Abstract
Objectives
The dual endothelin receptor antagonist bosentan improves pulmonary vascular resistance (PVR) in patients with primary pulmonary hypertension (PH). The effects of bosentan on secondary PH due to systolic heart failure (HF) are not well defined. This study evaluates the effect and tolerability of bosentan in patients with PH secondary to HF.
Methods
Seventeen adult HF patients with PH and New York Heart Association class III–IV symptoms were treated with bosentan, 62.5 mg twice daily, for 1 month, which was gradually increased to 125 mg twice daily thereafter. Right heart catheterization (RHC), a clinical evaluation and echocardiographic measurements were performed at baseline and at 4 ± 3 (mean ± SD) months of follow-up. Response to bosentan was defined as an improvement in clinical, echocardiographic and RHC parameters.
Results
Six patients did not complete the study (therapy was discontinued due to hypotension, elevated liver enzymes or acute decompensation of HF), 11 patients completed the follow-up; 9 patients responded to therapy. Systemic arterial pressures, pulmonary pressures, PVR and the transpulmonary gradient significantly decreased compared with baseline levels in 9 responders (p = 0.05, 0.05, 0.01 and 0.004, respectively), and 4 became eligible for heart transplantation and 3 for left ventricular assist device implantation.
Conclusions
Bosentan decreased pulmonary pressures and PVR in the majority of patients with PH secondary to systolic HF, thereby allowing them to be considered candidates for heart transplantation.
Keywords: Bosentan, Pulmonary hypertension, Systolic heart failure
Introduction
Pulmonary hypertension (PH) and elevated pulmonary vascular resistance (PVR) secondary to systolic heart failure (HF) are risk factors for increased mortality due to right ventricular failure after orthotopic heart transplantation [1–3]. Elevated plasma levels of endothelin alter pulmonary hemodynamics by its vasoconstrictive action, and they contribute to progression of HF and increase mortality in these patients [4].
Bosentan, a dual receptor endothelin antagonist, improves pulmonary hemodynamics and quality of life in patients with primary and postthromboembolic PH [5]. However, the effects of bosentan on pulmonary hemodynamics and eligibility for heart transplantation in patients with PH secondary to systolic HF are not well characterized. Patients with refractory HF treated with high-dose bosentan had improvement in New York Heart Association (NYHA) class, without survival benefit compared with standard therapies. However, this clinical trial was interrupted due to an increased incidence of adverse events (most commonly elevated liver transaminases and HF exacerbations) in patients receiving bosentan [6].
Interestingly, in patients who were deemed ineligible for heart transplantation due to severe irreversible PH, 6-week therapy with bosentan decreased PVR, thereby making the patients eligible for heart transplantation [7]. The present study assessed the effect and tolerability of bosentan regarding pulmonary hemodynamics and eligibility for heart transplantation in patients with severe PH secondary to systolic HF.
Methods
Study Population
Patients with NYHA class III or IV HF, left ventricular (LV) ejection fraction <35% and severe PH refractory to the nitroprusside challenge test at the right heart catheterization (RHC) were included in the study. All patients were aged >18 years. Exclusion criteria included: history of myocardial infarction within 8 weeks; unstable angina or angina-limited exercise; HF due to uncorrected valvular stenosis, obstructive cardiomyopathy, pericardial disease, amyloidosis, active myocarditis or a malfunctioning artificial heart valve; symptomatic chronic obstructive pulmonary disease or asthma; history of symptomatic ventricular tachycardia, ventricular fibrillation or sudden death unless treated with an implantable defibrillator; 2nd- or 3rd-degree atrioventricular block unless treated with a pacemaker; heart rate >115 bpm; and systolic blood pressure <90 mm Hg or >200 mm Hg.
All patients were treated with 62.5 mg bosentan twice daily for 1 month, which was gradually increased to 125 mg twice daily thereafter. All patients underwent medical and echocardiographic examinations as well as RHC 2 weeks prior to starting treatment and at follow-up after bosentan titration. The study protocol was approved by the institutional review committee at each study site, and all patients provided their written informed consent.
Hemodynamic Measurements
A pulmonary artery catheter was used to measure pulmonary artery pressures, mean right atrial pressure and mean pulmonary capillary wedge pressure (PCWP). The wedge position was verified by fluoroscopy, phasic changes in pressure waveforms and oxygen saturation. Cardiac output (CO) and the cardiac index (CI) were derived by the Fick equation through sampling of a mixed central venous blood gas taken from the pulmonary artery and radial artery blood gas. All patients underwent the nitroprusside challenge test. RHC was performed at baseline, before bosentan therapy, and repeated after 4 ± 3 (mean ± SD) months of follow-up, when the patients’ clinical condition was stable. All patients with a PVR of >3 Wood units (WU) and/or systolic pulmonary arterial pressure (SPAP) of >50 mm Hg and/or a transpulmonary gradient (TPG) of >10 mm Hg underwent a nitroprusside challenge test according to the International Lung and Heart Society of Transplantation Guidelines [8]. Patients were considered responders to the bosentan therapy if their PVR was <2.5 WU and/or their TPG was <10 mm Hg at the follow-up visit.
Echocardiographic Measurements
The examination was performed using a Vivid 7.0 (GE Healthcare, USA) ultrasound system. Standard linear measurements were obtained according to American Society of Echocardiography guidelines [9]. LV volumes and ejection fraction were calculated from the apical 4- and 2-chamber views using the modified Simpson rule. SPAP was estimated by measuring the peak tricuspid regurgitation velocity and estimating right atrial pressure in accordance with inferior vena cava size and respiratory collapse. Systolic right ventricular function was explored by measuring tricuspid annular plane systolic excursion (TAPSE).
Statistical Analysis
Continuous variables are expressed as means ± SD. Categorical variables are expressed as percentages. The paired t test and χ2 test were used for data comparisons as appropriate. A value of p ≤ 0.05 was considered evidence of statistical significance. The analysis was performed with SPSS for Windows (version 15; SPSS Inc., Chicago, Ill., USA).
Results
A total of 17 patients with severe PH and NYHA class III–IV HF symptoms were enrolled. The demographic characteristics of the study participants titrated with bosentan are shown in table 1. The mean follow-up time was 4 ± 3 months. Bosentan was discontinued prior to the 1-month follow-up visit in 6 patients (in 3 patients due to hypotension, in 2 patients due to a pathological increase in liver enzymes, and in 1 patient due to acute HF decompensation). Functional capacity at baseline was severely reduced in all of the remaining 11 patients despite optimal medical therapy.
Table 1.
Demographic characteristics of population treated with bosentan (n = 17)
| Age, years | 56±7 |
| Gender – male, n | 15 (88%) |
| BSA, m2 | 2±0.1 |
| NYHA class III, n | 15 (88%) |
| MVO2, l/kg/m2 | 12±3 |
| NT proBNP, ng/l | 2,011±1,011 |
| Etiology, n | |
| Ischemic | 11 (65%) |
| Idiopathic | 6 (35%) |
| Therapy, n | |
| ACEI/ARB | 16 (94%) |
| Beta-blockade | 15 (88%) |
| Diuretic | 17 (100%) |
| Amiodarone | 10 (58%) |
| Digoxin | 8 (47%) |
| Spironolactone | 16 (94%) |
| Statins | 14 (82%) |
| AICD/BIV | 17 (100%) |
All values are expressed as means ± SD unless specified otherwise. BSA = Body surface area; MVO2 = myocardial oxygen consumption; NT proBNP = N-terminal fragment of probrain natriuretic peptide; ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blockers; AICD/BIV = automatic implantable cardioverter defibrillator/biventricular pacemaker.
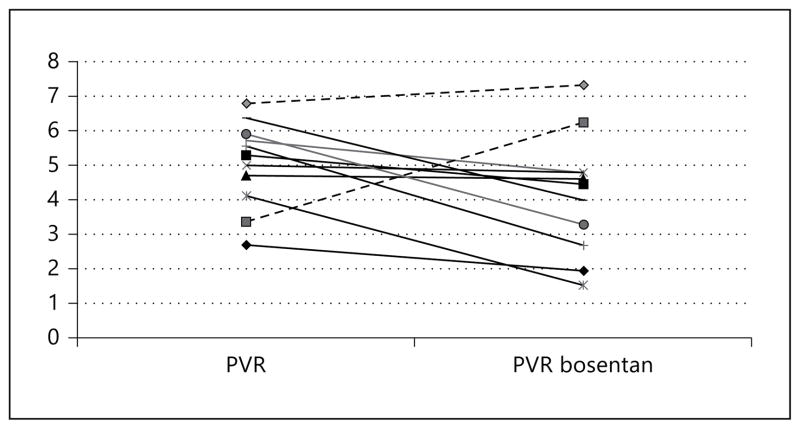
All patients had severe LV systolic dysfunction, with moderate-to-severe tricuspid and mitral regurgitation and decreased TAPSE. All patients had elevated pulmonary pressures at baseline despite the optimal medical therapy, and were resistant to the nitroprusside challenge test (table 2). Nine patients responded to bosentan therapy (fig. 1).
Table 2.
Swan-Ganz catheterization results before bosentan (at baseline and after nitroprusside challenge test) and after bosentan titration (at follow-up and after nitroprusside challenge test; n = 11)
| Baseline | Nitroprusside test | Bosentan follow-up | Nitro prusside test | |
|---|---|---|---|---|
| SBP, mm Hg | 120±19 | 94±13a | 110±16 | 99±14b, c |
| RAP, mm Hg | 8±4 | 5±2a | 8±3 | 4±3b, c |
| SPAP, mm Hg | 68±10 | 42±10a | 60±24 | 41±12b, c |
| MPAP, mm Hg | 41±7 | 26±7a | 39±15 | 25±8b, c |
| PCWP, mm Hg | 26±7 | 13±4a | 24±9 | 15±5b, c |
| TPG, mm Hg | 16±4 | 13±4a | 15±7 | 10±3b, c |
| CO, l/min | 3.2±0.7 | 4.0±0.7a | 3.3±0.7 | 4.1±0.9b, c |
| CI, l/min/m2 | 1.7±0.2 | 2.2±0.3a | 1.9±0.4 | 2.2±0.4b, c |
| PVR, WU | 5.0±1.2 | 2.1±0.3a | 4.2±1.7 | 2.5±0.9b, c |
SBP = Systolic blood pressure; RAP = right atrial pressure; MPAP = mean pulmonary arterial pressure.
Baseline vs. nitric oxide: p < 0.05.
Bosentan vs. bosentan nitric oxide: p < 0.05.
Baseline vs. postbosentan nitric oxide: p < 0.05.
Fig. 1.
PVR at baseline and at follow-up. Dashed lines: nonresponders to therapy.
At follow-up after bosentan titration, the responders showed improvement in hemodynamic variables (table 3). Mean pulmonary arterial pressure, SPAP, PVR, TPG and mean arterial pressure significantly decreased with bosentan (p = 0.05, 0.007, 0.01, 0.004 and 0.04, respectively), while CO, CI, PCWP and right arterial pressure remained unchanged. Echocardiographic variables did not differ significantly at follow-up compared with baseline levels. No significant changes were observed on echo Doppler examination, including mitral, aortic and tricuspid valve function (table 4).
Table 3.
Swan-Ganz catheterization results before bosentan (at baseline and after nitroprusside challenge test) and after bosentan titration (at follow-up and after nitroprusside challenge test) for responders to the therapy (n = 9)
| Baseline | Nitroprusside test | Bosentan | Nitroprusside test | |
|---|---|---|---|---|
| SBP, mm Hg | 119±20 | 90±11b | 108±17 | 97±12c |
| RAP, mm Hg | 9±5 | 4±3b | 8±4 | 3±2c |
| SPAP, mm Hg | 68±12 | 41±9b | 52±14a | 40±13c |
| MPAP, mm Hg | 41±8 | 25±7b | 34±10a | 24±8c |
| PCWP, mm Hg | 26±8 | 12±6b | 22±7 | 14±6c |
| TPG, mm Hg | 16±4 | 12±4b | 12±4a | 8±3c |
| CO, l/min | 3.1±0.7 | 3.8±0.8b | 3.3±0.8 | 3.9±0.8c |
| CI, l/min/m2 | 1.7±0.2 | 2.1±0.3b | 1.7±0.4 | 2.1±0.4c |
| PVR, WU | 5.3±1.2 | 3.1±0.7b | 3.9±1.8a | 2.6±1.0c |
SBP = Systolic blood pressure; RAP = right atrial pressure; MPAP = mean pulmonary arterial pressure.
Baseline vs. bosentan: p < 0.05.
Baseline vs. nitroprusside: p < 0.05.
Bosentan vs. bosentan nitroprusside: p < 0.05.
Table 4.
Echocardiographic parameters before and after bosentan titration in responders to therapy (n = 9)
| Baseline | Follow-up | p | |
|---|---|---|---|
| LV TDD, mm | 67±6 | 70±6 | 0.14 |
| LV TSD, mm | 55±4 | 56±6 | 0.78 |
| EF, % | 23±4 | 24±6 | 0.39 |
| SPAP, mm Hg | 71±14 | 54±25 | 0.16 |
| TAPSE, mm | 13±6 | 16±4 | 0.25 |
TDD = Telediastolic diameter; TSD = telesystolic diameter; EF = ejection fraction.
The 9 patients who responded to bosentan therapy were considered eligible for heart transplantation. At the 1-year follow-up, 3 patients received an LV assist device. Bosentan was discontinued after implantation in those 3 patients. All patients who responded to bosentan therapy were maintained on bosentan up to the 1-year follow-up visit. All patients remained on the transplant list. No patient received a heart transplant at the 1-year follow-up visit. One patient had bosentan discontinued after hospitalization for uncontrolled PH. The nonresponders remained ineligible for heart transplantation, and 2 of them died during the 1-year follow-up period. In the nonresponders, bosentan was discontinued after the first catheterization.
Limitations
We conducted nonrandomized, small-sample observational study in a single center. The number of dropouts due to adverse events or symptomatic hypotension was similar to that in other studies [6, 10].
Discussion
The major findings of this study are that bosentan improves pulmonary and systemic hemodynamics in HF patients with PH who are able to tolerate such a therapy, and that treatment with bosentan may have a beneficial impact on the eligibility of these patients for heart transplantation.
The improvement in pulmonary and systemic hemodynamics among patients with HF and PH who tolerated bosentan therapy well suggests that such a therapy may be beneficial in at least half of these patients. More importantly, addition of bosentan to a standard HF therapy led to a significant decrease in systemic blood pressure and pulmonary resistance, which enabled these patients to become eligible for heart transplantation. This is in agreement with previous reports on the beneficial effect of bosentan therapy on PVR and diastolic pulmonary pressure, which enabled a subset of responders to bosentan to become eligible for heart transplantation [7, 11]. After bosentan titration, PVR decreased by 60% compared with baseline values at the 12-month follow-up in heart transplant candidates with severe PH [12]. The reduction in PVR with bosentan therapy is associated with favorable outcome after transplantation and LV assist device implantation [12, 13].
In our study, the bosentan therapy significantly lowered mean pulmonary arterial pressure, SPAP, PVR and TPG, but it did not affect CO and CI. In contrast, other investigators reported that addition of bosentan to optimal medical therapy improves all hemodynamic variables after 24 h, including CO, with such a trend being maintained at the 2-week follow-up [14]. This discrepancy can be explained by the administration of higher dosages such as 1,000 mg twice daily, which may have enhanced bosentan vasodilation. Such high dosages of bosentan have been shown to be unsafe in long-term studies [6, 14]. Bosentan dosed at 500 mg twice daily was associated with adverse effects, including liver function abnormalities, in almost half of patients with severe HF [6]. The number of dropouts was similar to that in other studies; however, our patients had severe HF, which rendered a third of them intolerant to bosentan therapy [6, 10]. Bosentan therapy did not affect LV diameter and ejection fraction or systolic right ventricular function measured by TAPSE and SPAP in our patients. In contrast, Galiè et al. [10] reported that 16 weeks of bosentan therapy reduced right ventricular dilation and increased left ventricle size, although within a range of normality, due to a decrease in the septal displacement from the right ventricle toward the left ventricle, stroke volume, CI, and right ventricular ejection and LV early diastolic filling characteristics compared with placebo in patients with primary PH. We did not observe such changes, possibly due to the fact that all of our patients had dilated left ventricles and severely depressed ejection fraction, whereas the participants in the study by Galiè et al. [10] had a small left ventricle with preserved LV function. In conclusion, bosentan therapy may improve pulmonary and systemic hemodynamics in patients with PH and systolic HF who are able to tolerate it, thereby making such patients eligible for heart transplantation.
Footnotes
Disclosure Statement
No conflict of interest declared.
References
- 1.Chang PP, Longenecker JC, Wang NY, Baughman KL, Conte JV, Hare JM, Kasper EK. Mild vs severe pulmonary hypertension before heart transplantation: different effects on posttransplantation pulmonary hypertension and mortality. J Heart Lung Transplant. 2005;24:998–1007. doi: 10.1016/j.healun.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 2.Costard-Jäckle A, Fowler MB. Influence of preoperative pulmonary artery pressure on mortality after heart transplantation: testing of potential reversibility of pulmonary hypertension with nitroprusside is useful in defining a high risk group. J Am Coll Cardiol. 1992;19:48–54. doi: 10.1016/0735-1097(92)90050-w. [DOI] [PubMed] [Google Scholar]
- 3.Bourge RC, Naftel DC, Costanzo MR, Kirklin JK, Young JB, Kubo SH, Olivari MT, Kasper EK. Pretransplantation risk factors for death after heart transplantation: a multiinstitutional study. J Heart Lung Transplant. 1993;12:549–562. [PubMed] [Google Scholar]
- 4.van Beneden R, Gurné O, Selvais PL, Ahn SA, Robert AR, Ketelslegers JM, Pouleur HG, Rousseau MF. Superiority of big endothelin-1 and endothelin-1 over natriuretic peptides in predicting survival in severe congestive heart failure: a 7-year follow-up study. J Card Fail. 2004;10:490–495. doi: 10.1016/j.cardfail.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Galiè N, Rubin LJ, Hoeper M, Jansa P, Al-Hiti H, Meyer G, Chiossi E, Kusic-Pajic A, Simonneau G. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomised controlled trial. Lancet. 2008;71:2093–2100. doi: 10.1016/S0140-6736(08)60919-8. [DOI] [PubMed] [Google Scholar]
- 6.Packer M, McMurray J, Massie BM, Caspi A, Charlon V, Cohen-Solal A, Kiowski W, Kostuk W, Krum H, Levine B, Rizzon P, Soler J, Swedberg K, Anderson S, Demets DL. Clinical effects of endothelin receptor antagonism with bosentan in patients with severe chronic heart failure: results of a pilot study. J Card Fail. 2005;11:12–20. doi: 10.1016/j.cardfail.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Perez-Villa F, Cuppoletti A, Rossel V, et al. Initial experience with bosentan therapy in patients considered ineligible for heart transplantation because of severe pulmonary hypertension. Clin Transplant. 2006;20:239–244. doi: 10.1111/j.1399-0012.2005.00475.x. [DOI] [PubMed] [Google Scholar]
- 8.Mehra MR, Kobashigawa J, Starling R, Russell S, Uber PA, Parameshwar J, et al. Listing criteria for heart transplantation: International Society for Heart and Lung Transplantation guidelines for the care of cardiac transplant candidates – 2006. J Heart Lung Transplant. 2006;25:1024–1042. doi: 10.1016/j.healun.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J, Solomon S, Spencer KT, St John Sutton M, Stewart W American Society of Echocardiography’s Nomenclature and Standards Committee, Task Force on Chamber Quantification, American College of Cardiology Echocardiography Committee, American Heart Association, European Association of Echocardiography, European Society of Cardiology: Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 10.Galiè N, Hinderliter AL, Torbicki A, Fourme T, Simonneau G, Pulido T, Espinola-Zavaleta N, Rocchi G, Manes A, Frantz R, Kurzyna M, Nagueh SF, Barst R, Channick R, Dujardin K, Kronenberg A, Leconte I, Rainisio M, Rubin L. Effects of the oral endothelin-receptor antagonist bosentan on echocardiographic and Doppler measures in patients with pulmonary arterial hypertension. J Am Coll Cardiol. 2003;41:1380–1386. doi: 10.1016/s0735-1097(03)00121-9. [DOI] [PubMed] [Google Scholar]
- 11.Daftari B, Alejos JC, Perens G. Initial experience with sildenafil, bosentan, and nitric oxide for pediatric cardiomyopathy patients with elevated pulmonary vascular resistance before and after orthotopic heart transplantation. J Transplant. 2010;2010:656984. doi: 10.1155/2010/656984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez-Villa F, Farrero M, Cardona M, Castel MA, Tatjer I, Penela D, Vallejos I. Bosentan in heart transplantation candidates with severe pulmonary hypertension: efficacy, safety and outcome after transplantation. Clin Transplant. 2013;27:25–31. doi: 10.1111/j.1399-0012.2012.01689.x. [DOI] [PubMed] [Google Scholar]
- 13.Imamura T, Kinugawa K, Hatano M, Kato N, Minatsuki S, Muraoka H, Inaba T, Maki H, Shiga T, Yao A, Kyo S, Ono M, Nagai R. Bosentan improved persistent pulmonary hypertension in a case after implantation of a left ventricular assist device. J Artif Organs. 2013;16:101–104. doi: 10.1007/s10047-012-0662-4. [DOI] [PubMed] [Google Scholar]
- 14.Sütsch G, Kiowski W, Yan XW, Hunziker P, Christen S, Strobel W, Kim JH, Rickenbacher P, Bertel O. Short-term oral endothelin-receptor antagonist therapy in conventionally treated patients with symptomatic severe chronic heart failure. Circulation. 1998;98:2262–2268. doi: 10.1161/01.cir.98.21.2262. [DOI] [PubMed] [Google Scholar]