Increased frequency, coverage or sensitivity of HIV testing among MSM is unlikely reduce HIV incidence unless men diagnosed through enhanced testing programs also achieve viral suppression by engaging in effective HIV care at higher rates than currently observed.
Keywords: HIV serodiagnosis, HIV serosorting, mathematical model
Abstract
Background. In the United States, public health recommendations for men who have sex with men (MSM) include testing for human immunodeficiency virus (HIV) at least annually. We model the impact of different possible HIV testing policies on HIV incidence in a simulated population parameterized to represent US MSM.
Methods. We used exponential random graph models to explore, among MSM, the short-term impact on baseline (under current HIV testing practices and care linkage) HIV incidence of the following: (1) increasing frequency of testing; (2) increasing the proportion who ever test; (3) increasing test sensitivity; (4) increasing the proportion of the diagnosed population achieving viral suppression; and combinations of 1–4. We simulated each scenario 20 times and calculated the median and interquartile range of 3-year cumulative incidence of HIV infection.
Results. The only intervention that reduced HIV incidence on its own was increasing the proportion of the diagnosed population achieving viral suppression; increasing frequency of testing, the proportion that ever test or test sensitivity did not appreciably reduce estimated incidence. However, in an optimal scenario in which viral suppression improved to 100%, HIV incidence could be reduced by an additional 17% compared with baseline by increasing testing frequency to every 90 days and test sensitivity to 22 days postinfection.
Conclusions. Increased frequency, coverage, or sensitivity of HIV testing among MSM is unlikely to result in reduced HIV incidence unless men diagnosed through enhanced testing programs are also engaged in effective HIV care resulting in viral suppression at higher rates than currently observed.
In the US human immunodeficiency virus (HIV) epidemic, men who have sex with men (MSM) have been, and continue to be, the most heavily impacted HIV risk group [1, 2]. Recent estimates of HIV incidence among young black MSM have led to calls [3, 4] for immediate action to improve HIV prevention in the United States. HIV-infected persons who are not aware of their HIV infection are more likely to engage in behaviors that place their partners at risk of HIV transmission, and both primary studies of recently infected MSM [5, 6] and a recent meta-analysis commissioned by the World Health Organization [7] document evidence that most persons who learn of their HIV-positive status take steps to reduce the risk of HIV transmission to others.
The US Centers for Disease Control (CDC) recommends that MSM should test for HIV at least annually [8, 9]. However, recent reports suggest that most men are not testing for HIV this frequently, with 1 study finding that only 37% of a US sample of MSM had tested in the past 12 months [4, 9, 10]. How many US MSM have ever tested, how frequently they test, and whether MSM should test more frequently than once a year are still under debate [11–17]. In addition to behavioral changes that result from an HIV diagnosis, this diagnosis serves as a necessary but insufficient first step to receiving HIV medical care and antiretroviral treatment, which can result in reduced viral load and associated decreases in infectiousness [18]. In the United States, a minority (43.4%) of those diagnosed with HIV actually achieve viral suppression [19–21]. The proportion of all infections that are diagnosed and the testing frequency among MSM are increasing [9], but there has been relatively little change in the proportion of all diagnosed persons achieving viral suppression [19–21], with some estimates suggesting that this proportion has recently decreased to 35% of the diagnosed population and is even lower in some subgroups [20, 21]. It is unclear whether the potential behavioral changes associated with increased awareness of HIV infection resulting from increased testing frequency are sufficient to result in reduced incidence in the absence of increased viral suppression in the population.
We developed interaction and transmission models, parameterized using data from a national online survey of MSM [22], to assess the impact of increases in testing frequency on 3-year HIV incidence and to determine how testing frequency interacts with viral suppression, test sensitivity, and the proportion of the population seeking testing.
METHODS
Modeling Strategy and Source of Parameter Values
A detailed description of how our model was developed and parameterized is included in Supplementary Figure 1. We developed individual-based models (IBMs) using the time-varying extensions of exponential random graph models (ERGMs), called Separable Temporal ERGMs (STERGMs) [23]. Exponential random graph models are used to describe statistical properties of cross-sectional information about networks, in this case sexual partnerships among MSM. Separable Temporal ERGMs allow the structure of these networks to vary over time by modeling partnership formation and break-up separately at each time point within the IBM simulation. In our case, partnership formation is a function (fully described in the Supplementary Data) of the total number of partnerships in the network, the number of partners each of 2 men who could become partners already have, and their self-perceived HIV status (including a group that would say they do not know their HIV status). Existing partnerships end with a probability dependent on how long they have existed, which varies by the perceived serostatus of the couple, eg, men who do not know each other's HIV status have shorter partnerships and are more likely to break up than men who say they both know they are HIV negative or both know they are HIV positive. Separable Temporal ERGMs have the ability to capture statistical properties of sexual networks such as the total number of partnerships and overall degree distribution, as well as how these vary by other variables, such as the serostatus of both men in the partnership, features that cannot be described directly in either IBMs alone or within compartmental (eg, susceptible-infected) models for HIV transmission [24]. Separable Temporal ERGMs were fit using the statnet [23] suite of packages in R, and we used customized extensions to the R package EpiModel [25] to control testing intervention parameters within our simulations.
A national online survey of MSM [22] was used to parameterize the STERGM portions of our IBM. The survey sample included men from around the United States, and although it was slightly more diverse than the overall US population, the majority of participants were white [22]. The survey collected information on behaviors with up to 5 partners in the 6 months prior to interview [22, 26], as well as partnership type (main, casual, and one-time), current partners (number at the time of interview) and in the last 6 months, partnership duration, and self-reported HIV serostatus of the participant and each reported partner. Based on the survey data, we took a different approach than others [11–17] because we have found that many nonmain partnerships are recurring, if not ongoing, relationships, so we did not treat them as one-time events. Instead, we captured the duration of all ongoing partnerships (ie, those that were not one-time events) and used these data to describe the duration of partnerships using different rates of the ending of partnerships by perceived HIV serostatus. One-time partnerships were captured separately as a probability of an additional unprotected sex act in each 5-day increment, which differs by serostatus of the pair in an existing partnership. For the transmission model, per-act probability of HIV transmission was modeled as a function of changes in HIV viral load after infection, similar to other IBM models of MSM HIV transmission [16–18]. Access to HIV treatment was included as a variable of interest (Panel 1) and modeled using a probability of accessing treatment for each newly diagnosed man, to produce a population mean of 43% of the diagnosed population achieving viral suppression as reported by CDC in early 2014 [19]. The probability of testing on any given day was varied based both on the proportion of the population that never test and the time since last test for those who do. For the test sensitivity parameter, we used a 45-day window from infection to detection, meaning that for men infected less than 45-days ago, they would “be told” that their HIV test was negative in the simulation. This number is based on data [27] for blood-based rapid HIV antibody tests, and it is longer than the 22-day estimate used by others [16–17] to emulate 3rd- or 4th-generation laboratory testing for HIV. We include both a test with a 22-day window and a hypothetical perfect test with no window in alternative scenarios (Panel 1). The simulation included 5250 MSM, chosen to maximize efficiency of the simulations, which is limited by the software's use of computer memory [23–25]. Additional detail about the structure of our STERGM model and other IBM parameters that were held constant in all simulations is included in the Supplementary Data. The fitted baseline model was validated using data from a 735-person subset of study participants who were tested for HIV at baseline, and, if HIV-negative, enrolled in follow-up with an HIV-test performed 12 months after completion of the baseline questionnaire [28].
Statistical Analysis of Outcomes From Model Simulations
The model was used to assess the impact of hypothetical manipulations of HIV testing on HIV incidence, circulating virus, and time to HIV diagnosis. These simulated interventions, including changes in testing frequency distribution and other testing intervention parameters, were varied as described in Panel 1 and the Supplementary Data, and complete cohorts were simulated 20 times to capture the stochastic variation inherent in the model and develop a sample of populations in which 3-year incidence of HIV infection could be summarized. Supplementary Figures 7 and 8 describe the rationale for presenting data on 20 simulations of each scenario. When we investigated additional (up to 100) simulations for the comparison of the baseline testing frequency to a scenario in which each MSM tests on average every 90 days, neither the standard error for estimates of HIV incidence nor the interpretation of the primary comparison of 3-year incidence in the scenarios changed as more simulations were added. Thus, we choose to report data based on 20 simulations for each of 17 possible combinations of our 4 HIV testing interventions (Panel 1). We calculated the median and interquartile range (IQR), used these observed incidence measures to calculate incidence rates, then compared these values for the baseline model and models that modified key factors related to HIV testing as described in Panel 1.
Investigation of the Effects of Changes in Testing Frequency on Transmission Dynamics
As in all individual-based models, our STERGM approach allow us to (1) track each MSM throughout the 3-year simulation; (2) calculate summary measures such as of the number of infected men, their individual contribution to the total circulating viral load in the simulated population, and the number of onward transmissions each man contributes; and (3) stratify each of these measures by serostatus awareness and testing status. Calculation details for each of these intermediate outcome measures appear in the Supplementary Data. The model records how often each man tests for HIV and calculates the time between infection and diagnosis. To assess the impact on HIV incidence, we compare the distributions of these intermediate factors and their impact on HIV transmission dynamics in the population across levels of testing frequency and other testing interventions.
Investigation of Population Level Interactions of Testing Intervention Components
To address how the effect of increasing testing frequency varied depending on the values for parameters of other testing interventions under our control in the simulations, we evaluated the effect of testing frequency with higher levels of viral suppression subsequent to diagnosis, with more sensitive tests, and with more complete coverage of testing interventions (ie, with fewer MSM who never test for HIV) as described in Panel 1. We modified these variables to represent typical US situations as well as an ideal situation, and we assess the effect of increasing testing frequency under each of these counterfactual scenarios. By varying 1 facet of a testing intervention at a time and then combining interventions, we can describe any synergy across interventions, where the effect of interventions in combination is more (or less) than the sum of each intervention implemented on its own.
RESULTS
Baseline Model and the Effects of Increases in Testing Frequency
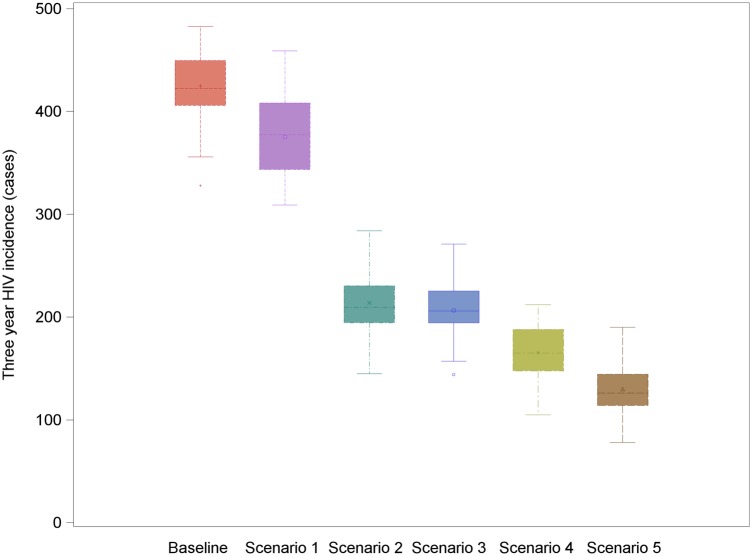
Our baseline scenario—in which 80% of the MSM population test on average annually, 20% never test, and with 43.4% of the diagnosed population achieving viral suppression (Panel 1)—resulted in a median of 422.5 infections over 3 years, corresponding to an incidence rate of 2.87 per 100 person-years (Table 1). In this scenario, over 11 000 HIV tests were performed over the 3-year study period among the 5250 MSM chosen as the simulation size for the model. Both the total number of tests performed and the mean number of tests per person (Supplementary Table 4) increased as testing frequency increased. Under the scenario in which participants tested every 90 days, we observed a >3-fold increase in testing compared with baseline. Both the median time from infection to diagnosis and the variance around that time decreased with more frequent testing (Supplementary Figure 2). Cumulative HIV incidence decreased slightly (Figure 1) but not meaningfully with increasing testing frequency.
Table 1.
Median and Interquartile Range of Incidence Observed in 20 Simulations of 3 Years of Follow-up in a Simulated Population of 5250 MSM Under Baseline and 12 Alternative Scenarios for HIV Testing Interventions in the United States
| Model Scenario | Value | New Infections/100 Person Years |
|
|---|---|---|---|
| Median | IQR | ||
| Baselinea | 2.87 | 2.76–3.06 | |
| Increasing test frequency (distribution's for each scenario reported in Supplementary Table 3) | Test every | ||
| 5 d | 2.63 | 2.32–2.75 | |
| 90 d | 2.57 | 2.34–2.78 | |
| 180 d | 2.80 | 2.50–2.92 | |
| 365 d | 2.98 | 2.64–3.07 | |
| 1095 d | 3.27 | 3.10–3.57 | |
| Reduce the proportion of MSM who have never tested for HIV (baseline 20%) | 7% | 2.67 | 2.58–2.86 |
| 0.2% | 2.60 | 2.44–2.86 | |
| Increasing viral suppression through linkage to care and treatment (baseline 43%) | 68.5% | 2.19 | 2.03–2.47 |
| 100% | 1.43 | 1.32–1.57 | |
| Using tests with different sensitivity for early infection (baseline 45 d) | 70 d | 2.89 | 2.83–3.12 |
| 22 d | 2.68 | 2.53–3.02 | |
| 0 d | 2.84 | 2.64–3.17 | |
Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range; MSM, men who have sex with men.
a Baseline testing frequency is assigned based on data collected from an online survey; under this scenario, MSM test almost annually on average, but many test more (9.7% test every 90 days) and less (14.7% test once every 3 years). A complete description of the baseline testing frequency distribution and how testing was implemented is included in the Supplementary Data. Baseline values for other testing intervention parameters include 20% of MSM never testing, 43% of the population with diagnosed infection achieving viral suppression, and a test capable of detecting infection 45 days after it occurs. Data were analyzed as if the complete cohort of 4900 HIV-negative persons were observed for 3 years and contributed a total of 14 700 person-years of follow-up, thus these rates are averages over the 3-year cumulative period of the simulation.
Figure 1.
Population-level impact of increasing testing frequency on 3-year human immunodeficiency virus (HIV) incidence in a simulated population of 5250 men who have sex with men in the United States. Estimates are the median and ranges of total cases of HIV observed over 20 simulations of each scenario, where the frequency of testing was set to either the baseline distribution observed in our study population or the time interval indicated on the x-axis.
Effects of Testing Interventions Other Than Increases in Testing Frequency
In simulations that reflected other possible interventions related to HIV testing, incidence did not change from baseline with more sensitive HIV tests (detection at 22 or even 0 days) or with decreasing proportions of the population that does not ever test (Table 1). In contrast, increasing the proportion of the diagnosed population achieving viral suppression to 68.5% (corresponding to a scenario where all those currently estimated to be receiving HIV care are suppressed [19, Panel 1]) reduced the median incidence by 24%, to 2.19 per 100 person-years (IQR, 2.03–2.47). Increasing the proportion virally suppressed to an ideal goal of 100% of those diagnosed reduced incidence to a median of 1.43 per 100 person-years (IQR, 1.32–1.57), a reduction of 50% relative to baseline.
Effects of Increases in Testing Frequency Combined With Improved Viral Suppression
To investigate why increasing the frequency of testing had little impact on HIV incidence, whereas HIV care had a large impact, we estimated the proportion of the total infections and circulating viral load in each stage of serostatus awareness and infection, under varied testing and care scenarios. Figures 2 and 3 and Supplementary Figure 5 show how increasing testing frequency can only have a limited impact on the total circulating viral load (Figure 2) and, as a result, on transmitted infections (Figure 3), because diagnosis alone only moves those previously undiagnosed to the diagnosed group and has a small impact on circulating viral load overall.
Figure 2.
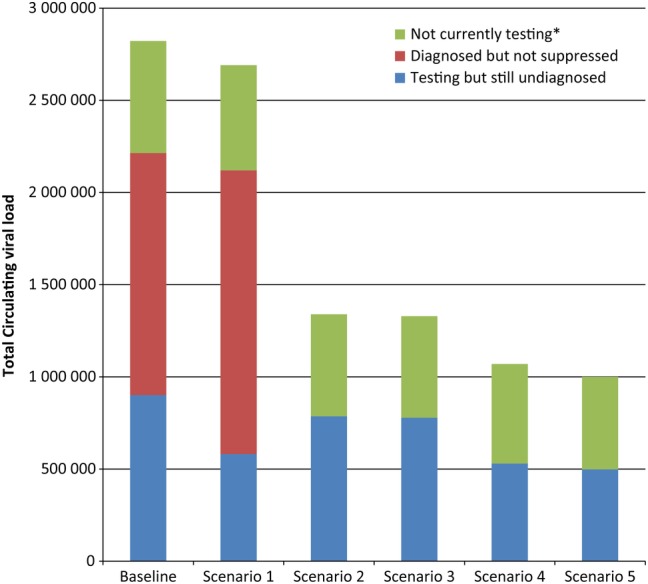
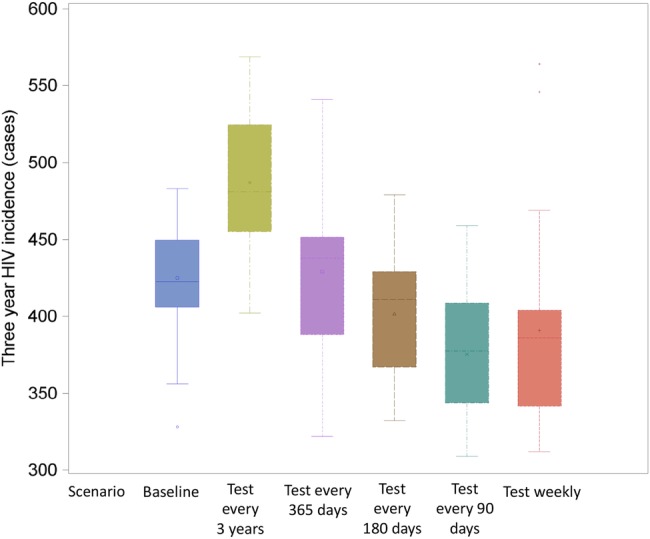
Synergistic effects of human immunodeficiency virus (HIV) testing frequency, test sensitivity, and viral suppression among the diagnosed population on the median 3-year total of circulating viral load, by HIV testing and diagnosis group of men who have sex with men in a simulated population. Scenario 1 increased HIV testing frequency to once every 90 days and held the percentage of the diagnosed population that achieve viral suppression to 43.4% and test sensitivity to 45 days as in the baseline scenario. Scenario 2 increases the proportion achieving viral suppression to 100% of the diagnosed population, while keeping the baseline scenario for testing frequency and test sensitivity. Scenario 3 increases both viral suppression (to 100% of the diagnosed population) and test sensitivity (to 22 days), while holding testing frequency to the baseline distribution. Scenario 4 increases testing frequency to every 90 days and increases viral suppression to 100%, while holding test sensitivity to 45 days. Scenario 5 optimizes all 3 strategies at once, with viral suppression increased to 100% of the diagnosed population, testing every 90 days, and a test with a 22-day window period.
Figure 3.
Synergistic effects of human immunodeficiency virus (HIV) testing frequency, test sensitivity, and viral suppression among the diagnosed population on 3-year HIV incidence across 20 simulations of a population of 5250 men who have sex with men in the United States. Scenario 1 increased HIV testing frequency to once every 90 days and held the percentage of the diagnosed population that achieve viral suppression to 43.4% and test sensitivity to 45 days as in the baseline scenario. Scenario 2 increases the proportion achieving viral suppression to 100% of the diagnosed population, while keeping the baseline scenario for testing frequency and test sensitivity. Scenario 3 increases both viral suppression (to 100% of the diagnosed population) and test sensitivity (to 22 days), while hold testing frequency to the baseline distribution. Scenario 4 increases testing frequency to every 90 days and increases viral suppression to 100%, while holding test sensitivity to 45 days. Scenario 5 optimizes all 3 strategies at once, with viral suppression increased to 100% of the diagnosed population, testing every 90 days, and a test with a 22-day window period.
However, if treatment coverage can be increased after diagnosis, then changes in testing frequency begin to have a greater impact on HIV transmission. Figures 2 and 3 show that increasing viral suppression among those whose infection is diagnosed reduces total circulating viral load (Figure 2) and onward transmission (Figure 3) substantially within both the baseline testing scenario and a scenario in which MSM test every 90 days. In Figure 2, when the diagnosed population no longer contributes to the total circulating viral load, increasing testing frequency to once every 90 days and using a test that detects HIV within 22 days of infection removes an additional 26% of the circulating viral load compared with improving viral suppression alone; in this scenario, the total viral load observed in the simulated population is reduced to only 35% of the baseline estimate. In Figure 3, under the baseline testing scenario, but again with the highly optimistic counterfactual of 100% viral suppression among those with diagnosed infection, a change to a more sensitive test alone also does not have any impact on incidence (Scenarios 2 and 3 in Figure 3) . However, when treatment coverage was 100% and testing frequency also increased to once every 90 days (Scenarios 4 and 5 in Figure 3), changing to a more sensitive test (Scenario 5) appears to decrease incidence even further than increasing treatment coverage and testing frequency alone (Scenario 4). Supplementary Figure 6 shows the proportion of all infections averted for each component of Scenario 5. With 3 testing interventions combined, there is an additional 7% reduction in incidence over the 3-year simulation, over and above what was observed when each intervention was applied to the population individually. A similar synergistic effect was observed when viral suppression among the diagnosed population was 100% and more (93% compared with 80% at baseline) MSM test for HIV at least annually (Supplementary Figure 3).
DISCUSSION
We developed a simulation model for MSM sexual partnerships parameterized, in part, by data from an online survey of MSM, and we evaluated 4 hypothetical interventions related to HIV testing in terms of their effects on resulting HIV incidence in our simulated population over a short (3-year) period. We found that, at current rates of HIV viral suppression in the United States, increasing the frequency of HIV testing by MSM increased the numbers of test performed, but it did little to affect 3-year incidence of HIV. The only scenario that led to reduced HIV incidence was increasing the proportion of MSM who proceed from diagnosis to achieve HIV viral suppression. Under the assumption that 100% of those who are diagnosed with HIV achieve viral suppression, circulating viral load and, ultimately, HIV transmissions could be reduced if MSM tested every 90 days instead of testing annually. Thus, increased HIV testing frequency for MSM has a role to play as part of a comprehensive package of services that culminates in effective linkage and care, but, according to our data, it would not lead to reductions in HIV incidence if implemented without improvements in downstream continuum steps.
Our baseline scenario was parameterized using behavioral data from a prospective HIV incidence cohort of US MSM [22, 28], and it produced an annualized estimate of HIV incidence of 2.87/100 person-years. The cohort study itself observed a very similar HIV incidence (2.4/100 person-years; 95% confidence interval [CI], 1.4–4.1) [28], which suggests our model was reasonably calibrated. The model estimates of incidence and the cohort estimate are consistent with meta-analysis results describing the annual HIV incidence among MSM recruited from community-based studies in the United States (mean, 2.25; 95% CI, 2.05–2.45) [29]. In addition, we achieved a baseline distribution of HIV testing frequency that was consistent with our survey data and which produced relatively stable estimates of HIV testing over time, without having to assume a constant rate of testing in the population [11–17].
Our finding that testing frequency had little impact on 3-year HIV incidence is consistent with several other studies. Gray [11] found that increasing testing to twice or 4 times annually resulted in a nonsignificant trend in infections averted, with reductions in 10-year incidence of 8.5% (range, −5.7% to 20%) and 13.8% (range, −4.2% to 20.6%), respectively. For the baseline scenario in her deterministic model of HIV transmission, Long [14] assumed that only 23% of high-risk individuals (including MSM) test annually; the counterfactual scenarios were to deploy high-sensitivity testing strategies (ie, able to identify persons at 22, 17, and 11 days after infection). She found a large reduction in HIV incidence if more high-risk people tested annually, but she reported only small marginal benefits from testing every 6 months. Semiannual testing of MSM with a test with a 17-day window period was estimated to provide a 1.9% reduction in incidence at a cost of $4.9 billion dollars for additional testing [14]. Likewise, Lucas [12] found that testing MSM every 3 months would be the “optimal” strategy, being cost effective ($45 074/quality-adjusted life-year), but at a cost of $8.1 billion per year. It is unclear whether the publically and privately funded healthcare systems responsible for such testing are prepared to absorb these costs [30]. Khanna studied a scenario in which testing frequency was tailored to personal risk (based on number of nonmain sex partners with whom condomless anal intercourse occurs), and he found this strategy to significantly reduce incidence compared with both annual and less frequent (testing every 2 and 10 years on average) testing. The Centers for Disease Control and Prevention recommends that highest risk MSM, which would include men with more than 3 casual sex partners in 3 months as modeled by Khanna [17], consider pre-exposure prophylaxis, which also requires frequent retesting for HIV [31]. Strategies that can encourage testing with each new partner [32, 33] may be an alternative that is appealing to lower risk MSM.
We broaden previous reports by examining the intermediate impacts of HIV testing on transmission. Given our current situation, with large gaps in viral suppression for those MSM with diagnosed HIV infection [20, 21], the interaction between increasing testing frequency and achieving viral suppression given a positive test result is important. Even without additional testing, in our model increasing the proportion of HIV-infected men on treatment reduced incidence dramatically. In the absence of treatment and viral suppression for those who are aware of their infection, the only mechanism for reducing incidence after diagnosis is through behavioral changes due to serostatus awareness [5–7, 16]. When only 43% of the diagnosed population achieves viral suppression (reported by the CDC in early 2014 [19], but recently updated estimates are even lower [20, 21]), the majority of the effect of testing is to move small percentages of men from undiagnosed to “diagnosed but not suppressed”, leading to only small reductions in the circulating viral load in the population overall. In contrast, if universal and prompt suppression of viral load after HIV diagnosis were achieved, increasing testing frequency and increasing test sensitivity could further reduce the remaining circulating viral load by a relatively larger percentage, ultimately reducing transmission. This interaction of the impacts of testing programs and treatment efforts has been observed in other models [12, 15, 17, 34, 35] but not always identified as such. For example, Lucas and Armbruster [12] accounted for this concept by assuming immediate access to therapy for all those who were diagnosed when determining that the “optimal” strategy was for MSM to test every 90 days, but did not present a counterfactual situation in which testing frequency was increased without perfect follow-up care. In a model parameterized based on the HIV testing, care and treatment, and risk profiles for MSM in Seattle, Washington, Katz et al [34] also found that test sensitivity was an important consideration when all men test at least annually and the proportion of the diagnosed population receiving treatment was 74%. Our findings are also consistent with recently published models of HIV transmission in the United Kingdom, where the authors estimated both that 84% of diagnosed MSM received treatment and that the majority (64%) of ongoing transmission arose from undiagnosed MSM [35]. The Centers for Disease Control and Prevention estimates that the proportion of all infected individuals who are aware of their infection has increased in the last 3 years, but this proportion will likely not reach the National HIV/AIDS Strategy (NHAS) 2015 goal of 90% [4, 9]. The current estimates of the proportion of MSM that have achieved viral suppression fall even shorter of the 2015 NHAS target [20, 21]. Efforts to improve both outcomes simultaneously are needed.
Our analysis has several potential limitations. In our model, although we parameterized test sensitivity to allow for false-negative results to occur, we did not consider the impact of false-positive results. With a low prevalence of HIV in the population overall, increasing the frequency of testing among the uninfected population will likely increase the number of false-positive results given to MSM annually, which could impact future testing behavior. We assume testing behaviors are periodic, and we do not account for risk-based episodic HIV testing (for example, testing based on recent exposure or symptoms of HIV seroconversion illness). The extent to which such testing could change the transmission dynamics of HIV will again be limited by how quickly men newly identified as being infected are able to access treatment and suppress their viral load. There are differences in both (1) proportion of diagnosed infections and (2) access to HIV care and treatment by race and age [21], and these differences appear to be more important to explaining observed disparities in HIV prevalence and incidence than factors such as age mixing [36, 37]. Although we hope to address this issue in future work, our current analysis essentially averages over these factors to produce an overall estimate of the effects in a population of MSM with racial and age distributions reported by Khosropuor et al. [20]. Nonetheless, our data support maximizing viral suppression as a strategy for reducing incidence in all race and age groups. Similar to the model developed by Lucas and Armbruster [12], our model presumes that treatment and viral suppression are started by MSM immediately after diagnosis. This approach is in contrast with other recent models [11, 13–17], which instead assumed that treatment will commence based on time since infection and disease progression. However, in the United States, it is recommended that all persons infected with HIV be offered treatment as soon as possible after diagnosis [38], a recommendation that was recently validated by results from a large randomized trial [39]. Even if this is not happening currently throughout the United States, for our most optimistic scenario, we wanted to model viral suppression as being available to all as soon as possible after diagnosis.
CONCLUSIONS
According to our data, more frequent HIV testing by US MSM will not result in reduced HIV incidence unless combined with improvements in effective HIV care. With limited resources available for reducing onward transmission of HIV, efforts focused on increasing the proportion of infected men who achieve and maintain viral suppression would have a larger impact on HIV transmission. Only after viral suppression becomes the normative outcome of HIV diagnosis does additional focus on increasing HIV testing as the gateway to this outcome become warranted.
Supplementary Data
Supplementary material is available online at Open Forum Infectious Diseases online (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Acknowledgments
Author contributors. K. P. D. conceived the idea for the study and led the analysis and writing of the manuscript. E. S. R. and P. S. S. contributed to the concept development, analysis, and writing. M. R. K. and L. A. W. contributed to the concept development and writing of the manuscript. All authors have seen and approved the final version of the manuscript for publication.
Financial support. This work was funded by the Emory Center for AIDS Research (grant P30-AI050409), and data used in the analysis were collected with support from the National Institute on Minority Health and Health Disparities (grant RC1MD004370) and National Institute of Mental Health (grant R01MH085600) at the National Institutes of Health.
Potential conflicts of interests. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
APPENDIX
Panel 1: Description of Testing Interventions
Increase the Frequency of Testing
Our modeling of human immunodeficiency virus (HIV) testing frequency is fully described in the Supplementary Data. At baseline, on average, men who have sex with men (MSM) test approximately annually, but some test more or less frequently (9.3% test every 90 days, 14.3% test only once every 3 years). We modified this so that all men actually test annually and also more (every 180 days, every 90 days, every 5 days) or less (every 3 years) frequently.
Improve the Proportion That Ever Test
To consider the effects of a strategy that would get more MSM to test at least once (such as a social media campaign, routine testing in a hospital or other clinical setting, or other outreach to the MSM population that is not currently testing), we increased the proportion of the population that ever tests for HIV from the baseline value of 80% to 93% and 99%.
Changing the Type of Test Used for Human Immunodeficiency Virus Screening
We implemented a test sensitivity parameter by varying the number of days after infection when a test, if conducted on the infected individual, would return a (correct) HIV-positive result. We used 22, 45, and 70 days to correspond to estimates of sensitivity for the newest laboratory tests, standard rapid blood tests, and oral fluid rapid tests that are also available for home use. We also include a hypothetical “perfect” HIV test which can detect infection the day it occurs.
Improve Human Immunodeficiency Virus Treatment Access and Viral Suppression
In the United States, there is a large proportion of the HIV-infected population that is aware of their infection but not receiving effective therapy to suppress their virus and thereby reduce the risk of transmission [19–21]. However, although the national average in the United States is low (43.4%), there are some cities with higher proportions of the population achieving viral suppression, as high as the 68.5% of all HIV-infected persons estimated to be in care for HIV [19], and guidelines for HIV treatment in the United States recommend universal treatment for all HIV-infected persons [38]. Thus, we varied the proportion of the population suppressed, setting this parameter to 43.4%, 68.5%, and 100% to assess the effect of increased testing frequency in situations where a higher proportion of the population follows their diagnosis with effective treatment.
Combinations of These Strategies
In addition to looking at the impact of each of the 12 intervention strategies above individually, we developed 4 additional scenarios in which interventions were combined. The first 2 additional scenarios combined increasing testing frequency (increasing testing from baseline to every 90 days) with increasing coverage of viral suppression (increasing suppression to 100% of the diagnosed population) and increasing test sensitivity (from 45 days at baseline to 22 days representing today's best laboratory tests). We separately considered a scenario where testing frequency was held at baseline levels, but the proportion of MSM who never test was decreased to 7% while increasing suppression and test sensitivity as described above. Finally, we report results of the scenario where testing frequency (every 90 days), viral suppression (100% of the diagnosed population), and test sensitivity (22 days after infection) were all increased simultaneously.
References
- 1.Centers for Disease Control and Prevention. Estimated HIV incidence in the United States, 2007–2010. HIV Surveillance Supplemental Report 2012; Vol. 17 (No. 4). Available at: http://www.cdc.gov/hiv/pdf/statistics_hssr_vol_17_no_4.pdf. Accessed 28 October 2015.
- 2.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data: United States and 6 U.S. dependent areas—2011. HIV Surveillance Supplemental Report, 2013; Vol. 18 (No. 5). Available at: http://www.cdc.gov/hiv/pdf/2011_monitoring_hiv_indicators_hssr_final.pdf. Accessed 28 October 2015.
- 3.Rosenberg ES, Sullivan PS, Kelley CF et al. . Race and age disparities in HIV incidence and prevalence among MSM in Atlanta GA. Available at: http://www.croiconference.org/sessions/race-and-age-disparities-hiv-incidence-and-prevalence-among-msm-atlanta-ga Accessed 9 February 2015. [Google Scholar]
- 4.Office of National AIDS Policy. National HIV/AIDS Strategy for the United States. Available at: http://aids.gov/federal-resources/national-hiv-aids-strategy/nhas.pdf. Accessed 28 October 2015. [Google Scholar]
- 5.Gorbach PM, Drumright LN, Daar ES, Little SJ. Transmission behaviors of recently HIV-infected men who have sex with men. J Acquir Immune Defic Syndr 2006; 42:80–5. [DOI] [PubMed] [Google Scholar]
- 6.Gorbach PM, Weiss RE, Jeffries R et al. . Behaviors of recently HIV-infected men who have sex with men in the year postdiagnosis: effects of drug use and partner types. J Acquir Immune Defic Syndr 2011; 56:176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization . Prevention and treatment of HIV and other sexually transmitted infections among men who have sex with men and transgender people: recommendations for a public health approach. Available at: http://www.who.int/hiv/pub/guidelines/msm_guidelines2011/en/ Accessed 20 November 2014. [PubMed]
- 8.Branson BM, Handsfield HH, Lampe MA et al. . Revised recommendations for HIV testing of adults, adolescents and pregnant women in health-care settings. MMWR Morb Mortal Wkly Rep 2006; 55 (RR-14):1–17. [PubMed] [Google Scholar]
- 9.Cooley LA, Oster AM, Rose CE et al. . Increases in HIV testing among men who have sex with men--National HIV Behavioral Surveillance System, 20 U.S. Metropolitan Statistical Areas, 2008 and 2011. PLoS One 2014; 9:e104162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Copen CE, Chandra A, Febo-Vazquez I. HIV testing in the past year among the U.S. household population aged 15–44: 2011–2013. NCHS Data Brief 2015; 202:1–8. Hyattsville, MD: National Center for Health Statistics. [PubMed] [Google Scholar]
- 11.Gray RT, Prestage GP, Down I et al. . Increased HIV testing will modestly reduce HIV incidence among gay men in NSW and would be acceptable if HIV testing becomes convenient. PLoS One 2013; 8:e55449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lucas A, Armbruster B. The cost-effectiveness of expanded HIV screening in the United States. AIDS 2013; 27:795–801. [DOI] [PubMed] [Google Scholar]
- 13.Wilson DP, Hoare A, Regan DG, Law MG. Importance of promoting HIV testing for preventing secondary transmissions: modelling the Australian HIV epidemic among men who have sex with men. Sex Health 2009; 6:19–33. [DOI] [PubMed] [Google Scholar]
- 14.Long EF. HIV screening via fourth-generation immunoassay or nucleic acid amplification test in the United States: a cost-effectiveness analysis. PLoS One 2011; 6:e27625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brookmeyer R, Boren D, Baral SD et al. . Combination HIV prevention among MSM in South Africa: results from agent-based modeling. PLoS One 2014; 9:e112668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khanna AS, Goodreau SM, Gorbach PM et al. . Modeling the impact of post-diagnosis behavior change on HIV prevalence in Southern California men who have sex with men (MSM). AIDS Behav 2014; 18:1523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khanna AS, Goodreau SM, Wohlfeiler D et al. . Individualized diagnosis interventions can add significant effectiveness in reducing human immunodeficiency virus incidence among men who have sex with men: insights from Southern California. Ann Epidemiol 2015; 1:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sullivan PS, Carballo-Dieguez A, Coates T et al. . Successes and challenges of HIV prevention in men who have sex with men. Lancet 2012; 380:388–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gray KM, Cohen SM, Hu X et al. . Jurisdiction level differences in HIV diagnosis, retention in care, and viral suppression in the United States. J Acquir Immune Defic Syndr 2014; 65:129–32. [DOI] [PubMed] [Google Scholar]
- 20.Bradley H, Hall HI, Wolitski RJ et al. . Vital signs: HIV diagnosis, care, and treatment among persons living with HIV--United States, 2011. MMWR Morb Mortal Wkly Rep 2014; 63:1113–7. [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenberg ES, Millett GA, Sullivan PS et al. . Understanding the HIV disparities between black and white men who have sex with men in the USA using the HIV care continuum: a modeling study. Lancet HIV 2014; 1:e112–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khosropour CM, Johnson BA, Ricca AV, Sullivan PS. Enhancing retention of an Internet-based cohort study of men who have sex with men (MSM) via text messaging: randomized controlled trial. J Med Internet Res 2013; 15:e194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Handcock MS, Hunter DR, Butts CT et al. . statnet: software tools for the representation, visualization, analysis and simulation of network data. J Stat Softw 2008; 24:1548–7660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris M, Kurth AE, Hamilton DT et al. . Concurrent partnerships and HIV prevalence disparities by race: linking science and public health practice. Am J Public Health 2009; 99:1023–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jenness SM, Goodreau SM, Morris M.. EpiModel. Mathematical Modeling of Infectious Disease Version 1.1.1, A Tutorial. Available at: www.epimodel.org Accessed 20 November 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sullivan PS, Peterson J, Rosenberg ES et al. . Understanding racial HIV/STI disparities in black and white men who have sex with men: a multilevel approach. PLoS One 2014; 9:e90514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masciotra S, Luo W, Youngpairoj AS et al. . Performance of the Alere Determine™ HIV-1/2 Ag/Ab Combo Rapid Test with specimens from HIV-1 seroconverters from the US and HIV-2 infected individuals from Ivory Coast. J Clin Virol 2013; 58 (Suppl 1):e54–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ricca A, Khosropour C, Sullivan PS. HIV incidence among men who have sex with men enrolled in an online behavioral risk study. XIX International AIDS Conference Washington, DC, USA July 22–27, 2011 (Abstract: TUPE205). Available at: https://www.aids2014.org/Default.aspx?pageId=12&abstractId=200745638. Accessed 28 October 2015. [Google Scholar]
- 29.Stall R, Duran L, Wisniewski SR et al. . Running in place: implications of HIV incidence estimates among urban men who have sex with men in the United States and other industrialized countries. AIDS Behav 2009; 13:615–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin EG, Schackman BR. Updating the HIV-testing guidelines--a modest change with major consequences. N Engl J Med 2013; 368:884–6. [DOI] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. Pre-exposure prophylaxis for the prevention of HIV infection in the United States—2014: a clinical practice guideline. Available at: www.cdc.gov/hiv/pdf/guidelines/PrEPguidelines2014.pdf. Accessed 28 October 2015.
- 32.Chakravarty D, Hoff CC, Neilands TB, Darbes LA. Rates of testing for HIV in the presence of serodiscordant UAI among HIV-negative gay men in committed relationships. AIDS Behav 2012; 16:1944–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sullivan PS, Wall KM, O'Hara B et al. . The prevalence of undiagnosed HIV serodiscordance among male couples presenting for HIV testing. Arch Sex Behav 2014; 43:173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katz DA, Cassels SL, Stekler JD. Replacing clinic-based tests with home-use tests may increase HIV prevalence among Seattle men who have sex with men: evidence from a mathematical model. Sex Transm Dis 2014; 41:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Punyacharoensin N, Edmunds WJ, De Angelis D et al. . Modelling the HIV epidemic among MSM in the United Kingdom: quantifying the contributions to HIV transmission to better inform prevention initiatives. AIDS 2015; 29:339–49. [DOI] [PubMed] [Google Scholar]
- 36.Grey JA, Rothenberg RB, Sullivan PS, Rosenberg ES. Disassortative age-mixing does not explain differences in HIV prevalence between young white and black MSM: findings from four studies. PLoS One 2015; 10:e0129877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hernández-Romieu AC, Sullivan PS, Rothenberg R et al. . Heterogeneity of HIV prevalence among the sexual networks of black and white men who have sex with men in Atlanta: illuminating a mechanism for increased HIV risk for young black men who have sex with men. Sex Transm Dis 2015; 42:505–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.AIDSinfo. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Available at: http://www.aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf Accessed 27 May 2015. [Google Scholar]
- 39.National Institutes of Health. Starting antiretroviral treatment early improves outcomes for HIV-infected individuals. Available at: http://www.nih.gov/news/health/may2015/niaid-27.htm Accessed 27 May 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.