Abstract
Alzheimer’s disease (AD) is increasingly recognized as a complex neurodegenerative disease beginning decades prior to the cognitive decline. While cognitive deficits remain the cardinal manifestation of AD, metabolic and non-cognitive abnormalities, such as alterations in body weight and neuroendocrine functions are also present, often preceding the cognitive decline. Furthermore, hypothalamic dysfunction can also be a driver of AD pathology. Here we offer a brief appraisal of hypothalamic dysfunction in AD, and provide insight into an underappreciated dual role of the hypothalamus as both a culprit and target of AD pathology, as well as into new opportunities for therapeutic interventions and biomarker development.
Introduction
Alzheimer’s disease (AD), the most common cause of dementia in the elderly, is an incurable and devastating disease that has emerged as one of the major public health threats of our times (Alzheimer's Association, 2015). The pathogenesis of AD remains elusive, but the abnormal accumulation in the brain of amyloid-beta (Aβ), a peptide derived from the amyloid precursor protein (APP), and of the microtubule associated protein tau is believed to lead to the synaptic dysfunction and neurodegeneration underlying the dementia (Musiek and Holtzman, 2015). AD pathology develops decades prior to the initial cognitive symptoms in a preclinical or presymptomatic stage, in which Aβ and tau start to accumulate in brain, as amyloid plaques and neurofibrillary tangles (Sperling et al., 2011). Cerebrovascular function is also impaired in patients with early AD or at risk for AD, leading to a mismatch between the delivery of oxygen and glucose through blood flow and the energy demands of the active brain (Iadecola, 2013). In addition, cerebrovascular dysfunction may impair the vascular clearance of Aβ and promote AD pathology (Gupta and Iadecola, 2015). Therefore, AD is now believed to be a continuum with gradually worsening pathological changes in the brain that are the consequences of Aβ, tau, vascular dysfunctions, and other changes that take several years to decades before manifesting with clinical symptoms. Thus, an early preclinical stage is followed by mild cognitive symptoms (minimal cognitive impairment or MCI) before progressing to symptomatic AD and eventually terminal dementia (Albert et al., 2011; McKhann et al., 2011).
Since cognitive deficits are the most prominent feature of the disease, AD research has placed emphasis on brain regions associated with cognition and memory, such as the hippocampus and entorhinal cortex (Musiek and Holtzman, 2015). However, AD patients exhibit significant non-cognitive deficits such as weight loss, sleep-wake disorders and neuroendocrine alterations attributable to hypothalamic dysfunction (Csernansky et al., 2006; Prinz et al., 1982; White et al., 1996). These alterations can occur prior to the initial mental decline and progressively worsen as the disease advances (Johnson et al., 2006; Ju et al., 2013; White et al., 1996), suggesting that they are an intrinsic feature of AD pathophysiology. Although recent reviews have addressed selected features of AD attributable to hypothalamic dysfunction, such as systemic metabolic deficits or the sleep abnormalities (Kiliaan et al., 2014; Musiek et al., 2015), an appraisal of how AD affects the hypothalamus in light of recent advances in both hypothalamic physiology and AD pathobiology is conspicuously missing from the recent literature. Therefore, in the present Perspective we sought to provide an integrated view of the bidirectional relationships between hypothalamic dysfunction and AD, building a case for the hypothalamus as a contributor and a target of AD pathology and emphasizing its implications for the early diagnosis and treatment of the disease.
Alterations in the structure of the hypothalamus in AD
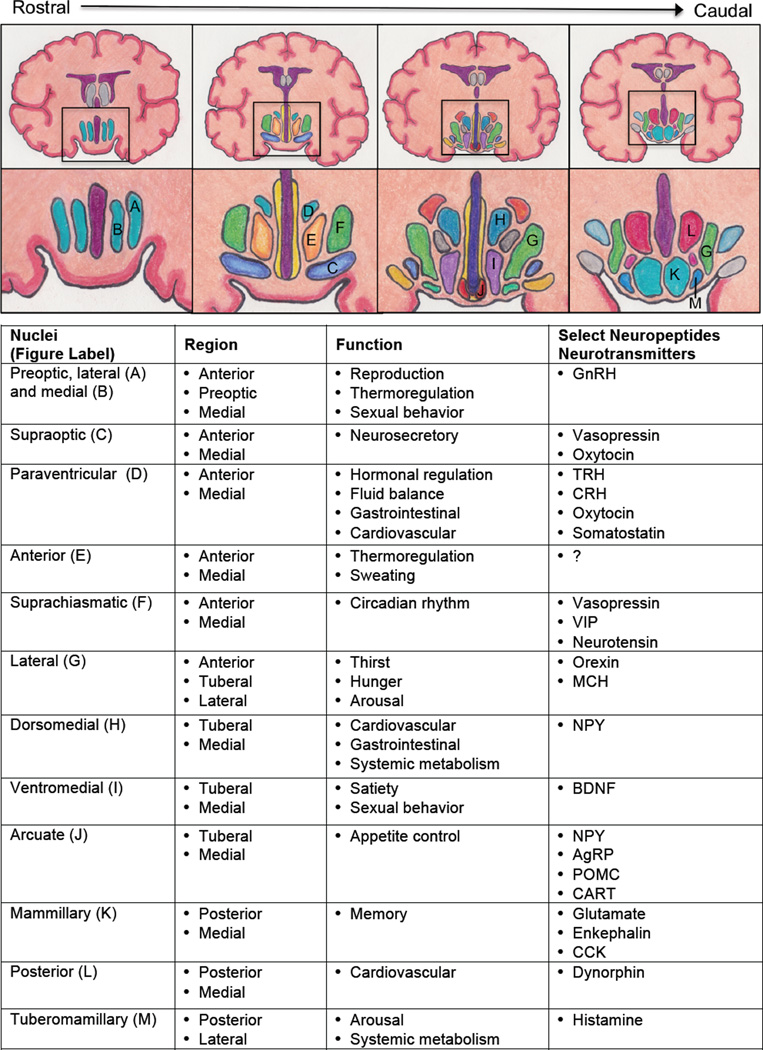
Despite occupying only 4 cm3 of the brain, about the size of an almond, the hypothalamus is the master coordinator of a myriad of homeostatic functions essential for life such as growth, reproduction, sleep, metabolism, and autonomic homeostasis (Swaab, 1997). The hypothalamus is divided into different regions, each having specific nuclei or clustering of neurons with distinct behavioral and/or physiological roles (Figure 1) (Swaab, 1997). In the following sections, we will describe the evidence for alterations in the hypothalamus in AD from classical neuropathological to more recent neuroimaging studies.
Figure 1. Hypothalamic nuclei: structure and function.
Coronal brain images illustrate the hypothalamus and the location of its nuclei (lettered) in order from most rostral (left) to caudal (right) with a description of key functions and neurotransmitters/neuropeptides associated with each hypothalamic nuclei. Abbreviations: AgRP – agouti related peptide; BDNF – brain-derived neurotrophic factor; CART – cocaine and amphetamine related transcript; CRH – corticotropin releasing hormone; GI: gastrointestinal; GnRH – gonadotropin releasing hormone; MCH – melanin concentrating hormone; NPY – neuropeptide Y; POMC – proopiomelanocortin; TRH – thyrotropin releasing hormone; VIP – vasoactive intestinal peptide.
Neuropathology
Several autopsy studies have reported amyloid plaques and neurofibrillary tangles in the hypothalamus of AD subjects (Table 1). One of the earliest studies (Stief, 1927), described a 70 year-old woman with dementia and a striking loss in body weight (from 34.5 kg on presentation to 28 kg at time of death). At autopsy, the body was noted to be completely devoid of fat tissue, and, in the brain, plaques and tangles were found not only in the cortex but also in the paraventricular nucleus of the hypothalamus and mammillary bodies (Stief, 1927). Since then, several other studies have reported plaques and tangles throughout the hypothalamus in patients with AD, including the landmark paper of Braak and Braak on staging of AD pathology (Braak and Braak, 1991) (Table 1). More recently, studies using immunohistochemistry in AD brains confirmed the presence of neuronal loss, amyloid and tau in the hypothalamus (Table 1). These studies also found reductions in various neuropeptides and neurotransmitters, e.g., vasopressin and neurotensin in the hypothalamus (Table 1). Therefore, the hypothalamus is a target of AD pathology like the cortex and hippocampus.
Table 1.
Hypothalamus and Alzheimer’s disease: select pathological studies
| Study Subjects (n) | Pathological Studies |
Hypothalamic Findings | Key References |
|---|---|---|---|
| 70 year-old woman with AD |
|
|
(Stief, 1927) |
| AD (28) |
|
|
(McDuff and Sumi, 1985) |
| AD (4) Controls (28) |
|
|
(Swaab et al., 1985) |
| AD (3) Controls (3) |
|
|
(Saper and German, 1987) |
| AD (17) Down syndrome (4) |
|
|
(Braak and Braak, 1991) |
| AD (5) Down syndrome (7) Controls (4) |
|
|
(Iwatsubo et al., 1996) |
| AD (11) AD with LBD (3) FTD (4) Controls (8) |
|
|
(Harper et al., 2008) |
| AD (12) Controls (12) |
|
|
(Baloyannis et al., 2014) |
Abbreviations: DLB – dementia with Lewy bodies; DMH – dorsomedial nucleus; EM – electron microscopy; NFT – neurofibrillary tangles; LH – lateral hypothalamic area; PVN – paraventricular nucleus; SCN – suprachiasmatic nucleus; SON – supraoptic nucleus; TMN – tuberomammillary nucleus; VIP – vasoactive intenstinal peptide; VMN – ventromedial nucleus.
Neuroimaging
Recent advances in neuroimaging have enabled the investigation of AD pathology in the living brain in a longitudinal fashion that was simply not possible with postmortem analyses. Volumetric analysis using magnetic resonance imaging (MRI) demonstrated that the brain atrophy well known to occur in cortex and hippocampus is also found in the hypothalamus and can be seen in the early stages of AD (Table 2). Perfusion studies using single-photon emission computed tomography (SPECT) showed a non-significant trend towards decreased perfusion in the hypothalamus (Table 2); however, limitations with the study including partial voluming and analysis of an overall small volume of brain may have resulted in the statistically negative result (Callen et al., 2002; 2004). Neuroimaging studies with positron emission tomography (PET) using 18F-fluorodeoxyglucose (18FDG) as a tracer have reported deficits in glucose metabolism in the hypothalamus of MCI or AD subjects (Table 2). Substantial reductions in hypothalamic glucose metabolisms have also been reported in “presymptomatic” transgenic mice overexpressing APP (Tg2576) (Niwa et al., 2002). More recently, PET imaging using amyloid or tau tracers has enabled investigators to study AD pathology in vivo (Fodero-Tavoletti et al., 2011; Klunk et al., 2004). However, to the best of our knowledge, no studies have thus far performed amyloid or tau imaging in the hypothalamus. Complicating factors include the limited spatial resolution of PET and off-target lipophilic binding of amyloid tracers, which may make it difficult to accurately assess a small brain region like the hypothalamus (Johnson et al., 2013; Su et al., 2015).
Table 2.
Hypothalamus and Alzheimer’s disease: select neuroimaging studies
| MRI(volume) | |||
| Study Subjects (n) | Degree of cognitive dysfunction | Findings | Key References |
| Mild AD (19) Controls (16) |
MMSE
|
|
(Baron et al., 2001) |
| AD (40) Controls (40) |
MMSE
|
|
(Callen et al., 2001) |
| Male AD (20) Female AD (20) Male controls (20) Female controls (20) |
MMSE
|
|
(Callen et al., 2004) |
| Cognitive symptoms (20) MCI (20) AD (16) Controls (20) |
MMSE
|
|
(Copenhaver et al., 2006) |
| AD (73) DLB (73) Controls (73) |
MMSE
|
|
(Whitwell et al., 2007) |
| AD (26) Normal to AD (21)Controls (127) |
3MS
|
|
(Hall et al., 2008) |
| Mild AD (63) Controls (55) |
MMSE
|
|
(Loskutova et al., 2010) |
| SPECT (perfusion) | |||
| Study Subjects (n) | Degree of cognitive dysfunction | Findings | Key References |
| AD (40) Controls (18) |
MMSE
|
|
(Callen et al., 2002) |
| Male AD (20) Female AD (20) Male controls (20) Female controls (20) |
MMSE
|
|
(Callen et al., 2004) |
| 18FDG-PET (glucose metabolism) | |||
| Study Subjects (n) | Degree of cognitive dysfunction | Findings | Key References |
| AD (10) MCI (10) Controls (15) |
MMSE
|
|
(Nestor et al., 2003) |
| MCI (12) Controls (23) |
MMSE
|
|
(Cross et al., 2013) |
Abbreviations: MCI – mild cognitive impairment, BMD – bone mineral density; DLB – dementia with Lewy bodies; MMSE – mini mental state examination (scale: 0 to 30 with normal range: 27 to 30); 3MS – modified mini-mental state (scale: 0 to 100 with dementia/cognitive impairment < 79).
Alterations in hypothalamic function in AD
In addition to the structural abnormalities reviewed above, functional studies suggest that hypothalamic dysfunction is a common manifestation of AD, often early in the disease course. While the exact mechanisms underlying the various hypothalamic dysfunction remain to be elucidated, it is likely to include not only direct effects of Aβ and tau on the hypothalamus but also the vascular changes that are commonly seen in AD (Iadecola, 2013), since the release of many of the hormones and factors from the hypothalamus and the downstream pituitary gland are particularly sensitive to changes in the circulation (Fekete and Lechan, 2014; Hrabovszky and Liposits, 2013). In the following sections, we will discuss some of the common hypothalamic abnormalities associated with AD (Table 3) including dysfunction of the classic hypothalamic-pituitary pathways (Figure 2), body weight and systemic metabolism, bone metabolism, sleep-wake/circadian rhythm, and aggression/sundown syndrome and will review selected studies from animal models (Table S1) and AD subjects.
Table 3.
Representative non-cognitive deficits suggesting hypothalamic dysfunction in Alzheimer’s disease
| Physiological parameter |
Abnormality | Hypothalamic nuclei |
Neuropeptides/ neurotransmitters |
Key References |
|---|---|---|---|---|
| HPA – Cortisol |
|
|
|
(Csernansky et al., 2006; Davis et al., 1986; Swanwick et al., 1996) |
| HPT – Thyroid |
|
|
|
(Tan and Vasan, 2009; Yong-Hong et al., 2013) |
| HPG – Estrogen and Testosterone |
|
|
|
(Manly et al., 2000; Moffat et al., 2004; Rodrigues et al., 2008; Short et al., 2001) |
| Body weight |
|
|
|
(Barrett-Connor et al., 1996; Ewers et al., 2012; Johnson et al., 2006; Vidoni et al., 2011; White et al., 1998; Whitmer et al., 2008) |
| Circadian rhythm |
|
|
|
(Harper et al., 2001; 2008; Hu et al., 2013; van Someren et al., 1996) |
| Sleep |
|
|
|
(Ju et al., 2013; Liguori et al., 2014; Lim et al., 2014; Prinz et al., 1982; Schmidt et al., 2013) |
| Bone metabolism |
|
|
|
(Loskutova et al., 2010; Yaffe et al., 1999) |
Abbreviations: HPA – hypothalamic-pituitary-adrenal axis; HPT – hypothalamic-pituitary-thyroid axis; HPG – hypothalamic-pituitary-gonadal axis; αMSH – alpha melanocyte stimulating hormone; AgRP – agouti related peptide; NPY – neuropeptide Y; POMC – proopiomelanocortin; CRH – corticotropin releasing hormone; MCH – melanin concentrating hormone; TRH – thyrotropin releasing hormone; GnRH – gonadotropin releasing hormone; VIP – vasoactive intestinal peptide.
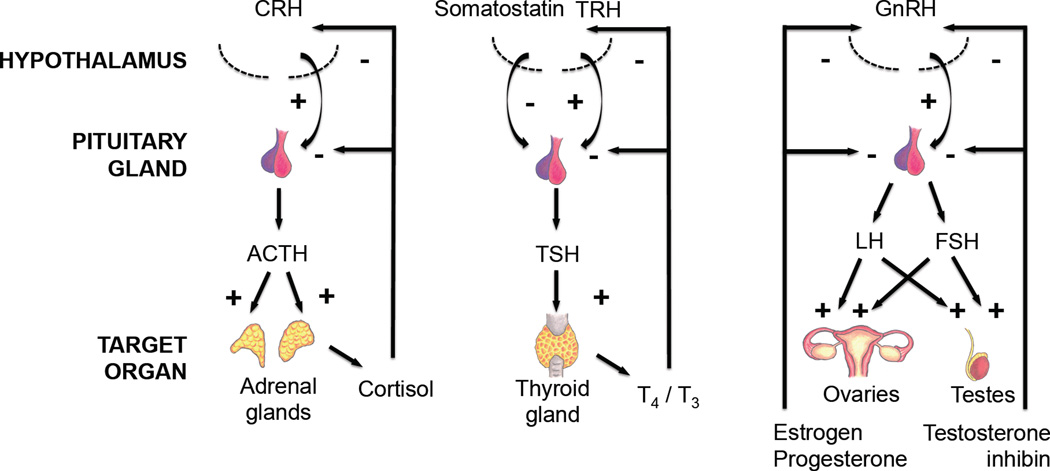
Figure 2. Hypothalamic-pituitary pathways affected in Alzheimer’s disease.
The normal homeostatic regulation of the hypothalamic-pituitary pathways due to stimulatory (+) and inhibitory (−) signals is shown. i.e., for the hypothalamic-pituitary-adrenal pathway, the hypothalamus releases CRH, which stimulates (+) the secretion of ACTH from the pituitary gland. The ACTH then stimulates (+) release of cortisol from the adrenal glands. The cortisol would then send negative feedback signals (−) to both the hypothalamus and pituitary gland to inhibit the any further release of CRH and ACTH respectively. Similar homeostastic regulation occurs with the hypothalamic-pituitary-thyroid and hypothalamic-pituitary-gonadal axes. Abbreviations: ACTH - adrenocorticotroic hormone, CRH - corticotropin-releasing hormone, FSH – follicle-stimulating hormone, LH - luteinizing hormone, TSH - thyroid-stimulating hormone, T3 - triiodothyronine, T4 - thyroxine. Figure was adapted and modified from original (Ishii, 2014).
Hypothalamic-pituitary-adrenal axis
Disruption of the hypothalamic-pituitary-adrenal axis (HPA, Figure 2) has been consistently demonstrated in AD. Increased basal cortisol levels and overall insensitivity to glucocorticoid feedback have been reported for all stages of AD even in early AD (Csernansky et al., 2006; Davis et al., 1986; Greenwald et al., 1986; Rasmuson et al., 2001), and may be associated with a more rapid disease progression (Csernansky et al., 2006; Swanwick et al., 1996). Various transgenic mice with increased brain Aβ deposition, e.g., TgCRND8, Tg2576, exhibited evidence of increased HPA axis activation at an early age (Carroll et al., 2011; Touma et al., 2004). Additionally, acute intracerebroventricular administration of Aβ25–35 peptides in rats was reported to induce memory impairment, anxiety and HPA axis hyperactivity (Brureau et al., 2013), suggesting that HPA axis dysfunction is an early consequence of the amyloid presence in the brain.
A major regulator of the HPA axis is the paraventricular nucleus of the hypothalamus through the release of the neuropeptide corticotropin-releasing hormone (CRH), which in turn stimulates pituitary secretion of ACTH and subsequently adrenal secretion of glucocorticoids (Figure 2). CRH in the CSF has been found to be increased (Banki et al., 1992), decreased (May et al., 1987), or unchanged (Pomara et al., 1989) in AD subjects. Relatively small postmortem studies of AD brains have also yielded mixed results (Bissette et al., 1985; Nemeroff et al., 1989; Powers et al., 1987; Raadsheer et al., 1995), calling for new investigations that are appropriately powered. However, in a transgenic mouse model of AD chronic stress can induce tau pathology, neurodegeneration, and learning impairments, which can be blocked by CRH receptor 1 antagonists and enhanced by CRH overexpression (Carroll et al., 2011). These data suggest that the increases in CRH and the HPA axis could contribute to brain dysfunction in AD, but supportive evidence in humans is still missing.
Hypothalamic-pituitary-thyroid axis
Although both hypothyroidism or hyperthyroidism have long been associated with cognitive impairment and increased risk of AD (Tan and Vasan, 2009), the link between the hypothalamic-pituitary-thyroid (HPT) axis and AD remains not well understood. With an intact HPT axis, low circulating thyroid hormones stimulate production of thyrotropin releasing hormone (TRH) from the hypothalamus and thyroid stimulating hormone (TSH) from the pituitary to increase thyroid hormone production (Figure 2). Studies measuring TRH levels in the CSF have reported either increases (Banki et al., 1992) or decreases (Oram et al., 1981) in AD subjects, while a postmortem study found no differences in the hypothalamus and extrahypothalamic areas (Yates et al., 1983). Similarly, studies in which TSH and thyroid hormones were measured reported increases, decreases, or no differences in AD (Ganguli et al., 1996; Johansson et al., 2013; Parsaik et al., 2014; Quinlan et al., 2010; Tan et al., 2008; van Osch et al., 2004). More appropriately, one study evaluated the function of the entire HPT axis by measuring serum TRH, TSH, and thyroid hormones in AD subjects (Yong-Hong et al., 2013) and found levels lower than controls. Since no TRH or TSH increases were seen, the findings suggest a dysfunction at the hypothalamic and/or pituitary levels resulting in a hypothyroid state. Based on these results, AD pathology could lead to neuronal dysfunction in the hypothalamus causing a decrease in TRH, or insensitivity to low thyroid levels, which, in turn, would decrease TSH secretion from the pituitary, and ultimately reduce thyroid hormone causing hypothyroidism. On the other hand, the hypothyroid state could exacerbate AD pathology (Tan and Vasan, 2009). There is evidence that TRH and thyroid hormones are neuroprotective against AD pathology. For example, TRH administration to hippocampal neurons resulted in a decrease in GSK-3β and tau phosphorylation, while TRH knockdown increases both (Luo and Stopa, 2004). Analogs of TRH were neuroprotective in glutamate and Aβ toxicity in neuronal cultures (Faden et al., 2005). Administration of L-thyroxine in mice in which Aβ was microinjected in the hippocampus prevented the resulting cognitive and memory impairment (Fu et al., 2010). Therefore, HPT dysfunction could be both a cause and a consequence of AD pathology.
Hypothalamic-pituitary-gonadal axis
The natural aging process results in alterations of the hypothalamic-pituitary-gonadal (HPG) axis (Figure 2) leading to a dramatic decline in estrogen in women and a gradual decline in testosterone in men (Vest and Pike, 2013). It has been hypothesized that these alterations of the HPG axis, particularly low estrogen in postmenopausal women, could precipitate AD or contribute to its progression (Vest and Pike, 2013). Both low estrogen in women (Manly et al., 2000) and low testosterone in men have been consistently associated with poor cognitive performance and increased risk of AD (Moffat et al., 2004; Yaffe et al., 2002). Conversely, several studies have supported a role of testosterone and estrogen in protecting against neurodegeneration and cognitive decline by improving synaptic function, preventing neuronal death, and inhibiting Aβ accumulation and/or tau hyperphosphorylation (Vest and Pike, 2013). Unfortunately, testosterone or estrogen supplementation trials have not demonstrated consistent improvements in cognitive function or delay in AD progression (Emmelot-Vonk et al., 2008; Espeland et al., 2013; Lu et al., 2006; Shumaker et al., 2003). One possible explanation for the failure particularly of estrogen replacement in early trials is that the estrogen treatment began too late. It has been suggested that there is a “critical period” or “window of opportunity," where estrogen replacement would have to occur in close temporal proximity to menopause to be beneficial (McCarrey and Resnick, 2015). Recently, two large clinical trials tested this “critical period” hypothesis, but these studies found that hormonal replacement neither harmed nor provided any benefit to cognition in women with early menopause (Espeland et al., 2013; Gleason et al., 2015). Additionally, the enthusiasm for testosterone or estrogen supplementation has waned as the potential harmful consequences of long term sex hormone therapy in older men and women have become increasingly recognized (Rossouw et al., 2002; Vigen et al., 2013).
While age-related changes in the HPG axis have focused on the sharp decline in estrogen and testosterone, loss of the hypothalamic negative feedback from the sex hormones has also been implicated in AD (Rossmanith et al., 1994). Furthermore, hypothalamic dysfunction leading to gonadotropin releasing factor (GnRH) signaling deficits could also play a role. Several reports have demonstrated cognitive improvement with modulation of GnRH signaling in animal models and AD subjects (Bowen et al., 2015; Zhang et al., 2013). In one study, CSF GnRH levels were found to be significantly reduced in AD (Oram et al., 1981). However, a study in transgenic mice with amyloid pathology found dramatic increases in GnRH expression in the hippocampus, which were attenuated by Lupron, a GnRH superagonist that downregulates GnRH receptors (Nuruddin et al., 2014). Unfortunately, the GnRH levels in the hypothalamus were not reported in this study. Therefore, it remains unclear if dysfunction of the feedback loop in the HPG axis contributes to AD pathophysiology.
A recent study in mice found that aging causes hypothalamic NF-κβ-driven inflammation leading to decrease in GnRH signaling and cognitive dysfunction, which was rescued by GnRH replacement therapy (Zhang et al., 2013). This important study highlights a potential role of reduced hypothalamic GnRH in aging and possibly AD, but the downstream effectors of its beneficial actions remain unclear. For example, luteinizing hormone (LH), a gonadotropin secreted by the pituitary in response to GnRH, may exacerbate AD pathology. Thus, several studies have suggested an association between increased LH levels and cognitive decline and AD (Rodrigues et al., 2008; Short et al., 2001). In animal studies, exogenous LH potentiates both amyloidosis and cognitive impairment (Berry et al., 2008; Bowen et al., 2004), while LH receptor deficiency in transgenic mice overexpressing APP significantly decreased amyloid load and tau hyperphosphorylation (Lin et al., 2010). Recently, a Phase 2 clinical trial found that high doses of Lupron, which decreases LH, in combination with an acetylcholinesterase inhibitor stabilized the cognitive decline in mild to moderate AD (Bowen et al., 2015). Thus, modulation of the HPG axis and in particular GnRH signaling are potential therapeutic targets in AD, but a better appreciations of the downstream effector mechanisms is needed, e.g., LH, since they may be harmful.
Body weight and systemic metabolism
Weight loss is commonly seen in AD patients and correlates with disease severity and mortality (White et al., 1996; 1998). Furthermore, low body mass index (BMI) has been associated with worsening AD pathology in postmortem brains (Buchman et al., 2006; Vidoni et al., 2011) and worsening CSF biomarkers (tau and Aβ1–42) in vivo (Ewers et al., 2012). Importantly, there is substantial evidence that late life weight loss or low body weight can be seen prior to the cognitive decline (Barrett-Connor et al., 1996; Buchman et al., 2005; Gao et al., 2011; Johnson et al., 2006; Stewart et al., 2005). Furthermore, once cognitive impairment or dementia develops, low body weight or a further decrease in body weight is associated with worsening morbidity and mortality, while increased body weight may be protective (White et al., 1998). In contrast to late life weight loss, mid-life weight gain and obesity are associated with an increased risk of AD (Whitmer et al., 2005) independent of other cardiovascular risk factors (Whitmer et al., 2008; Xu et al., 2011). However, a recent large UK population study found that mid-life obesity decreases AD risk, while low body weight in mid-life is associated with an increased risk (Qizilbash et al., 2015), suggesting a complex interaction between body weight and AD. Clearly, a better understanding of the pathways by which systemic metabolism and body weight can influence AD risk is needed (Kiliaan et al., 2014). However, that late life weight loss can portend AD has been consistently reported in several different study populations (Barrett-Connor et al., 1996; Buchman et al., 2005; Gao et al., 2011; Johnson et al., 2006; Stewart et al., 2005) making it likely to be an early manifestation of AD linked to the pathophysiology of the disease.
The exact mechanisms underlying the body weight loss in AD are not known. Owing to its major role as integrator of peripheral metabolic signals, e.g., glucose, insulin, leptin, etc., and as regulator of body weight and systemic metabolism (Williams and Elmquist, 2012), the hypothalamus is likely to be a major driver of the weight loss in AD. Increasing evidence suggests that leptin, an adipocyte-derived hormone that acts on the hypothalamus as a negative afferent signal to maintain body weight and energy homeostasis (Friedman and Mantzoros, 2015), is involved in AD. Low plasma leptin levels have been associated with increased risk of AD and cognitive decline (Holden et al., 2009; Lieb et al., 2009). Similar to the early weight loss seen in AD, transgenic mice overexpressing APP (Tg2576) exhibited low body weight, due to reduced fat mass, and low plasma leptin levels prior to amyloid plaque formation and cognitive deficits (Ishii et al., 2014). In hypothalamic slices from Tg2576 and or wild type slices exposed to Aβ1–42, the electrophysiological responses to leptin were abnormal in leptin-responsive NPY-expressing arcuate neurons, suggesting that Aβ-induced hypothalamic dysfunction may underlie the early body weight and systemic metabolic deficits (Ishii et al., 2014). Conversely, other studies found that leptin supplementation ameliorates, while leptin receptor deficiency exacerbates, the cognitive deficits and amyloid pathology in transgenic mice (Greco et al., 2010; Pérez-González et al., 2014; Takeda et al., 2010). Collectively, these studies suggest that early in AD, Aβ1–42 can potentially cause hypothalamic dysfunction in cells critical for leptin signaling resulting in low body weight and low adiposity. The hypothalamic dysfunction caused by Aβ1–42 could lead to an inability to sense the low plasma leptin levels resulting from low adiposity. This lack of response to the normal feedback signals would lead to the persistence of the low body weight and pathologically low leptin state. The low leptin levels may also contribute to cognitive deficits in AD, because leptin has beneficial effects on cognition by improving synaptic and hippocampal function, decreasing Aβ load, and reducing tau hyperphosphorylation in cell and animal models (Doherty et al., 2013; Greco et al., 2010). Beside leptin, other peripheral hormones involved in regulating systemic metabolism and body weight, e.g., adiponectin, ghrelin, and insulin (see below) have their main site of action in the hypothalamus and as such may also play a role (Kiliaan et al., 2014; Moon et al., 2011). Alternatively, since olfactory and taste deficits are common in AD (Doty et al., 1987; Velayudhan, 2015), the weight loss could be due to decreased appetite secondary to these deficits. However, at least in mouse models, the early body weight deficits were found to be from increased metabolism not reduced food intake (Ishii et al., 2014). Longitudinal study in humans investigating how the olfactory/taste changes in AD affects appetite and body weight, are clearly needed to evaluate the contribution of changes in olfaction and taste to the weight loss in AD.
In addition to changes in body weight, recent epidemiological studies have found that diabetes, particularly in mid-life, is associated with an increased risk of developing AD (Biessels et al., 2006; Kim and Feldman, 2015; Ott et al., 1999). There is also accumulating evidence to suggest that insulin resistance in the brain may have a significant role in AD pathobiology with some labeling sporadic AD as “type 3 diabetes” (la Monte, 2014). Insulin receptors are widely expressed in the brain including the olfactory bulb, cortex, hippocampus, amygdala and the hypothalamus (Ghasemi et al., 2013; Kim and Feldman, 2015; Unger et al., 1991), and insulin signaling has important roles in the regulation of body weight and systemic metabolism, reproduction, cognition and memory (Ghasemi et al., 2013). Importantly, there is significant evidence that insulin regulates Aβ and tau, while Aβ can disrupt insulin signaling (Kim and Feldman, 2015; la Monte, 2014). Based on these observations, insulin signaling has been proposed as a therapeutic target of AD, and treatment with insulin has been demonstrated to have beneficial effects on AD pathology and cognition in animal and human studies (Claxton et al., 2015; Kim and Feldman, 2015; Vandal et al., 2014). Due to the widespread expression of insulin receptors in the hypothalamus and the critical importance of the hypothalamus in brain insulin signaling, the hypothalamus is well-positioned to serve as the critical link between diabetes/impaired insulin signaling and AD.
Diet or nutritional intake can also significantly impact AD. High-fat diets, particularly those high in saturated fatty acids which are often associated with obesity and insulin-resistant diabetes, have been associated with increased AD risk and exacerbation of AD pathology in several animal and human studies (Julien et al., 2010; Morris and Tangney, 2014; Refolo et al., 2000). As high-fat diets, mid-life obesity, and diabetes have all been associated with an increased risk of developing AD, it is perhaps not surprising that caloric restriction particularly earlier in life has been shown to be protective against AD pathology and aging-related disorders in various animal models (Halagappa et al., 2007; Mattson, 2012; Patel et al., 2005). For example, reducing caloric intake to 60% of ad libitum fed mice in AD mouse models (APP+PS1 and J20) significantly decreased the accumulation of amyloid plaques and astrocytic inflammation (Patel et al., 2005). Similarly, reduction of caloric intake in the 3xTg triple-transgenic mouse model of AD significantly improved the performance in the water maze and had lower levels of Aβ1–42, Aβ1–40, and phospho-tau in the hippocampus (Halagappa et al., 2007). Interestingly, intermittent fasting by depriving food for 24 hours every other day in the 3xTg mice improved the performance of the mice in the water maze, but did not alter levels of Aβ1–42, Aβ1–40, and phospho-tau, suggesting that the beneficial effects of intermittent fasting may operate by a different mechanism than chronically reducing caloric intake (Halagappa et al., 2007). In addition to mouse models with increased amyloid deposition, caloric restriction in a mouse model of increased tau deposition (Tg4510) performed significantly better in the novel object recognition test and contextual fear conditioning compared to ad libitum fed mice; however, tau and phospho-tau levels were unchanged as were astrocyte and microglia activation markers (Brownlow et al., 2014). Collectively, these and other studies have consistently shown that caloric restriction can reduce AD-like pathology and improve cognitive behavior; however, the mechanisms underlying this beneficial effect are not known, but may include reducing cellular stress, altering systemic metabolism, and changing hormonal balance e.g., increased insulin sensitivity (Mattson, 2012). It has been proposed that the hypothalamus has a vital role in the benefits seen with caloric restriction (Dacks et al., 2013). Evidence to support a hypothalamic role include the loss of caloric restriction-mediated anti-tumor effects in NPY knockout mice and mice with lesions of the hypothalamic arcuate nuclei by monosodium glutamate, suggesting the importance of hypothalamic pathways that regulate appetite and systemic metabolism in mediating the beneficial effects of caloric restriction (Minor et al., 2011). Caloric restriction also upregulates the mammalian nicotinamide adenine dinucleotide (NAD)-dependent deacetylase SIRT1, a key mediator of metabolism and life span, in the dorsomedial and lateral hypothalamus, where it is hypothesized to play an important role in regulating aging and longevity (Satoh et al., 2010; 2013). The exact mechanisms underlying the beneficial effects of caloric restriction still remain to be elucidated, but the current evidence strongly suggests that the hypothalamus is likely to play a significant role.
Bone metabolism
Bone loss as measured by bone mineral density has been associated with both age-associated cognitive decline and increased risk of developing AD (Tan et al., 2005; Yaffe et al., 1999). Decreased bone density has also been found in the early stages of AD, and this decline was associated with brain atrophy and cognitive decline (Loskutova et al., 2009). A hypothalamic involvement has been suggested as the lower whole body bone mineral density found in early AD was positively associated with low grey matter volume of the hypothalamus (Loskutova et al., 2010). Therefore, hypothalamic regulation of bone metabolism is emerging as another key early feature of AD. The mechanisms underlying the bone metabolism deficits in AD are unclear but may involve the hypothalamic-gonadal pathways, leptin, NPY or other factors implicated in the hypothalamic regulation of bone metabolism (Sharan and Yadav, 2014).
Disruption of circadian rhythm
Circadian rhythm disorders are commonly reported in AD (Musiek et al., 2015). Although the underlying mechanisms remain to be elucidated, the suprachiasmatic nucleus (SCN) of the hypothalamus has long been recognized as the main biological regulator of circadian rhythms (Saper, 2013) and, as reviewed below, is likely to be involved in AD.
Early studies using rest-activity monitors found marked disruption of the normal circadian rest-activity rhythm in AD patients, which correlated with disease severity (van Someren et al., 1996). In addition, body temperature rhythms are also abnormal in AD (Harper et al., 2001). A prospective cohort study using wrist actigraphy found that a decreased circadian activity rhythm amplitude and delayed rhythms were associated with an increased risk of MCI and dementia (Tranah et al., 2011). In addition, detailed analysis of circadian motor activity in subjects with mild AD found that scale-invariance/fractal patterns of locomotor activity fluctuations, i.e., the temporal characteristics and properties of fluctuations that remain similar over time, were reduced in AD (Hu et al., 2009). The same group also found that the degree of scale-invariance or fractal activity disruption was strongly associated with the reduction of two major circadian neurotransmitters vasopressin and neurotensin in the SCN in postmortem AD brains (Hu et al., 2013), confirming previous results by others (Harper et al., 2008; Stopa et al., 1999). In addition, the amyloid plaque burden in SCN was significantly associated with decreasing density of neurotensin-expressing neurons (Hu et al., 2013). In contrast, another study found no changes in SCN vasopressin levels in AD brains (Wang et al., 2015). Irrespective of the neurotransmitters involved, these findings implicate the SCN in the circadian rhythm disruption in AD (Table 1).
As in humans, abnormal circadian rhythm patterns, i.e., increased daytime and decreased nocturnal activity levels, have been found in TgCRND8 mice (Ambrée et al., 2006) and exaggerated amplitude and alterations in core body temperature rhythms in 3xTg mice (Knight et al., 2013). However, there have been inconsistencies between animal models, which are likely due to the differences in the strains, genetic makeup, and testing conditions used in these studies (Coogan et al., 2013). Nevertheless, the functional and neuropathological studies in humans with AD have provided strong evidence that circadian rhythm abnormalities are commonly present in AD and linked to SCN dysfunction.
Sleep-wake cycle regulation
Closely related to the alterations in circadian rhythm are sleep disorders such as fragmented sleep and reduced sleep, which have been described at all stages of AD (Musiek et al., 2015; Prinz et al., 1982). EEG studies of the sleep architecture of AD subjects have found decreased rapid eye movement (REM) and slow wave (delta) sleep (Prinz et al., 1982). In a cross-sectional study of cognitively-intact community-dwelling older adults, self-reported shorter sleep duration and poorer sleep quality were associated with greater Aβ burden as measured by amyloid imaging (Spira et al., 2013). A similar longitudinal study found that self-reported reduced sleep was associated with an increased risk of developing dementia and AD (Hahn et al., 2014). Finally, a cross-sectional analysis of cognitively-intact normal individuals found that those who had CSF Aβ1–42 levels consistent with preclinical AD had worse sleep quality, compared to cognitively-intact CSF negative controls (Ju et al., 2013). In agreement with human studies, transgenic mice with increased amyloid deposition exhibited sleep disturbances including decreased total sleep time with fragmented sleep, decreased non-REM sleep, and altered nocturnal activity (Roh et al., 2012; Sethi et al., 2015; Wisor et al., 2005). Collectively, these studies highlight that sleep disturbances can occur prior to the cognitive decline in AD.
Recently, it was hypothesized that sleep disturbances could actually increase Aβ deposition and thereby potentiate AD progression. Kang et al., used in vivo cerebral microdialysis to measure Aβ levels from brain interstitial fluid (ISF) in awake, freely moving mice and found that ISF Aβ levels have a significant diurnal pattern with highest levels found during the dark (awake) phase (Kang et al., 2009). Studies of Aβ dynamics in humans found a similar diurnal pattern in CSF Aβ levels (Huang et al., 2012). Furthermore, sleep deprivation worsened Aβ pathology in transgenic mouse models, while administering an orexin antagonist to increase sleep reduced amyloid plaque burden (Kang et al., 2009). These studies established that Aβ levels are tightly associated with the sleep-wake cycle, but the exact mechanisms involved are not known. One possibility is that increased neuronal activity during the awake state increases Aβ levels and drive Aβ aggregation (Cirrito et al., 2005). Alternatively, sleep may be important in clearing Aβ from the brain, since the clearance of exogenously injected Aβ in the mouse brain was dependent on sleep (Xie et al., 2013). Collectively, these studies raise the intriguing possibility that the sleep disorders caused by the AD pathology in the early stages of the disease may potentiate amyloid deposition and accelerate AD progression.
Although various brain areas and neurotransmitters/neuropeptides are involved in the sleep-wake cycle, the hypothalamic neuropeptide orexin (or hypocretin) plays a major role (Sakurai, 2013). APP/PS1 transgenic mice with the orexin gene knocked-out have marked decreases in Aβ pathology in the brain, while sleep deprivation or increasing wakefulness increased Aβ pathology (Roh et al., 2014). Therefore, alterations in hypothalamic orexin signaling can modulate Aβ pathology presumably by affecting sleep-wake cycles. However, recent studies in small cohorts of AD subjects gave conflicting results. A postmortem study found lower orexin levels in the CSF and hypothalamus in AD (Fronczek et al., 2012), while other studies found increased or no significant changes (Liguori et al., 2014; Schmidt et al., 2013). Interestingly, a recent case-control study investigating genetic variants in the orexin or orexin receptor genes found that a polymorphism in the orexin receptor 2 gene increased the risk for AD (OR 2.53, 95%CI 1.10–5.80), suggesting that genetic variations in orexin signaling could contribute to AD susceptibility either through deficits in sleep regulation or another currently unknown mechanism (Gallone et al., 2014). One study interestingly found no significant difference in CSF orexin levels between AD and healthy control subjects, but CSF levels of melanin-concentrating hormone (MCH) were significantly elevated in AD subjects and correlated with CSF tau levels and severity of cognitive impairment (Schmidt et al., 2013). This is of particular interest because MCH is synthesized in the lateral hypothalamic area and may be a key regulator of sleep-wake states (Jego et al., 2013; Tsunematsu et al., 2014). In addition to orexin and MCH, galanin-expressing neurons are reduced in the intermediate nucleus or ventrolateral preoptic area of AD brains, a lower number of neurons correlating with worsening sleep fragmentation in both demented and non-demented older individuals (Lim et al., 2014). Therefore, loss of this neuropeptide may also be a factor in the sleep dysfunction. Finally, several studies have found elevated levels of CSF epinephrine and norepinephrine in AD, which may lead to increased arousal and agitation that is commonly seen in AD (Elrod et al., 1997; Peskind et al., 1998; Raskind et al., 1999).
Agitation/aggressive behaviors and sundown syndrome
Agitation and aggressive behaviors are common manifestations in AD affecting approximately 20% of those living in the community and 40–60% of those living in institutional care settings (Ballard and Corbett, 2013). They often lead to significant challenges in caretaking and are commonly a major factor in the decision to move a person with AD to an institutional care setting. While the exact etiology underlying the aggressive and agitation behaviors in AD are not known, they are likely to involve the hypothalamus. Since the early 20th century when electrical stimulation of the hypothalamus in cats were demonstrated to elicit aggressive behaviors, the hypothalamus has been long recognized as an integral brain region regulating aggressive behavior (Haller, 2013; Hess and Akert, 1955). Subsequent studies have found that the hypothalamic stimulation results in aggressive behaviors in nearly all animal species studied and have identified “hypothalamic attack areas” or specific regions of the hypothalamus including the lateral hypothalamic area and ventromedial hypothalamic nucleus that when stimulated leads to different aggressive behaviors (Haller, 2013). Furthermore, while there is supporting data in animal studies to suggest that serotonin, vasopressin, and other neurotransmitters such as norepinephrine and Substance P may have an etiological role in agitation and aggressive behaviors, human studies particularly in AD are unfortunately scarce (Haller, 2013).
Sundown syndrome or “sundowning” is a particular form of agitation found commonly in demented individuals where there is emergence or increase in neuropsychiatric symptoms such as agitation, confusion, anxiety, and aggression in the late afternoon, evening or at night (Khachiyants et al., 2011). The overall reported rate of sundowning among demented patients ranges from as low as 2.4% to as high as 66% depending on the study (Khachiyants et al., 2011). As sundowning has features of circadian, sleep, and agitation behavior disorders, the underlying neurobiological mechanism has been hypothesized to be due to dysfunction of the hypothalamus particularly the SCN (Bedrosian and Nelson, 2013; Khachiyants et al., 2011). Thus, the sundowning seen in AD patients is likely a reflection of the neurodegeneration and pathological changes commonly seen in the SCN in AD (Table 2). In addition, one recent report found that AD subjects with sundowning had higher salivary cortisol levels than AD subjects without sundowning or healthy controls, suggesting that chronic exposure to high levels of glucocorticoids due to HPA axis dysregulation could play a role (Venturelli et al., 2013). Unfortunately, current clinical management strategies commonly rely on medications such as anti-psychotics, which may have detrimental consequences, and non-pharmacological treatments, which may not be effective in severe cases (Ballard and Corbett, 2013), and a better mechanistic understanding of these disturbances would help with the development of more rational management strategies.
Bench-to-bedside: challenges and opportunities
The evidence summarized in the previous sections indicates that hypothalamic dysfunction plays an important role in AD, and could ultimately lead to the identification of novel biomarkers and therapeutic targets. However, there are several issues that remain to be addressed.
There is controversy about the exact nature of the neuroendocrine dysfunction in AD. For example, although the age-related decline in estrogen and testosterone are consistently associated with cognitive decline and AD (Manly et al., 2000; Moffat et al., 2004; Yaffe et al., 2002), the role of the HPG axis, GnRH signaling in particular, remains controversial. This is not surprising since most human studies on neuroendocrine dysfunction in AD were underpowered and did not explore the entire hypothalamic endocrine axis, but only selected hormones. Another drawback of these studies is that the diagnosis for AD often relied exclusively on clinical criteria, which led to the possibility of misdiagnosing and misclassification particularly in subjects with milder symptoms (Sutphen et al., 2014). Appropriately powered studies using neuroimaging and/or CSF biomarkers for AD diagnosis should help address these problems. Studies in animals are also problematic, because there is no single animal model that recapitulates the full spectrum of pathological abnormalities seen in AD (Webster et al., 2014). Therefore, key findings in one model need to be verified in other models and ultimately in humans.
An important open question concerns whether the hypothalamic dysfunction in AD actually contributes to AD pathogenesis or is simply a consequence of the underlying disease process. The answer may be that it is both. One possibility is that, similar to the hippocampus and cortex, abnormal accumulation of Aβ and/or tau in the hypothalamus disrupts normal hypothalamic function leading to impairment in several downstream signals that are important for maintaining vital physiological processes, e.g., body weight regulation and neuroendocrine function (Figure 3). In this case the hypothalamic dysfunction would be a consequence of the underlying disease process of AD and manifests itself based on the hypothalamic pathways affected, e.g., neuroendocrine systems, body weight and systemic metabolism, circadian/sleep cycles, and bone metabolism. Another possibility is that hypothalamic dysfunction contributes to the pathogenesis of AD. Many of the hypothalamic signaling pathways that are affected in AD, such as thyroid hormones, sex hormones, leptin signaling, etc., have been shown to improve synaptic function, protect against neuronal death, and inhibit Aβ accumulation and/or tau hyperphosphorylation (Fu et al., 2010; Greco et al., 2010; Tan and Vasan, 2009; Vest and Pike, 2013). Therefore, we propose that hypothalamic dysfunction could be both a target of AD pathology and a contributor to its underlying causes. Whereas the initial AD pathology may lead to hypothalamic dysfunction and its numerous disparate manifestations, the hypothalamic dysfunction, in turn, suppresses neuroendocrine hormones and brain signaling mechanisms that are important for maintaining cognitive function, and for protection against AD pathology (Figure 3). This model highlighting the importance of hypothalamic dysfunction in AD can therefore explain not only the progressive nature of the metabolic and non-cognitive manifestations seen in AD, but also suggests that the disruption of the underlying hypothalamic pathways leading to the metabolic and non-cognitive manifestations are important etiological factors of AD.
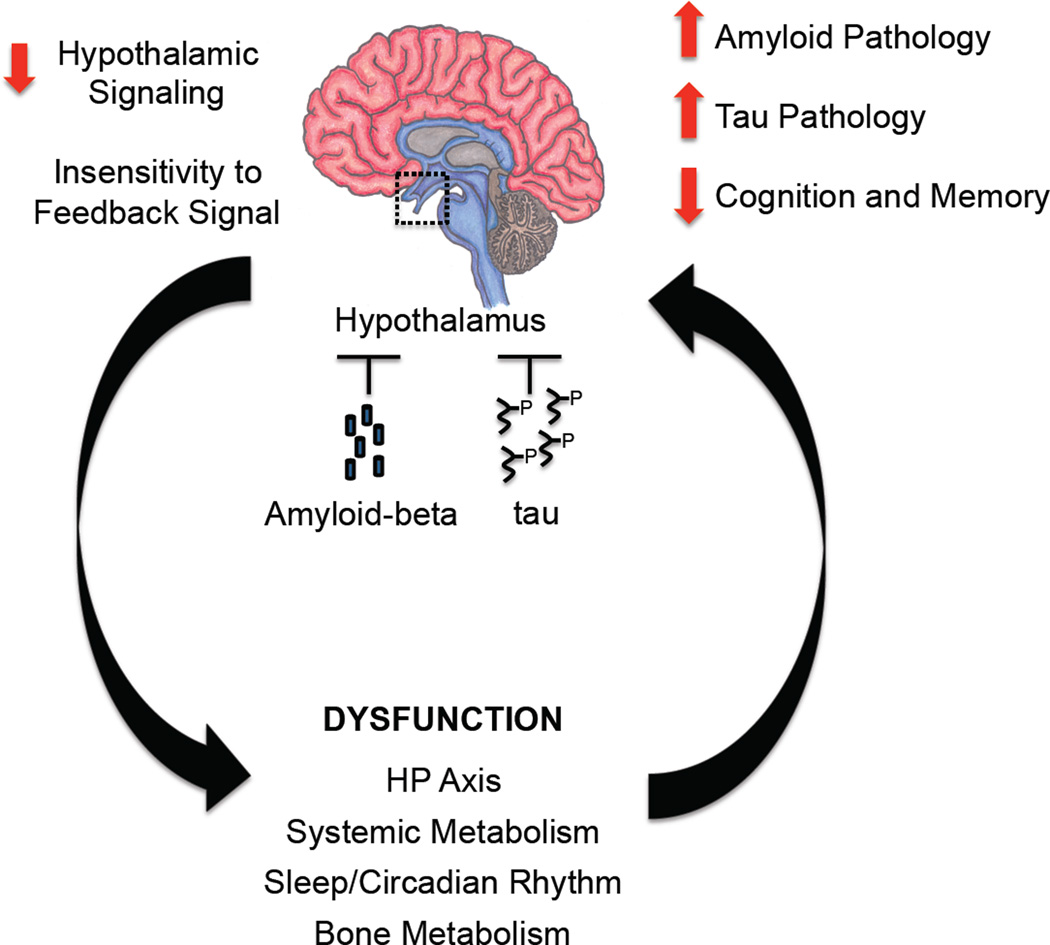
Figure 3. The hypothalamus: target and culprit of AD pathology?
Proposed mechanism depicting how abnormal accumulation of amyloid-beta and tau in the hypothalamus can result in decreased hypothalamic signaling and insensitivity to the normal hormonal feedback signals. Reduced hypothalamic signaling in turn leads to alterations in critical physiological functions including neuroendocrine axis, systemic metabolism, and sleep/circadian rhythm, which can potentially contribute to worsening amyloid and tau pathology and cognitive/mental decline.
The dual significance of hypothalamic dysfunction in AD has important implications both for identifying additional biomarkers and for developing new therapies. As an early manifestation of AD, hypothalamic dysfunction could be an attractive area to improve AD diagnosis. First, hypothalamic deficits are often seen in the preclinical stages of AD. This could enable earlier diagnosis of AD and thereby increase the potential options for possible therapeutic intervention. Second, while neuroimaging and CSF biomarkers are rapidly increasing in sensitivity and specificity, these modalities are expensive, somewhat invasive, or simply not widely available. Therefore, examination of hypothalamic function by EEG sleep studies, or plasma neuroendocrine markers, may be a desirable alternative or an adjunct to other approaches for AD diagnosis. Finally, biomarkers based on hypothalamic function could potentially improve therapeutic trials in preclinical AD. Current trials rely on cognitive endpoints to assess therapeutic benefit, which are disrupted relatively later in AD (Kryscio, 2014; Sperling et al., 2011). Since the hypothalamic dysfunction seen in AD, such as body weight loss and sleep/circadian rhythm disturbances, occur early in the disease and worsen with disease progression, assessing hypothalamic function could provide additional information regarding the effectiveness of the drug early in the course of the trial.
If hypothalamic dysfunction promotes AD pathogenesis, then restoration of hypothalamic function should lead to improvements not only in the specific hypothalamic disorder but also in cognitive and memory deficits. Perhaps a striking example of how modulating hypothalamic function could help in AD was serendipitously provided by deep brain stimulation (DBS) studies of the fornix/lateral hypothalamus in humans for treatment of obesity. It was soon appreciated that the stimulation could evoke autobiographic memories by driving activity in mesial temporal lobe structures (Hamani et al., 2008). A phase I trial further supported that DBS of the hypothalamus could improve or at least slow down the cognitive decline in AD subjects without any significant adverse events (Laxton et al., 2010). These preliminary studies provide an important proof of concept that modulation of hypothalamic function could not only improve any neuroendocrine and non-cognitive deficits, but may also have direct therapeutic benefits on cognitive function and memory in AD. Future clinical studies are needed to determine if targeting select hypothalamic pathways affected in AD (e.g., HPG axis, sleep pathways, leptin etc.), by itself or in a combinatorial fashion, leads to similar results.
Conclusion
In this Perspective, we briefly reviewed several decades of AD research to evaluate the evidence for hypothalamic dysfunction in AD. These studies span from early pathological studies identifying the characteristic plaques and tangles in the hypothalamus of postmortem AD brains, to more recent investigations using genetic mouse models to elucidate the specific hypothalamic cell populations affected by amyloid toxicity. Although evidence pointing to hypothalamic dysfunction has long been provided, there has been a relative lack of attention to the hypothalamus in AD research. While the cognitive deficits are the cardinal manifestations of AD, it has become clear that metabolic and non-cognitive deficits are an integral part of the disease as well, and may contribute to its pathogenesis. Focusing on the hypothalamus has allowed us to tie together seemingly disparate manifestations of AD to a single brain region that has been demonstrated to be a pathological target of AD. Thus, exploring further the role of the hypothalamus in AD, may provide new directions in biomarker research and new therapeutic approaches. Since the non-cognitive deficits attributable to hypothalamic dysfunction involve a wide variety of organ systems traditionally associated with different disciplines, multidisciplinary approaches by basic scientists and clinicians with diverse interests and expertise would be required to move the field forward. Efforts in this direction promise to advance our understanding of an understudied but vitally important aspect of the pathobiology of AD.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge research support from the Leon Levy Foundation (MI), BrightFocus Foundation (MI), and NS37853 (CI). Dr. Anja Kahl provided assistance in translating the original 1927 German article by Stief to English. Chihiro Saka provided original drawings used in the figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging- Alzheimer‘s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer's Association. 2015 Alzheimer's disease facts and figures. Alzheimers Dement. 2015;11:332–384. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Ambrée O, Touma C, Görtz N, Keyvani K, Paulus W, Palme R, Sachser N. Activity changes and marked stereotypic behavior precede Abeta pathology in TgCRND8 Alzheimer mice. Neurobiol. Aging. 2006;27:955–964. doi: 10.1016/j.neurobiolaging.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Ballard C, Corbett A. Agitation and aggression in people with Alzheimer's disease. Curr Opin Psychiatry. 2013;26:252–259. doi: 10.1097/YCO.0b013e32835f414b. [DOI] [PubMed] [Google Scholar]
- Baloyannis SJ, Mavroudis I, Mitilineos D, Baloyannis IS, Costa VG. The Hypothalamus in Alzheimer's Disease: A Golgi and Electron Microscope Study. Am J Alzheimers Dis Other Demen. 2014 doi: 10.1177/1533317514556876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banki CM, Karmacsi L, Bissette G, Nemeroff CB. Cerebrospinal fluid neuropeptides in dementia. Biol. Psychiatry. 1992;32:452–456. doi: 10.1016/0006-3223(92)90132-j. [DOI] [PubMed] [Google Scholar]
- Baron JC, Chételat G, Desgranges B, Perchey G, Landeau B, la Sayette de V, Eustache F. In vivo mapping of gray matter loss with voxel-based morphometry in mild Alzheimer's disease. Neuroimage. 2001;14:298–309. doi: 10.1006/nimg.2001.0848. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Edelstein SL, Corey-Bloom J, Wiederholt WC. Weight loss precedes dementia in community-dwelling older adults. J Am Geriatr Soc. 1996;44:1147–1152. doi: 10.1111/j.1532-5415.1996.tb01362.x. [DOI] [PubMed] [Google Scholar]
- Bedrosian TA, Nelson RJ. Sundowning syndrome in aging and dementia: research in mouse models. Exp. Neurol. 2013;243:67–73. doi: 10.1016/j.expneurol.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Berry A, Tomidokoro Y, Ghiso J, Thornton J. Human chorionic gonadotropin (a luteinizing hormone homologue) decreases spatial memory and increases brain amyloid-beta levels in female rats. Horm Behav. 2008;54:143–152. doi: 10.1016/j.yhbeh.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- Bissette G, Reynolds GP, Kilts CD, Widerlöv E, Nemeroff CB. Corticotropin-releasing factor-like immunoreactivity in senile dementia of the Alzheimer type. Reduced cortical and striatal concentrations. JAMA. 1985;254:3067–3069. [PubMed] [Google Scholar]
- Bowen RL, Perry G, Xiong C, Smith MA, Atwood CS. A clinical study of lupron depot in the treatment of women with Alzheimer's disease: preservation of cognitive function in patients taking an acetylcholinesterase inhibitor and treated with high dose lupron over 48 weeks. Journal of Alzheimer's Disease : JAD. 2015;44:549–560. doi: 10.3233/JAD-141626. [DOI] [PubMed] [Google Scholar]
- Bowen RL, Verdile G, Liu T, Parlow AF, Perry G, Smith MA, Martins RN, Atwood CS. Luteinizing hormone, a reproductive regulator that modulates the processing of amyloid-beta precursor protein and amyloid-beta deposition. J. Biol. Chem. 2004;279:20539–20545. doi: 10.1074/jbc.M311993200. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Brownlow ML, Joly-Amado A, Azam S, Elza M, Selenica M-L, Pappas C, Small B, Engelman R, Gordon MN, Morgan D. Partial rescue of memory deficits induced by calorie restriction in a mouse model of tau deposition. Behav. Brain Res. 2014;271:79–88. doi: 10.1016/j.bbr.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Brureau A, Zussy C, Delair B, Ogier C, Ixart G, Maurice T, Givalois L. Deregulation of hypothalamic-pituitary-adrenal axis functions in an Alzheimer's disease rat model. Neurobiol. Aging. 2013;34:1426–1439. doi: 10.1016/j.neurobiolaging.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Buchman AS, Schneider JA, Wilson RS, Bienias JL, Bennett DA. Body mass index in older persons is associated with Alzheimer disease pathology. Neurology. 2006;67:1949–1954. doi: 10.1212/01.wnl.0000247046.90574.0f. [DOI] [PubMed] [Google Scholar]
- Buchman AS, Wilson RS, Bienias JL, Shah RC, Evans DA, Bennett DA. Change in body mass index and risk of incident Alzheimer disease. Neurology. 2005;65:892–897. doi: 10.1212/01.wnl.0000176061.33817.90. [DOI] [PubMed] [Google Scholar]
- Callen DJ, Black SE, Gao F, Caldwell CB, Szalai JP. Beyond the hippocampus: MRI volumetry confirms widespread limbic atrophy in AD. Neurology. 2001;57:1669–1674. doi: 10.1212/wnl.57.9.1669. [DOI] [PubMed] [Google Scholar]
- Callen DJA, Black SE, Caldwell CB. Limbic system perfusion in Alzheimer's disease measured by MRI-coregistered HMPAO SPET. Eur. J. Nucl. Med. Mol. Imaging. 2002;29:899–906. doi: 10.1007/s00259-002-0816-3. [DOI] [PubMed] [Google Scholar]
- Callen DJA, Black SE, Caldwell CB, Grady CL. The influence of sex on limbic volume and perfusion in AD. Neurobiol. Aging. 2004;25:761–770. doi: 10.1016/j.neurobiolaging.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Carroll JC, Iba M, Bangasser DA, Valentino RJ, James MJ, Brunden KR, Lee VM-Y, Trojanowski JQ. Chronic stress exacerbates tau pathology, neurodegeneration, and cognitive performance through a corticotropin-releasing factor receptor-dependent mechanism in a transgenic mouse model of tauopathy. Journal of Neuroscience. 2011;31:14436–14449. doi: 10.1523/JNEUROSCI.3836-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Claxton A, Baker LD, Hanson A, Trittschuh EH, Cholerton B, Morgan A, Callaghan M, Arbuckle M, Behl C, Craft S. Long-acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer's disease dementia. Journal of Alzheimer's Disease : JAD. 2015;44:897–906. doi: 10.3233/JAD-141791. [DOI] [PubMed] [Google Scholar]
- Coogan AN, Schutová B, Husung S, Furczyk K, Baune BT, Kropp P, Häβler F, Thome J. The circadian system in Alzheimer's disease: disturbances, mechanisms, and opportunities. Biol. Psychiatry. 2013;74:333–339. doi: 10.1016/j.biopsych.2012.11.021. [DOI] [PubMed] [Google Scholar]
- Copenhaver BR, Rabin LA, Saykin AJ, Roth RM, Wishart HA, Flashman LA, Santulli RB, McHugh TL, Mamourian AC. The fornix and mammillary bodies in older adults with Alzheimer's disease, mild cognitive impairment, and cognitive complaints: a volumetric MRI study. Psychiatry Res. 2006;147:93–103. doi: 10.1016/j.pscychresns.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Cross DJ, Anzai Y, Petrie EC, Martin N, Richards TL, Maravilla KR, Peskind ER, Minoshima S. Loss of olfactory tract integrity affects cortical metabolism in the brain and olfactory regions in aging and mild cognitive impairment. J. Nucl. Med. 2013;54:1278–1284. doi: 10.2967/jnumed.112.116558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernansky JG, Dong H, Fagan AM, Wang L, Xiong C, Holtzman DM, Morris JC. Plasma cortisol and progression of dementia in subjects with Alzheimer-type dementia. Am J Psychiatry. 2006;163:2164–2169. doi: 10.1176/appi.ajp.163.12.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacks PA, Moreno CL, Kim ES, Marcellino BK, Mobbs CV. Role of the hypothalamus in mediating protective effects of dietary restriction during aging. Front Neuroendocrinol. 2013;34:95–106. doi: 10.1016/j.yfrne.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KL, Davis BM, Greenwald BS, Mohs RC, Mathé AA, Johns CA, Horvath TB. Cortisol and Alzheimer's disease, I: Basal studies. Am J Psychiatry. 1986;143:300–305. doi: 10.1176/ajp.143.3.300. [DOI] [PubMed] [Google Scholar]
- Doherty GH, Beccano-Kelly D, Yan SD, Gunn-Moore FJ, Harvey J. Leptin prevents hippocampal synaptic disruption and neuronal cell death induced by amyloid β. Neurobiol. Aging. 2013;34:226–237. doi: 10.1016/j.neurobiolaging.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Doty RL, Reyes PF, Gregor T. Presence of both odor identification and detection deficits in Alzheimer's disease. Brain Res. Bull. 1987;18:597–600. doi: 10.1016/0361-9230(87)90129-8. [DOI] [PubMed] [Google Scholar]
- Elrod R, Peskind ER, DiGiacomo L, Brodkin KI, Veith RC, Raskind MA. Effects of Alzheimer's disease severity on cerebrospinal fluid norepinephrine concentration. Am J Psychiatry. 1997;154:25–30. doi: 10.1176/ajp.154.1.25. [DOI] [PubMed] [Google Scholar]
- Emmelot-Vonk MH, Verhaar HJJ, Pour HRN, Aleman A, Lock TMTW, Bosch JLHR, Grobbee DE, van der Schouw YT. Effect of Testosterone Supplementation on Functional Mobility, Cognition, and Other Parameters in Older Men A Randomized Controlled Trial. JAMA. 2008;299:39–52. doi: 10.1001/jama.2007.51. [DOI] [PubMed] [Google Scholar]
- Espeland MA, Shumaker SA, Leng I, Manson JE, Brown CM, Leblanc ES, Vaughan L, Robinson J, Rapp SR, Goveas JS, et al. Long-Term Effects on Cognitive Function of Postmenopausal Hormone Therapy Prescribed to Women Aged 50 to 55 Years. JAMA Intern Med. 2013:1–8. doi: 10.1001/jamainternmed.2013.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewers M, Schmitz S, Hansson O, Walsh C, Fitzpatrick A, Bennett D, Minthon L, Trojanowski JQ, Shaw LM, Faluyi YO, et al. Body mass index is associated with biological CSF markers of core brain pathology of Alzheimer's disease. Neurobiol. Aging. 2012;33:1599–1608. doi: 10.1016/j.neurobiolaging.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faden AI, Movsesyan VA, Knoblach SM, Ahmed F, Cernak I. Neuroprotective effects of novel small peptides in vitro and after brain injury. Neuropharmacology. 2005;49:410–424. doi: 10.1016/j.neuropharm.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Fekete C, Lechan RM. Central regulation of hypothalamic-pituitary-thyroid axis under physiological and pathophysiological conditions. Endocr. Rev. 2014;35:159–194. doi: 10.1210/er.2013-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodero-Tavoletti MT, Okamura N, Furumoto S, Mulligan RS, Connor AR, McLean CA, Cao D, Rigopoulos A, Cartwright GA, O'Keefe G, et al. 18F-THK523: a novel in vivo tau imaging ligand for Alzheimer's disease. Brain. 2011;134:1089–1100. doi: 10.1093/brain/awr038. [DOI] [PubMed] [Google Scholar]
- Friedman JM, Mantzoros CS. 20 years of leptin: From the discovery of the leptin gene to leptin in our therapeutic armamentarium. Metab. Clin. Exp. 2015;64:1–4. doi: 10.1016/j.metabol.2014.10.023. [DOI] [PubMed] [Google Scholar]
- Fronczek R, van Geest S, Frölich M, Overeem S, Roelandse FWC, Lammers GJ, Swaab DF. Hypocretin (orexin) loss in Alzheimer's disease. Neurobiol. Aging. 2012;33:1642–1650. doi: 10.1016/j.neurobiolaging.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Fu AL, Zhou CY, Chen X. Thyroid hormone prevents cognitive deficit in a mouse model of Alzheimer's disease. Neuropharmacology. 2010;58:722–729. doi: 10.1016/j.neuropharm.2009.12.020. [DOI] [PubMed] [Google Scholar]
- Gallone S, Boschi S, Rubino E, De Martino P, Scarpini E, Galimberti D, Fenoglio C, Acutis PL, Maniaci MG, Pinessi L, et al. Is HCRTR2 a genetic risk factor for Alzheimer's disease? Dement Geriatr Cogn Disord. 2014;38:245–253. doi: 10.1159/000359964. [DOI] [PubMed] [Google Scholar]
- Ganguli M, Burmeister LA, Seaberg EC, Belle S, DeKosky ST. Association between dementia and elevated TSH: a community-based study. Biol. Psychiatry. 1996;40:714–725. doi: 10.1016/0006-3223(95)00489-0. [DOI] [PubMed] [Google Scholar]
- Gao S, Nguyen JT, Hendrie HC, Unverzagt FW, Hake A, Smith-Gamble V, Hall K. Accelerated weight loss and incident dementia in an elderly African-American cohort. J Am Geriatr Soc. 2011;59:18–25. doi: 10.1111/j.1532-5415.2010.03169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemi R, Haeri A, Dargahi L, Mohamed Z, Ahmadiani A. Insulin in the brain: sources, localization and functions. Mol. Neurobiol. 2013;47:145–171. doi: 10.1007/s12035-012-8339-9. [DOI] [PubMed] [Google Scholar]
- Gleason CE, Dowling NM, Wharton W, Manson JE, Miller VM, Atwood CS, Brinton EA, Cedars MI, Lobo RA, Merriam GR, et al. Effects of Hormone Therapy on Cognition and Mood in Recently Postmenopausal Women: Findings from the Randomized, Controlled KEEPS-Cognitive and Affective Study. PLoS Med. 2015;12:e1001833. doi: 10.1371/journal.pmed.1001833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco SJ, Bryan KJ, Sarkar S, Zhu X, Smith MA, Ashford JW, Johnston JM, Tezapsidis N, Casadesus G. Leptin reduces pathology and improves memory in a transgenic mouse model of Alzheimer's disease. Journal of Alzheimer's Disease : JAD. 2010;19:1155–1167. doi: 10.3233/JAD-2010-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald BS, Mathé AA, Mohs RC, Levy MI, Johns CA, Davis KL. Cortisol and Alzheimer's disease, II: Dexamethasone suppression, dementia severity, and affective symptoms. Am J Psychiatry. 1986;143:442–446. doi: 10.1176/ajp.143.4.442. [DOI] [PubMed] [Google Scholar]
- Gupta A, Iadecola C. Impaired Aβ clearance: a potential link between atherosclerosis and Alzheimer's disease. Front Aging Neurosci. 2015;7:115. doi: 10.3389/fnagi.2015.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn EA, Wang H-X, Andel R, Fratiglioni L. A change in sleep pattern may predict Alzheimer disease. Am J Geriatr Psychiatry. 2014;22:1262–1271. doi: 10.1016/j.jagp.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Halagappa VKM, Guo Z, Pearson M, Matsuoka Y, Cutler RG, LaFerla FM, Mattson MP. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer's disease. Neurobiol. Dis. 2007;26:212–220. doi: 10.1016/j.nbd.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Hall AM, Moore RY, Lopez OL, Kuller L, Becker JT. Basal forebrain atrophy is a presymptomatic marker for Alzheimer's disease. Alzheimers Dement. 2008;4:271–279. doi: 10.1016/j.jalz.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J. The neurobiology of abnormal manifestations of aggression--a review of hypothalamic mechanisms in cats, rodents, and humans. Brain Res. Bull. 2013;93:97–109. doi: 10.1016/j.brainresbull.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Hamani C, McAndrews MP, Cohn M, Oh M, Zumsteg D, Shapiro CM, Wennberg RA, Lozano AM. Memory enhancement induced by hypothalamic/fornix deep brain stimulation. Ann. Neurol. 2008;63:119–123. doi: 10.1002/ana.21295. [DOI] [PubMed] [Google Scholar]
- Harper DG, Stopa EG, McKee AC, Satlin A, Harlan PC, Goldstein R, Volicer L. Differential circadian rhythm disturbances in men with Alzheimer disease and frontotemporal degeneration. Arch. Gen. Psychiatry. 2001;58:353–360. doi: 10.1001/archpsyc.58.4.353. [DOI] [PubMed] [Google Scholar]
- Harper DG, Stopa EG, Kuo-Leblanc V, McKee AC, Asayama K, Volicer L, Kowall N, Satlin A. Dorsomedial SCN neuronal subpopulations subserve different functions in human dementia. Brain. 2008;131:1609–1617. doi: 10.1093/brain/awn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess WR, Akert K. Experimental data on role of hypothalamus in mechanism of emotional behavior. AMA Arch Neurol Psychiatry. 1955;73:127–129. doi: 10.1001/archneurpsyc.1955.02330080005003. [DOI] [PubMed] [Google Scholar]
- Holden KF, Lindquist K, Tylavsky FA, Rosano C, Harris TB, Yaffe K Health ABC study. Serum leptin level and cognition in the elderly: Findings from the Health ABC Study. Neurobiol. Aging. 2009;30:1483–1489. doi: 10.1016/j.neurobiolaging.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabovszky E, Liposits Z. Afferent neuronal control of type-I gonadotropin releasing hormone neurons in the human. Front Endocrinol (Lausanne) 2013;4:130. doi: 10.3389/fendo.2013.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Harper DG, Shea SA, Stopa EG, Scheer FAJL. Noninvasive fractal biomarker of clock neurotransmitter disturbance in humans with dementia. Sci Rep. 2013;3:2229. doi: 10.1038/srep02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Van Someren EJW, Shea SA, Scheer FAJL. Reduction of scale invariance of activity fluctuations with aging and Alzheimer's disease: Involvement of the circadian pacemaker. Proc. Natl. Acad. Sci. U.S.a. 2009;106:2490–2494. doi: 10.1073/pnas.0806087106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Potter R, Sigurdson W, Santacruz A, Shih S, Ju Y-E, Kasten T, Morris JC, Mintun M, Duntley S, et al. Effects of age and amyloid deposition on Aβ dynamics in the human central nervous system. Archives of Neurology. 2012;69:51–58. doi: 10.1001/archneurol.2011.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80:844–866. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii M, Wang G, Racchumi G, Dyke JP, Iadecola C. Transgenic Mice Overexpressing Amyloid Precursor Protein Exhibit Early Metabolic Deficits and a Pathologically Low Leptin State Associated with Hypothalamic Dysfunction in Arcuate Neuropeptide Y Neurons. Journal of Neuroscience. 2014;34:9096–9106. doi: 10.1523/JNEUROSCI.0872-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii M. Neurologic Complications of Nondiabetic Endocrine Disorders. Continuum (Minneap Minn) 2014;20:560–579. doi: 10.1212/01.CON.0000450966.68828.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsubo T, Saido TC, Mann DM, Lee VM, Trojanowski JQ. Full-length amyloid-beta (1–42(43)) and amino-terminally modified and truncated amyloid-beta 42(43) deposit in diffuse plaques. Am. J. Pathol. 1996;149:1823–1830. [PMC free article] [PubMed] [Google Scholar]
- Jego S, Glasgow SD, Herrera CG, Ekstrand M, Reed SJ, Boyce R, Friedman J, Burdakov D, Adamantidis AR. Optogenetic identification of a rapid eye movement sleep modulatory circuit in the hypothalamus. Nature Neuroscience. 2013;16:1637–1643. doi: 10.1038/nn.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson P, Almqvist EG, Johansson J-O, Mattsson N, Hansson O, Wallin A, Blennow K, Zetterberg H, Svensson J. Reduced cerebrospinal fluid level of thyroxine in patients with Alzheimer's disease. Psychoneuroendocrinology. 2013;38:1058–1066. doi: 10.1016/j.psyneuen.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Johnson DK, Wilkins CH, Morris JC. Accelerated weight loss may precede diagnosis in Alzheimer disease. Archives of Neurology. 2006;63:1312–1317. doi: 10.1001/archneur.63.9.1312. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Minoshima S, Bohnen NI, Donohoe KJ, Foster NL, Herscovitch P, Karlawish JH, Rowe CC, Carrillo MC, Hartley DM, et al. Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer's Association. Alzheimers Dement. 2013;9 doi: 10.1016/j.jalz.2013.01.002. e–1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju Y-ES, McLeland JS, Toedebusch CD, Xiong C, Fagan AM, Duntley SP, Morris JC, Holtzman DM. Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 2013;70:587–593. doi: 10.1001/jamaneurol.2013.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien C, Tremblay C, Phivilay A, Berthiaume L, Emond V, Julien P, Calon F. High-fat diet aggravates amyloid-beta and tau pathologies in the 3xTg-AD mouse model. Neurobiol. Aging. 2010;31:1516–1531. doi: 10.1016/j.neurobiolaging.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Kang J-E, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S, Holtzman DM. Amyloid-β Dynamics Are Regulated by Orexin and the Sleep-Wake Cycle. Science. 2009;326:1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khachiyants N, Trinkle D, Son SJ, Kim KY. Sundown syndrome in persons with dementia: an update. Psychiatry Investig. 2011;8:275–287. doi: 10.4306/pi.2011.8.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiliaan AJ, Arnoldussen IAC, Gustafson DR. Adipokines: a link between obesity and dementia? The Lancet Neurology. 2014;13:913–923. doi: 10.1016/S1474-4422(14)70085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Feldman EL. Insulin resistance as a key link for the increased risk of cognitive impairment in the metabolic syndrome. Exp. Mol. Med. 2015;47:e149. doi: 10.1038/emm.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergström M, Savitcheva I, Huang G-F, Estrada S, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann. Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Knight EM, Brown TM, Gümüsgöz S, Smith JCM, Waters EJ, Allan SM, Lawrence CB. Age-related changes in core body temperature and activity in tripletransgenic Alzheimer's disease (3xTgAD) mice. Dis Model Mech. 2013;6:160–170. doi: 10.1242/dmm.010173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryscio RJ. Secondary prevention trials in Alzheimer disease: the challenge of identifying a meaningful end point. JAMA Neurol. 2014;71:947–949. doi: 10.1001/jamaneurol.2014.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- la Monte, de SM. Type 3 diabetes is sporadic Alzheimer’s disease: mini-review. Eur Neuropsychopharmacol. 2014;24:1954–1960. doi: 10.1016/j.euroneuro.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxton AW, Tang-Wai DF, McAndrews MP, Zumsteg D, Wennberg R, Keren R, Wherrett J, Naglie G, Hamani C, Smith GS, et al. A phase I trial of deep brain stimulation of memory circuits in Alzheimer's disease. Ann. Neurol. 2010;68:521–534. doi: 10.1002/ana.22089. [DOI] [PubMed] [Google Scholar]
- Lieb W, Beiser AS, Vasan RS, Tan ZS, Au R, Harris TB, Roubenoff R, Auerbach S, DeCarli C, Wolf PA, et al. Association of Plasma Leptin Levels With Incident Alzheimer Disease and MRI Measures of Brain Aging. JAMA. 2009;302:2565–2572. doi: 10.1001/jama.2009.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liguori C, Romigi A, Nuccetelli M, Zannino S, Sancesario G, Martorana A, Albanese M, Mercuri NB, Izzi F, Bernardini S, et al. Orexinergic system dysregulation, sleep impairment, and cognitive decline in Alzheimer disease. JAMA Neurol. 2014;71:1498–1505. doi: 10.1001/jamaneurol.2014.2510. [DOI] [PubMed] [Google Scholar]
- Lim ASP, Ellison BA, Wang JL, Yu L, Schneider JA, Buchman AS, Bennett DA, Saper CB. Sleep is related to neuron numbers in the ventrolateral preoptic/intermediate nucleus in older adults with and without Alzheimer's disease. Brain. 2014;137:2847–2861. doi: 10.1093/brain/awu222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Li X, Yuan F, Lin L, Cook CL, Rao CV, Lei Z. Genetic ablation of luteinizing hormone receptor improves the amyloid pathology in a mouse model of Alzheimer disease. J. Neuropathol. Exp. Neurol. 2010;69:253–261. doi: 10.1097/NEN.0b013e3181d072cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loskutova N, Honea RA, Brooks WM, Burns JM. Reduced limbic and hypothalamic volumes correlate with bone density in early Alzheimer's disease. Journal of Alzheimer's Disease : JAD. 2010;20:313–322. doi: 10.3233/JAD-2010-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loskutova N, Honea RA, Vidoni ED, Brooks WM, Burns JM. Bone density and brain atrophy in early Alzheimer's disease. Journal of Alzheimer's Disease : JAD. 2009;18:777–785. doi: 10.3233/JAD-2009-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu PH, Masterman DA, Mulnard R, Cotman C, Miller B, Yaffe K, Reback E, Porter V, Swerdloff R, Cummings JL. Effects of testosterone on cognition and mood in male patients with mild Alzheimer disease and healthy elderly men. Archives of Neurology. 2006;63:177–185. doi: 10.1001/archneur.63.2.nct50002. [DOI] [PubMed] [Google Scholar]
- Luo L, Stopa EG. Thyrotropin releasing hormone inhibits tau phosphorylation by dual signaling pathways in hippocampal neurons. Journal of Alzheimer's Disease : JAD. 2004;6:527–536. doi: 10.3233/jad-2004-6510. [DOI] [PubMed] [Google Scholar]
- Manly JJ, Merchant CA, Jacobs DM, Small SA, Bell K, Ferin M, Mayeux R. Endogenous estrogen levels and Alzheimer's disease among postmenopausal women. Neurology. 2000;54:833–837. doi: 10.1212/wnl.54.4.833. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab. 2012;16:706–722. doi: 10.1016/j.cmet.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May C, Rapoport SI, Tomai TP, Chrousos GP, Gold PW. Cerebrospinal fluid concentrations of corticotropin-releasing hormone (CRH) and corticotropin (ACTH) are reduced in patients with Alzheimer's disease. Neurology. 1987;37:535–538. doi: 10.1212/wnl.37.3.535. [DOI] [PubMed] [Google Scholar]
- McCarrey AC, Resnick SM. Postmenopausal hormone therapy and cognition. Horm Behav. 2015 doi: 10.1016/j.yhbeh.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDuff T, Sumi SM. Subcortical degeneration in Alzheimer's disease. Neurology. 1985;35:123–126. doi: 10.1212/wnl.35.1.123. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer‘s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor RK, López M, Younts CM, Jones B, Pearson KJ, Anson RM, Dieguez C, de Cabo R. The arcuate nucleus and neuropeptide Y contribute to the antitumorigenic effect of calorie restriction. Aging Cell. 2011;10:483–492. doi: 10.1111/j.1474-9726.2011.00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat SD, Zonderman AB, Metter EJ, Kawas C, Blackman MR, Harman SM, Resnick SM. Free testosterone and risk for Alzheimer disease in older men. Neurology. 2004;62:188–193. doi: 10.1212/wnl.62.2.188. [DOI] [PubMed] [Google Scholar]
- Moon M, Choi JG, Nam DW, Hong H-S, Choi Y-J, Oh MS, Mook-Jung I. Ghrelin ameliorates cognitive dysfunction and neurodegeneration in intrahippocampal amyloid- β1–42 oligomer-injected mice. Journal of Alzheimer's Disease : JAD. 2011;23:147–159. doi: 10.3233/JAD-2010-101263. [DOI] [PubMed] [Google Scholar]
- Morris MC, Tangney CC. Dietary fat composition and dementia risk. Neurobiol. Aging. 2014;35(Suppl 2):S59–S64. doi: 10.1016/j.neurobiolaging.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek ES, Holtzman DM. Three dimensions of the amyloid hypothesis: time, space and “wingmen”. Nature Neuroscience. 2015;18:800–806. doi: 10.1038/nn.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek ES, Xiong DD, Holtzman DM. Sleep, circadian rhythms, and the pathogenesis of Alzheimer Disease. Exp. Mol. Med. 2015;47:e148. doi: 10.1038/emm.2014.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB, Kizer JS, Reynolds GP, Bissette G. Neuropeptides in Alzheimer's disease: a postmortem study. Regul. Pept. 1989;25:123–130. doi: 10.1016/0167-0115(89)90254-1. [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Fryer TD, Smielewski P, Hodges JR. Limbic hypometabolism in Alzheimer's disease and mild cognitive impairment. Ann. Neurol. 2003;54:343–351. doi: 10.1002/ana.10669. [DOI] [PubMed] [Google Scholar]
- Niwa K, Kazama K, Younkin SG, Carlson GA, Iadecola C. Alterations in cerebral blood flow and glucose utilization in mice overexpressing the amyloid precursor protein. Neurobiol. Dis. 2002;9:61–68. doi: 10.1006/nbdi.2001.0460. [DOI] [PubMed] [Google Scholar]
- Nuruddin S, Syverstad GHE, Lillehaug S, Leergaard TB, Nilsson LNG, Ropstad E, Krogenæs A, Haraldsen IRH, Torp R. Elevated mRNA-levels of gonadotropin-releasing hormone and its receptor in plaque-bearing Alzheimer's disease transgenic mice. PLoS ONE. 2014;9:e103607. doi: 10.1371/journal.pone.0103607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram JJ, Edwardson J, Millard PH. Investigation of cerebrospinal fluid neuropeptides in idiopathic senile dementia. Gerontology. 1981;27:216–223. doi: 10.1159/000212476. [DOI] [PubMed] [Google Scholar]
- Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53:1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- Parsaik AK, Singh B, Roberts RO, Pankratz S, Edwards KK, Geda YE, Gharib H, Boeve BF, Knopman DS, Petersen RC. Hypothyroidism and risk of mild cognitive impairment in elderly persons: a population-based study. JAMA Neurol. 2014;71:201–207. doi: 10.1001/jamaneurol.2013.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel NV, Gordon MN, Connor KE, Good RA, Engelman RW, Mason J, Morgan DG, Morgan TE, Finch CE. Caloric restriction attenuates Abeta-deposition in Alzheimer transgenic models. Neurobiol. Aging. 2005;26:995–1000. doi: 10.1016/j.neurobiolaging.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Peskind ER, Elrod R, Dobie DJ, Pascualy M, Petrie E, Jensen C, Brodkin K, Murray S, Veith RC, Raskind MA. Cerebrospinal fluid epinephrine in Alzheimer's disease and normal aging. Neuropsychopharmacology. 1998;19:465–471. doi: 10.1016/S0893-133X(98)00054-2. [DOI] [PubMed] [Google Scholar]
- Pérez-González R, Alvira-Botero MX, Robayo O, Antequera D, Garzón M, Martín-Moreno AM, Brera B, de Ceballos ML, Carro E. Leptin gene therapy attenuates neuronal damages evoked by amyloid-β and rescues memory deficits in APP/PS1 mice. Gene Ther. 2014 doi: 10.1038/gt.2013.85. [DOI] [PubMed] [Google Scholar]
- Pomara N, Singh RR, Deptula D, LeWitt PA, Bissette G, Stanley M, Nemeroff CB. CSF corticotropin-releasing factor (CRF) in Alzheimer's disease: its relationship to severity of dementia and monoamine metabolites. Biol. Psychiatry. 1989;26:500–504. doi: 10.1016/0006-3223(89)90071-1. [DOI] [PubMed] [Google Scholar]
- Powers RE, Walker LC, DeSouza EB, Vale WW, Struble RG, Whitehouse PJ, Price DL. Immunohistochemical study of neurons containing corticotropin-releasing factor in Alzheimer's disease. Synapse. 1987;1:405–410. doi: 10.1002/syn.890010504. [DOI] [PubMed] [Google Scholar]
- Prinz PN, Vitaliano PP, Vitiello MV, Bokan J, Raskind M, Peskind E, Gerber C. Sleep, EEG and mental function changes in senile dementia of the Alzheimer's type. Neurobiol. Aging. 1982;3:361–370. doi: 10.1016/0197-4580(82)90024-0. [DOI] [PubMed] [Google Scholar]
- Qizilbash N, Gregson J, Johnson ME, Pearce N, Douglas I, Wing K, Evans SJW, Pocock SJ. BMI and risk of dementia in two million people over two decades: a retrospective cohort study. Lancet Diabetes Endocrinol. 2015;3:431–436. doi: 10.1016/S2213-8587(15)00033-9. [DOI] [PubMed] [Google Scholar]
- Quinlan P, Nordlund A, Lind K, Gustafson D, Edman A, Wallin A. Thyroid Hormones Are Associated with Poorer Cognition in Mild Cognitive Impairment. Dement Geriatr Cogn Disord. 2010;30:205–211. doi: 10.1159/000319746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raadsheer FC, van Heerikhuize JJ, Lucassen PJ, Hoogendijk WJ, Tilders FJ, Swaab DF. Corticotropin-releasing hormone mRNA levels in the paraventricular nucleus of patients with Alzheimer's disease and depression. Am J Psychiatry. 1995;152:1372–1376. doi: 10.1176/ajp.152.9.1372. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Holmes C, Goldstein DS. Patterns of cerebrospinal fluid catechols support increased central noradrenergic responsiveness in aging and Alzheimer's disease. Biol. Psychiatry. 1999;46:756–765. doi: 10.1016/s0006-3223(99)00008-6. [DOI] [PubMed] [Google Scholar]
- Rasmuson S, Andrew R, Näsman B, Seckl JR, Walker BR, Olsson T. Increased glucocorticoid production and altered cortisol metabolism in women with mild to moderate Alzheimer's disease. Biol. Psychiatry. 2001;49:547–552. doi: 10.1016/s0006-3223(00)01015-5. [DOI] [PubMed] [Google Scholar]
- Refolo LM, Malester B, Lafrancois J, Bryant-Thomas T, Wang R, Tint GS, Sambamurti K, Duff K, Pappolla MA. Hypercholesterolemia accelerates the Alzheimer's amyloid pathology in a transgenic mouse model. Neurobiol. Dis. 2000;7:321–331. doi: 10.1006/nbdi.2000.0304. [DOI] [PubMed] [Google Scholar]
- Rodrigues MA, Verdile G, Foster JK, Hogervorst E, Joesbury K, Dhaliwal S, Corder EH, Laws SM, Hone E, Prince R, et al. Gonadotropins and cognition in older women. Journal of Alzheimer's Disease : JAD. 2008;13:267–274. doi: 10.3233/jad-2008-13304. [DOI] [PubMed] [Google Scholar]
- Roh JH, Finn MB, Stewart FR, Mahan TE, Cirrito JR, Heda A, Snider BJ, Li M, Yanagisawa M, de Lecea L, et al. Potential role of orexin and sleep modulation in the pathogenesis of Alzheimer's disease. J. Exp. Med. 2014 doi: 10.1084/jem.20141788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh JH, Huang Y, Bero AW, Kasten T, Stewart FR, Bateman RJ, Holtzman DM. Disruption of the sleep-wake cycle and diurnal fluctuation of β-amyloid in mice with Alzheimer's disease pathology. Sci Transl Med. 2012;4:150ra122. doi: 10.1126/scitranslmed.3004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossmanith WG, Reichelt C, Scherbaum WA. Neuroendocrinology of aging in humans: attenuated sensitivity to sex steroid feedback in elderly postmenopausal women. Neuroendocrinology. 1994;59:355–362. doi: 10.1159/000126678. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford S, Howard BV, Johnson KC, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women - Principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Sakurai T. Orexin deficiency and narcolepsy. Curr. Opin. Neurobiol. 2013;23:760–766. doi: 10.1016/j.conb.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Saper CB, German DC. Hypothalamic pathology in Alzheimer's disease. Neurosci. Lett. 1987;74:364–370. doi: 10.1016/0304-3940(87)90325-9. [DOI] [PubMed] [Google Scholar]
- Saper CB. The central circadian timing system. Curr. Opin. Neurobiol. 2013;23:747–751. doi: 10.1016/j.conb.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh A, Brace CS, Ben-Josef G, West T, Wozniak DF, Holtzman DM, Herzog ED, Imai S-I. SIRT1 promotes the central adaptive response to diet restriction through activation of the dorsomedial and lateral nuclei of the hypothalamus. Journal of Neuroscience. 2010;30:10220–10232. doi: 10.1523/JNEUROSCI.1385-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh A, Brace CS, Rensing N, Cliften P, Wozniak DF, Herzog ED, Yamada KA, Imai S-I. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 2013;18:416–430. doi: 10.1016/j.cmet.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt FM, Kratzsch J, Gertz H-J, Tittmann M, Jahn I, Pietsch U-C, Kaisers UX, Thiery J, Hegerl U, Schönknecht P. Cerebrospinal fluid melanin-concentrating hormone (MCH) and hypocretin-1 (HCRT-1, orexin-A) in Alzheimer's disease. PLoS ONE. 2013;8:e63136. doi: 10.1371/journal.pone.0063136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi M, Joshi SS, Webb RL, Beckett TL, Donohue KD, Murphy MP, O'Hara BF, Duncan MJ. Increased fragmentation of sleep-wake cycles in the 5XFAD mouse model of Alzheimer's disease. Neuroscience. 2015;290:80–89. doi: 10.1016/j.neuroscience.2015.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharan K, Yadav VK. Hypothalamic control of bone metabolism. Best Pract. Res. Clin. Endocrinol. Metab. 2014;28:713–723. doi: 10.1016/j.beem.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Short RA, Bowen RL, O'Brien PC, Graff-Radford NR. Elevated gonadotropin levels in patients with Alzheimer disease. Mayo Clinic Proceedings. 2001;76:906–909. doi: 10.4065/76.9.906. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, III, Assaf AR, Jackson RD, et al. Estrogen Plus Progestin and the Incidence of Dementia and Mild Cognitive Impairment in Postmenopausal WomenThe Women's Health Initiative Memory Study: A Randomized Controlled Trial. JAMA. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Kaye J, Montine TJ, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer‘s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, Zhou Y, Wong DF, Ferrucci L, Resnick SM. Self-reported Sleep and β-Amyloid Deposition in Community-Dwelling Older Adults. JAMA Neurol. 2013;70:1537–1543. doi: 10.1001/jamaneurol.2013.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart R, Masaki K, Xue Q-L, Peila R, Petrovitch H, White LR, Launer LJ. A 32-year prospective study of change in body weight and incident dementia: the Honolulu-Asia Aging Study. Archives of Neurology. 2005;62:55–60. doi: 10.1001/archneur.62.1.55. [DOI] [PubMed] [Google Scholar]
- Stief A. über die anatomischen Grundlagen der vegetativen Störungen bei Geisteskrankheiten. Deutsche Zeitschrift F. Nervenheilkunde. 1927;97:112–132. [Google Scholar]
- Stopa EG, Volicer L, Kuo-Leblanc V, Harper D, Lathi D, Tate B, Satlin A. Pathologic evaluation of the human suprachiasmatic nucleus in severe dementia. J. Neuropathol. Exp. Neurol. 1999;58:29–39. doi: 10.1097/00005072-199901000-00004. [DOI] [PubMed] [Google Scholar]
- Su Y, Blazey TM, Snyder AZ, Raichle ME, Marcus DS, Ances BM, Bateman RJ, Cairns NJ, Aldea P, Cash L, et al. Partial volume correction in quantitative amyloid imaging. Neuroimage. 2015;107:55–64. doi: 10.1016/j.neuroimage.2014.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutphen CL, Fagan AM, Holtzman DM. Progress update: fluid and imaging biomarkers in Alzheimer's disease. Biol. Psychiatry. 2014;75:520–526. doi: 10.1016/j.biopsych.2013.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaab DF. Chapter II Neurobiology and neuropathology of the human hypothalamus. Handbook of Chemical Neuroanatomy, (Elsevier) 1997:39–137. [Google Scholar]
- Swaab DF, Fliers E, Partiman TS. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res. 1985;342:37–44. doi: 10.1016/0006-8993(85)91350-2. [DOI] [PubMed] [Google Scholar]
- Swanwick GR, Coen RF, Walsh JB, Coakley D, Lawlor BA. The predictive value of hypothalamic-pituitary-adrenal axis dysfunction in Alzheimer's disease. Biol. Psychiatry. 1996;39:976–978. doi: 10.1016/0006-3223(95)00590-0. [DOI] [PubMed] [Google Scholar]
- Takeda S, Sato N, Uchio-Yamada K, Sawada K, Kunieda T, Takeuchi D, Kurinami H, Shinohara M, Rakugi H, Morishita R. Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Abeta deposition in an Alzheimer mouse model with diabetes. Proc. Natl. Acad. Sci. U.S.a. 2010;107:7036–7041. doi: 10.1073/pnas.1000645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan ZS, Vasan RS. Thyroid function and Alzheimer's disease. Journal of Alzheimer's Disease : JAD. 2009;16:503–507. doi: 10.3233/JAD-2009-0991. [DOI] [PubMed] [Google Scholar]
- Tan ZS, Beiser A, Vasan RS, Au R, Auerbach S, Kiel DP, Wolf PA, Seshadri S. Thyroid function and the risk of Alzheimer disease: the Framingham Study. Arch Intern Med. 2008;168:1514–1520. doi: 10.1001/archinte.168.14.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan ZS, Seshadri S, Beiser A, Zhang Y, Felson D, Hannan MT, Au R, Wolf PA, Kiel DP. Bone mineral density and the risk of Alzheimer disease. Archives of Neurology. 2005;62:107–111. doi: 10.1001/archneur.62.1.107. [DOI] [PubMed] [Google Scholar]
- Touma C, Ambrée O, Görtz N, Keyvani K, Lewejohann L, Palme R, Paulus W, Schwarze-Eicker K, Sachser N. Age- and sex-dependent development of adrenocortical hyperactivity in a transgenic mouse model of Alzheimer's disease. Neurobiol. Aging. 2004;25:893–904. doi: 10.1016/j.neurobiolaging.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Tranah GJ, Blackwell T, Stone KL, Ancoli-Israel S, Paudel ML, Ensrud KE, Cauley JA, Redline S, Hillier TA, Cummings SR, et al. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann. Neurol. 2011;70:722–732. doi: 10.1002/ana.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunematsu T, Ueno T, Tabuchi S, Inutsuka A, Tanaka KF, Hasuwa H, Kilduff TS, Terao A, Yamanaka A. Optogenetic manipulation of activity and temporally controlled cell-specific ablation reveal a role for MCH neurons in sleep/wake regulation. Journal of Neuroscience. 2014;34:6896–6909. doi: 10.1523/JNEUROSCI.5344-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger JW, Livingston JN, Moss AM. Insulin receptors in the central nervous system: localization, signalling mechanisms and functional aspects. Prog. Neurobiol. 1991;36:343–362. doi: 10.1016/0301-0082(91)90015-s. [DOI] [PubMed] [Google Scholar]
- van Osch LADM, Hogervorst E, Combrinck M, Smith AD. Low thyroid-stimulating hormone as an independent risk factor for Alzheimer disease. Neurology. 2004;62:1967–1971. doi: 10.1212/01.wnl.0000128134.84230.9f. [DOI] [PubMed] [Google Scholar]
- van Someren EJ, Hagebeuk EE, Lijzenga C, Scheltens P, de Rooij SE, Jonker C, Pot AM, Mirmiran M, Swaab DF. Circadian rest-activity rhythm disturbances in Alzheimer's disease. Biol. Psychiatry. 1996;40:259–270. doi: 10.1016/0006-3223(95)00370-3. [DOI] [PubMed] [Google Scholar]
- Vandal M, White PJ, Tremblay C, St-Amour I, Chevrier G, Emond V, Lefrançois D, Virgili J, Planel E, Giguere Y, et al. Insulin reverses the high-fat diet-induced increase in brain Aβ and improves memory in an animal model of Alzheimer disease. Diabetes. 2014;63:4291–4301. doi: 10.2337/db14-0375. [DOI] [PubMed] [Google Scholar]
- Velayudhan L. Smell identification function and Alzheimer's disease: a selective review. Curr Opin Psychiatry. 2015;28:173–179. doi: 10.1097/YCO.0000000000000146. [DOI] [PubMed] [Google Scholar]
- Venturelli M, Scarsini R, Muti E, Salvagno GL, Schena F. Sundowning syndrome and hypothalamic-pituitary-adrenal axis dysregulation in individuals with Alzheimer's disease: is there an association? J Am Geriatr Soc. 2013;61:2055–2056. doi: 10.1111/jgs.12491. [DOI] [PubMed] [Google Scholar]
- Vest RS, Pike CJ. Gender, sex steroid hormones, and Alzheimer's disease. Horm Behav. 2013;63:301–307. doi: 10.1016/j.yhbeh.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidoni ED, Townley RA, Honea RA, Burns JM Alzheimer’s Disease Neuroimaging Initiative. Alzheimer disease biomarkers are associated with body mass index. Neurology. 2011;77:1913–1920. doi: 10.1212/WNL.0b013e318238eec1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigen R, O'Donnell CI, Barón AE, Grunwald GK, Maddox TM, Bradley SM, Barqawi A, Woning G, Wierman ME, Plomondon ME, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829–1836. doi: 10.1001/jama.2013.280386. [DOI] [PubMed] [Google Scholar]
- Wang JL, Lim AS, Chiang W-Y, Hsieh W-H, Lo M-T, Schneider JA, Buchman AS, Bennett DA, Hu K, Saper CB. Suprachiasmaticvasopress neuron numbers and rest-activity circadian rhythms in older humans. Ann. Neurol. 2015 doi: 10.1002/ana.24432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster SJ, Bachstetter AD, Nelson PT, Schmitt FA, Van Eldik LJ. Using mice to model Alzheimer's dementia: an overview of the clinical disease and the preclinical behavioral changes in 10 mouse models. Frontiers in Genetics. 2014;5:88. doi: 10.3389/fgene.2014.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White H, Pieper C, Schmader K. The association of weight change in Alzheimer's disease with severity of disease and mortality: a longitudinal analysis. J Am Geriatr Soc. 1998;46:1223–1227. doi: 10.1111/j.1532-5415.1998.tb04537.x. [DOI] [PubMed] [Google Scholar]
- White H, Pieper C, Schmader K, Fillenbaum G. Weight change in Alzheimer's disease. J Am Geriatr Soc. 1996;44:265–272. doi: 10.1111/j.1532-5415.1996.tb00912.x. [DOI] [PubMed] [Google Scholar]
- Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71:1057–1064. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. Bmj. 2005;330:1360–1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Weigand SD, Shiung MM, Boeve BF, Ferman TJ, Smith GE, Knopman DS, Petersen RC, Benarroch EE, Josephs KA, et al. Focal atrophy in dementia with Lewy bodies on MRI: a distinct pattern from Alzheimer's disease. Brain. 2007;130:708–719. doi: 10.1093/brain/awl388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KW, Elmquist JK. From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior. Nature Neuroscience. 2012;15:1350–1355. doi: 10.1038/nn.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisor JP, Edgar DM, Yesavage J, Ryan HS, McCormick CM, Lapustea N, Murphy GM. Sleep and circadian abnormalities in a transgenic mouse model of Alzheimer's disease: a role for cholinergic transmission. Neuroscience. 2005;131:375–385. doi: 10.1016/j.neuroscience.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O'Donnell J, Christensen DJ, Nicholson C, Iliff JJ, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu WL, Atti AR, Gatz M, Pedersen NL, Johansson B, Fratiglioni L. Midlife overweight and obesity increase late-life dementia risk: a population-based twin study. Neurology. 2011;76:1568–1574. doi: 10.1212/WNL.0b013e3182190d09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Browner W, Cauley J, Launer L, Harris T. Association between bone mineral density and cognitive decline in older women. J Am Geriatr Soc. 1999;47:1176–1182. doi: 10.1111/j.1532-5415.1999.tb05196.x. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Lui L-Y, Zmuda J, Cauley J. Sex hormones and cognitive function in older men. J Am Geriatr Soc. 2002;50:707–712. doi: 10.1046/j.1532-5415.2002.50166.x. [DOI] [PubMed] [Google Scholar]
- Yates CM, Harmar AJ, Rosie R, Sheward J, Sanchez de Levy G, Simpson J, Maloney AFJ, Gordon A, Fink G. Thyrotropin-releasing hormone, luteinizing hormone-releasing hormone and substance P immuno-reactivity in post-mortem brain from cases of alzheimer-type dementia and Down's syndrome. Brain Res. 1983;258:45–52. doi: 10.1016/0006-8993(83)91224-6. [DOI] [PubMed] [Google Scholar]
- Yong-Hong L, Xiao-Dong P, Chang-Quan H, Bo Y, Qing-Xiu L. Hypothalamicpituitary-thyroid axis in patients with Alzheimer disease (AD) J. Investig. Med. 2013;61:578–581. doi: 10.2310/JIM.0b013e318280aafb. [DOI] [PubMed] [Google Scholar]
- Zhang G, Li J, Purkayastha S, Tang Y, Zhang H, Yin Y, Li B, Liu G, Cai D. Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH. Nature. 2013;497:211–216. doi: 10.1038/nature12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.