Abstract
X linked hypophosphataemia (XLH) is a rare condition with numerous musculoskeletal complications. It may mimic other more familiar conditions, such as vitamin D deficiency, ankylosing spondylitis or diffuse idiopathic skeletal hyperostosis. We describe two cases with Chiari type 1 malformations and syringomyelia, neither of which is well recognised in XLH. The first presented late with the additional complications of spinal cord compression, pseudofracture, renal stones and gross femoroacetabular impingement requiring hip replacement. The second also had bulbar palsy; the first case to be described in this condition, to the best of our knowledge. We wish to raise awareness of the important neurological complications of syringomyelia, Chiari malformation, spinal cord compression and bulbar palsy when treating these patients. We also wish to draw attention to the utility of family history and genetic testing when making the diagnosis of this rare but potentially treatable condition.
Background
X linked hypophosphataemia (XLH) is a rare genetic condition caused by mutations in the PHEX gene.1 2 Numerous musculoskeletal complications are recognised, including pseudofractures, enthesopathy, genu varum and dental abscesses.1 We present two cases illustrating the range of complications that can be associated with this condition, in particular syringomyelia, Chiari malformation and bulbar palsy, which are not well recognised in XLH. We hope that this will increase awareness of potential complications and, in particular, associated neurological problems, for clinicians treating these patients.
Case presentation: presenting features and medical/social/family history
Case 1
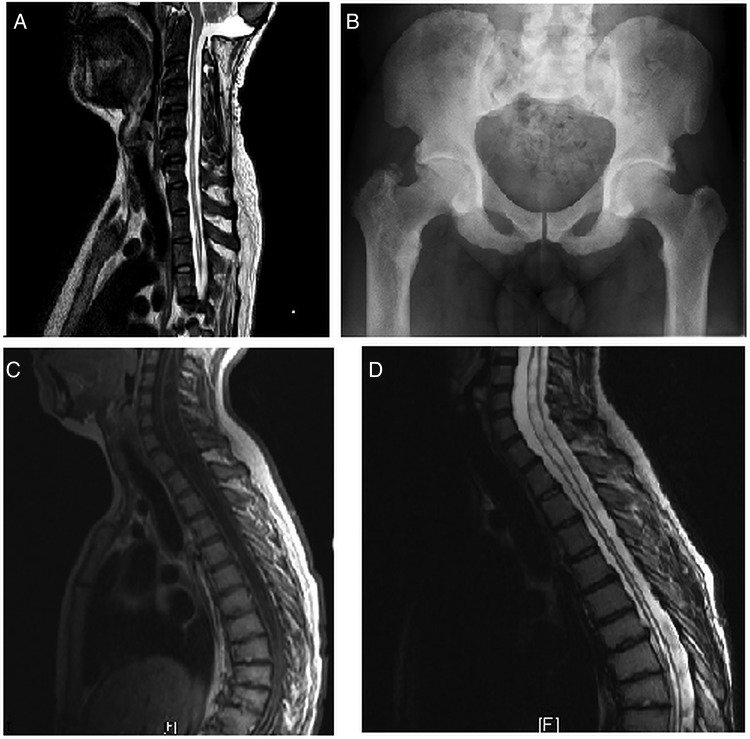
A 36-year-old man was diagnosed only after a family member was referred for investigation of short stature. The patient had, in his early 20s, developed decreased sensation, wasting and trophic changes of both hands caused by syringomyelia. On examination, tone in both arms was normal. There was weakness, worse in the left arm than the right, and worse distally than proximally. Grip strength was 3/5 on the Medical Research Council (MRC) scale in the left hand and wrist; 3–4/5 in the right wrist; 3–4/5 in the left elbow; 4/5 in the right elbow and 4–5/5 in both shoulders (mainly limited by shoulder pain rather than muscular weakness). Sensation was decreased in C6-T1 dermatomes bilaterally. There were no ulcers on either hand despite trophic changes and wasting bilaterally. Upper limb reflexes were absent bilaterally. Tone in both legs was mildly increased, with brisk lower limb reflexes. The syrinx extended through much of the patient's spinal cord (figure 1A) and was associated with a type 1 Chiari malformation treated by foramen magnum decompression. Before diagnosis, he had also required L2–5 laminectomy for spinal canal stenosis and had multiple procedures for dental abscesses, a well-recognised complication of XLH.
Figure 1.
Radiological images of cases 1 and 2 showing syringomyelia and enthesopathy (A) cervicothoracic MRI of case 1; (B) pelvic X-ray of case 1; (C) preoperative MRI of case 2 and (D) postoperative MRI of case 2.
At diagnosis, radiographs revealed widespread hyperostostic enthesopathic changes, particularly in the spine and hips, where there was dramatic ossification of the joint capsule and the trochanters (figure 1B). Biochemically, blood phosphate was low (<0.5 mmol/L, normal range 0.7–1.45) with elevated alkaline phosphatase (approximately 600 iu/L, normal <290). Genetic testing revealed a c.2066C>T (Ala689Val) PHEX mutation, confirming the diagnosis.
A pseudofracture was present in the proximal right femur. Ultrasonography revealed an asymptomatic left renal stone. Aged 39 years, the patient underwent successful hip replacement with a long-stemmed prosthesis to correct the impingement symptoms around his right hip and to stabilise the Looser zone in the femoral neck. Within a year he developed transient T9/10 paraparesis resulting from spinal stenosis due to florid ligamenta flava ossification. Following the previous syringomyelia, he was already known to have increased tone and brisk lower limb reflexes. New neurological findings on examination included an ataxic gait, decreased sensation in both lower limbs, a sensory level at the area of the umbilicus and decreased perianal sensation. MRI confirmed the severe stenosis, which was successfully treated by surgical decompression with full return of sensation and correction of the patient's ataxic gait.
Case 2
This case was recognised in infancy due to family history and was treated with calcitriol and oral phosphate during childhood and adolescence. Aged 10–11 years, the patient developed a thoracolumbar scoliosis that was managed conservatively. Aged 20 years, she developed paraesthesia and functional impairment in the hands, interfering with her ability to work. On examination, she had marked wasting of the small muscles of her hands bilaterally. There was decreased distal power, worse on the left than the right. Left finger abduction was 3/5, right finger abduction and other upper limb muscle groups had a power of 4/5 bilaterally. Triceps reflexes were absent bilaterally with decreased biceps reflexes. Sensory loss, particularly affecting pain and temperature, was found in both distal upper limbs in a non-dermatomal pattern. MRI revealed a type 1 Chiari malformation and extensive syrinx from C1 to the conus (figure 1C). Foramen magnum decompression and posterior C1 arch removal was performed. Postoperative MRI showed a reduction in syrinx size (figure 1D), but the patient derived little symptomatic benefit. Subsequent to this, she developed difficulty with speech and swallowing. On examination, she had a bulbar palsy with dysarthria, nasal speech, tongue weakness and wasting.
Differential diagnosis
In our first case, diagnosis was delayed until adulthood, only being suspected when a family member was diagnosed with XLH after initially being suspected of having hypochondroplasia (another rare skeletal dysplasia). The differential diagnosis includes vitamin D deficient rickets, necessitating measurement of vitamin D levels. The enthesopathy and spinal manifestations may mimic ankylosing spondylitis or diffuse idiopathic skeletal hyperostosis. In this case, measurement of serum phosphate levels was important in reaching the diagnosis—the finding of low serum phosphate in the context of the family history was highly suggestive. Given the adult presentation, genetic testing was used to confirm the diagnosis. In the second case, there was no difficulty in diagnosing the XLH due to the known family history and early use of genetic testing.
MRI was key to diagnosing the syringomyelia in each case, differentiating it from other more common causes of neurological symptoms, including multiple sclerosis. Our first case was already known to have a history of spinal stenosis, so the suspicion of spinal cord compression was high when he developed acute-onset neurological symptoms in the lower limbs.
In our second case, bulbar palsy developed after her syringomyelia had been treated. The initial symptoms were speech disturbance and dysphagia, typical of a bulbar palsy. With the known history of syringomyelia, we considered the risk of syringobulbia to be high, but this was not confirmed by MRI. Other, non-XLH causes of a bulbar palsy to be considered include motor neuron disease (not supported by the absence of other new motor signs and non-progressive nature), myasthaenia gravis (not supported as no fatigable weakness was detected), brainstem stroke (which would expect a more sudden onset) and myotonic dystrophy.
Treatment
Treatment for XLH typically involves calcitriol and phosphate during childhood until growth is complete. Whether adults should also be treated is unclear but treatment is usually reserved for those adults with overt bone disease, such as of the Looser zones, and/or fractures.3 Both our patients had received treatment at various points during adulthood. Case 1 was treated around the time of his hip replacement surgery in order to improve healing. Case 2 was treated in early adulthood due to increased fatigue when she stopped taking calcitriol and phosphate. By the time of presentation with their symptoms of syringomyelia, surgery was only of limited benefit. Timely surgical decompression when case 1 presented with spinal cord compression resulted in resolution of the gait and lower limb sensory disturbances.
Discussion
We present these two complex cases in order to draw attention to the range of complications that can be associated with this rare condition. In particular, these cases illustrate some of the neurological manifestations that clinicians (particularly orthopaedic surgeons, rheumatologists and endocrinologists) treating these patients should be aware of.
Fibroblast growth factor 23 (FGF23) plays a key role in renal phosphate handling by downregulating renal tubular sodium/phosphate transport, decreasing renal phosphate reabsorption4 and lowering blood phosphate.1 FGF23 is itself regulated by the catalytic enzyme PHEX (phosphate-regulating endopeptidase homologue, X linked), which is affected by various loss-of-function mutations in XLH, causing hypophosphataemia.2 5 The mutation in exon 20 in case 1 results in substitution of valine for a highly conserved alanine. This mutation does not appear among the more than 300 PHEX mutations that have been reported to date.5 Trials targeting FGF23 with therapeutic monoclonal antibodies are currently in progress,6 7 but it remains to be seen whether this form of treatment will have the dramatic effect of transforming the lives of individuals with this extremely troublesome disease.
We are not aware of previous descriptions of bulbar symptoms similar to those exhibited by case 2 in XLH. The exact cause for her symptoms is uncertain but may relate to brainstem compression from bony abnormalities associated with XLH. Symptoms could be consistent with syringobulbia, but the syrinx in this case did not appear to extend into the brainstem. Her symptoms have remained stable and there are no features to suggest an alternative cause of the bulbar palsy.
Although type 1 Chiari malformations and syringomyelia are not routinely considered among the complications of XLH, we are aware of at least one other case. There is one previous case report of syringomyelia and Chiari malformation,8 and a series of 16 cases where 44% had Chiari malformation on MRI.9 An increased risk of Chiari malformation could be explained by calvarial thickening,9 decreased size of the posterior fossa, or sagittal synostosis,10 all of which have been identified in association with XLH. There is also a recognised association between type 1 Chiari malformations and syringomyelia.11 The skull abnormalities in XLH therefore suggest that Chiari malformation and associated syringomyelia are to be expected as a potential complication.
Florid enthesopathy, which can lead to joint impingement, is well recognised in XLH.1 Despite understandable reluctance to operate on hips where the articular cartilage is relatively well preserved, the very limited reports on joint replacement suggest that, with careful patient selection, hip replacement can be beneficial.12 We are aware of another four patients in our practice who have undergone hip replacements satisfactorily.
Case 1 also demonstrates the risk of spinal cord compression in XLH due to a combination of ossification of the ligamenta flava, thickening of the laminae, hypertrophy of facet joints and intervertebral disc calcification.13 These two cases illustrate the wide range of musculoskeletal and neurological complications of XLH. We particularly draw attention to syringomyelia, Chiari malformation, bulbar palsy and spinal cord compression as important complications when treating these patients, and to the utility of the family history and genetic testing when making the diagnosis.
Learning points.
Those caring for patients with X linked hypophosphataemia (XLH) must be aware of the neurological complications that can be associated with this condition.
Syringomyelia and Chiari malformation with neurological symptoms appear to be under-recognised associations of XLH.
Family history and genetic testing can be useful when making the diagnosis.
Spinal cord compression is an important complication requiring urgent investigation and treatment.
Enthesopathy may result in joint impingement symptoms.
Footnotes
Contributors: PW had the original idea and identified the cases. LW collected the information and drafted the article. PW and LW critically revised the manuscript and approved the version to be published.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Reid IR, Hardy DC, Murphy WA et al. . X-linked hypophosphatemia: a clinical, biochemical, and histopathologic assessment of morbidity in adults. Medicine (Baltimore) 1989;68:336–52. [PubMed] [Google Scholar]
- 2.Francis F, Hennig S, Korn B et al. . A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. The HYP Consortium. Nat Genet 1995;11:130–6. 10.1038/ng1095-130 [DOI] [PubMed] [Google Scholar]
- 3.Carpenter TO, Imel EA, Holm IA et al. . A clinician's guide to X-linked hypophosphatemia. J Bone Miner Res 2011;26:1381–8. 10.1002/jbmr.340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JY, Imel EA. The changing face of hypophosphatemic disorders in the FGF-23 era. Pediatr Endocrinol Rev 2013;10(Suppl 2):367–79. [PMC free article] [PubMed] [Google Scholar]
- 5.PHEXdb. Secondary PHEXdb. http://www.phexdb.mcgill.ca/
- 6.Carpenter TO, Imel EA, Ruppe MD et al. . Randomized trial of the anti-FGF23 antibody KRN23 in X-linked hypophosphatemia. J Clin Invest 2014;124:1587–97. 10.1172/JCI72829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imel EA, Zhang X, Ruppe MD et al. . Prolonged correction of serum phosphorus in adults with X-linked hypophosphatemia using monthly doses of KRN23. J Clin Endocrinol Metab 2015;100:2565–73. 10.1210/jc.2015-1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuether TA, Piatt JH. Chiari malformation associated with vitamin D-resistant rickets: case report. Neurosurgery 1998;42:1168–71. 10.1097/00006123-199805000-00134 [DOI] [PubMed] [Google Scholar]
- 9.Caldemeyer KS, Boaz JC, Wappner RS et al. . Chiari I malformation: association with hypophosphatemic rickets and MR imaging appearance. Radiology 1995;195:733–8. 10.1148/radiology.195.3.7754003 [DOI] [PubMed] [Google Scholar]
- 10.Currarino G. Sagittal synostosis in X-linked hypophosphatemic rickets and related diseases. Pediatr Radiol 2007;37:805–12. 10.1007/s00247-007-0503-4 [DOI] [PubMed] [Google Scholar]
- 11.Arnautovic A, Splavski B, Boop FA et al. . Pediatric and adult Chiari malformation Type I surgical series 1965–2013: a review of demographics, operative treatment, and outcomes. J Neurosurg Pediatr 2015;15:161–77. 10.3171/2014.10.PEDS14295 [DOI] [PubMed] [Google Scholar]
- 12.Larson AN, Trousdale RT, Pagnano MW et al. . Hip and knee arthroplasty in hypophosphatemic rickets. J Arthroplasty 2010;25:1099–103. 10.1016/j.arth.2009.06.023 [DOI] [PubMed] [Google Scholar]
- 13.Soehle M, Casey AT. Cervical spinal cord compression attributable to a calcified intervertebral disc in a patient with X-linked hypophosphatemic rickets: case report and review of the literature. Neurosurgery 2002;51:239–42. 10.1097/00006123-200207000-00038 [DOI] [PubMed] [Google Scholar]