Abstract
Objective
To compare discontinuation rates of first and second biologics in rheumatoid arthritis (RA) by tumour-necrosis factor inhibitor (TNFi) status and identify predictors and reasons for discontinuation.
Methods
From 1998 to 2011, self-reported medication use for RA was assessed every 6 months via questionnaire in a longitudinal study in the USA. Time-on-drug analyses were conducted for individual biologics and groups, and annual rates reported. Time to discontinuation of TNFi and non-TNFi was compared, unadjusted and adjusted using propensity score analyses. Baseline and time-varying predictors of biologic discontinuation were derived through Cox regression.
Results
Of 2281 patients initiating their first biologic, 1100 (48%) discontinued and of 1097 initiating a second biologic, 537 (49%) discontinued. The annual discontinuation rate was 17% (median 4 years) for first biologic and 20% (median 3.3 years) for second biologic. TNFi had lower discontinuation rates than non-TNFi after propensity score adjustment: HR for first biologic 0.49 (0.34 to 0.71) and 0.68 (0.51 to 0.90) for second biologic. The annual discontinuation rate was significantly lower in patients starting their first biologic before January 2005 vs after (16 vs 25%, p=0.005). Predictors of discontinuation for the first biologic included smoking, higher comorbidity index, worse overall health and not using concomitant methotrexate.
Conclusions
In this large cohort, patients with RA tended to remain on their first and second biologics for relatively long periods suggesting the drugs’ effectiveness. Discontinuation rates were lower in patients using TNFi, and all rates increased after January 2005 when the number of biologics available increased.
Keywords: Rheumatoid Arthritis, DMARDs (biologic), Anti-TNF, Outcomes research
Key messages.
What is already known about this subject?
Recent studies reported a higher discontinuation rate of tumour-necrosis factor (TNF) inhibitors (compared to non-TNF inhibitors) as a second (or higher) biologic.
However, no other comparisons between drug classes are published.
In order to inform treatment choice, it is also important to identify predictors of discontinuation.
What does this study add?
Discontinuation rates were lower in patients using TNF inhibitors (compared to non-TNF inhibitors), a finding not previously reported.
Predictors of discontinuation of first biologic include smoking, comorbidities, worse overall health and a protective effect of concomitant methotrexate.
How might this impact on clinical practice?
Our findings help provide context for why patients stop their biologics and factors for rheumatologists to consider when making treatment decisions.
Introduction
Efficacy of a drug is usually established by randomised controlled trials (RCT) although data from RCTs may not directly translate to effectiveness in clinical practice.1 Effectiveness is better assessed using an observational study. Long-term effectiveness is particularly important when evaluating treatments for chronic conditions. The length of time a patient remains on a drug may be a reasonable proxy for effectiveness in a clinical setting when other measures are not available.1
Multiple large cohort studies with outcomes of patients with rheumatoid arthritis (RA) treated with biologics have been conducted. These have enabled us to evaluate long-term outcomes of these treatments in clinical practice, where patients are not selected based on RCT eligibility criteria. There are some data on discontinuation rates of biologics over the long-term from registries;2–7 however, most of the studies focused on the rates of the three earliest tumour-necrosis factor inhibitors (TNFi)2 3 5 6 with few including newer biologics.4 7 Furthermore, most prior analyses focused on either first or second biologic.2–4 6
An important effectiveness question that remains is whether there are differences in the discontinuation rate of TNFi compared to agents with other mechanisms of actions and whether the difference is the same when they are used first versus second line. Recent analyses from a Swiss4 and an Italian cohort8 reported a higher discontinuation rate of TNFi as a second (or higher) line. However, no other comparisons between drug classes are published. In order to inform treatment choice, it is also important to identify predictors of discontinuation.
In our study we assessed the rates and reasons for discontinuation of biologics for RA when used as first or second biologic in a clinical practice setting, identified predictors of discontinuation and compared discontinuation rates between biologics by mechanism of action.
Methods
Study patients were RA participants in the National Data Bank for Rheumatic Diseases (NDB), a longitudinal observational study of rheumatic disease outcomes.9 10 Patients are recruited primarily from US rheumatology practices and followed prospectively through self-reported semiannual questionnaires that collect demographics, clinical outcomes and treatment. The study was approved by Via Christi Institutional Review Board and all patients gave their informed consent before inclusion. This study required at least one assessment prior to initiating biologic treatment and one after during 1998 through 2011. Therefore, patients studied for discontinuation of their first biologic entered the NDB biologic naive whereas patients studied for discontinuation of their second biologic could have entered the NDB either biologic naïve or after starting their first biologic. NDB patients recruited in drug safety registries were excluded to have a more homogeneous sample.9
Definition of discontinuation
The main outcome is discontinuation of biologics. All eight biologics (TNFi: infliximab, etanercept, adalimumab, golimumab, certolizumab pegol; non-TNFi: rituximab, tocilizumab and abatacept) were included. Anakinra was not included in the presented analyses because of its rare use, highest discontinuation rate and associated impact on misinterpreting non-TNFi rates.
Discontinuation was defined subsequently. For patients who self-reported discontinuation, the reported date was used. For patients who switched to another biologic, start of the new biologic was considered the discontinuation date. If a patient did not report using their biologic for ≥2 consecutive 6-month periods or had ≥2 consecutive missing observations, discontinuation was assumed at the end of the 6-month period when the biologic was last reported. Since rituximab's dosing schedule is every 6–12 months, an additional 6-month gap was allowed. Patients were censored at the last observation available.
Discontinuation rates of first and second biologics were reported as a whole, by mechanism of action (TNFi vs non-TNFi), and by agent. Discontinuation rates were also assessed before and after 1 January 2005 (≥2005) when newer agents, including adalimumab, became more accessible. The reasons for discontinuation were assessed applying the following hierarchy when more than one reason was given: (1) Side effect; (2) Inefficacy; (3) Cost; (4) Other; and (5) No reason reported.
Predictors of discontinuation
We examined the predictors of discontinuation of the first biologic using baseline (ie, at the start of the biologic) and time-varying models. For clinical practice, both models can give complementary information. Baseline predictors enable a clinician to identify at the start of therapy patients more likely to discontinue. The time-varying model enables ongoing assessment of discontinuation risk throughout the course of therapy.
Factors assessed for their association with future biologic discontinuation included the following: age, sex, RA duration, ethnicity, body mass index (kg/m2), educational level (years), employment status (active employment vs not), household income (US$), insurance type (Medicare vs other), smoking status (ever vs never smoking) and comorbidities measured by the rheumatic disease comorbidity index (0–9).11 Several RA clinical and quality of life measurements were also assessed including: Health Assessment Questionnaire disability index (HAQ),12 13 RA disease activity index (RADAI, 0–10),14 pain visual analogue scale (VAS) (0–10), patient global assessment of disease severity VAS (0–10), SF-36 physical and mental component summary scores (PCS and MCS, 0–100),15 fatigue VAS (0–10), sleep quality VAS (0–10) and polysymptomatic distress scale (0–31).16 Finally, a number of medication factors were considered including number of previous DMARDs, concomitant therapy with DMARDs, glucocorticoid or NSAID use, and total duration of methotrexate and glucocorticoid therapies.
Statistical analysis
Rates of discontinuation and 95% CIs were obtained through survival analyses. Kaplan Meyer curves were obtained and compared unadjusted through the log-rank tests.
Discontinuation rates were calculated as the number of events (ie, discontinuations of biologics) per person-time and are presented as annual rates. Discontinuation rates of TNFi and non-TNFi were compared for both first and second biologics. Discontinuation of the second biologic was analysed for all patients that were started on a second biologic during follow-up, that is, not necessarily only for those who were included in the analysis of the discontinuation of the first biologic. The analysis in the restricted group was conducted as a sensitivity analysis. Discontinuation rates per reason of discontinuation were also calculated.
Analyses were conducted over the entire follow-up period and also restricted to biologics started ≥2005 due to 7 of the 9 biologics not being available in the USA until after this time. Rates with and without propensity score (PS) adjustment were reported. The PS was defined as the conditional probability of being treated with TNFi versus non-TNFi. This method is used in observational studies to balance the covariates in two groups that are being compared, and therefore reduce confounding by indication.17 PSs were calculated using logistic regression with biologic mechanism of action (TNFi vs other) as the dependent variable and the following independent variables: age, comorbidity, HAQ, concomitant methotrexate use. Total household income was included for first biologic. For second biologic, duration of methotrexate therapy and patient global assessment of disease severity were included (these variables significantly contributed to explain the outcome). The PSs were adjusted to have a balanced distribution of patients in each quintile for each treatment. Through Cox regression, we obtained the HR of discontinuing a TNFi versus non-TNFi.
We used Cox regression to identify baseline and time-varying predictors for discontinuation of first biologic. Entry to survival models was the start of first biologic and continued until either discontinuation or censorship. Models were adjusted for starting the biologic ≥2005 and for the biologic class used. Two prediction models were specified: a ‘research model’ which included all significant predictors after starting with all previously mentioned factors, and a ‘clinical model’ which was similar but excluded predictors less likely to be used in clinical practice (ie, SF-36, polysymptomatic distress, sleep and fatigue scales). Univariable analysis was followed by multivariable (variables with a p value<0.20 were included), and forward selection was performed, taking confounding effects into account.
Data were analysed as observed, without imputation, as individual measures had <5% missing data. Data were analysed using Stata, V.12.
Results
A total of 2281 patients initiated their first biologic with 97.5% starting a TNFi. Sociodemographic and clinical characteristics of these patients are presented in table 1. Almost a quarter of the patients started their first biologic ≥2005, with an expected higher proportion starting a non-TNFi in this period. Patients initiating TNFi were younger than patients starting a non-TNFi, had been exposed to fewer DMARDs, and had more comedication with non-steroidal anti-inflammatory drugs (NSAIDs).
Table 1.
Baseline sociodemographic and clinical characteristics
| Variables | Initiated first biologic† | Initiated TNF-inhibitor as first biologic† | Initiated non- TNF-inhibitor as first biologic† | Initiated second biologic‡ | Initiated TNF-inhibitor as second biologic‡ | Initiated non-TNF-inhibitor as second biologic‡ |
|---|---|---|---|---|---|---|
| N | 2281 | 2225 | 56 | 1097 | 988 | 109 |
| Started biologic ≥2005 (%) | 524 (23) | 470 (21)** | 54 (96)** | 520 (47) | 414 (42)** | 106 (97)** |
| Age (years) | 59.7 [12.4] | 59.6 [12.4]** | 65.3 [11.1]** | 60.8 [12.2] | 60.5 [12.0]* | 63.0 [13.7]* |
| Gender (% female) | 1830 (80) | 1784 (80) | 46 (82) | 871 (79) | 784 (79) | 87 (80) |
| Disease duration (years) | 15.0 [11.0] | 14.9 [11.0] | 16.4 [11.3] | 16.2 [11.3] | 16.0 [11.4] | 18.1 [11.0] |
| Ethnicity (% Caucasian) | 2090 (93) | 2051 (93)** | 39 (74)** | 975 (91) | 879 (91) | 96 (93) |
| Body mass index (kg/m2) | 27.5 [6.6] | 27.4 [6.6] | 28.8 [6.5] | 28.1 [6.3] | 28.1 [6.3] | 27.8 [5.6] |
| Married (%) | 1626 (71) | 1593 (72)* | 33 (59)* | 786 (72) | 709 (72) | 77 (71) |
| Educational level (years) | 13.8 [2.3] | 13.8 [2.3] | 14.2 [2.1] | 13.6 [2.2] | 13.6 [2.2] | 13.9 [2.1] |
| Employed (%) | 775 (34) | 760 (34) | 15 (27) | 337 (31) | 314 (32)* | 23 (21)* |
| Total income ($10 000) | 4.8 [3.0] | 4.9 [3.0] | 4.4 [3.1] | 4.8 [3.0] | 4.8 [3.0] | 4.7 [2.8] |
| Smoking (%) | 286 (13) | 283 (13) | 3 (5) | 126 (11) | 118 (12) | 53 (10) |
| Comorbidity index (0–9) | 1.5 [1.4] | 1.5 [1.4] | 1.8 [1.3] | 1.8 [1.6] | 1.7 [1.5]* | 2.1 [1.6]* |
| HAQ (0–3) | 1.2 [0.7] | 1.2 [0.7] | 1.1 [0.7] | 1.2 [0.7] | 1.2 [0.7] | 1.3 [0.8] |
| Pain scale (0–10) | 4.5 [2.8] | 4.5 [2.8] | 4.6 [2.9] | 4.4 [2.8] | 4.4 [2.8] | 4.6 [2.9] |
| Global severity (0–10) | 4.0 [2.4] | 4.0 [2.4] | 4.3 [2.4] | 4.2 [2.4] | 4.1 [2.4] | 4.6 [2.4] |
| Fatigue (0–10) | 4.8 [2.9] | 4.8 [2.9] | 4.8 [3.3] | 4.8 [2.9] | 4.8 [2.9] | 5.1 [2.8] |
| Sleep scale (0–10) | 4.0 [3.0] | 4.0 [3.0] | 3.7 [2.9] | 4.1 [3.0] | 4.2 [3.1] | 4.0 [2.9] |
| RADAI | 3.2 [1.6] | 3.2 [1.6] | 3.1 [1.8] | 3.1 [1.7] | 3.1 [1.7] | 3.1 [1.6] |
| SF-36 PCS (0–100) | 34.0 [10.5] | 34.0 [10.5] | 35.9 [10.6] | 33.9 [10.7] | 33.9 [10.8] | 33.1 [9.9] |
| SF-36 MCS (0–100) | 49.3 [11.1] | 49.3 [11.1] | 48.4 [12.6] | 48.6 [11.3] | 48.6 [11.1] | 48.2 [12.4] |
| Polysymptomatic distress (0–31) | 11.6 [7.2] | 11.7 [7.2] | 11.1 [7.4] | 11.9 [7.5] | 11.8 [7.5] | 13.0 [7.5] |
| Number of DMARDs | 3.0 [1.6] | 3.0 [1.6]* | 2.5 [1.4]* | 3.1 [1.7] | 3.1 [1.7] | 2.9 [1.7] |
| Concomitant DMARD (%) | 2084 (91) | 2035 (91) | 49 (88) | 883 (80) | 797 (81) | 86 (79) |
| Methotrexate (%) | 1567 (69) | 1534 (69) | 33 (59) | 661 (60) | 596 (60) | 65 (60) |
| Methotrexate duration (months) | 48.8 [54.7] | 48.8 [54.5] | 48.9 [60.4] | 58.4 [58.9] | 56.3 [56.8]* | 77.4 [72.8]* |
| Leflunomide (%) | 532 (23) | 516 (23) | 16 (29) | 185 (17) | 171 (17) | 14 (13) |
| Prednisone (%) | 1073 (47) | 1053 (47) | 20 (36) | 482 (44) | 433 (44) | 49 (45) |
| NSAIDs (%) | 1599 (70) | 1574 (71)** | 25 (45)** | 659 (60) | 600 (61) | 59 (54) |
| Biologic started (%) | ||||||
| Etanercept | 1018 (45) | 315 (29) | ||||
| Infliximab | 873 (38) | 427 (39) | ||||
| Adalimumab | 310 (14) | 218 (20) | ||||
| Golimumab | 8 (0.4) | 11 (1.0) | ||||
| Certolizumab pegol | 16 (0.7) | 17 (1.5) | ||||
| Abatacept | 28 (1.2) | 61 (6) | ||||
| Rituximab | 25 (1.1) | 42 (4) | ||||
| Tocilizumab | 3 (0.1) | 6 (0.5) |
Results are expressed in mean [SD] or n (%). Percentages refer to the total number of patients with information available on a specific variable, which might not match with dividing the n over the total group N due to eventual missing items
*p Value <0.05; **p value<0.001.
†Information corresponding to the observation when the first biologic was started.
‡Information corresponding to the observation when the second biologic was started.
DMARDs, disease-modifying antirheumatic drugs; HAQ, Health Assessment Questionnaire; NSAIDs, non-steroidal anti-inflammatory drugs; RADAI, rheumatoid arthritis disease activity index; SF-36 MCS and PCS, Short form-36, mental component summary and physical component summary.
A quarter of the patients starting a non-TNFi were non-Caucasian versus only 7% of those treated with a TNFi. We examined the location/region of the patients in these two groups and found that the non-TNFi patients were statistically more represented in larger population states (CA, TX, FL, etc) compared to the TNFi patients. We also found no differences in rural versus urban categories based on ZIP of these patient groups.
A total of 1097 initiated a second biologic, 90% of which were TNFi. Half of the patients started their second biologic ≥2005, and 97% of patients who started a non-TNFi did so ≥2005. Patients taking their second biologic were younger, had a lower comorbidity index and a shorter duration of methotrexate therapy.
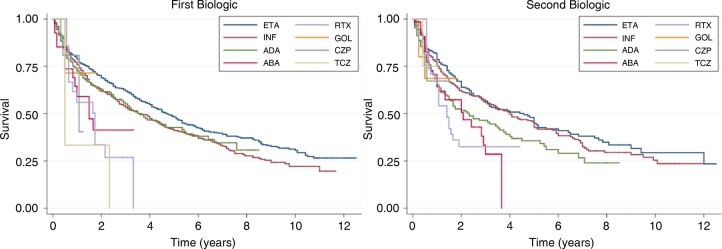
Out of the 2281 patients, 1100 (48%) discontinued their first biologic with median time to discontinuation of 4.1 years (figure 1 and table 2). Of the 1097 patients who started a second biologic, 537 (49%) discontinued and the median time to discontinuation was 3.3 years. There were significant differences in the discontinuation rates across biologics both for first and second biologics. These differences persisted even when the comparison was restricted to the three original TNFi (figure 1). However, there were no differences between the eight survival curves when the analysis was restricted to patients starting ≥2005 (p=0.185 all biologics, p=0.953 for the three initial TNFi). For the second biologic, there were no significant differences between the eight survival curves (p=0.239), but there was a significant difference between the three initial TNFi (p=0.044).
Figure 1.
Time to discontinuation of each of the biologic agents. (A) Time to discontinuation of first biologics; (B) Time to discontinuation of second biologics. Comparison between survival curves (logrank test): all 8 survival curves first biologic: p value <0.001; survival curves of 3 older TNF-inhibitors (etanercept, infliximab, adalimumab) first biologic p value=0.006; all 8 survival curves second biologic: p value 0.004; survival curves of 3 older TNF-inhibitors second biologic p value=0.007. ABA, abatacept; ADA, adalimumab; CZP, certolizumab pegol; ETA, etanercept; GOL, golimumab; INF, infliximab; RTX, rituximab; TCZ, tocilizumab.
Table 2.
Rates of discontinuation of first and second biologics
| First biologic |
Second biologic |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Number of discontinuations (n) | Annual rate of discontinuation (95% CI) | Survival time (years) | N | Number of discontinuations (n) | Annual rate of discontinuation (95% CI) | Survival time (years) |
|||||
| 25% | 50% | 75% | 25% | 50% | 75% | |||||||
| All biologics | 2281 | 1100 | 0.17 (0.16 to 0.18) | 1.08 | 4.08 | 10.42 | 1097 | 537 | 0.20 (0.18 to 0.22) | 1 | 3.33 | 9.92 |
| All biologics (onset <2005) | 1757 (77%) | 888 | 0.16 (0.15 to 0.18) | 1.25 | 4.33 | 10.50 | 577 (53%) | 301 | 0.16 (0.14 to 0.18) | 1.17 | 4.42 | 12 |
| All biologics (onset ≥2005) | 524 (23%) | 212 | 0.25 (0.22 to 0.29) | 0.67 | 3.08 | . | 520 (47%) | 236 | 0.31 (0.27 to 0.35) | 0.74 | 2.08 | . |
| TNF-inhibitors | 2225 (97.5%) | 1069 | 0.17 (0.16 to 0.18) | 1.17 | 4.08 | 10.42 | 988 (90%) | 481 | 0.19 (0.17 to 0.21) | 1 | 3.58 | 10.08 |
| Non-TNF inhibitors | 56 (2.5%) | 31 | 0.46 (0.33 to 0.66) | 0.66 | 1.66 | 3.32 | 109 (10%) | 56 | 0.38 (0.30 to 0.50) | 0.67 | 1.57 | 3.67 |
| Injectable biologics | 1352 (59%) | 611 | 0.16 (0.15 to 0.17) | 1.17 | 4.5 | . | 561 (51%) | 266 | 0.20 (0.18 to 0.22) | 1 | 3.17 | 12 |
| Infused biologics | 929 (41%) | 489 | 0.20 (0.18 to 0.21) | 1 | 3.33 | 8.75 | 536 (49%) | 271 | 0.20 (0.18 to 0.23) | 1 | 3.42 | 9.5 |
| Etanercept | 1018 (45%) | 467 | 0.15 (0.14 to 0.16) | 1.42 | 4.75 | . | 315 (29%) | 146 | 0.16 (0.14 to 0.19) | 1.42 | 4.58 | 12 |
| Infliximab | 873 (38%) | 458 | 0.19 (0.17 to 0.21) | 1.08 | 3.58 | 9 | 427 (39%) | 215 | 0.18 (0.16 to 0.20) | 1.08 | 4 | 10.08 |
| Adalimumab | 310 (14%) | 139 | 0.20 (0.17 to 0.23) | 0.83 | 3.58 | . | 218 (20%) | 112 | 0.26 (0.22 to 0.32) | 0.75 | 2.33 | 7.08 |
| Abatacept | 28 (1%) | 13 | 0.41 (0.24 to 0.70) | 0.5 | 1.5 | . | 61 (6%) | 31 | 0.37 (0.26 to 0.53) | 0.67 | 2.08 | 3.67 |
| Rituximab | 25 (1%) | 15 | 0.48 (0.29 to 0.79) | 0.66 | 1.74 | 3.32 | 42 (4%) | 24 | 0.40 (0.27 to 0.60) | 0.66 | 1.41 | . |
| Tocilizumab | 3 (0%) | 3 | 6 (1%) | 1 | ||||||||
| Certolizumab pegol | 16 (1%) | 3 | 17 (2%) | 5 | ||||||||
| Golimumab | 8 (0%) | 2 | 11 (1%) | 3 | ||||||||
TNF, tumour-necrosis factor.
Annual discontinuation rates for the first and second biologics are summarised in table 2. For the first biologic, the annual discontinuation rate was 17%, and was significantly higher in patients who started a biologic ≥2005 vs <2005 (25% vs 16%; p=0.005). For the second biologic, the annual discontinuation rate was higher at 20%, and also higher in the group starting ≥2005. The annual discontinuation rate was significantly lower with etanercept (14%) and adalimumab (18%) as compared to infliximab (26%). As previously described, anakinra was not included, as its discontinuation rate was 48% for first biologic and 106% for second biologic (ie, patients discontinued the drug in a period <1 year and hence a discontinuation rate>100%). Infused biologics had a slightly higher discontinuation rate than injectable ones, but the difference was small (20% vs 16%).
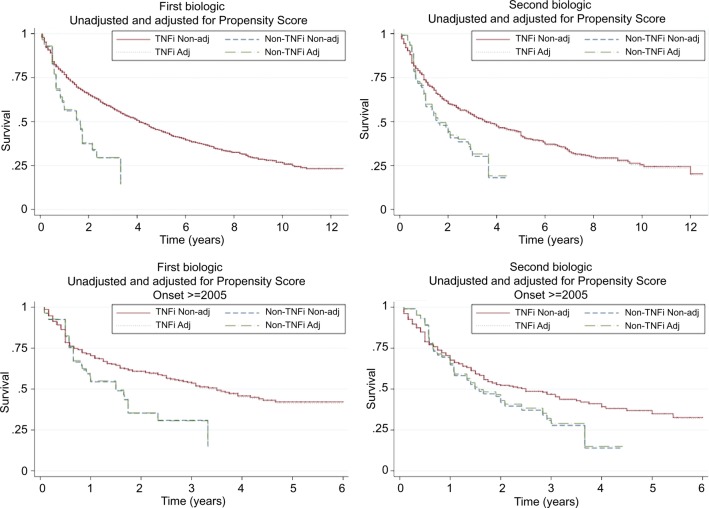
There was a lower probability of discontinuing a TNFi first biologic compared to a non-TNFi (unadjusted HR 0.48 (95% CI 0.34 to 0.69); PS-adjusted 0.49 (0.34 to 0.71); figure 2). The difference remained after restricting the analysis to biologics started ≥2005: unadjusted HR 0.59 (0.40 to 0.87) and PS-adjusted 0.60 (0.41 to 0.90) meaning that patients started on a TNFi had a 51% (or 41% if only ≥2005) lower discontinuation rate than those started on a non-TNFi. The corresponding HR for discontinuation of the second biologic was 0.64 (0.48 to 0.84) and, after adjustment, 0.68 (0.51 to 0.90), thus a 32% lower discontinuation rate for TNFi. When analyses were restricted to biologics started ≥2005, there was no longer a significant difference and the HR was 0.78 (0.58 to 1.06) and, after adjustment, 0.82 (0.60 to 1.12).
Figure 2.
Time to discontinuation of TNF-inhibitors (TNF-i) and non-TNF-inhibitors (non-TNFi), non-adjusted (non-adj) and adjusted (adj) for propensity score survival curves. (A) First biologic. (B) Second biologic. (C) First biologic with onset ≥2005. (D) Second biologic with onset ≥2005. TNF inhibitor versus non-TNF inhibitor (first biologic): Whole follow-up, unadjusted HR 0.48 (0.34 to 0.69), adjusted HR 0.49 (0.34 to 0.71); Started biologic ≥2005: unadjusted HR 0.59 (0.40 to 0.87), adjusted HR 0.60 (0.41 to 0.90). TNF inhibitor versus non-TNF inhibitor (second biologic): Whole follow-up, unadjusted HR 0.64 (0.48 to 0.84), adjusted HR 0.68 (0.51 to 0.90); Started biologic ≥2005: unadjusted HR 0.78 (0.58 to 1.06), adjusted HR 0.82 (0.60 to 1.12).
Of the patients who discontinued their first biologic and reported a reason for discontinuation (n=773), 40% discontinued due to a side effect, 27% due to inefficacy and 11% due to costs (table 3). Patients discontinuing due to a side effect had the highest annual discontinuation rate (63% discontinuation in 1 year), followed by those who discontinued due to inefficacy (47% discontinuation in 1 year). More patients discontinued their second biologic due to costs (16%), fewer due to side effects (32%) and overall discontinuation rates were higher (annual discontinuation rates were 81% for side effects and 57% for inefficacy).
Table 3.
Reasons for discontinuation of first and second-line biologics
| First Biologic |
Second Biologic |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of discontinuations (n) | Annual rate of discontinuation (95% CI) | Survival time (years) |
Number of discontinuations (n) | Annual rate of discontinuation (95% CI) | Survival time (years) |
|||||
| 25% | 50% | 75% | 25% | 50% | 75% | |||||
| Side effect | 309 | 0.63 (0.56 to 0.71) | 0.33 | 1 | 2 | 100 | 0.81 (0.67 to 0.99) | 0.25 | 0.67 | 1.67 |
| Inefficacy | 209 | 0.47 (0.41 to 0.54) | 0.5 | 1.17 | 3 | 93 | 0.57 (0.47 to 0.70) | 0.42 | 0.92 | 2.42 |
| Costs | 83 | 0.42 (0.34 to 0.52) | 0.58 | 1.67 | 4.08 | 51 | 0.51 (0.39 to 0.67) | 0.58 | 1.42 | 2.92 |
| Other | 172 | 0.39 (0.34 to 0.45) | 0.67 | 1.92 | 3.75 | 72 | 0.48 (0.38 to 0.61) | 0.5 | 1.25 | 2.42 |
| Not specified | 327 | 0.46 (0.41 to 0.51) | 0.5 | 1.08 | 3.33 | 221 | 0.51 (0.44 to 0.58) | 0.5 | 1 | 2.5 |
Baseline and time-varying predictors of first biologic discontinuation were identified (table 4). For the baseline predictors, the ‘research model’ (ie, model including all significant variables) included the following predictive factors: smoking status HR 1.22 (1.02 to 1.46), a higher comorbidity index HR 1.09 (1.04 to 1.13), older age HR 1.01 (1.00 to 1.01) and higher score on the polysymptomatic distress scale HR 1.02 (1.01 to 1.03). Comedication with methotrexate had a protective effect against discontinuation, HR 0.84 (0.74 to 0.95) and the SF-36 MCS had an HR just below 1. The ‘clinical model’ was similar to the research model, and discontinuation of biologics was further predicted by patient global VAS: the higher the global assessment of disease activity, HR 1.05 (1.03 to 1.08), the higher the discontinuation rate. Time-varying predictors were reasonably similar to the baseline predictors, except that smoking and comedication with methotrexate no longer predicted the outcome, but RADAI, as a disease activity assessment, was the strongest predictor.
Table 4.
Predictors of discontinuation of first biologic
| Baseline predictors |
Time-varying predictors |
|||||
|---|---|---|---|---|---|---|
| Univariable | Multivariable Research Model N=2279 |
Multivariable Clinical Model N=2279 |
Univariable | Multivariable Research Model N=2263 |
Multivariable Clinical Model N=2262 |
|
| Age (years) | 1.01 (1.00 to 1.01) | 1.01 (1.00 to 1.01) | 1.01 (1.00 to 1.01) | 1.01 (1.00 to 1.01) | 1.01 (1.00 (1.01) | 1.01 (1.00 to 1.01) |
| Patient global severity (0–10) | 1.07 (1.04 to 1.09) | * | 1.05 (1.03 to 1.08) | 1.07 (1.04 to 1.09) | * | * |
| Comorbidity index (0–9) | 1.14 (1.09 to 1.19) | 1.09 (1.04 to 1.13) | 1.11 (1.06 to 1.15) | 1.14 (1.10 to 1.18) | 1.06 (1.01 to 1.10) | 1.07 (1.03 to 1.11) |
| Smoking | 1.17 (0.98 to 1.40) | 1.22 (1.02 to 1.46) | 1.23 (1.03 to 1.47) | 1.17 (0.98 to 1.40) | * | * |
| MTX | 0.84 (0.74 to 0.95) | 0.84 (0.74 to 0.95) | 0.84 (0.74 to 0.95) | † | † | † |
| SF-36 MCS (0–100) | 0.99 (0.98 to 0.99) | 0.99 (0.98 to 0.99) | ‡ | 0.99 (0.98 to 0.99) | * | ‡ |
| SF-36 PCS (0–100) | 0.99 (0.98 to 0.99) | * | ‡ | 0.97 (0.97 to 0.98) | 0.99 (0.98 to 0.99) | ‡ |
| RADAI (0–10) | 1.09 (1.04 to 1.13) | * | * | 1.24 (1.19 to 1.29) | 1.23 (1.16 to 1.30) | 1.28 (1.22 to 1.35) |
| Fibromyalginess (0–31) | 1.03 (1.02 to 1.04) | 1.02 (1.01 to 1.03) | ‡ | 1.04 (1.03 to 1.05) | * | ‡ |
| Pain scale (0–10) | 1.04 (1.02 to 1.07) | * | * | 1.04 (1.02 to 1.07) | 0.95 (0.92 to 0.98) | 0.96 (0.93 to 0.98) |
All analyses were adjusted for biologic drug class (anti-TNF vs other) and analyses of whole study period were adjusted for onset of biologic therapy ≥2005.
Other variables that were tested in the univariable analysis and eventually included in the multivariable analysis, but that were not part of any final model: comedication with leflunomide, comedication with prednisone, employed status, HAQ, fatigue scale, sleep scale, educational level, comedication with DMARDs, comedication with NSAIDs, disease duration, number of DMARDs previously made, gender, marital status, body mass index, ethnicity, insurance type, income, biological drug.
*Not selected during multivariable regression analysis (p≥0.05).
†Not included in the multivariable model (p value in the univariable model ≥0.20).
‡Not included in this short ‘clinical’ model (to present a model with variables more used in clinical practice).
DMARDs, disease-modifying antirheumatic drugs; HAQ, Health Assessment Questionnaire; MTX, methotrexate; NSAIDs, non-steroidal anti-inflammatory drugs; RADAI, rheumatoid arthritis disease activity index; SF-36 MCS and PCS, Short Form 36 mental component summary and physical component summary; TNF, tumour-necrosis factor.
Discussion
The present study shows that patients with RA tend to remain on their initial biologic therapy for relatively long periods, median of 4.1 years and 3.1 years when starting ≥2005, suggesting the drugs’ effectiveness. Discontinuation rates were significantly lower in patients on a TNFi compared to a non-TNFi as first biologic, but there were no significant differences for second biologic. Smoking, comorbidities, worse health, increased polysymptomatic distress, non-use of methotrexate and age predicted biologic discontinuation. At the observation immediately before discontinuation, a higher disease activity (assessed by RADAI) predicted discontinuation.
Discontinuation rates of biologics have been reported in the literature. Nevertheless, comparison of the results is challenging as analyses have not been homogeneous in many ways including: different drugs; variable populations (biologic naïve or not); different definitions of discontinuation; individual or groups of drugs categorisation; various stratifications and covariates; annual discontinuation rates or median survival times are not consistently reported; different years and countries reflecting a variety of clinical practices with biologics. A Danish study2 reported slightly lower discontinuation rates. Our results are in line with the results from the Spanish registry,5 in which a 17–21% discontinuation rate is reported in the first year of treatment of the first TNFi (rate increasing with increasing calendar year).
Drug retention is inversely proportional to previous failure to biologics, which is in line with previous descriptions.3 Drug survival was also shown to be inversely proportional to later year of treatment initiation,3 18 as there are increasingly more treatment options, and more ambitious treatment targets. These seem achievable, if not with one drug, then possibly after trying another one19 and actually accumulated experience rheumatologists have with managing these drugs can lead to a higher discontinuation, in an attempt to seek better outcomes.
From the Swiss registry,4 an adjusted HR for the discontinuation rate of a second (or higher) line non-TNFi versus TNFi of 0.50 was reported, which corresponds to an HR of 2.0, from the perspective of TNFi versus non-TNFi. An Italian study reported an HR for the retention of a second non-TNFi versus TNFi of 2.26.8 Interestingly, these results are in the opposite direction of what we report in our cohort: PS-adjusted discontinuation of TNFi versus non-TNFI HR 0.68. This HR did not reach statistical significance, which may mean that there is no significant difference between the groups or that there was not sufficient power. In both the Swiss and Italian studies, almost half of the patients were on a non-TNFi. Despite the fact that these are often second line or later treatments, this is still very different from what we found (10% under non-TNFi). These findings warrant further confirmation and so far no other comparisons have been reported. The only other study reporting discontinuation rates of a non-TNFi is from Sakai et al,7 but comparisons were only established directly between the individual drugs and not at a drug-class level.
Similarly, we also compared the discontinuation of TNFi versus non-TNFi as a first biologic. We found that TNFi had a 51% lower discontinuation rate (after adjustment for PS) and 40% lower for start of therapy ≥2005. To our knowledge this is the first time this comparison is established and this finding warrants confirmation in other cohorts. This must be interpreted with caution, as it might also reflect different prescription patterns over time and the preponderance of TNFi in our cohort, which was in line with recommendations of starting with a TNFi.20 Nevertheless, differences persisted despite adjustment for PS and restriction of the analyses to ≥2005.
Predictors of discontinuation of biologics are also very heterogeneous across studies, reflecting the different methodologies. Most studies report baseline predictors. The protective effect of methotrexate3 and the predictive effect of self-reported disease activity (RADAI)6 for discontinuation of biologics had already been reported. Our findings underline the importance of combination of a biologic with methotrexate due to the higher effectiveness of this strategy compared to biologic as monotherapy. According to the recent recommendations for the management of RA, a biologic should preferentially be used in combination with methotrexate or, in case of intolerance, other synthetic DMARDs.21 Smoking has been identified as a predictor of discontinuation of therapy and to our knowledge this is the first report of this effect. Interestingly, Fagerli et al22 recently found the same in psoriatic arthritis and it seems that smoking may be associated with worse outcomes of inflammatory diseases,23 despite some contradictory findings.24 Despite the fact that some predictors have been identified in the literature and in our study, statistically these have low levels of association. Our HRs varied between 1.01 and 1.28 (or between 0.84 and 0.99 for the inverse relationships), which pragmatically are less useful in clinical practice. There is still the unmet need to clearly identify the patients who will not respond to a specific therapy and on whom, for instance, a different drug class would lead to a lower discontinuation rate, eventually reflecting a higher effectiveness.
Our study has some notable limitations. First of all, we do have a relatively low number of patients on a non-TNFi (both first and second biologics), making comparisons between these drug classes, even though adjusted for PSs, challenging. Our findings are relevant and contradicting the only two class comparisons reported so far, and warrant further confirmation, especially in larger cohorts. It may be the case that there are differences between European and American patients, due to different prescription patterns, reimbursement policies, patients’ comorbidities, etc. Nevertheless, our numbers are also smaller because of our strict inclusion criteria for the analysis, since we only included incident users of biologic therapy. In order to prevent exclusion of a large number of patients, those included in the second biologic discontinuation analysis were not necessarily in the first biologic analysis. When we repeated the analysis in the subgroup of 468 patients that were in both, the results were similar (data not shown). Information is self-reported, and discontinuation rates might therefore not be very precise compared to administrative reporting of an infusion. For the same reason, there were several cases of drug discontinuation without a known reason for discontinuation; therefore, we did not further examine just those patients who indicated discontinuation due to inefficacy (approximately 20%). Ideally, we would study drugs’ effectiveness through RCTs to avoid confounding by indication and any imbalance between the groups, both limitations of observational studies, but RCTs are not suitable for investigating long-term outcomes either and we were interested in the long-term treatment effectiveness. Furthermore, no substantial differences were found between treatment groups at baseline and PS adjustment was made throughout and did not change the results. Although we adjusted the analysis for potential confounders, we cannot exclude additional confounding by unmeasured factors (eg, patient preferences can be an example for this).
In conclusion, patients remain on their initial biologic for relatively long periods, a proxy for their effectiveness, though having increased options for biologic treatment is associated with increased discontinuation rates. These rates appear to be lower with TNFi, especially with the first biologic. Future studies addressing this question are needed to confirm or not our findings. Predictors of discontinuation of first biologic include smoking, comorbidities, worse overall health and a protective effect of concomitant methotrexate.
Footnotes
Twitter: Follow Kaleb Michaud at @Dr_K
Contributors: SR, KM, RL and DvdH designed the study. SR and KM collected the data. SR, KM, RL and DvdH analysed the data and all authors critically interpreted the results. SR prepared the first version of the manuscript. All the authors reviewed the draft versions and gave their approval of the final version of the manuscript.
Funding: This study was partially funded by Immunex Corporation, a wholly owned subsidiary of Amgen Inc, and by Wyeth, which was acquired by Pfizer Inc in October 2009.
Competing interests: This study was partially funded by Immunex Corporation, a wholly owned subsidiary of Amgen Inc, and by Wyeth, which was acquired by Pfizer Inc in October 2009. SR was supported by the Fundação para a Ciência e Tecnologia (FCT) grant SFRH/BD/68684/2010. DvdH received consulting fees and/or research grants from Abbott, Amgen, AstraZeneca, BMS, Centocor, Chugai, Eli-Lilly, GSK, Merck, Novartis, Otsuka, Pfizer, Roche, Sanofi-Aventis, Schering-Plough, UCB, Wyeth. Owner of Imaging Rheumatology bv. RL received consulting fees and/or research grants from Abbott, Amgen, AstraZeneca, BMS, Centocor, GSK, Merck, Novartis, Pfizer, Roche, Schering-Plough, UCB, Wyeth. Owner of Rheumatology Consultancy Bv. DH and DC are employees of Amgen, Inc. KM was supported by the Rheumatology Research Foundation Investigator Award.
Patient consent: Obtained.
Ethics approval: Via Christi Institutional Review Board .
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Wolfe F. The epidemiology of drug treatment failure in rheumatoid arthritis. Baillieres Clin Rheumatol 1995;9:619–32. 10.1016/S0950-3579(05)80305-X [DOI] [PubMed] [Google Scholar]
- 2.Hetland ML, Christensen IJ, Tarp U et al. . Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum 2010;62:22–32. 10.1002/art.27227 [DOI] [PubMed] [Google Scholar]
- 3.Du Pan SM, Dehler S, Ciurea A et al. . Comparison of drug retention rates and causes of drug discontinuation between anti-tumor necrosis factor agents in rheumatoid arthritis. Arthritis Rheum 2009;61:560–8. 10.1002/art.24463 [DOI] [PubMed] [Google Scholar]
- 4.Du Pan SM, Scherer A, Gabay C et al. . Differential drug retention between anti-TNF agents and alternative biological agents after inadequate response to an anti-TNF agent in rheumatoid arthritis patients. Ann Rheum Dis 2012;71:997–9. 10.1136/annrheumdis-2011-200882 [DOI] [PubMed] [Google Scholar]
- 5.Gomez-Reino JJ, Carmona L. Switching TNF antagonists in patients with chronic arthritis: an observational study of 488 patients over a four-year period. Arthritis Res Ther 2006;8:R29 10.1186/ar1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agarwal SK, Glass RJ, Shadick NA et al. . Predictors of discontinuation of tumor necrosis factor inhibitors in patients with rheumatoid arthritis. J Rheumatol 2008;35:1737–44. [PMC free article] [PubMed] [Google Scholar]
- 7.Sakai R, Tanaka M, Nanki T et al. . Drug retention rates and relevant risk factors for drug discontinuation due to adverse events in rheumatoid arthritis patients receiving anticytokine therapy with different target molecules. Ann Rheum Dis 2012;71:1820–6. 10.1136/annrheumdis-2011-200838 [DOI] [PubMed] [Google Scholar]
- 8.Favalli EG, Biggioggero M, Marchesoni A et al. . Survival on treatment with second-line biologic therapy: a cohort study comparing cycling and swap strategies. Rheumatology (Oxford) 2014;53:1664–8. 10.1093/rheumatology/keu158 [DOI] [PubMed] [Google Scholar]
- 9.Wolfe F, Michaud K. The National Data Bank for rheumatic diseases: a multi-registry rheumatic disease data bank. Rheumatology (Oxford) 2011;50:16–24. 10.1093/rheumatology/keq155 [DOI] [PubMed] [Google Scholar]
- 10.Wolfe F, Michaud K. A brief introduction to the National Data Bank for rheumatic diseases. Clin Exp Rheumatol 2005;23:S168–71. [PubMed] [Google Scholar]
- 11.Wolfe F, Michaud K, Li T et al. . Chronic conditions and health problems in rheumatic diseases: comparisons with rheumatoid arthritis, noninflammatory rheumatic disorders, systemic lupus erythematosus, and fibromyalgia. J Rheumatol 2010;37:305–15. 10.3899/jrheum.090781 [DOI] [PubMed] [Google Scholar]
- 12.Fries JF, Spitz P, Kraines RG et al. . Measurement of patient outcome in arthritis. Arthritis Rheum 1980;23:137–45. 10.1002/art.1780230202 [DOI] [PubMed] [Google Scholar]
- 13.Wolfe F. A reappraisal of HAQ disability in rheumatoid arthritis. Arthritis Rheum 2000;43:2751–61. [DOI] [PubMed] [Google Scholar]
- 14.Mason JH, Anderson JJ, Meenan RF et al. . The rapid assessment of disease activity in rheumatology (radar) questionnaire. Validity and sensitivity to change of a patient self-report measure of joint count and clinical status. Arthritis Rheum 1992;35:156–62. 10.1002/art.1780350206 [DOI] [PubMed] [Google Scholar]
- 15.Stewart AL, Hays RD, Ware JE Jr. The MOS short-form general health survey. Reliability and validity in a patient population. Med Care 1988;26:724–35. 10.1097/00005650-198807000-00007 [DOI] [PubMed] [Google Scholar]
- 16.Wolfe F, Brähler E, Hinz A et al. . Fibromyalgia prevalence, somatic symptom reporting, and the dimensionality of polysymptomatic distress: results from a survey of the general population. Arthritis Care Res (Hoboken) 2013;65:777–85 10.1002/acr.21931 [DOI] [PubMed] [Google Scholar]
- 17.D'Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998;17:2265–81. [DOI] [PubMed] [Google Scholar]
- 18.Gómez-Reino JJ, Rodríguez-Lozano C, Campos-Fernández C et al. . Change in the discontinuation pattern of tumour necrosis factor antagonists in rheumatoid arthritis over 10 years: data from the Spanish registry BIOBADASER 2.0. Ann Rheum Dis 2012;71:382–5. 10.1136/annrheumdis-2011-200302 [DOI] [PubMed] [Google Scholar]
- 19.Ramiro S, Machado P, Singh JA et al. . Applying science in practice: the optimization of biological therapy in rheumatoid arthritis. Arthritis Res Ther 2010;12:220 10.1186/ar3149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smolen JS, Landewé R, Breedveld FC et al. . EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis 2010;69:964–75. 10.1136/ard.2009.126532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smolen JS, Landewé R, Breedveld FC et al. . EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 2014;73:492–509. 10.1136/annrheumdis-2013-204573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fagerli KM, Lie E, van der Heijde D et al. . The role of methotrexate co-medication in TNF-inhibitor treatment in patients with psoriatic arthritis: results from 440 patients included in the NOR-DMARD study. Ann Rheum Dis 2014;73:132–7. 10.1136/annrheumdis-2012-202347 [DOI] [PubMed] [Google Scholar]
- 23.Chung HY, Machado P, van der Heijde D et al. . Smokers in early axial spondyloarthritis have earlier disease onset, more disease activity, inflammation and damage, and poorer function and health-related quality of life: results from the DESIR cohort. Ann Rheum Dis 2012;71:809–16. 10.1136/annrheumdis-2011-200180 [DOI] [PubMed] [Google Scholar]
- 24.Maska LB, Sayles HR, O'Dell JR et al. . Serum cotinine as a biomarker of tobacco exposure and the association with treatment response in early rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:1804–10. 10.1002/acr.21758 [DOI] [PMC free article] [PubMed] [Google Scholar]