Summary
Current therapies for Alzheimer’s disease do not modify the course of disease and are not universally beneficial.
Clinical trials of drugs targeting amyloid and tau in established Alzheimer’s disease have been unsuccessful as it is thought that treating established disease may be too late.
Research has moved to the prodromal and pre-symptomatic phases of Alzheimer’s disease, with a greater emphasis on the role of biomarkers in defining cases and monitoring response to therapy.
Mixed pathologies predominate in the older population. The associations between biomarkers, neuropathology and clinical syndromes are weaker in older people and this is likely to pose a greater challenge in identifying effective therapies.
Key words: Alzheimer's disease, cholinesterase inhibitors, dementia, memantine
Introduction
Alzheimer’s disease is the most prevalent of the dementias. There are no disease-modifying therapies and the condition is progressive, significantly impacting on cognition, function, lifespan and healthcare use. Any pharmacological management must be done in tandem with optimising the management of comorbidities, including behavioural symptoms, rationalising other medicines, and ensuring adequate education, carer support and provision of services.
Current therapies
There have been many unsuccessful therapeutic trials in Alzheimer’s disease. Failed therapies include anti-inflammatories, statins, hormonal therapies and chelators (drugs that bind metals that are thought to promote abnormal amyloid beta aggregation).
Cholinesterase inhibitors
Cholinergic neurotransmitter activity is low in Alzheimer’s disease. Cholinesterase inhibitors are thought to work by reducing the breakdown of the neurotransmitter acetylcholine. Donepezil, galantamine and rivastigmine are currently approved for use in mild to moderate Alzheimer’s disease, with rivastigmine also available as a transdermal patch. The three are equally efficacious and may temporarily improve cognition.
Pooled trials of cholinesterase inhibitors identified an improvement of 1.4 points on a Mini-Mental State Examination (MMSE) over six months. Small but statistically significant improvements in activities of daily living and behavioural symptoms, such as apathy, have also been identified. However, these improvements represent an average from thousands of trial participants, with individual responses varying.1 Only one-third of people show a clinically measurable benefit. Another third show clinical worsening during the first six months of therapy, and drop-out rates of 29% due to adverse effects are observed. The common adverse effects associated with cholinesterase inhibitors include nausea, vomiting, diarrhoea, abdominal pain, loss of appetite, muscle cramps, insomnia and nightmares. Relative contraindications to their use include heart block, bradyarrhythmias, epilepsy, active peptic ulcer disease, obstructive urinary disease and significant airway disease.
Cost–benefit studies of cholinesterase inhibitors, although limited, have failed to identify any economic benefit. There are no randomised double-blind placebo controlled trials showing that cholinesterase inhibitors delay entry into residential care. Weak evidence suggesting a delay is from less robust open-label studies and extrapolation of data from short-term trials. Although cholinesterase inhibitors are the current mainstay of Alzheimer’s disease therapy, objective and measurable benefit is not seen in most patients. These drugs do not modify disease and their economic benefits are uncertain.
Memantine
Memantine is a glutaminergic N-methyl-D-aspartate (NMDA) receptor antagonist currently thought to reduce NMDA receptor-mediated neurotoxicity. It is approved for moderate to severe Alzheimer’s disease.
Memantine has a statistically significant effect on cognition, behaviour and the ability to perform activities of daily living.2 A small reduction in agitation has been consistently observed. However, the trials examining memantine were limited by high drop-out rates, and the benefits identified, although statistically significant, were of small magnitude. A recent two-year trial has provided further evidence that memantine does not modify disease progression and is ineffective in mild Alzheimer’s disease.3
Data showing an economic benefit are limited. Given the small clinical benefits and the lack of effect on progression, memantine, like the cholinesterase inhibitors, provides symptomatic relief to some but has failed to provide universal benefit in Alzheimer’s disease.
Alternative therapies
Souvenaid is a nutritional supplement which combines vitamins and lipids. In two positive phase II trials of 12 and 24 weeks’ duration in people with mild Alzheimer’s disease (MMSE ≥20), the supplement was given to people not taking a cholinesterase inhibitor. The 12-week study found a statistically significant benefit on a delayed verbal recall task but no benefit on other cognitive, behavioural or functional measures. In the 24-week trial, the Neuropsychological Test battery failed to show any statistically significant improvement. However, when the memory test sub-score within the battery was examined, there was a statistically significant benefit that was predominantly driven by improvements between weeks 12 and 24 of the study.
In a third 24-week study of mild to moderate Alzheimer’s disease (MMSE 14−24), Souvenaid was used in combination with either a cholinesterase inhibitor, memantine or both. No evidence for cognitive or functional benefit was found.
In all three studies, the supplement was well tolerated but there was no evidence that Souvenaid slowed cognitive or functional decline. However, it may improve memory in the early stages of the disease in those who have not previously taken cholinesterase inhibitors.4 The potential small benefits need to be balanced with the cost of therapy − approximately $4 daily.
Numerous complementary and alternative medicines are used by patients with Alzheimer’s disease, including Ginkgo biloba, acetyl-L-carnitine, curcumin and coconut oil. Although many of these compounds are associated with plausible hypothetical effects and encouraging results from basic research, randomised trial data have not confirmed their benefit.
Therapeutic directions
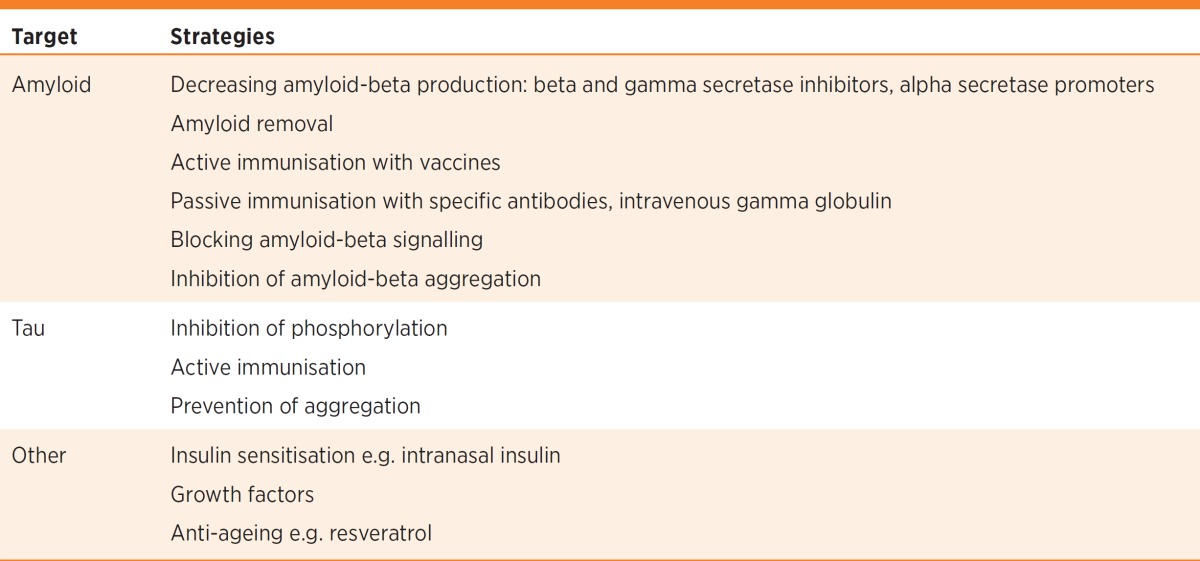
Current research is focusing on drugs that may slow or prevent disease progression (Table). Neurodegenerative conditions such as Alzheimer’s disease are thought to be proteinopathies. These are diseases caused by the deposition of abnormally folded or processed proteins that lead to cell death. Factors including inflammation, excitotoxicity (excessive release of stimulatory neurotransmitters resulting in neuronal death), mitochondrial dysfunction and free radical damage, perhaps induced by these proteins, are thought to promote the neurodegenerative process.
Table. Therapeutic strategies in the treatment of Alzheimer’s disease.
Alzheimer’s disease is characterised by the deposition of two proteins − amyloid-beta which is the predominant component of plaques, and hyperphosphorylated tau. Measuring the concentrations of these proteins in the brain and cerebrospinal fluid through labeled positron emission tomography (PET) and quantitative assessment is now possible and has become an integral component of therapeutic trials.
Proteinopathy-based therapies in established Alzheimer’s disease
Anti-amyloid drugs
The autosomal dominant forms of Alzheimer’s disease are caused by mutations that result in abnormal amyloid precursor protein processing and deposition. These findings have given rise to the amyloid cascade hypothesis which postulates that the transmembrane protein, amyloid precursor protein, is abnormally cleaved by beta and gamma secretase enzymes. This results in an over-production of amyloid-beta peptides that aggregate to ultimately form the characteristic amyloid plaques of Alzheimer’s disease. The abnormal amyloid-beta fragments are thought to be toxic, promoting the neurodegenerative process.
Anti-amyloid drugs aim to either decrease amyloid-beta production, increase its clearance or reduce aggregation of the protein. Amyloid-beta production can be inhibited by modifying the activity of the beta and gamma secretase enzymes that cleave the amyloid precursor protein. Despite evidence of efficacy for these drugs in vitro and in mice, drugs that inhibit the beta and gamma secretases have failed to show any benefit in established disease in clinical trials.
Immunotherapy has been used to increase clearance of amyloid-beta through active (vaccination) or passive (monoclonal antibodies, gamma globulin) immunisation. Initial vaccination trials were ceased due to meningoencephalitis, with no cognitive benefit found. Results are awaited from a phase II trial of a new vaccine.
Passive immunotherapy is based on the administration of monoclonal or polyclonal antibodies against amyloid-beta. Recently published phase III trials of two monoclonal antibodies have failed to show any benefit, with vasogenic oedema and microhaemorrhages identified as a potentially serious adverse event.5 Intravenous immunoglobulins have also failed to show benefit. Of the limited trials of drugs that inhibit the aggregation of amyloid, none have been positive to date.
Anti-tau drugs
The amyloid hypothesis has dominated much of the research into effective therapies for Alzheimer’s disease. However, recent failures for amyloid-based therapies have led some to question whether amyloid-beta is the consequence rather than the cause of Alzheimer’s disease.
Tau is a protein that stabilises microtubules. It is abundant in neurons but in Alzheimer’s disease it is hyperphosphorylated in the form of tangles. Anti-tau therapies have focused on inhibition of tau aggregation and phosphorylation. Trials of anti-tau therapies in established Alzheimer’s disease have not yet been successful.
Other drugs
There are numerous other drugs currently being trialled in established Alzheimer’s disease including intranasal insulin and resveratrol. Resveratrol stimulates the sirtuin pathway. Sirtuin proteins are thought to have an anti-ageing effect and have been found to promote the activity of alpha secretase. In contrast to beta and gamma secretase, alpha secretase cleaves the amyloid precursor protein into peptides that do not aggregate. Promoting alpha secretase through the sirtuin pathway may potentially reduce the formation of amyloid plaques. A phase II trial of a gene therapy, which is thought to stimulate nerve growth factor, is currently in progress.
Current research directions
In trials to date, treatment of mild to moderate Alzheimer’s disease with anti-amyloid therapies has resulted in the removal of amyloid, but the cognitive deficits of the disease have persisted. The current hypothesis is that anti-amyloid therapy is too late once there is established dementia as it is known that amyloid deposits precede cognitive changes by 10–15 years. Trials have therefore shifted to what is believed to be the two precursor states of Alzheimer’s disease. The prodromal phase, termed prodromal Alzheimer’s disease or mild cognitive impairment, is characterised by mild cognitive deficits in the absence of dementia with or without biomarker positivity. The hypothesised earliest phase of Alzheimer’s disease is called pre-symptomatic Alzheimer’s disease and is characterised by intact cognition in association with amyloid deposition as detected by biomarkers or a known genetic inheritance.
Trials in prodromal (or mild cognitive impairment) and pre-symptomatic Alzheimer’s disease are in progress with amyloid being the major target. Trials in pre-symptomatic Alzheimer’s disease are using monoclonal drugs targeting amyloid6 and include:
A4 study (Anti-Amyloid Treatment in Asymptomatic Alzheimer’s) in cognitively normal 65–85 year olds who have abnormal amyloid deposition as determined by PET scans
Dominantly Inherited Alzheimer’s Network study in pre-symptomatic individuals with dominantly inherited Alzheimer’s disease
Alzheimer’s Prevention Initiative in a large familial cohort with a known autosomal dominant Alzheimer’s disease mutation.
These studies will be vital for understanding the role of amyloid in Alzheimer’s disease pathogenesis. The A4 study will shed light on whether late onset sporadic Alzheimer’s disease behaves in a biologically similar way to inherited early onset Alzheimer’s disease.
Cautions and challenges for the future
Many believe that a single modality for treating Alzheimer’s disease will not be possible and that future therapies will need to address multiple aspects in the pathogenesis of Alzheimer’s disease. A major issue facing trials is the convergence of symptoms that may occur within the dementia spectrum. Dementias with the same clinical features may in fact be caused by different pathologies. Although biomarker studies may assist in identifying the associated proteinopathy, to date these studies have focused on well-defined and evaluated clinic-based populations. It is unknown how this will extrapolate to patients in the community where mixed pathologies may predominate.
One of the greatest future challenges lies in the epidemiology of Alzheimer’s disease. Age is the strongest predictor of disease and significantly outweighs all other risk factors and biomarkers. Older people suffer from more medical comorbidities. They have higher rates of sensory loss, psychoactive medicine use and frailty. These factors can have a significant impact on cognition, leading some to postulate that dementia in the ‘old-old’ also reflects these multiple coexistent pathologies. Amyloid scans are positive in about 65% of people over the age of 80, but are not predictive of cognitive function. Neuropathological post-mortem studies of brains have identified that in those aged over 85, the prevalence of pathological Alzheimer’s disease is similar between people with and without dementia, and about half of those over 90 with clinical dementia do not have sufficient neuropathology to account for their dementia. Therefore, although phenotypically similar to younger sufferers, older people suffer from a greater diversity of pathologies and it seems far less likely that they will respond in a uniform manner to proteinopathy-based therapies.
Conclusion
Currently available treatments provide symptomatic relief for Alzheimer’s disease but the benefits are not universal. The recent history of drug trials in Alzheimer’s disease suggests that a cautious approach in announcing a ‘cure’ is prudent. The inability to translate results from in vitro and animal studies to success in human trials has beset Alzheimer’s disease research and reflects the complexity of the disease pathogenesis. A large number of therapies with a plausible scientific basis and positive phase II trials have failed when progressed to phase III trials.
Proteinopathy-based therapies in established Alzheimer’s disease have failed and studies are now focusing on pre-symptomatic individuals as defined by biomarkers or genetic inheritance. Should these trials be successful, screening, escalating healthcare costs and translation to older dementia sufferers are likely to create new challenges.
Footnotes
Conflict of interest: none declared
References
- 1.Birks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev 2006;(1):CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McShane R, Areosa Sastre A, Minakaran N. Memantine for dementia. Cochrane Database Syst Rev 2006;(2):CD003154. [DOI] [PubMed] [Google Scholar]
- 3.Dysken MW, Sano M, Asthana S, Vertrees JE, Pallaki M, Llorente M, et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM-AD VA cooperative randomized trial. JAMA 2014;311:33-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NPS MedicineWise. 2014. Souvenaid: Help for people with Alzheimer’s disease? www.nps.org.au/publications/health-professional/health-news-evidence/2014/souvenaid [cited 2015 Mar 3]
- 5.Karran E, Hardy J. Antiamyloid therapy for Alzheimer’s disease--are we on the right road? N Engl J Med 2014;370:377-8. [DOI] [PubMed] [Google Scholar]
- 6.Friedrich MJ. Researchers test strategies to prevent Alzheimer disease. JAMA 2014;311:1596-8. [DOI] [PubMed] [Google Scholar]