Abstract
Umbilical venous catheterisation (UVC) insertion is a common procedure performed in most neonatal units. We report a case of a neonate who developed pleural and pericardial effusions and ascites due to a malpositioned UVC causing diaphragmatic perforation. Timely diagnosis using bedside sonography and prompt removal of the catheter resulted in resolution of the effusions without undue complications.
Background
Umbilical venous catheter (UVC) insertion is a common procedure performed in most neonatal units. UVC is preferred over peripherally inserted central lines, because the insertion is technically easy and the catheter is less expensive. Even though the procedure is relatively safe, many complications have been reported in the literature. We report a case of pleural and pericardial effusions and ascites due to a malpositioned UVC causing diaphragmatic perforation. To the best of our knowledge, this is the first case report of an UVC causing diaphragmatic perforation and pleural effusion.
Case presentation
A baby girl was born to a 29-year-old primigravida mother at 35 weeks gestation by emergency caesarean section for severe preeclampsia and breech presentation. The baby was small for gestational age with a birth weight of 1420 g. She cried at birth and had an APGAR score of 9 and 10 at 1 and 5 min, respectively.
The baby developed respiratory distress with grunting at 2 h of life. She also had signs of poor perfusion in the form of abnormal colour and cold, dusky peripheries. Blood pressure was normal. Blood gas showed mild metabolic acidosis with a pH of 7.3, bicarbonate of 18 mmol/litre and base deficit of 6 mmol/litre. Hence a normal saline bolus was given. Bedside echocardiogram showed poor cardiac contractility, so she was started on dobutamine infusion at 10 µg/kg/min. An UVC was inserted for the inotrope and fluid (10% dextrose) infusion. The UVC was inserted up to 7.5 cm, the length of insertion calculated from birth weight using the Shukla method (1/2×(3×birth weight+9)+1).1 As respiratory distress with grunting persisted, the baby was also started on continuous positive airway pressure support at 4 h of life with positive end-expiratory pressure of 5 cm H2O and FiO2 of 0.3, following which the grunting and respiratory distress improved.
Chest X-ray (figure 1) was taken to confirm the UVC position. The UVC tip was seen at the level of the diaphragm and the T9 vertebra; malposition of the catheter was not recognised. There was no pleural effusion in the X-ray or pericardial effusion in the echocardiogram, initially. The X-ray was reviewed only by the treating neonatologist; no radiologist was involved.
Figure 1.
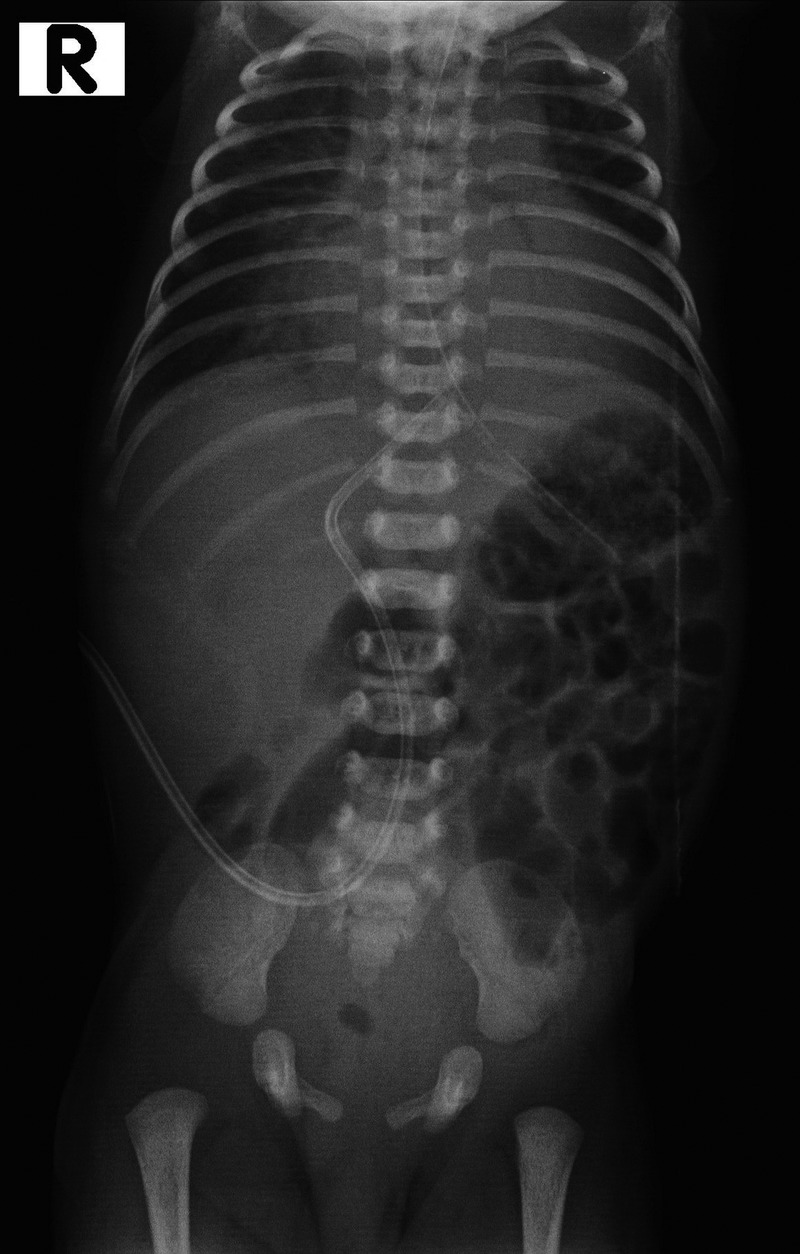
X-ray showing umbilical venous catheter at the level of diaphragm and T9 vertebra.
At 12 h of life, the baby's FiO2 requirement started to acutely increase. She was not maintaining saturation even with 100% FiO2 and started having gasping respiration. Hence she was intubated and started on conventional ventilation. Transillumination was positive in the right hemithorax. Hence a right pneumothorax was suspected and an emergency needle aspiration was performed in the right second intercostal space. However, instead of air, 6 mL of a serous yellow fluid was aspirated.
Investigations
Repeat chest X-ray (figure 2) showed bilateral pleural effusion with more fluid on the right than left. The UVC tip was seen at the level of the diaphragm, reaching the left border of the vertebral column, and there was a sharp angulation in the course of the UVC just above the lower border of the liver. Bedside sonography showed right pleural effusion of maximum depth 1 cm (video 1), left pleural effusion of depth 6 mm, mild pericardial effusion (3 mm) and minimal ascites. The UVC tip was not seen at the junction of the inferior vena cava (IVC) and right atrium (video 2). It was instead seen traversing the liver and perforating the diaphragm with the tip in the right pleural cavity adjacent to the pericardium (video 3). Pleural fluid analysis showed white cell counts 140 cells/mm3 with polymorphs 40% and lymphocytes 60% and 3500 red blood cells/mm3, glucose 355 mg/dL and protein 0.7 g/dL.
Figure 2.
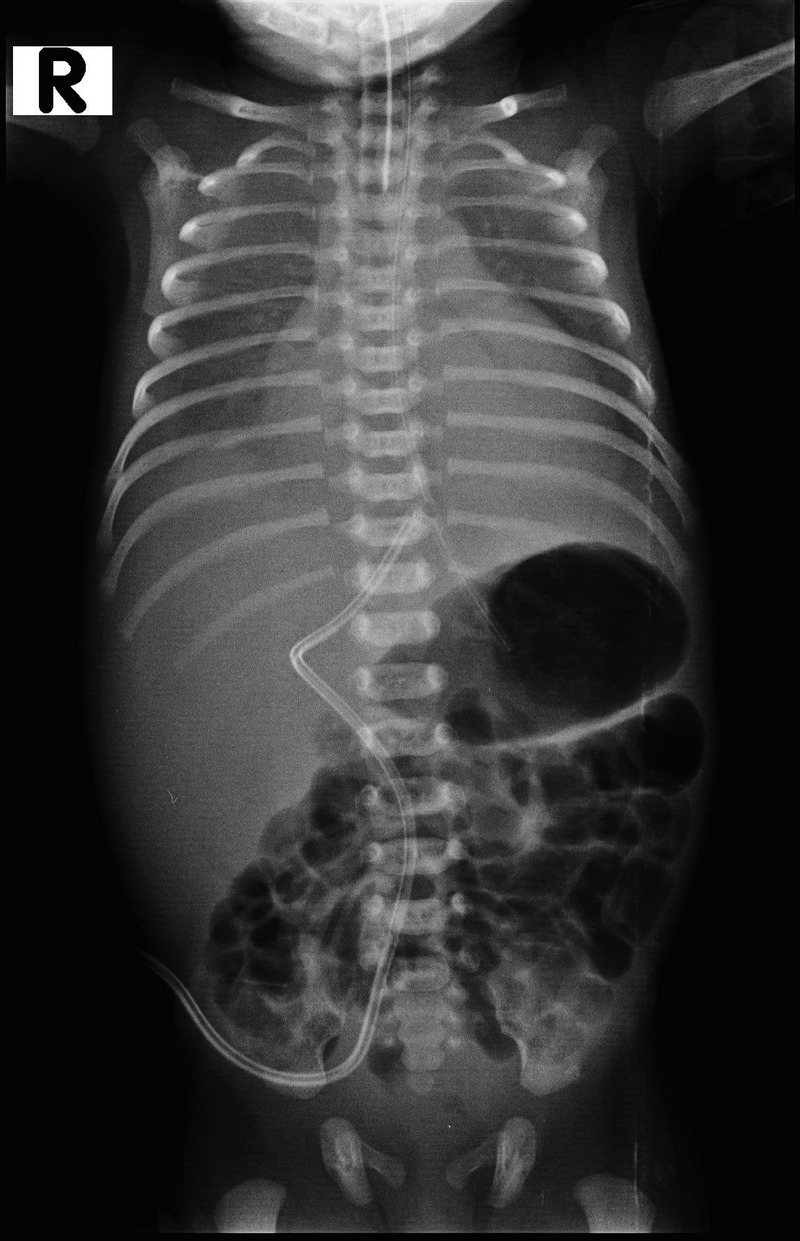
X-ray showing bilateral pleural effusion (right>left), umbilical venous catheter (UVC) tip reaching the left border of vertebral column and sharp angulation in the course of UVC.
Video 1.
Right pleural effusion.
Video 2.
Inferior vena cava right atrial junction—umbilical venous catheter tip was not seen here.
Video 3.
Umbilical venous catheter traversing the liver and perforating the diaphragm.
Treatment
The UVC was removed and an intercostal drainage tube was inserted in the right side. Needle aspiration was carried out on the left side and 40 mL of fluid was removed. Pericardial effusion and ascites were left behind as they were small.
Outcome and follow-up
The baby improved clinically and repeat chest X-ray showed no reaccumulation of pleural effusions. Repeat sonography after 24 h showed resolution of pleural and pericardial effusions and the ascites, following which she was weaned and extubated, and the intercostal drainage tube was removed. She was discharged on day 24 of life, on exclusive breast feeds.
Discussion
UVC has become a valuable source of central venous access in neonates ever since it was first reported in 1951.2 Ideal position of the UVC tip is at the level of the IVC-right atrial junction or the thoracic IVC.3 Two methods are commonly used to decide the length of catheter insertion. The Dunn method uses a nomogram based on the shoulder umbilical length and the Shukla method uses a regression equation based on birth weight.1 4
Anteroposterior radiography of the abdomen is the most frequent technique used to verify the position of the catheter. The desired location of the tip is at the level of T8–9 vertebrae, which usually corresponds to the IVC-right atrial junction. Other techniques such as lateral chest X-ray, bedside sonography and contrast studies, are less commonly used.
The UVC travels through the umbilical vein in the mid-sagittal plane anteriorly and enters the left portal vein at the porta hepatis. There is a focal dilation at the end of the umbilical vein, called the umbilical recess. The UVC then passes into the ductus venosus, which originates cephalad from the left portal vein, opposite to or slightly to the right of the umbilical vein. The slight convexity to the right seen normally in the UVC course in an anteroposterior radiograph is at the point of entry into the ductus venosus. From the ductus venosus, the UVC travels through the middle or the left hepatic vein and enters the IVC, directly before the IVC-right atrial junction. In a lateral radiograph, the course of UVC resembles a ‘Hogarthian’ curve, extending from above the posterior portion of the right hemidiaphragm to the umbilicus. The point of direction change in the curve represents the point of entry of the UVC into the ductus venosus.5
Abnormal positioning of the UVC occurs frequently, as the catheter is inserted without imaging guidance. If the UVC is advanced too far, the catheter may reach various chambers of the heart or the great vessels. This occurs usually due to wrong calculation of the length of insertion or because of later migration of the catheter tip. Prematurity is a known risk factor for wrong estimation of UVC length, as the methods used to calculate length of insertion are not accurate in preterm infants.6 Weight loss and decrease in abdominal girth, repeated flushing, cardiopulmonary resuscitation and thoracic surgeries are some of the known risk factors for later migration of the catheter tip.7–9
The UVC can be misdirected before reaching the expected location of the IVC—the right atrial junction. The UVC can coil in the umbilical recess and travel retrograde in the umbilical vein towards the umbilicus. The catheter tip may enter the left or right portal vein, when an acute angulation and further transverse course of the UVC is seen in the radiograph. Rarely, the UVC can travel hepatofugally into the main portal vein and farther into the splenic or superior mesenteric vein.
In all the three cases of UVC-associated pleural effusion reported so far, the effusion has occurred later in the course.10–12 The possible mechanisms are erosion of the vessel wall or myocardium by the infused hyperosmolar fluid or because of the migration of the catheter through the vessel wall or myocardium. However, they may also occur due to direct perforation of a vessel or myocardium at the time of insertion.
In our baby, the catheter had travelled through the left portal vein and then breached the diaphragm to enter the thorax. The catheter would have failed to enter the ductus venosus, the site of difficult negotiation. As the pleural and pericardial compartments are not water-tight, fluid may enter from one cavity to the other, thus causing bilateral pleural and pericardial effusions. Ascites could have also resulted from the trickling of fluid from the thorax into the peritoneal cavity through the breach in the diaphragm created by the perforating UVC, and other diaphragmatic openings.
The tip of the UVC seeming to cross the midline and the more than usual convexity in the course of the catheter in the initial radiograph should have been given importance and evaluated. This case emphasises the importance of confirming the normal position of the catheter. The normal position should never be assumed, it should, rather, be proved. Even the slightest suspicion should prompt us to evaluate further with bedside sonography to confirm the UVC tip position.
Bedside sonography is becoming the gold standard to confirm UVC position.13 Radiography has been found to be unreliable, because the level of the IVC right atrial junction was seen to vary widely from T6-T11.6 Hoellering et al14 found that the cardiac silhouette method was superior to the vertebral level method to confirm the UVC position in the abdominal radiograph, but the diagnostic accuracy of both the methods was found to be less when compared to sonography. Studies have shown that sonography identified malposition of the catheter in cases in which the position of the catheter on the X-ray was found to be normal.6 13 However, the unavailability of a good ultrasound machine with appropriate probes and lack of expertise limit the use of sonography to confirm UVC position in many centres. Lateral abdominal radiograph, however, may not identify malposition of the UVC in all cases, and may still be used in those centres that do not have ready access to sonography.
Learning points.
The normal catheter tip position in an anteroposterior radiograph should never be assumed, but should, instead, be proved.
Even the slightest suspicion should prompt us to evaluate further using lateral X-ray and/or bedside sonography to confirm the catheter tip position.
Neonatologists should be trained to identify the catheter position in bedside sonography.
Any baby with umbilical catheters should be kept under constant monitoring for the development of complications as long as the catheter is in place.
Footnotes
Contributors: TA and MPS were involved in the conceptualisation of the manuscript, collecting patient data, conducting the literature search and drafting the manuscript. MK supervised the data collection, helped in the literature search and revised the manuscript for scientific content. All the authors were involved in clinical management of the patient.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Shukla H, Ferrara A. Rapid estimation of insertional length of umbilical catheters in newborns. Am J Dis Child 1986;140:786–8. [DOI] [PubMed] [Google Scholar]
- 2.Diamond LK, Allen FH, Thomas WO. Erythroblastosis fetalis VII. Treatment with exchange transfusion. N Engl J Med 1951;244:39–49. 10.1056/NEJM195101112440201 [DOI] [PubMed] [Google Scholar]
- 3.Paster S, Middleton P. Roentgenographic evaluation of umbilical artery and vein catheters. JAMA 1975;231:742–6. 10.1001/jama.1975.03240190046020 [DOI] [PubMed] [Google Scholar]
- 4.Dunn PM. Localization of the umbilical catheter by post-mortem measurement. Arch Dis Child 1966;41:69–75. 10.1136/adc.41.215.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker DB, Berdon VE, James LS. Proper localization of umbilical arterial and venous catheters by lateral roentgenograms. Pediatrics 1969;43:34–9. [PubMed] [Google Scholar]
- 6.Ades A, Sable C, Cummings S et al. . Echocardiographic evaluation of umbilical venous catheter placement. J Perinatol 2003;23:24–8. 10.1038/sj.jp.7210851 [DOI] [PubMed] [Google Scholar]
- 7.Hong EJ, Lee KA, Bae H et al. . Umbilical venous line-related pleural and pericardial effusion causing cardiac tamponade in a premature neonate: a case report. Korean J Pediatr 2006;49:686–90. 10.3345/kjp.2006.49.6.686 [DOI] [Google Scholar]
- 8.Salvadori S, Piva D, Filippone M. Umbilical venous line displacement as a consequence of abdominal girth variation. J Pediatr 2002;141:737 10.1067/mpd.2002.128111 [DOI] [PubMed] [Google Scholar]
- 9.Nowlen TT, Rosenthal GL, Johnson GL et al. . Pericardial effusion and tamponade in infants with central catheters. Pediatrics 2002;110:137–42. 10.1542/peds.110.1.137 [DOI] [PubMed] [Google Scholar]
- 10.Kumar N, Murki S. Bilateral pleural effusion complicating umbilical venous catheterization. Indian Pediatr 2013;50:1157–8. 10.1007/s13312-013-0286-7 [DOI] [PubMed] [Google Scholar]
- 11.Kua KL, Whitehurst RM, Alrifai W et al. . Pleural effusion as a complication of a remotely placed catheter in a preterm infant. J Perinatol 2013;33:982–4. 10.1038/jp.2013.76 [DOI] [PubMed] [Google Scholar]
- 12.Pabalan MJ, Wynn RJ, Reynolds AM et al. . Pleural effusion with parenteral nutrition solution: an unusual complication of an “appropriately” placed umbilical venous catheter. Am J Perinatol 2007;24:581–5. 10.1055/s-2007-992174 [DOI] [PubMed] [Google Scholar]
- 13.Pulickal AS, Charlagorla PK, Tume SC et al. . Superiority of targeted neonatal echocardiography for umbilical venous catheter tip localization: accuracy of a clinician performance model. J Perinatol 2013;33:950–3. 10.1038/jp.2013.96 [DOI] [PubMed] [Google Scholar]
- 14.Hoellering AB, Koorts PJ, Cartwright DW et al. . Determination of umbilical venous catheter tip position with radiograph. Pediatr Crit Care Med 2014;15:56–61. 10.1097/PCC.0b013e31829f5efa [DOI] [PubMed] [Google Scholar]


