Abstract
Background and purpose
Endovascular sampling and characterization from patients can provide very useful information about the pathogenesis of different vascular diseases, but it has been limited by the lack of an effective method of endothelial cell (EC) enrichment. We optimized the EC yield and enrichment from conventional guide wires by laser capture microdissection (LCM) and fluorescence activated cell sorting (FACS) technique, and addressed the feasibility of using these enriched ECs for downstream gene expression detection.
Methods
Iliac artery endovascular samples from 10 patients undergoing routine catheter angiography were collected using conventional 0.038 in. J-shape guide wires. Each of these samples was equally divided into two parts, which were respectively used for EC enrichment by immunocytochemistry-coupled LCM or multiple color FACS. After RNA extraction and reverse transcription, the amplified cDNA was used for quantitative polymerase chain reaction (qPCR).
Results
Fixed ECs, with positive CD31 or vWF fluorescent signal and endothelial like nucleus, were successfully separated by LCM and live single ECs were sorted on FACS by a seven color staining panel. EC yields by LCM and FACS were 51 ± 22 and 149 ± 56 respectively (P < 0.001). The minimum number of fixed ECs from ICC-coupled LCM for acceptable qPCR results of endothelial marker genes was 30, while acceptable qPCR results as enriched by FACS were attainable from a single live EC.
Conclusion
Both LCM and FACS can be used to enrich ECs from conventional guide wires and the enriched ECs can be used for downstream gene expression detection. FACS generated a higher EC yield and the sorted live ECs may be used for single cell gene expression detection.
Keywords: Endothelial cell, Laser capture microdissection, Fluorescence activated cell sorting
1. Introduction
Many studies showed that endothelium plays a critical role in cerebrovascular diseases (Chalouhi et al., 2012; Sammons et al., 2011) and other vascular diseases (Landmesser and Drexler, 2005). Our collective understanding of how endothelial dysfunction is involved in the pathophysiological disturbance of different vascular diseases is, however, limited by the lack of a means to sample the endothelium in vivo because of potential procedure-related morbidity, particularly within the cerebrovasculature.
Several research groups (Colombo et al., 2002, 2005; Donato et al., 2007; Eskurza et al., 2006; Feng et al., 1999, 2005; Gates et al., 2007; Onat et al., 2007; Silver et al., 2007, 2010; Yu et al., 2009) have used endothelial sampling by using guide wires in large vessels including the aorta and the iliac, carotid, radial or branchial arteries, as well as veins of the forearm or antecubial fossa. These reports used similar endothelial cell enrichment and identification protocols, i.e., endothelial dissociation from guide wires, RBC lysis and verification of endothelial cell morphology and immunoreactivity of endothelial cell markers, although their endothelial cell yields have wide variation from dozens to hundreds. These reports used reverse transcript PCR (Feng et al., 1999, 2005), quantitative PCR (Onat et al., 2007) and quantitative immunofluorescence on single cells (Colombo et al., 2002, 2005; Donato et al., 2007; Eskurza et al., 2006; Gates et al., 2007; Silver et al., 2007, 2010; Yu et al., 2009) to address gene expression changes in some disease situations. Two EC enrichment methods were reported by these studies micropipette picking-up (Feng et al., 1999) and CD146 antibody magnetic beads (Feng et al., 2005; Onat et al., 2007; Yu et al., 2009). The efficiency of micropipette picking-up obviously depends on skill of the operator and as such may be difficult to standardize. Meanwhile, magnetic beads collect ECs only by one marker, CD146, without other antigen–antibody verification and the commercial beads may be cost restrictive for the high-throughput of clinical sampling. These shortcomings limit the generalization of these two EC enrichment methods in clinical research.
Laser capture microdissection (LCM) is a contemporary technology that has high efficiency for cell and tissue separation from both tissue slides and cell samples and has been widely used in basic and clinical research. Fluorescence activated cells sorting (FACS) is another powerful cell enrichment apparatus that can sort out single live cells from different kinds of cell samples by multiple cell surface markers for further culture and detection.
Recently we reported that conventional detachable coils can be used to harvest endothelial cells from pig iliac artery, and the endothelial cell yields correlated with the diameter of the coil (Cooke et al., 2013). Based on this and the works of the other groups, we aimed to address how effective LCM and FACS are for EC enrichment from guide wires collected from patients undergoing routine catheter angiography for diagnosis or treatment purposes, and if the enriched ECs can be used for quantitative PCR gene expression detection.
2. Materials and methods
2.1. Patient selection and endothelial cell harvest
Under an approved human research protocol, 10 patients undergoing cerebral angiography at the University of California, San Francisco were randomly recruited in this study. All patients underwent standard written surgical consent including the removal and use of tissue for diagnostic and research purposes. To perform biopsy of iliac endothelium, two coaxial curved stainless steel wires with a 0.038-in. diameter, 3-mm curve radius, and heparin coating (J-wire; Cook Inc., Bloomington, IN) were sequentially inserted into the right iliac artery through a 5F femoral sheath (Cook Inc.). The wire was then pulled back through the femoral sheath, and the tips of the wires were cut at about 3 in. (7.6 cm) and transferred in sterile fashion to a 15 mL Falcon Tube (Thermo Fisher Scientific, Rochester, NY) with 10 mL enzyme free cell dissociation buffer (Gibco, Grand Island, NY). After stretching the guide wires, the tubes were shaken on vortex mixer (Thermo Fisher Scientific, Rochester, NY) for 10 s before the wires were removed. Then the dissociation buffer with dislodged cells was centrifuged at 1500 rpm for 10 min at RT and the supernatant was aspirated. After lysing RBC by ACK Lysing Buffer (Gibco, Grand Island, NY), the sample was centrifuged again at 1500 rpm for 10 min at RT and re-suspended in FACS buffer. Then the resuspended sample was separated into two parts of equal volume for following immunofluorescence staining for LCM or multiple antibodies staining for FACS respectively.
2.2. Immunocytochemistry for endothelial cell identification
For endothelial cell identification before LCM, cells were seeded into DuplexDish 35 (Carl Zeiss AG, Oberkochen, Germany), a culture dish especially adapted for non-contact LCM. Cells were fixed with 4% PFA for 10 min and then blocked with 5% goat serum (Jackson ImmunoResearch Laboratories, West Grove, PA) in 1× PBS for 30 min. Then cells were incubated with primary antibodies diluted in 5% goat serum in 1× PBS overnight at 4 °C at the following concentrations: mouse anti-human CD31 (Dako, Glostrup, Denmark) at 1:50 dilution or mouse anti-human Von Willebrand factor (vWF) antibody (Dako, Glostrup, Denmark) at 1:200 dilution, followed by incubation with 1:400 diluted Alexa Fluor 488-conjugated goat anti-mouse IgG (Molecular probes, Eugene, OR) for 1 h at RT. Then the cell nucleus was stained by either 4′,6-diamidino-2-phenylindole (DAPI) in Vectashield Hard Set Mounting Medium (Vector Laboratories, Burlinggame, CA) or 1:100 diluted propidium iodide (Abcam, San Francisco, CA). Both CD31 and vWF antibodies for EC immunofluorescent staining were tested and got positive signal at the beginning of this study. Because CD31 gave better cell surface signal which is easier to identify than the cytosol vWF signal under microscopy, we used CD31 as the identifying EC marker for LCM throughout this study.
2.3. Endothelial cell enrichment by LCM and RNA purification
Laser microdissection and laser pressure catapulting (LPC) were performed on the PALM MicroBeam system (P.A.L.M. Micro-laser Technologies AG) equipped with an Axiovert 200 Zeiss inverted microscope and a SN3103 color camera (Carl Zeiss AG, Oberkochen, Germany). PALM RoboSoftware (v2.2) was used to selected endothelial cells. The following settings have been used with a 40× objective: after identified by positive CD31 or vWF staining and endothelial like nucleus by propidium iodide staining, ECs were microdissected using a laser UV energy setting of 65 and a cut setting of 40 in the PALM Robosoftware. The microdissected ECs were catapulted into the cap of AdhesiveCap 500 opaque (Carl Zeiss Microscopy GmbH, Gottingen, Germany). After LCM, total RNA of collected endothelial cells is purified by RNeasy FFPE Kit (Qiagen, Valencia, CA) which is specially designed for purification of total RNA from formalin-fixed, paraffin-embedded (FFPE) tissue sections. Then the extracted RNA was used for reverse transcription, cDNA amplification and Duplex Real-time PCR by the Ambion Single Cell-to-CT Kit (Life Technologies, Carlsbad, CA).
2.4. Endothelial cell enrichment by FACS
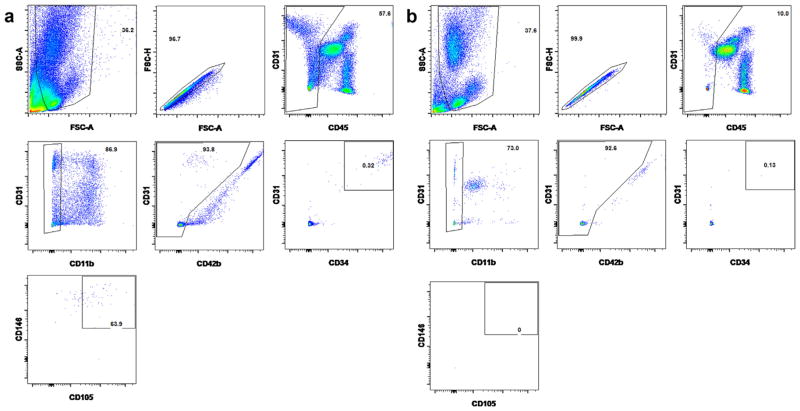
Dislodged cells from wires were stained with fluorescently conjugated monoclonal antibodies to identify and sort CD45−CD11b−CD42b−CD31+CD34+CD105+CD146+ECs. 100 μL peripheral blood was also taken from each patient to do the same staining and FACS sorting to make sure that no similar cell subgroup can be sorted from blood. After RBC lysis and washing described above, cells were stained with monoclonal antibodies to identify leukocytes (1:50 anti CD45-Alex 700, Life Technologies, Grand Island, NY), macrophages (1:50 anti CD11b-PacBlue, Biolegend, San Diego, CA) and platelets (1:50 anti CD42-FITC, BD Biosciences, San Jose, CA) and four EC markers were to identify ECs, 1:50 anti CD34-PE-Cy7 (Biolegend, San Diego, CA), 1:500 anti CD31-Alex 647, 1:100 anti CD105-PE-CF594 and 1:50 anti CD146 PE (all BD Biosciences, San Jose, CA). BD CompBead Anti-Mouse Ig Kappa (BD Biosciences, San Jose, CA) incubated with each antibody was used for compensation. ECs were sorted on a FACS Aria II (BD Biosciences, San Jose, CA) using DIVA V6.1.3 by single cell sort mode (BD Biosciences). The gating strategy used for sorting is illustrated in Fig. 4. Several different gating strategies were used throughout the course of this study in an attempt to increase the purity of viable ECs, the strategy illustrated in Fig. 4 was the most successful. Cells were first gated to exclude debris and doublets. Negative gates were then set to remove CD45+ leucocytes, CD11b+ myeloid cells and CD42b+ platelets, positive gates for CD31, CD105, CD34 and CD146 were set on the remaining cells to define the EC population for sorting. In initial experiments FMO controls were used to help define the positive gate and whole blood samples, stained with the same panel, were run in some experiments as a positive control for the negative gates and a negative control for the EC gates. From each sorted sample data was also acquired and subsequently analyzed with FlowJo V10 (Treestar, Ashland, OR). Each sorted EC was directly shot into one well of the Eppendorf twin.tec PCR Plate 96 (Eppendorf, Hauppauge, NY) which had 10 μL single cell lysis buffer composed of 9 μL Single Cell Lysis Solution and 1 μL Single Cell DNase I. After terminating the lysis reaction by adding 1 μL Single Cell Stop Solution and incubating at RT for 2 min, the samples are stored at −20 °C for reverse transcription and duplex quantitative PCR by the Ambion Single Cell-to-CT Kit.
Fig. 4.
Representative figures of staining and gating strategy for live ECs on FACS. After gating on the single cells, the leukocytes (CD45+), macrophages (CD11b+) and platelets (CD42b+) were eliminated before four endothelial markers (CD31+CD34+CD105+CD146+) were used to identify and sort out the ECs. (a) and (b) Representative figures from one patient’s wire sample and peripheral blood.
2.5. Duplex quantitative reverse transcription PCR
Gene expressions of both LCM separated ECs and FACS sorted single ECs were detected by the Ambion Single Cell-to-CT Kit (Life Technologies, Carlsbad, CA) and Duplex Real-time PCR. Briefly, 3.0 μL Single Cell VILO RT Mix and 1.5 μL Single Cell SuperScript RT were then added to 10 μL cell lysis samples to make the RT reaction mix. Reverse transcription was performed in a thermal cycler at 25 °C for 10 min, 42 °C for 60 min and then 85 °C for 5 min. For cDNA pre-amplification, 5 μL Single Cell PreAmp Mix plus 6 μL five-time diluted TaqMan primers were added to each sample and incubated in a thermal cycler at 95 °C for 10 min, then 14 cycles of 95 °C for 15 s to denature and 60 °C for 4 min for annealing and extension, then 99 °C for 10 min to deactivate the enzyme. For two color real-time PCR, 10 μL 10 times diluted pre-amplified cDNA product by 1× TE Buffer (pH 8.0) was mixed with 25 μL TaqMan Gene Exp Master Mix, 5 μL TaqMan primers (2.5 μL each for both the FAM and VIC dye labeled primers for Duplex qPCR) and 10 μL nuclease-free water to make a 50 μL PCR system. The qPCRs were performed on the Agilent Mx3005P QPCR Systems (Agilent Technologies, Santa Clara, CA) by the program of 50 °C for 2 min, 95 °C for 10 min, 40 cycles of 95 °C 5 s and 60 °C 1 min (anneal/extend). QPCR results are analyzed by the MxPro QPCR Software by the same company. Three TaqMan gene-specific primers were purchased from Applied Biosystems (Foster City, CA), Von Willebrand factor (vWF, Hs01109446 m1) and Tie-2 (Hs00945146 m1) were labeled with FAM dye and used as EC markers, and primer limited GAPDH (Hs03929097 g1) was labeled with VIC dye and used as the internal control. For the LCM separated ECs, the real-time PCR results were considered as acceptable when the cycle threshold (Ct) value was between 17 and 32 cycles. qPCR Human Reference Total RNA (Clontech Laboratories Inc, Mountain View, CA) was used to justify the qPCR system. For 30 cell and 20 cell qPCR, both were carried out from 5 different samples. For statistical analysis, 6 endothelial cells with acceptable Ct value of GAPDH from each of the 10 patients were used to collect their vWF and Tie-2 Ct values, and mean, standard deviation (SD) and P values were calculated in the GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA). 10 endothelial cells were randomly picked from each patient’s sample for single cell qPCR analysis of GAPDH, vWF and Tie-2 genes. The acceptable Ct values (17–32) of each gene were pooled together to get mean ± SD, and the cell numbers with acceptable Ct value out of the total 100 cells are also shown in Table 1.
Table 1.
Ct values of duplex qPCR on single FACS enriched ECs and different number of LCM enriched fixed ECs.
| Single cell by FACS | 30 cells by LCM | 20 cells by LCM | 10 cells by LCM | |
|---|---|---|---|---|
| vWF | 19.60 ± 1.84, 46 (100) | 18.42 ± 0.99 | 32.56 ± 0.96 | UD |
| Tie-2 | 25.58 ± 2.48, 31 (100) | 21.85 ± 1.52 | 33.47 ± 1.24 | UD |
| GAPDH | 28.89 ± 2.07, 39 (100) | 26.68 ± 2.52 | UD | UD |
qPCR results were considered as acceptable only when cycle threshold (Ct) value was between 17 and 32 cycles. The number of cells which gave acceptable Ct value (X) in 100 FACS sorted single ECs is also presented as X (100). UD means Ct value are undetectable.
2.6. Statistical methods
The EC yield data and qPCR data are shown as mean ± SD. Statistical significance of EC yield between LCM and FACS groups was calculated by paired t-test on GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA). A P value <0.05 was considered as statistical significance.
3. Results
3.1. Harvesting efficiency
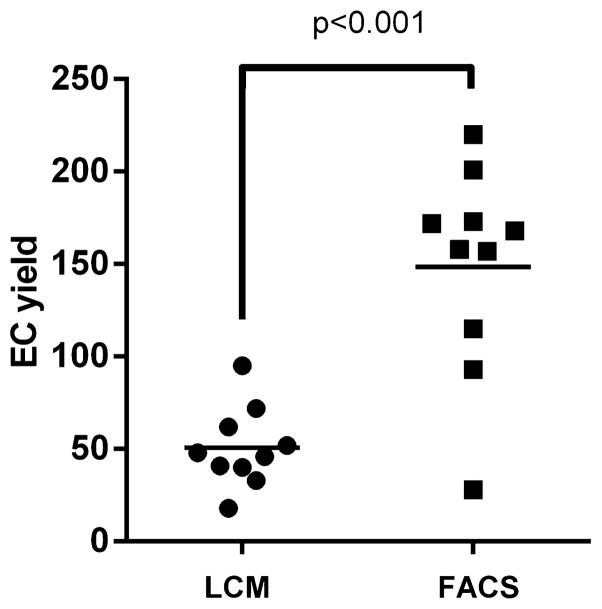
Two curved guide wires were successfully placed and retrieved into the right iliac artery in each of the 10 patients in successive order and cellular material was detected along the winds of the coils under light microscopy (for figure see our published paper, Cooke et al., 2013). Cellular material was collected from all patients’ samples and all of them yielded endothelial cells. The average EC yield from FACS is 149 ± 56, was significantly higher than the EC yield of LCM, 51 ± 22 (P < 0.001, Fig. 1).
Fig. 1.
The average EC yield by LCM and FACS.
3.2. Characterization of harvested ECs by LCM
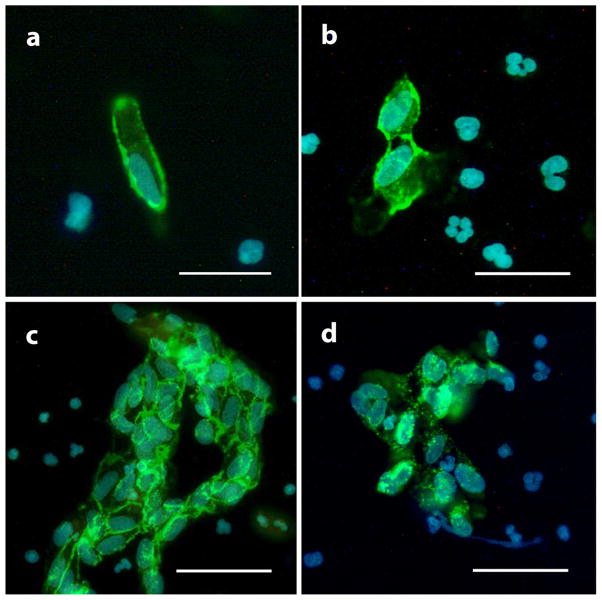
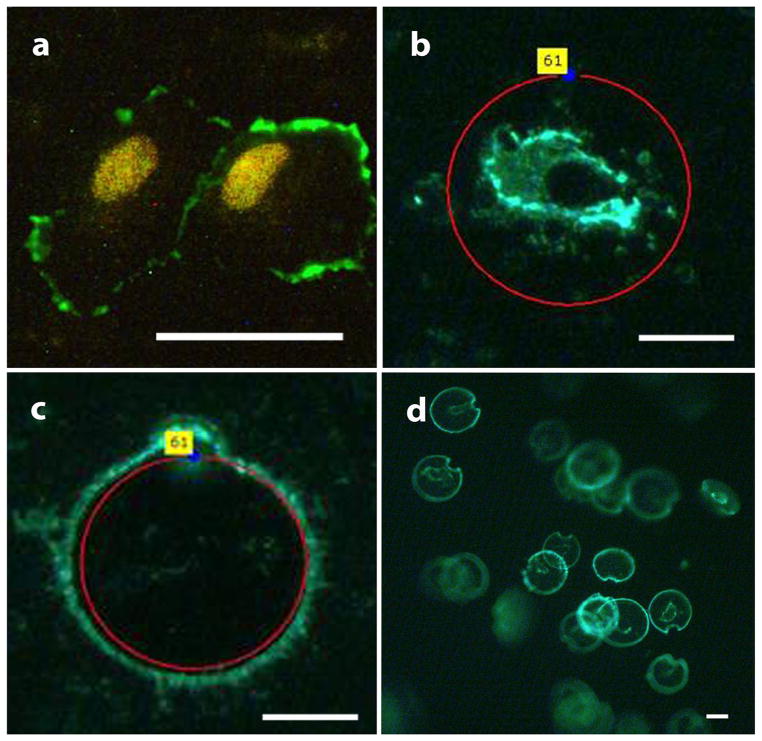
For LCM enrichment, ECs were first identified by both oval and kidney-shaped endothelial-like nucleus shown by DAPI or propidium iodide and positive CD31 membrane staining signal or vWF cytosol staining signal (Fig. 2). Endothelial cells were present in three ways: (a) single cell (Fig. 2a); (b) small aggregates which were two to four cells together (Fig. 2b); and (c) larger sheets which are five or more cells together (Fig. 2c). The ECs also showed positive vWF cytosol staining (Fig. 2d). Intense and intact membrane CD31 signal was always seen when endothelial cells came in sheets (Fig. 2c). Laser capture microdissection was used to isolate individual endothelial cells that had positive CD31 membrane staining signal and an endothelial-like nucleus (Fig. 3a). After identification under fluorescent microscopy, these endothelial-like cells were cut and catapulted (Fig. 3b and c) by laser one by one into AdhesiveCap 500 collection cap (Fig. 3d) with RNA recovery buffer.
Fig. 2.
Endothelial cells identification by immunocytochemistry. ECs were identified by big size (≥20 μM), endothelial-like oval or kidney-shaped nucleus (a) and positive CD31 membrane staining (a, b and c) or vWF cytosol staining (d). Single cell (a), small aggregates of two to four cells (b) and bigger sheets of five or more cells (c) can be seen under fluorescent microscopy. Scale bar is 20 μm in (a) and (b) or 40 μm in (c) and (d).
Fig. 3.
Representative figures of EC enrichment by LCM. ECs were first identified by endothelial like nucleus with propidium iodide staining and positive CD31 membrane staining (a), then the chosen ECs were microdissected (b) one by one by laser UV and capultated (c) into collection cap with RNA recovery buffer (d). The scale bar is 25 μm.
3.3. EC enrichment from wire sample and blood by FACS
Fig. 4 shows representative FACS sorting results from both the wire dislodging cells and the peripheral blood from one same patient. A subgroup of CD45−CD11b−CD42b−CD31+CD34+CD105+CD146+ cells can be sorted from wire dislodging cells (Fig. 4a). At the same time, no such cells could be sorted from 100 μL peripheral blood of the patients (Fig. 4b). This result excluded the possibility that some of the sorted ECs may be the circulating endothelial cells which may stick to the wires when they were deployed in the iliac artery.
3.4. Quantitative real-time PCR results of enriched ECs
Quantitative PCR results from single FACS enriched ECs and 30, 20, and 10 LCM harvested fixed ECs are shown in Table 1. Because the GAPDH primer is labeled by VIC and its concentration is decreased by the company for better duplex qPCR result, the cycle threshold (Ct) values of GAPDH are higher than FAM labeled vWF and Tie-2. The qPCR data indicated that only up to 30 fixed ECs from LCM yielded acceptable Ct values, yet 20 and 10 such fixed ECs did not generate acceptable or detectable Ct values, which may suggest that the proteinase K in RNeasy FFPE Kit can only partly be recovered by 4% PFA fixed mRNA in harvested endothelial cells and that the minimum number of gene detection for LCM harvested ECs is about 30. For the single cell qPCR, only 39 cells out of the 100 FACS sorted ECs gave acceptable Ct for GAPDH gene, and this number for vWF and Tie-2 genes are 46 and 31. Among them, some single ECs had positive results for vWF or Tie-2, but not for GAPDH. This may indicate the variable mRNA level detected on single cell level. The more diluted GAPDH primer used for the duplex qPCR may be another possible reason. Some “cells” had no mRNA expressions for all these three genes, it may indicate that either the cells were in bad condition or no cell was sorted into that well on the 96 PCR plate.
4. Discussion
Because the guide wire is such a widely used device in catheter angiography, most of the previous studies of EC biopsy used them as their sampling tool, the variation being the guide wire numbers used and the deployment location. Wire numbers have ranged from one (Feng et al., 1999) to five (Onat et al., 2007), and the deployment locations included large vessels such as the iliac, carotid, brachial and radial, arteries, smaller vessels such as the coronary arteries (Yu et al., 2009), and the superficial conduit veins of the arm. Similar to the work up by Feng et al., we used guide wires during femoral access to collect the endovascular sample. This sampling location is easy to access and has long standing safety profile.
Five of the 11 previous studies showed endothelial cell yield data (Colombo et al., 2002, 2005; Feng et al., 1999; Gates et al., 2007; Yu et al., 2009). These five papers used different numbers of guide wires to sample endothelial cells at varying locations, and, not surprisingly, the endothelial cell yields reported ranged from dozens to hundreds. Our study showed both LCM and FACS can get at capture at least dozens of ECs. Theoretically, FACS is designed to collect single endothelial cells and may miss the endothelial cell sheets, while LCM may be able collect such cell aggregates. Our data, however, demonstrated FACS generated a higher EC yield than the LCM. A possible reason is that the immmunofluorescent staining before LCM requires several steps of antibody incubations and rinsing, during which more ECs may be lost prior to LCM. Another reason may be that by LCM all the ECs need to be identified individually under microscopy by a researcher’s eye, and that this may be less efficient than the automatic singular cell sorting of FACS. Meanwhile, we only claimed EC high-enrichment but not EC purification in this study because of the limitation of LCM and FACS. Also, a second confirmatory technique, e.g. qPCR, may be needed to exclude the possible contamination of leukocyte and vascular smooth muscle cells inside the sampling vessel.
Because the prior EC yields from arterial samples collected numbers into the hundreds cells of ECs, the previous studies used qualitative or quantitative RT-PCR to detect the mRNA transcription level of different target genes or used single cell quantitative immunofluorescent staining analysis to observe the protein expression of these genes. In this study, we focused on what the minimum enriched ECs might be used to get acceptable quantitative real-time PCR data by either LCM or FACS, because this minimum EC number may limit how many target genes can be detected for their gene expression from each patient’s sample at one time. According to our data, FACS enrichment methods were more efficient than LCM-based methods, the former capable of single cell real-time PCR. This may reflect that FACS can sort out live or active ECs that yield higher quality mRNA, whereas LCM can only collect fixed ECs which need special enzyme treatment to retrieve mRNA from cross-linked protein. Although according to this study and previous reports, the maximum EC yields from guide wires are up to several hundreds, our recent study indicated that EC sampling by conventional coils, which are in vivo device, gave even fewer EC yields on the scale of single to tens of cells (Cooke et al., 2013). Our single cell qPCR indicated that less than half of FACS sorted ECs gave good qPCR results not only for endothelial markers vWF and Tie-2, but also the house keeping gene GAPDH, and some ECs even only expressed the mRNAs of vWF and (or) Tie-2 but not GAPDH. These results are consistent with the previous reports on the nature of single cell transcription, which found that single eukaryotic cells show cell to cell variation of up to hundreds folds in mRNA amounts including the house keeping gene β actin (Bengtsson et al., 2005). This phenomenon may represent the “burst and decay” behavior of eukaryotic transcripts. (Chubb et al., 2006). All these may suggest that better single cell mRNA detection techniques and especially better statistical methods are needed for analyzing such kind of single cell data. With the FACS enrichment method reported in this study and the advent of new single cell PCR amplification techniques, we believe that this limited number of ECs harvested by in situ devices, such as coils and stents, may provide us enough cell material to perform robust genetic analysis and in turn a better understanding of the pathogenesis of vascular diseases.
5. Conclusion
Our study showed that both LCM and FACS can be used for enriching ECs from endovascular samples harvested by guide wires from iliac artery of patients undergoing catheter angiography. FACS not only provided better EC yield, but live ECs with high quality mRNA scalable for analysis by quantitative PCR on the single cell level.
Abbreviations
- EC
endothelial cell
- LCM
laser capture microdissection
- FACS
fluorescence activated cell sorting
- qPCR
quantitative polymerase chain reaction
- vWF
Von Willebrand factor
- DAPI
6-diamidino-2-phenylindole
- LPC
laser pressure catapulting
- FFPE
formalin-fixed, paraffin-embedded
- FMO
fluorescence minus one
References
- Bengtsson M, Stahlberg A, Rorsman P, Kubista M. Gene expression pro-filing in single cells from the pancreatic islets of Langerhans reveals lognormal distribution of mRNA levels. Genome Res. 2005;15:1388–1392. doi: 10.1101/gr.3820805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalouhi N, Ali MS, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, Koch WJ, Dumont AS. Biology of intracranial aneurysms: role of inflammation. J Cereb Blood Flow Metab. 2012;32:1659–1676. doi: 10.1038/jcbfm.2012.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubb JR, Trcek T, Shenoy SM, Singer RH. Transcriptional pulsing of a developmental gene. Curr Biol. 2006;16:1018–1025. doi: 10.1016/j.cub.2006.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo PC, Ashton AW, Celaj S, Talreja A, Banchs JE, Dubois NB, Marinaccio M, Malla S, Lachmann J, Ware JA, Le Jemtel TH. Biopsy coupled to quantitative immunofluorescence: a new method to study the human vascular endothelium. J Appl Physiol (1985) 2002;92:1331–1338. doi: 10.1152/japplphysiol.00680.2001. [DOI] [PubMed] [Google Scholar]
- Colombo PC, Banchs JE, Celaj S, Talreja A, Lachmann J, Malla S, DuBois NB, Ashton AW, Latif F, Jorde UP, Ware JA, LeJemtel TH. Endothelial cell activation in patients with decompensated heart failure. Circulation. 2005;111:58–62. doi: 10.1161/01.CIR.0000151611.89232.3B. [DOI] [PubMed] [Google Scholar]
- Cooke DL, Su H, Sun Z, Guo Y, Guo D, Saeed MM, Hetts SW, Higashida RT, Dowd CF, Young WL, Halbach VV. Endovascular biopsy: evaluating the feasibility of harvesting endothelial cells using detachable coils. Interv Neuroradiol. 2013;19:399–408. doi: 10.1177/159101991301900401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007;100:1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- Eskurza I, Kahn ZD, Seals DR. Xanthine oxidase does not contribute to impaired peripheral conduit artery endothelium-dependent dilatation with ageing. J Physiol. 2006;571:661–668. doi: 10.1113/jphysiol.2005.102566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Matsumoto C, Schwartz A, Schmidt AM, Stern DM, Pile-Spellman J. Chronic vascular inflammation in patients with type 2 diabetes: endothelial biopsy and RT-PCR analysis. Diabetes Care. 2005;28:379–384. doi: 10.2337/diacare.28.2.379. [DOI] [PubMed] [Google Scholar]
- Feng L, Stern DM, Pile-Spellman J. Human endothelium: endovascular biopsy and molecular analysis. Radiology. 1999;212:655–664. doi: 10.1148/radiology.212.3.r99au28655. [DOI] [PubMed] [Google Scholar]
- Gates PE, Boucher ML, Silver AE, Monahan KD, Seals DR. Impaired flow-mediated dilation with age is not explained by L-arginine bioavailability or endothelial asymmetric dimethylarginine protein expression. J Appl Physiol (1985) 2007;102:63–71. doi: 10.1152/japplphysiol.00660.2006. [DOI] [PubMed] [Google Scholar]
- Landmesser U, Drexler H. The clinical significance of endothelial dysfunction. Curr Opin Cardiol. 2005;20:547–551. doi: 10.1097/01.hco.0000179821.11071.79. [DOI] [PubMed] [Google Scholar]
- Onat D, Jelic S, Schmidt AM, Pile-Spellman J, Homma S, Padeletti M, Jin Z, Le Jemtel TH, Colombo PC, Feng L. Vascular endothelial sampling and analysis of gene transcripts: a new quantitative approach to monitor vascular inflammation. J Appl Physiol (1985) 2007;103:1873–1878. doi: 10.1152/japplphysiol.00367.2007. [DOI] [PubMed] [Google Scholar]
- Sammons V, Davidson A, Tu J, Stoodley MA. Endothelial cells in the context of brain arteriovenous malformations. J Clin Neurosci. 2011;18:165–170. doi: 10.1016/j.jocn.2010.04.045. [DOI] [PubMed] [Google Scholar]
- Silver AE, Beske SD, Christou DD, Donato AJ, Moreau KL, Eskurza I, Gates PE, Seals DR. Overweight and obese humans demonstrate increased vascular endothelial NAD(P)H oxidase-p47(phox) expression and evidence of endothelial oxidative stress. Circulation. 2007;115:627–637. doi: 10.1161/CIRCULATIONAHA.106.657486. [DOI] [PubMed] [Google Scholar]
- Silver AE, Christou DD, Donato AJ, Beske SD, Moreau KL, Magerko KA, Seals DR. Protein expression in vascular endothelial cells obtained from human peripheral arteries and veins. J Vasc Res. 2010;47:1–8. doi: 10.1159/000231715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SY, Song YM, Li AM, Yu XJ, Zhao G, Song MB, Lin CM, Tao CR, Huang L. Isolation and characterization of human coronary artery-derived endothelial cells in vivo from patients undergoing percutaneous coronary interventions. J Vasc Res. 2009;46:487–494. doi: 10.1159/000200964. [DOI] [PubMed] [Google Scholar]