007/s11606-013-2693-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nemeth LS, Miller PM, Nietert PJ, Ornstein SM, Wessell AM, Jenkins RG. Organizational attributes and screening and brief intervention in primary care. Addict Behav. 2013;38(11):2639–2642. doi: 10.1016/j.addbeh.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaner EFS, Brown N, Jackson K. A systematic review of the impact of brief interventions on substance use and co-morbid physical and mental health conditions. Ment Health Subst Use. 2011;4(1):38–61. doi: 10.1080/17523281.2011.533449. [DOI] [Google Scholar]
- 72.Klatsky AL. Alcohol-associated hypertension: when one drinks makes a difference. Hypertension. 2004;44(6):805–806. doi: 10.1161/01.HYP.0000146538.26193.60. [DOI] [PubMed] [Google Scholar]
- 73.Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67(3):220–229. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- 74.Peikes DN, Reid RJ, Day TJ, Cornwell DD, Dale SB, Baron RJ, et al. Staffing patterns of primary care practices in the comprehensive primary care initiative. Ann Fam Med. 2014;12(2):142–149. doi: 10.1370/afm.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fleming MF, Barry KL, Manwell LB, Johnson K, London R. Brief physician advice for problem alcohol drinkers: a randomized controlled trial in community-based primary care practices. JAMA. 1997;277(13):1030–1045. doi: 10.1001/jama.1997.03540370029032. [DOI] [PubMed] [Google Scholar]
- 76.Williams EC, Rubinsky AD, Chavez LJ, Lapham GT, Rittmueller SE, Achtmeyer CE, et al. An early evaluation of implementation of brief intervention for unhealthy alcohol use in the US Veterans Health Administration. Addiction. 2014;109(9):1472–1481. doi: 10.1111/add.12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dawson DA, Grant BF, Chou SP, Pickering RP. Subgroup variation in US drinking patterns: results of the 1992 national longitudinal alcohol epidemiologic study. J Subst Abuse. 1995;7(3):331–344. doi: 10.1016/0899-3289(95)90026-8. [DOI] [PubMed] [Google Scholar]
- 78.Mertens JR, Weisner C, Ray GT, Fireman B, Walsh K. Hazardous drinkers and drug users in HMO primary care: prevalence, medical conditions, and costs. Alcohol Clin Exp Res. 2005;29(6):989–998. doi: 10.1097/01.ALC.0000167958.68586.3D. [DOI] [PubMed] [Google Scholar]
- 79.D’Amico EJ, Paddock SM, Burnam A, Kung FY. Identification of and guidance for problem drinking by general medical providers: results from a national survey. Med Care. 2005;43(3):229–236. doi: 10.1097/00005650-200503000-00005. [DOI] [PubMed] [Google Scholar]
- 80.Weisner C, Matzger H. Missed opportunities in screening for alcohol problems in medical and mental health services. Alcohol Clin Exp Res. 2003;27(7):1132–1141. doi: 10.1097/01.ALC.0000075546.38349.69. [DOI] [PubMed] [Google Scholar]
- 81.Volk RJ, Steinbauer JR, Cantor SB. Patient factors influencing variation in the use of preventive interventions for alcohol abuse by primary care physicians. J Stud Alcohol. 1996;57(2):203–209. doi: 10.15288/jsa.1996.57.203. [DOI] [PubMed] [Google Scholar]
- 82.Greenfield SF, Back SE, Lawson K, Brady KT. Substance abuse in women. Psychiatr Clin North Am. 2010;33(2):339–355. doi: 10.1016/j.psc.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bryson CL, Au DH, Sun H, Williams EC, Kivlahan DR, Bradley KA. Alcohol screening scores and medication nonadherence. Ann Intern Med. 2008;149(11):795–804. doi: 10.7326/0003-4819-149-11-200812020-00004. [DOI] [PubMed] [Google Scholar]
- 84.National Center on Addiction and Substance Abuse at Columbia University. Missed opportunity: National Survey of Primary Care Physicians and Patients on Substance Abuse. Survey Research Laboratory, University of Illinois at Chicago, Chicago. 2000. http://www.casacolumbia.org/articlefiles/380-Missed%20Opportunity%20Physicians%20and%20Patients.pdf. Accessed 8 Oct 2015.
- 85.Caetano R, Baruah J, Chartier KG. Ten-year trends (1992 to 2002) in sociodemographic predictors and indicators of alcohol abuse and dependence among whites, blacks, and Hispanics in the United States. Alcohol Clin Exp Res. 2011;35(8):1458–1466. doi: 10.1111/j.1530-0277.2011.01482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug Alcohol Depend. 2004;74(3):223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 87.Frank E, Carrera JS, Elon L, Hertzberg VS. Predictors of US medical students’ prevention counseling practices. Prev Med. 2007;44(1):76–81. doi: 10.1016/j.ypmed.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 88.Satre DD, McCance-Katz EF, Moreno-John G, Julian KA, O’Sullivan PS, Satterfield JM. Using needs assessment to develop curricula for Screening, Brief Intervention and Referral to Treatment (SBIRT) in academic and community health settings. Subst Abus. 2012;33(3):298–302. doi: 10.1080/08897077.2011.640100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Anderson P, Laurant M, Kaner E, Wensing M, Grol R. Engaging general practitioners in the management of hazardous and harmful alcohol consumption: results of a meta-analysis. J Stud Alcohol. 2004;65(2):191–199. doi: 10.15288/jsa.2004.65.191. [DOI] [PubMed] [Google Scholar]
- 90.Barnett NP, Monti PM, Cherpitel C, Bendtsen P, Borges G, Colby SM, et al. Identification and brief treatment of alcohol problems with medical patients: an international perspective. Alcohol Clin Exp Res. 2003;27(2):262–270. doi: 10.1097/01.ALC.0000057123.36127.8B. [DOI] [PubMed] [Google Scholar]
- 91.Funk M, Wutzke S, Kaner E, Anderson P, Pas L, McCormick R, et al. A multicountry controlled trial of strategies to promote dissemination and implementation of brief alcohol intervention in primary health care: findings of a World Health Organization collaborative study. J Stud Alcohol. 2005;66(3):379–388. doi: 10.15288/jsa.2005.66.379. [DOI] [PubMed] [Google Scholar]
- 92.Institute of Medicine. Crossing the quality chasm: a new health system for the 21st Century. Quality Chasm Series. Washington, DC: National Academy Press; 2001.
- 93.Kaner EF, Heather N, Brodie J, Lock CA, McAvoy BR. Patient and practitioner characteristics predict brief alcohol intervention in primary care. Br J Gen Pract. 2001;51(471):822–827. [PMC free article] [PubMed] [Google Scholar]
- 94.Lock CA, Kaner EF. Implementation of brief alcohol interventions by nurses in primary care: do non-clinical factors influence practice? Fam Pract. 2004;21(3):270–275. doi: 10.1093/fampra/cmh310. [DOI] [PubMed] [Google Scholar]
- 95.Nilsen P, Aalto M, Bendtsen P, Seppa K. Effectiveness of strategies to implement brief alcohol intervention in primary healthcare. A systematic review. Scand J Prim Health Care. 2006;24(1):5–15. doi: 10.1080/02813430500475282. [DOI] [PubMed] [Google Scholar]
- 96.Ockene JK, Adams A, Hurley TG, Wheeler EV, Hebert JR. Brief physician- and nurse practitioner-delivered counseling for high-risk drinkers: does it work? Arch Intern Med. 1999;159(18):2198–2205. doi: 10.1001/archinte.159.18.2198. [DOI] [PubMed] [Google Scholar]
- 97.Ohman-Strickland PA, John Orzano A, Nutting PA, Perry Dickinson W, Scott-Cawiezell J, Hahn K, et al. Measuring organizational attributes of primary care practices: development of a new instrument. Health Serv Res. 2007;42(3 Pt 1):1257–1273. doi: 10.1111/j.1475-6773.2006.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chaudhry B, Wang J, Wu S, Maglione M, Mojica W, Roth E, et al. Systematic review: impact of health information technology on quality, efficiency, and costs of medical care. Ann Intern Med. 2006;144(10):742–752. doi: 10.7326/0003-4819-144-10-200605160-00125. [DOI] [PubMed] [Google Scholar]
- 99.Zhou YY, Kanter MH, Wang JJ, Garrido T. Improved quality at Kaiser Permanente through e-mail between physicians and patients. Health Aff (Millwood) 2010;29(7):1370–1375. doi: 10.1377/hlthaff.2010.0048. [DOI] [PubMed] [Google Scholar]
- 100.Hillestad R, Bigelow J, Bower A, Girosi F, Meili R, Scoville R, et al. Can electronic medical record systems transform health care? Potential health benefits, savings, and costs. Health Aff (Millwood) 2005;24(5):1103–1117. doi: 10.1377/hlthaff.24.5.1103. [DOI] [PubMed] [Google Scholar]
- 101.Holroyd-Leduc JM, Lorenzetti D, Straus SE, Sykes L, Quan H. The impact of the electronic medical record on structure, process, and outcomes within primary care: a systematic review of the evidence. J Am Med Inform Assoc. 2011;18(6):732–737. doi: 10.1136/amiajnl-2010-000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.HealthIT.gov. How to attain meaningful use. EHR Incentives and Certification. http://www.healthit.gov/providers-professionals/how-attain-meaningful-use. Accessed 8 Oct 2015.
- 103.McKnight-Eily L. Communication between health professionals and their patients about alcohol use. Vital Signs Town Hall Teleconference sponsored by Centers for Disease Control and Prevention Office for State, Tribal, Local and Territorial Support. 2014. http://www.cdc.gov/stltpublichealth/townhall/presentations/2014/vs-january.pdf. Accessed 8 Oct 2015.


