Abstract
Objective
To investigate the question of whether serum leptin levels might be associated with post-stroke depression.
Methods
We studied 130 patients who experienced a first episode of stroke of more than three months' duration, without any previous history of depression or speech disorders. Data were collected regarding the patient demographics, depressive mood (Diagnostic and Statistical Manual of Mental Disorders 4th edition [DSM-IV] criteria and Beck Depression Inventory) and serum leptin levels measured by an enzyme-linked immunosorbent assay (ELISA). In addition, the Korean version of Modified Barthel Index (K-MBI) and Korean version of Mini-Mental State Examination (K-MMSE) were used to assess the subjects' independence, in regard to the activities of daily living and cognition. A statistical analysis was performed to determine differences the serum leptin levels between patients with depression and those without depression, and to determine the difference in the MBI and K-MMSE scores between the groups separated according to the serum leptin levels.
Results
Higher serum leptin levels were observed in patients with depression, compared with those without depression (38.5 ng/mL [range, 25.1-59.2 ng/mL] vs. 8.2 ng/mL [range, 4.9-17.8 ng/mL]; p<0.01. The serum leptin level showed an association with depression (odds ratio, 1.21; 95% confidence interval, 1.01-1.45; p=0.021). The K-MMSE and K-MBI improvement scores were lower, with statistical significance, in the group with the highest leptin level (>30 mg/dL), compared to the other two groups.
Conclusion
High serum leptin levels are associated with depression after stroke, and patients with elevated serum leptin levels were disadvantaged in regard to functional and cognitive outcomes.
Keywords: Stroke, Depression, Leptin, Outcome measure
INTRODUCTION
A stroke is a suddenly occurring functional disorder caused by a blockage of the blood supply to the brain, and is the second leading cause of death in developed countries [1]. Depression is the most frequent affective disorder that occurs after stroke, affecting approximately 33% of stroke patients. The degree of impairment related to activities of daily living is associated with whether or not a stroke patient suffers from depression [2]. Moreover, post-stroke depression (PSD) may exacerbate cognitive dysfunction and functional dependence, and increase mortality [2,3,4,5]. Therefore, the early diagnosis of depressive symptoms and introduction of pharmacological treatment is of great importance in the reduction of stroke complications and mortality, as well as for better functional outcomes.
The pathophysiology of PSD is complex and multifactorial in origin. Stroke lesion, stroke recurrence, mood regulating neural factors, and diseases that are comorbid with stroke (such as hypertension, artherosclerotic heart disease, and diabetes) are known risk factors for PSD [6]. Recently, chronic inflammation has been suggested as an important mechanism related to depression, and reduced anti-inflammatory cytokine function was associated with PSD [7,8]. Subtypes of intermediate-density lipoprotein and adipokines secreted from adipose tissue have been suggested as biomarkers related to depression [7,8,9].
Leptin, a 16-kDa adipokine of the obese (ob) gene product, is transported across the blood-brain barrier and affects neurotransmitters such as dopamine [10]. Although the exact neurobiological mechanisms of leptin associated with depression are not yet known, several clinical and experimental studies reported that leptin has been associated with the development of depression [11,12,13,14,15]. The effect of leptin in developing depression has been proposed via its modulatory effect on the hypothalamo-pituitary-adrenal (HPA) axis [10,12]. The concentration of the leptin levels in depression varies according to specific conditions, and the proper concentration is not measured. In cases of patients with simple depression, the serum leptin level is shown to be lower than the healthy controls. However, patients presenting atypical depression associated with a high risk for cardiovascular disease (such as metabolic syndrome) showed higher leptin levels compared with the healthy controls [13,14]. The function of leptin in PSD has not been studied yet. Clinical and experimental studies have shown that leptin is associated with the development of depression [15,16,17,18,19,20]; therefore, we investigated the association between serum leptin levels and PSD in 130 patients with ischemic stroke, and compare the serum leptin levels in stroke patients with and without depression.
MATERIALS AND METHODS
Study population
A total of 130 patients with a first episode of ischemic stroke were admitted to the Department of Rehabilitation Medicine. This study investigated patients with ischemic or hemorrhagic stroke, at least 3 months from onset. They all experienced subjective cognitive deterioration, and had a score on the Korean version of the Mini-Mental Status Examination (K-MMSE) that was ≥20 points and <27 points. Patients with a previous history of depression, immunologic diseases, thyroid disorders, any active infectious or inflammatory diseases, or uncontrolled diabetes were excluded. Patients who received antidepressant drugs and/or mood-influencing drugs were also excluded.
The protocol was approved by the Institutional Review Board and informed consent was given by patients, or their relatives.
Study method
The association between serum leptin levels and depression in stroke patients
In 130 adult stroke patients, depression was defined in accordance with the Diagnostic and Statistical Manual of Mental Disorders 4th edition (DSM-IV) criteria as 'major depression' and having a Beck Depression Inventory (BDI) score of more than 15 points. Then, stroke patients were classified as those with depression and without depression, according to those psychological evaluations. The leptin level and assessment of depression were checked within two weeks from the time of admission. The stroke patients were classified according to the presence of depression and we compared the leptin level using an enzyme-linked immunosorbent assay (ELISA).
The functional level assessment according to serum leptin level in stroke patients
The stroke patients were divided into three experimental groups: the upper tertile group, the middle tertile group, and the lower tertile group vs. >30 ng/mL, 20-30 ng/mL, and <20 ng/mL. The stroke severity, cognitive state, BDI, and functional dependency were measured for each group.
The stroke severity was measured using the Korean version of National Institute of Health Stroke Scale (K-NIHSS). The cognitive state was measured using the K-MMSE, which consisted of 12 questions (regarding disorientation, memory registration, memory recall, attention and calculation, language ability, understanding, and judgement), with a total possible score of 30. Functional dependency was evaluated using the Korean version of Modified Barthel Index (K-MBI; <74, functional dependence). The assessment of the functional outcome was measured using the K-MBI and the scores evaluation was performed at the initial admission and the final discharge date. The K-MBI consists of 10 items developed for an objective assessment of the activities of daily living. The testing of all subjects was administered by the same trained physician.
Statistical analysis
Values of p<0.05 were considered statistically significant in all tests. A statistical analysis was performed using SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA) for Windows 7. For the general characteristics of the patients, descriptive statistics were used, and all data were expressed as the mean (standard deviation) or median (interquartile range). Groups of stroke patients, with depression and without depression, were compared using the Student t-test or the Mann-Whitney test. Logistic regression modeling was performed to determine the influence of the serum leptin level on the PSD. Factors that were found to be related to PSD in the univariate analysis were entered into the model. In the three tertile groups divided according to the serum leptin level for a comparison and analysis of stroke severity, functional dependence and independence were used with the post-hoc Tukey's b-test of the ANOVA.
RESULTS
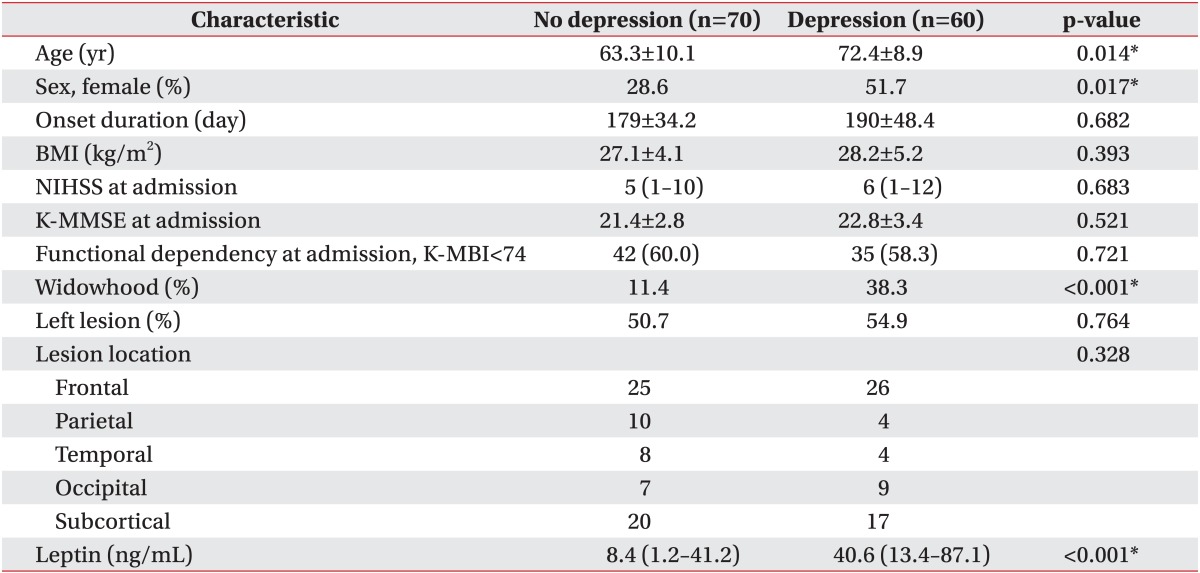
The 130 stroke patients were mostly elderly patients over the age of 60. Sixty of the patients (46.2%) showed depression, of which 29 were male and 31 were female. Significant differences in age, sex, functional dependency at discharge and widowhood were observed between the groups, but no difference in the NIHSS at admission, the K-MMSE at admission, and the functional dependency at admission. More than half of our patients had damage to the left hemisphere in various specific locations, but without statistical significance (Table 1).
Table 1. Biomedical characteristics at admission.
Values are presented as mean±standard deviation or median (interquartile range) or number (%).
BMI, body mass index; NIHSS, National Institutes of Health Stroke Scale; K-MMSE, Korean version of Mini-Mental Status Examination; K-MBI, Korean version of modified Barthel Index.
*p<0.05.
A comparison of the serum leptin level in stroke patients with and without depression
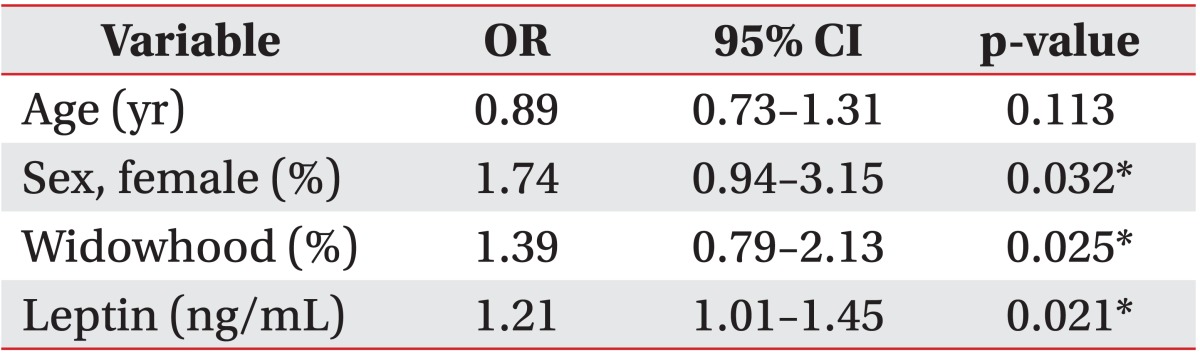
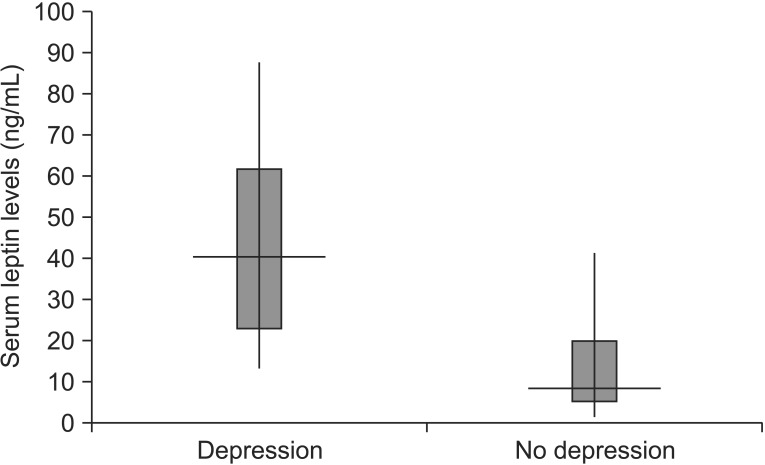
A comparison of the median value of the serum leptin levels between patients with depression and those without showed a significant difference (Table 1). After adjusting the model for all significant variables (age, sex, widowhood), the serum leptin level was found to be associated with depression (odds ratio [OR], 1.21; 95% confidence interval [CI], 1.01-1.45; p=0.021) (Table 2). In other words, the serum leptin levels were higher in patients with PSD than those without PSD (OR, 40.6; 95% CI, 13.4-87.1 vs. OR, 8.4; 95% CI, 1.2-41.2; p<0.05) (Fig. 1).
Table 2. Multivariable adjusted ORs, 95% CIs and p-values for the risk of post stroke depression.
OR, odds ratio; CI, confidence interval.
*p<0.05.
Fig. 1. The serum leptin levels of patients with and without post-stroke depression. The boxplots show the median values (horizontal line), quartiles (box boundaries; depression interquartile range, 22.7-60.8 and no depression interquartile range, 5.0-19.5), and the maximum and minimum values (lines shown above and below the box) of the leptin levels.
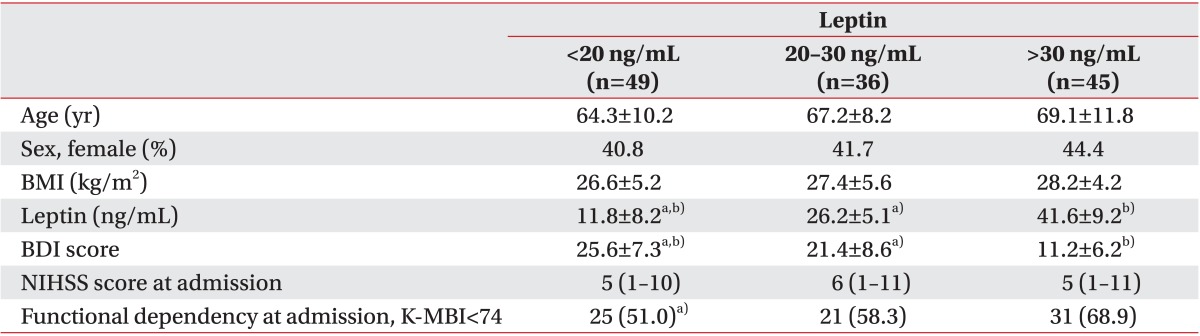
The general characteristics of the three tertile groups divided according to the serum leptin level
In the BDI scores, there was a significant difference between the groups (25.6±7.3 vs. 21.4±8.6 vs. 11.2±6.2; p<0.05) (Table 3). There was a significant difference only between the lower and upper tertile groups in functional dependency at admission (25 [51.0%] vs. 31 [68.9%]; p<0.05) (Table 3).
Table 3. Patients' characteristics according to the leptin tertiles at admission.
Values are presented as mean±standard deviation or median (interquartile range) or number (%).
BMI, body mass index; NIHSS, National Institutes of Health Stroke Scale; K-MBI, Korean version of modified Barthel Index.
a)p<0.05 vs. upper tertile group. b)p<0.05 vs. middle tertile group.
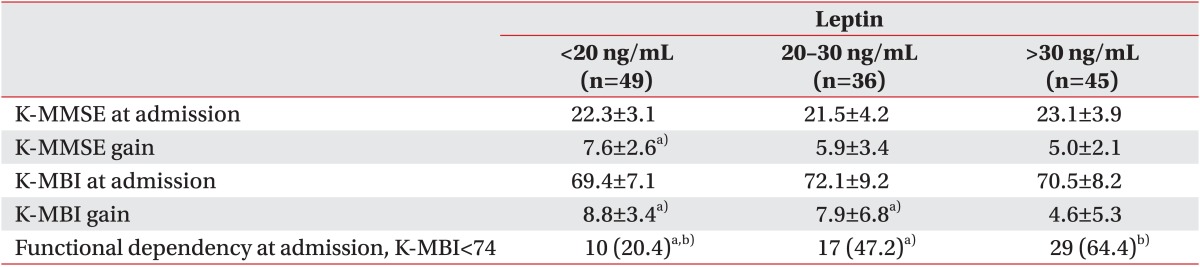
The cognitive function and functional independency among the three tertile groups divided according to the serum leptin level
In the K-MMSE mental status evaluation in the three groups, lower cognitive function was observed for the upper tertile group than for the lower tertile group (5.0±2.1 vs. 7.6±2.6; p<0.05). In this study, we evaluated cognitive and motor function improvement with the K-MBI score gain during the admission state. The score showed no statistically significant differences between the three groups at the admission state (69.4±7.1 vs. 72.1±9.2 vs. 70.5±8.2; p<0.05); however, the total K-MBI score gain in the upper tertile group was inferior to that of other groups and was considered to be statistically significant (4.6±5.3 vs. 7.9±6.8 vs. 8.8±3.4; p<0.05) (Table 4).
Table 4. Differences in functional outcomes according to leptin tertiles.
Values are presented as mean±standard deviation or number (%).
K-MMSE, Korean version of Mini-Mental Status Examination; K-MBI, Korean version of modified Barthel Index.
a)p<0.05 vs. upper tertile group. b)p<0.05 vs. middle tertile group.
In a comparison of functional dependency at the time of discharge, the upper tertile group was found to be more dependent than the other groups, and the middle tertile group was found to be more dependent than the lower tertile group statistically (29 [64.4%] vs. 17 [47.2%] vs. 10 [20.4%]; p<0.05] (Table 4).
DISCUSSION
The purpose of this study is to investigate and compare the serum leptin level in stroke patients with and without depression. The results of this study indicate that higher serum leptin levels are correlated with PSD.
The hypothalamo-pituitary-adrenal axis is crucial for the adaptation response to stress. A chronically overactive HPA axis can result in severe psychiatric conditions, such as depression and anxiety disorders [10,12]. Recently, several studies have considered the relationship between leptin levels and depression, but with contradictory results. Some clinical studies have reported that depression-like behavior was accompanied by a leptin level reduction in simple depression and bipolar disorder [15,16]. However, the leptin levels were significantly higher in atypical depression patients than in healthy subjects, after adjusting for age, gender, body mass index, and relative risk for cardiovascular disease [13,17,18,19]. The association between the serum leptin level and depression can vary with the patient's metabolic and neural stressful conditions.
The serum leptin level was elevated in many neural stressful conditions (such as stroke), and might be related to depressive symptoms via several possible mechanisms. The leptin signaling in the hypothalamus is related to the phosphorylation of the extracellular signal-regulated kinase (ERK), and is neuroprotective against ischemic neuronal injury, showing a rapid increase after ischemic and reperfusion [21]. In the state of increased leptin level, the leptin signaling system induces a suppressor of the cytokine signaling-3 (SOCS-3), the SOCS-3 suppresses the Janus-activated kinase-2 (JAK-2) and an autophosphorylation of the leptin receptor. The increased leptin receptors may be expressed in the hippocampus and the amygdala, suggesting a potential neuroactive function [14]. In addition, increased leptin has been shown to have an abnormal effect on the hippocampal and cortical structure, with neurogenesis, axon growth, synaptogenesis and dendritic morphology regulation [22]. The leptin resistance condition, with increased leptin receptors, may contribute to the psychologically abnormal state in PSD.
Furthermore, abnormally increased serum leptin might promote corticotropin-releasing hormone (CRH) release, contributing to HPA hyperactivity, and supporting the hypothesis that it is an effect of a leptin resistance condition [23]. Lastly, increased leptin may partially mediate the pathway from adipose tissue to a pro-inflammatory biomarkers increase [24].
This study has several limitations. First, the cross sectional design of our study cannot determine a causal relationship between serum leptin levels and depression in post stroke patients, and we cannot assume the relationship with leptin and cognitive and functional outcomes. Second, although the correlation was statistically significant, the low power and the small sample size were another limitation.
In conclusion, post-stroke patients with depression have elevated serum leptin levels, compared to post-stroke patients without depression, and elevated leptin levels are associated with the depression scale after controlling for potential confounding factors. Although the causal direction of the relationship between leptin levels and depression cannot be determined, our study collectively suggests that leptin may play a significant role in depression in post stroke patients, and could be a potential therapeutic target for the prevention and treatment of depression in post stroke patients. Further, large scale longitudinal studies are required to better understand our findings.
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 2.Brown C, Hasson H, Thyselius V, Almborg AH. Post-stroke depression and functional independence: a conundrum. Acta Neurol Scand. 2012;126:45–51. doi: 10.1111/j.1600-0404.2011.01595.x. [DOI] [PubMed] [Google Scholar]
- 3.Schulz R, Beach SR, Ives DG, Martire LM, Ariyo AA, Kop WJ. Association between depression and mortality in older adults: the Cardiovascular Health Study. Arch Intern Med. 2000;160:1761–1768. doi: 10.1001/archinte.160.12.1761. [DOI] [PubMed] [Google Scholar]
- 4.Pohjasvaara T, Vataja R, Leppavuori A, Kaste M, Erkinjuntti T. Depression is an independent predictor of poor long-term functional outcome post-stroke. Eur J Neurol. 2001;8:315–319. doi: 10.1046/j.1468-1331.2001.00182.x. [DOI] [PubMed] [Google Scholar]
- 5.House A, Knapp P, Bamford J, Vail A. Mortality at 12 and 24 months after stroke may be associated with depressive symptoms at 1 month. Stroke. 2001;32:696–701. doi: 10.1161/01.str.32.3.696. [DOI] [PubMed] [Google Scholar]
- 6.Flaster M, Sharma A, Rao M. Poststroke depression: a review emphasizing the role of prophylactic treatment and synergy with treatment for motor recovery. Top Stroke Rehabil. 2013;20:139–150. doi: 10.1310/tsr2002-139. [DOI] [PubMed] [Google Scholar]
- 7.Carvalho AF, Rocha DQ, McIntyre RS, Mesquita LM, Kohler CA, Hyphantis TN, et al. Adipokines as emerging depression biomarkers: a systematic review and meta-analysis. J Psychiatr Res. 2014;59:28–37. doi: 10.1016/j.jpsychires.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Altar CA. Neurotrophins and depression. Trends Pharmacol Sci. 1999;20:59–61. doi: 10.1016/s0165-6147(99)01309-7. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z, Mu J, Li J, Li W, Song J. Aberrant apolipoprotein E expression and cognitive dysfunction in patients with poststroke depression. Genet Test Mol Biomarkers. 2013;17:47–51. doi: 10.1089/gtmb.2012.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishibashi K, Berman SM, Paz-Filho G, Lee B, Robertson C, Mandelkern MA, et al. Dopamine D2/D3 receptor availability in genetically leptin-deficient patients after long-term leptin replacement. Mol Psychiatry. 2012;17:352–353. doi: 10.1038/mp.2011.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Disner SG, Beevers CG, Haigh EA, Beck AT. Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci. 2011;12:467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- 12.Holsboer F, Ising M. Stress hormone regulation: biological role and translation into therapy. Annu Rev Psychol. 2010;61:81–109. doi: 10.1146/annurev.psych.093008.100321. [DOI] [PubMed] [Google Scholar]
- 13.Stieg MR, Sievers C, Farr O, Stalla GK, Mantzoros CS. Leptin: a hormone linking activation of neuroendocrine axes with neuropathology. Psychoneuroendocrinology. 2015;51:47–57. doi: 10.1016/j.psyneuen.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Shanley LJ, Irving AJ, Harvey J. Leptin enhances NMDA receptor function and modulates hippocampal synaptic plasticity. J Neurosci. 2001;21:RC186. doi: 10.1523/JNEUROSCI.21-24-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cordas G, Gazal M, Schuch EM, Spessato BC, Branco J, Jansen K, et al. Leptin in depressive episodes: is there a difference between unipolar and bipolar depression? Neuroendocrinology. 2015;101:82–86. doi: 10.1159/000371803. [DOI] [PubMed] [Google Scholar]
- 16.Kraus T, Haack M, Schuld A, Hinze-Selch D, Pollmacher T. Low leptin levels but normal body mass indices in patients with depression or schizophrenia. Neuroendocrinology. 2001;73:243–247. doi: 10.1159/000054641. [DOI] [PubMed] [Google Scholar]
- 17.Antonijevic IA, Murck H, Frieboes RM, Horn R, Brabant G, Steiger A. Elevated nocturnal profiles of serum leptin in patients with depression. J Psychiatr Res. 1998;32:403–410. doi: 10.1016/s0022-3956(98)00032-6. [DOI] [PubMed] [Google Scholar]
- 18.Penninx BW, Kritchevsky SB, Yaffe K, Newman AB, Simonsick EM, Rubin S, et al. Inflammatory markers and depressed mood in older persons: results from the Health, Aging and Body Composition study. Biol Psychiatry. 2003;54:566–572. doi: 10.1016/s0006-3223(02)01811-5. [DOI] [PubMed] [Google Scholar]
- 19.Marazziti D, Rutigliano G, Baroni S, Landi P, Dell'Osso L. Metabolic syndrome and major depression. CNS Spectr. 2014;19:293–304. doi: 10.1017/S1092852913000667. [DOI] [PubMed] [Google Scholar]
- 20.Kim YW. Leptin resistance. J Korean Endocr Soc. 2007;22:311–316. [Google Scholar]
- 21.Spalletta G, Bossu P, Ciaramella A, Bria P, Caltagirone C, Robinson RG. The etiology of poststroke depression: a review of the literature and a new hypothesis involving inflammatory cytokines. Mol Psychiatry. 2006;11:984–991. doi: 10.1038/sj.mp.4001879. [DOI] [PubMed] [Google Scholar]
- 22.Bouret SG. Neurodevelopmental actions of leptin. Brain Res. 2010;1350:2–9. doi: 10.1016/j.brainres.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krishnan V, Nestler EJ. Linking molecules to mood: new insight into the biology of depression. Am J Psychiatry. 2010;167:1305–1320. doi: 10.1176/appi.ajp.2009.10030434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milaneschi Y, Sutin AR, Terracciano A, Canepa M, Gravenstein KS, Egan JM, et al. The association between leptin and depressive symptoms is modulated by abdominal adiposity. Psychoneuroendocrinology. 2014;42:1–10. doi: 10.1016/j.psyneuen.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]