Abstract
Diffusion-weighted magnetic resonance imaging is a useful tool to differentiate between acute ischemic and demyelinating lesions. In ischemic optic neuropathy the diffusion of water is impaired, but in optic neuritis it usually remains normal. We describe a patient with optic neuritis and aquaporin-4 autoantibodies, in whom imaging revealed prolonged reduction of the apparent diffusion coefficient. Immune-mediated destruction of water channels apparently decreased movement of fluid through the inflamed nerve. Diffusion-weighted imaging may distinguish optic neuritis associated with aquaporin-4 antibodies from the more common form that occurs in conjunction with multiple sclerosis.
Keywords: Neuromyelitis optica, diffusion-weighted imaging, optic neuritis, aquaporin, demyelination, apparent diffusion coefficient, ischemic optic neuropathy
Introduction
A serum immunoglobulin G (IgG) autoantibody directed against the aquaporin-4 (AQP4) channel is present in the majority of patients with neuromyelitis optica (NMO), or Devic's syndrome. AQP4 is the principal water channel in the central nervous system, allowing water to cross plasma membranes far more efficiently than by simple diffusion. Patients who harbor AQP4-specific IgG are susceptible to acute attacks of optic neuritis and transverse myelitis. It is believed that anti-AQP4 IgG binding occurs to the outer face of water channels located in the foot processes of astrocytes, triggering an inflammatory event. Immunohistochemical analysis in patients with NMO has shown local depletion of AQP4 channels within lesions (1,2). Loss of AQP4 channels might be expected to have a major impact on water movement within fresh NMO lesions. With diffusion-weighted magnetic resonance (MR) imaging it is possible to measure the movement of water through brain tissue (3). Here we provide the first account of diffusion-weighted MR imaging in the optic nerve of a patient with NMO-associated vision loss.
Report of Case
A 21-year-old man developed vision loss in the right eye that was diagnosed as optic neuritis. After a delay of five months, he was referred for neuro-ophthalmology evaluation. The visual acuity was 20/400 in the right eye and 20/20 in the left eye. Visual field testing showed severe depression in the right eye and normal function in the left eye (Fig. 1A). The right optic disc was pale. Magnetic resonance (MR) imaging revealed slight atrophy of the right optic nerve, but no demyelinating plaques. A year later the patient experienced rapidly progressive vision loss in the left eye, accompanied by an aching sensation over the brow. On examination four weeks after onset of vision loss, the acuity was 20/400 in the right eye and 20/800 in the left eye. Visual field testing in the right eye yielded a mean deviation of -33.21 dB and a foveal sensitivity of 0 dB (Fig 1B). In the left eye there was a mean deviation of -24.66 dB and a foveal sensitivity of 0 dB. The right optic disc appeared pale from the old attack of optic neuritis and the left optic disc showed mild, fresh edema. The patient was treated intravenously with methylprednisolone at a dose of 250 mg four times per day for three days. A lumbar puncture yielded no red blood cells, one white blood cell per μL (85% lymphocytes, 15% monocytes), protein 22 mg/dL, glucose 91 mg/dL (serum glucose 189 mg/dL), no oligoclonal bands, and a nonreactive VDRL. The serum and cerebrospinal fluid were positive for anti-AQP4 IgG, as determined by a cell-based fluorescence assay (Mayo Medical Laboratories, Rochester, MN) (4). A serum anti-nuclear antibody titer was elevated at a titer of 1:160, with a diffuse pattern. A serum SSA antibody was moderately positive at 66 units (negative < 20 units) and an SSB antibody was negative. Serum angiotensin converting enzyme, lysozyme, sedimentation rate, rapid plasma reagin, C-reactive protein, and human immunodeficiency virus antibody tests were negative. After discharge from the hospital the patient was treated orally with 60 mg of prednisone daily for 11 days. Three weeks later the acuity in the left eye had improved to 20/80. Visual field testing showed a mean deviation of only -5.91 dB in the left eye, but foveal sensitivity remained depressed at 19 dB (Fig. 1C). Currently the patient is being treated with mycophenolate mofetil at a dose of 1000 mg twice per day to prevent future inflammatory episodes.
Figure 1.
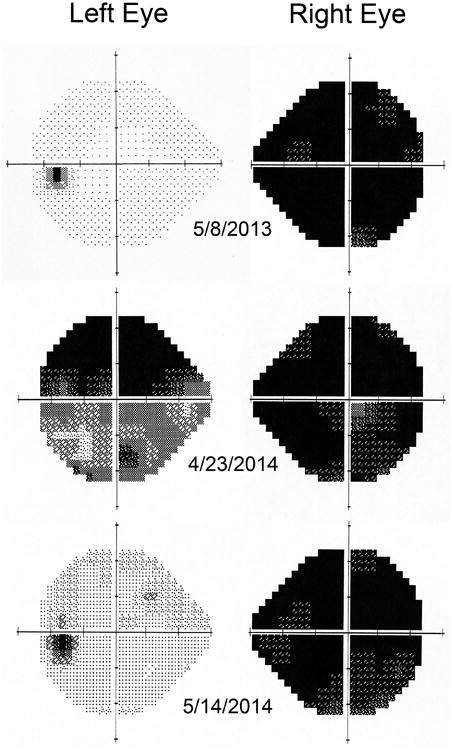
8 May 2013: five months after an attack of optic neuritis in the right eye the Humphrey 24-2 visual fields were normal in the left eye and severely depressed in the right eye. 23 April 2014: a month after an attack of optic neuritis in the left eye the visual fields showed reduced function in both eyes. 14 May 2014: three weeks later the function had improved in the left eye.
MR imaging of the brain and orbits was performed on a 3-Tesla clinical scanner (Signa Excite HDxt; GE Healthcare) using an 8 channel head coil. The following sequences were acquired: axial diffusion-weighted images (TR 9032 ms; TE 81 ms; slice thickness 2 mm; b value 0 and 1000 sec/mm2), coronal Cube fluid-attenuated inversion recovery images (TR 5850 ms; TE 123.9 ms; TI 1530 ms), axial fast spin echo T2 weighted images (TR 4695 ms; TE 91.05 ms, slice thickness 3 mm), axial post-contrast T1weighted fat-saturated images (TR 560 ms; TE 14 ms, slice thickness 3 mm), and coronal post-contrast T1-weighted fat-saturated images (TR 585 ms; TE 14 ms; slice thickness 4 mm). Apparent diffusion coefficient maps were generated from the diffusion-weighted images. Regions of interest within the optic nerves were drawn on the apparent diffusion coefficient maps.
MR imaging of the orbits four weeks after the onset of vision loss showed gadolinium enhancement of the left optic nerve, beginning behind the globe and extending through the optic canal (Fig. 2A). Although there was no history of xerostomia or keratoconjunctivitis sicca, the parotid glands had a granular texture typically seen in patients with Sjögren's syndrome. Imaging of the spinal cord was negative.
Figure 2.
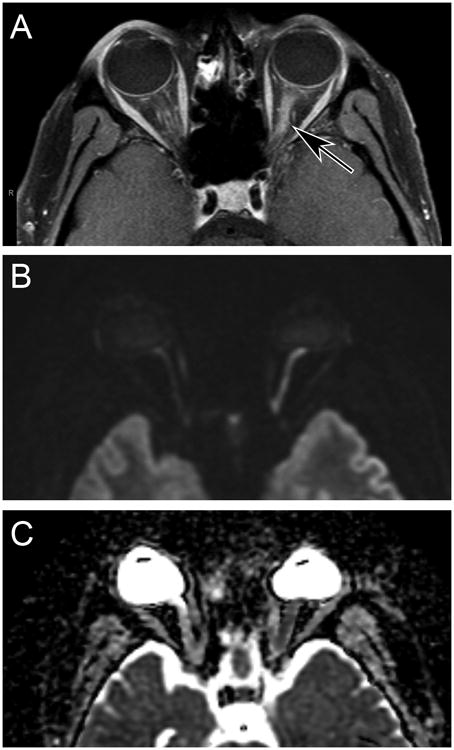
A) Axial T1-weighed magnetic resonance scan with fat saturation showing gadolinium enhancement of the left optic nerve (arrow). B) Diffusion-weighted image showing bright signal in the left optic nerve, corresponding to the gadolinium enhancement in (A). C) Apparent diffusion coefficient image, showing darker signal in the left optic nerve than the right optic nerve.
Diffusion-weighted imaging showed increased signal along the course of the left optic nerve, from the globe to the optic canal (Fig. 2B). This abnormality corresponded to the portion of the nerve demonstrating gadolinium enhancement. The apparent diffusion coefficient map revealed hypointense signal in the left optic nerve (Fig. 2C). The apparent diffusion coefficient measured along a representative 45.6 mm2 segment of the left optic nerve was 0.875 × 10-3 mm2/s. The apparent diffusion coefficient along a 48.4 mm2 segment of the right optic nerve was 1.63 × 10-3 mm2/s. Fortuitously, diffusion-weighted imaging of the optic nerves had been obtained when an MR scan was done a year before, five months after the original attack of optic neuritis in the right eye. At that time, the apparent diffusion coefficient along a 35.0 mm2 portion of the left optic nerve was 1.03 × 10-3 mm2/s.
Discussion
Cerebral ischemia impairs energy-dependent cellular functions, leading to intracellular edema, loss of fluid from the extracellular space, and reduced diffusion of free water. Bright signal appears rapidly in diffusion-weighted images, allowing early and sensitive identification of brain regions affected by stroke. Diffusion-weighted imaging is also valuable in the differentiation of ischemia from demyelination. In a study of 56 patients, the apparent diffusion coefficient was significantly lower in acute infarct (0.76 × 10-3 mm2/s) compared with acute plaque (1.11 × 10-3 mm2/s) (5).
More than a dozen case reports have described abnormal diffusion-weighted imaging in patients with ischemic optic neuropathy, especially in cases where the orbital portion of the nerve is affected. It has been proposed that diffusion-weighted MR imaging may differentiate ischemic optic neuropathy from optic neuritis (6). In a recent study, diffusion-weighted imaging was abnormal in 5/5 patients with acute ischemic optic neuropathy, but only 2/25 patients with acute optic neuritis (7). The mean apparent diffusion coefficient was 0.55 × 10-3 mm2/s in acute ischemic optic neuropathy and 1.10 × 10-3 mm2/s in acute optic neuritis.
Diffusion-weighted imaging was obtained when our patient was admitted to the hospital for intravenous corticosteroid therapy. The discovery of reduced diffusivity in the left optic nerve suggested a diagnosis of acute ischemic optic neuropathy rather than optic neuritis. The diagnosis of NMO-associated optic neuropathy was established only later, when testing revealed anti-AQP4-IgG antibodies. About 5% of patients with optic neuritis harbor AQP4 autoantibodies (8). Often, as in our patient, there is evidence of Sjogren syndrome.
There is controversy regarding which patients with optic neuritis should undergo testing for the presence of anti-AQP4 antibodies (9). Patients with NMO are more likely to have bilateral optic neuritis and generally have more severe vision loss. However, no difference has been found in the degree of optic nerve enlargement or the intensity of gadolinium enhancement between NMO and multiple sclerosis patients (10,11). Our patient's findings indicate that diffusion-weighted imaging might allow early diagnosis of optic neuritis occurring in the context of NMO. His acutely inflamed left optic nerve had a lower apparent diffusion coefficient than reported in acute optic neuritis or in normal optic nerves (12). It was also lower than the value measured a year earlier, before the attack of optic neuritis. Acute inflammation in typical cases of optic neuritis usually results in little or no reduction in the apparent diffusion coefficient (12). However, the situation may be different in NMO-associated optic neuropathy, because aquaporin channels are targeted for immune-mediated attack. Loss of aquaporin channels may impede water movement into cells, causing more severe reduction in the apparent diffusion coefficient than is found in optic neuritis associated with multiple sclerosis.
In the chronic phase of optic neuritis, myelin loss and axonal damage eventually increase the mean diffusivity of water (13,14). In optic neuritis it is unclear how long it takes for the apparent diffusion coefficient to rise, but in stroke it occurs in about two weeks (15). In our patient, the apparent diffusion coefficient was still low four weeks after the onset of vision loss in the left eye, presumably from lasting depletion of water channels. The prolonged time course for reduction of the apparent diffusion coefficient may be a characteristic feature of inflammatory events triggered by IgG autoantibodies directed against aquaporin channels.
Acknowledgments
Funding Support: National Eye Institute and Research to Prevent Blindness.
Footnotes
There are no conflicts of interest pertaining to this study
Contribution of Each Author: Jonathan C. Horton wrote the manuscript and cared for the patient. Vanja C. Douglas obtained the diffusion-weighted imaging, cared for the patient during hospitalization, made the diagnosis of NMO-associated optic neuropathy, and edited the manuscript. Soonmee Cha interpreted the magnetic resonance imaging data and edited the manuscript.
References
- 1.Misu T, Fujihara K, Kakita A, Konno H, Nakamura M, Watanabe S, Takahashi T, Nakashima I, Takahashi H, Itoyama Y. Loss of aquaporin 4 in lesions of neuromyelitis optica: distinction from multiple sclerosis. Brain. 2007;130:1224–1234. doi: 10.1093/brain/awm047. [DOI] [PubMed] [Google Scholar]
- 2.Roemer SF, Parisi JE, Lennon VA, Beanrroch EE, Lassmann H, Bruck W, Mandler RN, Weinshenker BG, Pittock SJ, Wingerchuck DM, Lucchinetti CF. Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain. 2007;130:1194–1205. doi: 10.1093/brain/awl371. [DOI] [PubMed] [Google Scholar]
- 3.Barker GJ. Diffusion-weighted imaging of the spinal cord and optic nerve. J Neurol Sci. 2001;186:S45–49. doi: 10.1016/s0022-510x(01)00490-7. [DOI] [PubMed] [Google Scholar]
- 4.Fryer JP, Lennon VA, Pittock SJ, Jenkins SM, Fallier-Becker P, Clardy SL, Horta E, Jedynak EA, Lucchinetti CF, Shuster EA, Weinshenker BG, Wingerchuk DM, McKeon A. AQP4 autoantibody assay performance in clinical lab service. Neurol Neuroimmunol Neuroinflammation. 2014;1:e11. doi: 10.1212/NXI.0000000000000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoque R, Ledbetter C, Gonzalez-Toledo E, Misra V, Menon U, Kenner M, Rabinstein AA, Kelley RE, Zivadinov R, Minagar A. The role of quantitative neuroimaging indices in the differentiation of ischemia from demyelination: an analytical study with case presentation. Int Rev Neurobiol. 2007;79:491–519. doi: 10.1016/S0074-7742(07)79022-0. [DOI] [PubMed] [Google Scholar]
- 6.He M, Cestari D, Cunnane MB, Rizzo JF., 3rd The use of diffusion MRI in ischemic optic neuropathy and optic neuritis. Semin Ophthalmol. 2010;25:225–232. doi: 10.3109/08820538.2010.518450. [DOI] [PubMed] [Google Scholar]
- 7.Bender B, Heine C, Danz S, Bischof F, Reimann K, Bender M, Nӓgele T, Ernemann U, Korn A. Diffusion restriction of the optic nerve in patients with acute visual deficit. J Magn Reson Imaging. 2014;40:334–340. doi: 10.1002/jmri.24367. [DOI] [PubMed] [Google Scholar]
- 8.Petzold A, Pittock S, Lennon V, Maggiore C, Weinshenker BG, Plant GT. Neuromyelitis optica-IgG (aquaporin-4) autoantibodies in immune mediated optic neuritis. J Neurol Neurosurg Psychiatry. 2010;81:109–111. doi: 10.1136/jnnp.2008.146894. [DOI] [PubMed] [Google Scholar]
- 9.Morrow MJ, Wingerchuk D. Neuromyelitis optica. J Neuroophthalmol. 2012;32:154–166. doi: 10.1097/WNO.0b013e31825662f1. [DOI] [PubMed] [Google Scholar]
- 10.Lim YM, Pyun SY, Lim HT, Jeong IH, Kim KK. First-ever optic neuritis: distinguishing subsequent neuromyelitis optica from multiple sclerosis. Neurol Sci. 2014;35:781–783. doi: 10.1007/s10072-014-1635-6. [DOI] [PubMed] [Google Scholar]
- 11.Tackley G, Kuker W, Palace J. Magnetic resonance imaging in neuromyelitis optica. Mult Scler. 2014 May 14; doi: 10.1177/1352458514531087. [DOI] [PubMed] [Google Scholar]
- 12.Fatima Z, Motosugi U, Muhi A, Hori M, Ishigame K, Araki T. Diffusion-weighted imaging in optic neuritis. Can Assoc Radiol J. 2013;64:51–55. doi: 10.1016/j.carj.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Kolbe S, Chapman C, Nguyen T, Bajraszewski C, Johnston L, Kean M, Mitchell P, Paine M, Butzkueven H, Kilpatrick T, Egan G. Optic nerve diffusion changes and atrophy jointly predict visual dysfunction after optic neuritis. Neuroimage. 2009;45:679–686. doi: 10.1016/j.neuroimage.2008.12.047. [DOI] [PubMed] [Google Scholar]
- 14.Hickman SJ, Wheeler-Kingshott CA, Jones SJ, Miszkiel KA, Barker GJ, Plant GT, Miller DH. Optic nerve diffusion measurement from diffusion-weighted imaging in optic neuritis. AJNR Am J Neuroradiol. 2005;26:951–956. [PMC free article] [PubMed] [Google Scholar]
- 15.Lansberg MG, Thijs VN, O'Brien MW, Ali JO, de Crespigny AJ, Tong DC, Moseley ME, Albers GW. Evolution of apparent diffusion coefficient, diffusion-weighted, and T2-weighted signal intensity of acute stroke. AJNR Am J Neuroradiol. 2001;22:637–644. [PMC free article] [PubMed] [Google Scholar]


