Abstract
Background
Although an early decrease in proteinuria has been correlated with good long-term renal outcome in lupus nephritis (LN), studies aimed at defining a cut-off proteinuria value are missing, except a recent analysis performed on patients randomised in the Euro-Lupus Nephritis Trial, demonstrating that a target value of 0.8 g/day at month 12 optimised sensitivity and specificity for the prediction of good renal outcome. The objective of the current work is to validate this target in another LN study, namely the MAINTAIN Nephritis Trial (MNT).
Methods
Long-term (at least 7 years) renal function data were available for 90 patients randomised in the MNT. Receiver operating characteristic curves were built to test the performance of proteinuria measured within the 1st year as short-term predictor of long-term renal outcome. We calculated the positive and negative predictive values (PPV, NPV).
Results
After 12 months of treatment, achievement of a proteinuria <0.7 g/day best predicted good renal outcome, with a sensitivity and a specificity of 71% and 75%, respectively. The PPV was high (94%) but the NPV low (29%). Addition of the requirement of urine red blood cells ≤5/hpf as response criteria at month 12 reduced sensitivity from 71% to 41%.
Conclusions
In this cohort of mainly Caucasian patients suffering from a first episode of LN in most cases, achievement of a proteinuria <0.7 g/day at month 12 best predicts good outcome at 7 years and inclusion of haematuria in the set of criteria at month 12 undermines the sensitivity of early proteinuria decrease for the prediction of good outcome. The robustness of these conclusions stems from the very similar results obtained in two distinct LN cohorts.
Trial registration number:
Keywords: Lupus Nephritis, proteinuria, Outcomes research
Introduction
Despite significant therapeutic advances, renal involvement remains a threatening disease manifestation of systemic lupus erythematosus, impacting quality of life and survival.1 Identification of short-term prognostic factors predictive of poor long-term outcome in lupus nephritis (LN) would be most welcome, especially to fine-tune the intensity and duration of immunosuppressive (IS) therapy. Another compelling reason is to determine optimal end points for clinical trials so that new therapies can be evaluated efficiently and accurately. In this respect, several reports have demonstrated that an early decrease of proteinuria after IS treatment is predictive of a good long-term renal outcome.2–5
Taking advantage of the long-term follow-up data from the Euro-Lupus Nephritis Trial (ELNT), we were able to determine the optimal proteinuria target that maximises sensitivity and specificity for prediction of good long-term renal outcome. Thus, we demonstrated that a proteinuria of <0.8 g/day 12 months after randomisation was the single best predictor of good long-term renal function, with a sensitivity and specificity of 81% and 78%, respectively.6
The current analysis is aimed at testing the validity of this proteinuria target in another patient population with LN, taking advantage of the long-term MAINTAIN Nephritis Trial (MNT) data set.5 7
Subjects and methods
The MAINTAIN Nephritis Trial
The MNT is a European multicentre randomised trial comparing azathioprine and mycophenolate mofetil as maintenance IS treatment of LN, after induction with low-dose Euro-Lupus intravenous cyclophosphamide. After long-term follow-up, renal relapse rates were similar in the two arms, as published elsewhere.5 7
Patient selection
One hundred and five patients suffering from LN were included in the MNT. For the purpose of this study, we applied the same patient selection as for the corresponding ELNT analysis,6 in order to make the two cohorts comparable. Thus, 15 MNT patients were excluded because serum creatine (sCr) measurement was not available at or after 7 years of follow-up. Of note, patients having achieved end-stage renal disease at any time (n=4; at month 30, month 36, month 41 and month 74) were included in the analysis. On the whole, data from 90 MNT patients were studied.
Definition of good long-term renal outcome
Good long-term outcome was defined as sCr ≤1.0 mg/dL at least 7 years after entry into the trial, again in accordance with the criteria used for the ELNT analysis.6 Conversely, patients with sCr >1.0 mg/dL and those who developed end-stage renal disease at any time were considered as having had a poor renal outcome.
Statistical analyses
We built receiver operating characteristics (ROC) curves, through MedCalc, and calculated their area under the curve (AUC; CIs) to test the performance of each proteinuria level measured at month 3, month 6 and month 12 as a predictor of long-term renal outcome. Briefly, ROC curves plot sensitivity (true positive rate) on the y axis against 1−specificity (false positive rate) on the x axis. The point on the curve closest to the upper left-hand corner, identified by the Youden index, corresponds to the cut-off proteinuria value that optimises sensitivity and specificity. The AUC summarises the overall accuracy of a diagnostic parameter. AUC values >0.9, >0.7 to 0.9, >0.5 to 0.7 and 0.5 are highly accurate, moderately accurate, low accurate or equal to chance, respectively.
The optimal proteinuria target value at month 12, as defined supra, was used to calculate the positive and negative predictive values (PPV and NPV).
We also calculated the sensitivity, specificity, PPV and NPV with the addition of other clinical variables to proteinuria, including renal function and urinalysis.
Results
Proteinuria levels that optimise sensitivity and specificity for prediction of good long-term renal outcome
We built ROC curves with proteinuria values measured at different time points within the 1st year of treatment in order to identify the target that best predicts good long-term renal outcome. Figure 1 depicts the ROC curves for proteinuria levels achieved at month 3, month 6 and month 12, their AUCs (CIs) and the proteinuria cut-off values maximising sensitivity (CIs) and specificity (CIs). After 12 months of treatment, achievement of a proteinuria <0.7 g/day predicted good outcome, with a sensitivity and a specificity of 71% and 75%, respectively.
Figure 1.
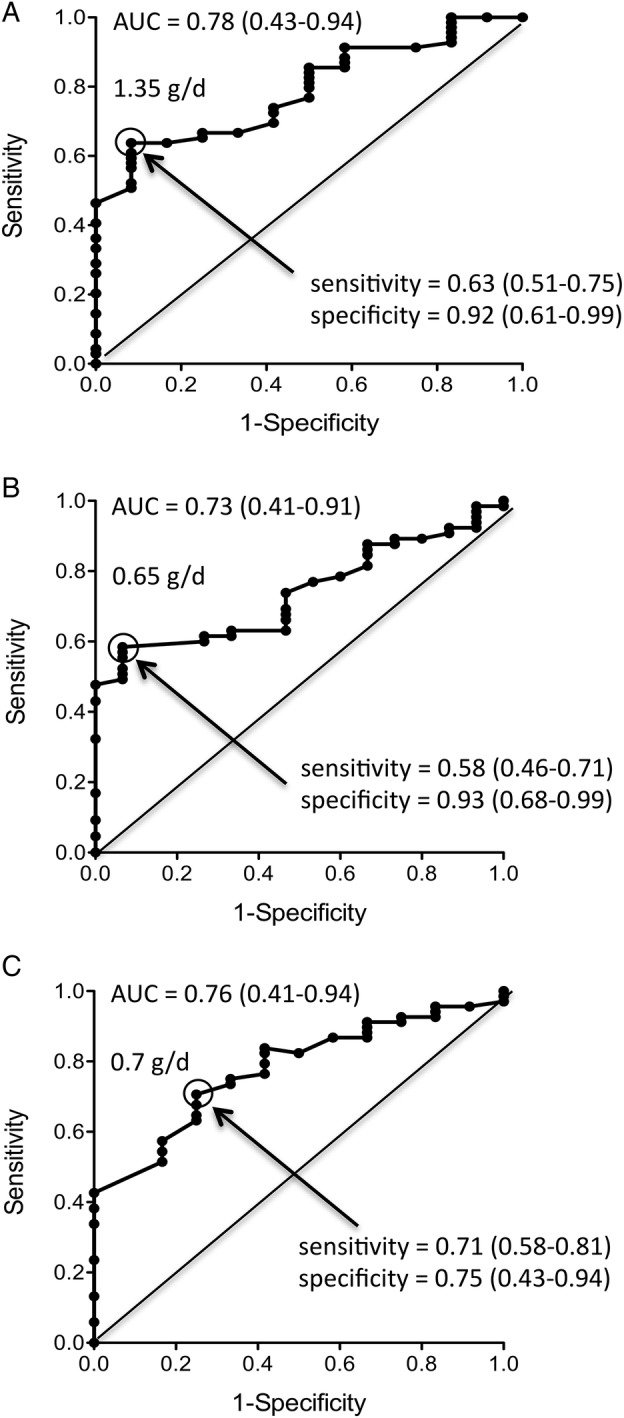
Receiver operating characteristic curves for predictive value of 24 h proteinuria at month 3 (A), month 6 (B) and month 12 (C) of patients randomised in the MAINTAIN Nephritis Trial. Sensitivity (true positive rate; y axis) is plotted against 1−specificity (false positive rate; x axis). The proteinuria (g/day) values indicated in the graphs optimise sensitivity and specificity. Figures in brackets are 95% CIs. AUC, area under the curve.
Sensitivity, specificity, PPV and NPV
As indicated in table 1, the PPV for a good long-term renal outcome of achieving a proteinuria <0.7 g/day at 12 months is very high (94%), thereby indicating that proteinuria decrease alone drives long-term renal prognosis. By contrast, the NPV is very low (31%), which means that more than two-thirds of the patients not meeting this target at month 12 will still experience a good long-term renal outcome.
Table 1.
Sensitivity, specificity, PPV and NPV for good long-term renal outcome according to target definition
| Target at 12 months | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| Proteinuria <0.7 g/day | 71 (48/68) | 75 (9/12) | 94 (48/51) | 31 (9/29) |
| Proteinuria <0.7 g/day and sCr ≤1 mg/dL | 63 (43/68) | 83 (10/12) | 96 (43/45) | 29 (10/31) |
| Proteinuria <0.7 g/day and sCr ≤1 mg/dL and RBC ≤5/hpf | 41 (28/68) | 67 (8/12) | 97 (28/29) | 21 (8/38) |
Figures in brackets are number of patients: for the sensitivity, patients with good outcome among those achieving the target/total patients with good outcome; for the specificity, patients with poor outcome among those not achieving the target/total patients with poor outcome; for the PPV, patients with good outcome/target achievers; for the NPV, patients with poor outcome/target non-achievers. Of note, only 80 of the 90 patients had proteinuria values available at month 12.
hpf, high power field; NPV, negative predictive value; PPV, positive predictive value; RBC, red blood cells; sCr, serum creatine.
Next, we wondered whether adding renal function and results of urinalyses would modify the sensitivity, specificity, PPV and NPV. As shown in table 1, addition of a normal renal function (sCr ≤1 mg/dL) to the target criteria, did not significantly alter the results. By contrast, requiring urine red blood cells (RBCs) to be ≤5/high power field (hpf), in addition to proteinuria <0.7 g/day and sCr ≤1 mg/dL as a response criteria reduced the sensitivity from 71% to 41%, implying that 59% of the patients who will experience a good long-term renal outcome would not be identified at month 12 if persistent haematuria is part of the target, while only 29% would be missed if proteinuria alone is used as criteria.
Last, we tested whether the NPV of proteinuria <0.7 g/day at month 12 would be higher in patients presenting with lower proteinuria levels at baseline. In these patients, non-achievement of the target should be associated with a poorer prognosis. We selected the mean (3.3 g/day) and the median (2.4 g/day) proteinuria values of the MNT cohort as cut-offs. As indicated in table 2, the NPV is indeed higher in patients with a lower baseline proteinuria level.
Table 2.
NPV of a proteinuria <0.7 g/day at 12 months according to baseline proteinuria
| Baseline proteinuria (g/day) | NPV (%) |
|---|---|
| According to mean | |
| <3.3 (n=48) | 44 (7/16) |
| ≥3.3 (n=32) | 15 (2/13) |
| According to median | |
| <2.4 (n=37) | 46 (6/13) |
| ≥2.4 (n=43) | 11 (3/16) |
Figures in brackets are numbers of patients, in casu poor outcomers/target non-achievers. Of note, only 80 of the 90 patients had proteinuria values available at month 12.
NPV, negative predictive value.
Discussion
Since permanent renal impairment is mostly a late event in LN, only trials or cohorts with long-term follow-up can unmask patients with poor renal outcome and allow us to test the validity of short-term predictors, such as the kinetics of proteinuria decrease during the 1st year of treatment. The data shown here, computed from the MNT, demonstrate (1) that achievement of a proteinuria value <0.7 g/day at month 12 best predicts good renal outcome at 7 years; and (2) that inclusion of microscopic haematuria in the set of outcome criteria at month 12 undermines the sensitivity of early proteinuria decrease for the detection of patients with good outcome.
These observations are consistent with those derived from the ELNT. Thus, Dall'Era et al demonstrated that a cut-off proteinuria value <0.8 g/day at month 12 maximised sensitivity and specificity for the prediction of good renal outcome and that addition of the results of urinalysis negatively impacted early identification of patients with good outcome, with a very comparable drop in sensitivity from 81% to 47%.7 We suggest that such a consistency across two distinct trials strengthens the validity of our conclusions.
The finding that haematuria did not contribute as a surrogate for long-term kidney outcomes is not so surprising. In a multicentre trial, it is difficult to ensure that the analysis of the urine sediment is done uniformly. The number of RBCs/hpf depends on the volume in which the pellet of sediment is resuspended. This is not standardised and if the volume is large the cellular elements in the pellet will be diluted and the counts inaccurate. The time of urine collection is important, and how long urine sits before urinalysis may affect the sediment, especially RBC casts. Additionally, there are many reasons for haematuria in a population of mainly young women. The red cells specific for glomerular bleeding, and thus indicative of LN, are dysmorphic and are called acanthocytes. While these cells are enumerated in centres that specialise in glomerular diseases, in clinical trials all RBCs are counted, and these are often eumorphic and from the lower urinary tract. We do not discount the value of urine sediment examination. If urine handling could be standardised and the reader focused on the elements of the urine that truly indicate glomerular injury, urinalysis may contribute to a surrogate end point of renal response.
The first lesson learned from these analyses is that assessment of microscopic haematuria should not be part of the response criteria used in LN trials, in contrast to the American College of Rheumatology (ACR) recommendations,8 more so as interpretation of urinalyses is complicated by delays in sample examination, the use of automated techniques instead of microscopic reading and interference by menstrual-related haematuria.
The second implication deals with treatment options at the bedside after 1 year. Patients meeting the 0.7 g/day proteinuria target at month 12 (ie, 64% of the MNT cohort) can be reassured regarding their final renal outcome, based on the very high PPV (94%). In such patients, glucocorticoid tapering and withdrawal could be considered, based on their well known contribution to damage accrual,9 10 although only a controlled trial could test the safety of this proposal in terms of renal relapse rates. On the other hand, treatment decision will be more difficult in patients not achieving the 0.7 g/day target at month 12, due to the low NPV: two-thirds of these patients will still experience a good outcome in the long run. In this respect, the fact that the NPV is twice as high in patients with a low baseline proteinuria compared with those with higher values suggests that a treatment switch might be more appropriate for the former compared with the latter. The kinetics of proteinuria decrease is obviously pivotal in this decision process. Again, only a controlled trial could test the possibility that optimising therapy in partial responders would be beneficial, a hypothesis tested in the RING trial (RItuximab in lupus Nephritis with remission as a Goal).
We acknowledge the limitations of our work. Data were acquired in a small patient population. Most subjects were Caucasians (79%) and suffered from their first episode of LN (87%). Further work is needed to improve the NPV which was not ameliorated by the use of a more stringent target, taking into account additional criteria, such as renal function or urinalysis. Yet, the robustness of our conclusions stems from the very similar results obtained in two prospectively followed cohorts.
Footnotes
Collaborators: This analysis was conducted as a collaborative work between the MAINTAIN Nephritis Trial investigators and the Lupus Nephritis Trials Network. The MAINTAIN investigators were: Daniel Abramowicz, Nephrology Department, Hôpital Erasme, Université Libre de Bruxelles, Brussels, Belgium; Fabiola Atzeni, Unita Operativa di Reumatologia, Ospedale Luigi Sacco, Milan, Italy; Daniel Blockmans, General Internal Medicine Department, UZ Gasthuisberg, Katholieke Universiteit Leuven, Leuven, Belgium; Maria Giovanna Danieli, Istituto di Clinica Medica Generale, Universia Degli Study di Ancona, Torrette di Ancona, Italy; Luc De Clercq, Rheumatology Department, Sint-Augustinus Ziekenhuis, Wilrijk, Belgium; David D'Cruz, Louise Coote Lupus Unit, St Thomas’ Hospital, London, UK; Maria del Mar Ayala Guttierez, General Internal Medicine, Hospital Regional Universitario Carlos Haya, Malaga, Spain; Enrique de Ramon Garrido, General Internal Medicine, Hospital Regional Universitario Carlos Haya, Malaga, Spain; Inge-Magrethe Gilboe, Rheumatology Department, Rikshospitalet University Hospital, Oslo, Norway; Filip de Keyser, Rheumatology Department, UZ Gent, University of Ghent, Ghent, Belgium ; Michel Delahousse, Service de Nephrologie, Hôpital Foch, Paris, France; Gerard Espinosa, Department of Autoimmune Diseases, Hospital Clinic, Barcelona, Catalonia, Spain; Christoph Fiehn, ACURA Center for Rheumatic Diseases, Baden-Baden, Germany; Marc Golstein, Service de Rheumatologie, Cliniques Saint-Jean, Brussels, Belgium; Loïc Guillevin, General Internal Medicine Department, Hôpital Cochin, Paris, France; Marco Hirsch, Luxembourg, Grand Duchy of Luxembourg; Alexandre Karras, Service de Néphrologie, Hôpital Européen Georges Pompidou, Paris, France; Philippe Lang, Nephrology Department, Hôpital Henri Mondor, Créteil, France; Véronique le Guern, General Internal Medicine Department, Hôpital Cochin, Paris, France; Martine Marchal, Service de Néphrologie, Hôpital de Tivoli, La Louvière, Belgium; Antonio Marinho, Clinical Immunology Unit, Hospital Santo Antonio, ICBAS, Porto, Portugal; Regina Max, Department of Internal Medicine V, University of Heidelberg, Heidelberg, Germany; Patrick Peeters, Nephrology Department, UZ Gent, University of Ghent, Ghent, Belgium; Peter Petera, Zentrum für Diagnostik und Therapie rheumatischer Erkrankungen, Krankenhaus Lainz, Wien, Austria; Radmila Petrovic, Institute of Rheumatology, University of Belgrade, Belgrade, Serbia; Thomas Quémeneur, Centre Hospitalier Régional Universitaire de Lille, Lille, France; Frank Raeman, Rheumatology Department, Jan Palfijn Hospital, Merksem, Belgium; Philippe Remy, Nephrology Department, Hôpital Henri Mondor, Créteil, France; Isabelle Ravelingien, Rheumatology Department, Onze-Lieve-Vrouw Ziekenhuis, Aalst, Belgium; Piercarlo Sarzi-Puttini, Unita Operativa di Reumatologia, Ospedale Luigi Sacco, Milan, Italy; Shirish Sangle, Louise Coote Lupus Unit, St Thomas’ Hospital, London, UK; Maria Tektonidou, First Department of Internal Medicine, National University of Athens, Athens, Greece; Lucia Valiente de Santis, General Internal Medicine, Hospital Regional Universitario Carlos Haya, Malaga, Spain; Carlos Vasconcelos, Clinical Immunology Unit, Hospital Santo Antonio, ICBAS, Porto, Portugal; Luc Verresen, Nephrology Department, Ziekenhuis Oost-Limburg, Genk, Belgium; Laurence Weiss, Département d'Immunologie, Hôpital Européen Georges Pompidou, Paris, France; René Westhovens, Rheumatology Department, UZ Gasthuisberg, Katholieke Universiteit Leuven, Leuven, Belgium.
Contributors: All coauthors have contributed to the study design, data acquisition, data organisation, manuscript writing and reviewing.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: Commission d'Ethique Hospitalo-Facultaire de l'Université catholique de Louvain.
Provenance and peer review: Not commissioned; externally peer reviewed.
Contributor Information
Collaborators: Daniel Abramowicz, Libre de Bruxelles, Fabiola Atzeni, Daniel Blockmans, Maria Giovanna Danieli, Luc De Clercq, David D'Cruz, Maria del Mar Ayala Guttierez, Enrique de Ramon Garrido, Inge-Magrethe Gilboe, Filip de Keyser, Michel Delahousse, Gerard Espinosa, Christoph Fiehn, Marc Golstein, Loïc Guillevin, Marco Hirsch, Alexandre Karras, Philippe Lang, Véronique le Guern, Martine Marchal, Antonio Marinho, Regina Max, Patrick Peeters, Peter Petera, Radmila Petrovic, Thomas Quémeneur, Frank Raeman, Philippe Remy, Isabelle Ravelingien, Piercarlo Sarzi-Puttini, Shirish Sangle, Maria Tektonidou, Lucia Valiente de Santis, Carlos Vasconcelos, Luc Verresen, Laurence Weiss, and René Westhovens
References
- 1.Costenbader KH, Desai A, Alarcón GS et al. Trends in the incidence, demographics, and outcomes of end-stage renal disease due to lupus nephritis in the US from 1995 to 2006. Arthritis Rheum 2011;63:1681–8. doi:10.1002/art.30293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Houssiau FA, Vasconcelos C, D'Cruz D et al. Early response to immunosuppressive therapy predicts good renal outcome in lupus nephritis: lessons from long-term followup of patients in the Euro-Lupus Nephriti Trial. Arthritis Rheum 2004;50:3934–40. doi:10.1002/art.20666 [DOI] [PubMed] [Google Scholar]
- 3.Houssiau FA, Vasconcelos C, D'Cruz D et al. The 10-year follow-up data of the Euro-Lupus Nephritis Trial comparing low-dose and high-dose intravenous cyclophosphamide. Ann Rheum Dis 2010;69:61–4. doi:10.1136/ard.2008.102533 [DOI] [PubMed] [Google Scholar]
- 4.Korbet SM, Lewis EJ, Collaborative Study Group. Severe lupus nephritis: the predictive value of a ≥50% reduction in proteinuria at 6 months. Nephrol Dial Transplant 2013;28:2313–18. doi:10.1093/ndt/gft201 [DOI] [PubMed] [Google Scholar]
- 5.Tamirou F, D'Cruz D, Sangle S et al. Long-term follow-up of the MAINTAIN Nephritis Trial, comparing azathioprine and mycophenolate mofetil as maintenance therapy of lupus nephritis. Ann Rheum Dis 2015;▪▪▪ doi:10.1136/annrheumdis-2014-206897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dall'Era M, Cisternas MG, Smilek DE et al. Predictors of long-term renal outcome in lupus nephritis trials: lessons learned from the Euro-Lupus Nephritis cohort. Arthritis Rheumatol 2015;67:1305–13. doi:10.1002/art.39026 [DOI] [PubMed] [Google Scholar]
- 7.Houssiau FA, D'Cruz D, Sangle S et al. Azathioprine versus mycophenolate mofetil for long-term immunosuppression in lupus nephritis: result from the MAINTAIN Nephritis Trial. Ann Rheum Dis 2010;69:2083–9. doi:10.1136/ard.2010.131995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Renal Disease Subcommittee of the American College of Rheumatology Ad Hoc Committee on Systemic Lupus Erythematosus Response Criteria. The American College of Rheumatology response criteria for proliferative and membranous renal disease in systemic lupus erythematosus clinical trials. Arthritis Rheum 2006;54:421–32. doi:10.1002/art.21625 [DOI] [PubMed] [Google Scholar]
- 9.Gladman DD, Urowitz MB, Rahman P et al. Accrual of organ damage over time in patients with systemic lupus erythematosus. J Rheumatol 2003;30:1955–9. [PubMed] [Google Scholar]
- 10.Thamer M, Hernán MA, Zhang Y et al. Prednisone, lupus activity, and permanent organ damage. J Rheumatol 2009;36:560–4. doi:10.3899/jrheum.080828 [DOI] [PMC free article] [PubMed] [Google Scholar]


