Abstract
Objectives
Annually, tens of thousands of children are brought to emergency departments for unsupervised medicine ingestions. We assessed whether adding flow restrictors to liquid medicine bottles can provide additional protection against unsupervised medicine ingestions by young children, even when the child-resistant closure is not fully secured.
Study Design
From April – May 2012, we conducted a block randomized trial with a convenience sample of 110 3- and 4-year-old children from 5 local preschools. Participants attempted to remove test liquid from an uncapped bottle with a flow restrictor and a control bottle without a flow restrictor (with either no cap or an incompletely-closed cap).
Results
Ninety-six percent (25/26) of open controls and 82% of incompletely-closed control bottles (68/83) were emptied within 2 minutes. Only 6% (7/110) of bottles with flow restrictors were emptied during the 10-minute testing period, none before 6 minutes. Overall, children removed less liquid from bottles with flow restrictors than from open or incompletely-closed controls (both P < .001). All children assigned open controls and 90% assigned incompletely-closed controls removed ≥25 mL liquid. In contrast, 11% of children removed ≥25 mL liquid from uncapped bottles with flow restrictors. Older children (54 – 59 months) were more successful than younger children at removing ≥25 mL liquid (P = .002) from bottles with flow restrictors.
Conclusions
Findings suggest that adding flow restrictors to liquid medicine bottles limits the accessibility of their contents to young children and could complement the safety provided by current child-resistant packaging.
Keywords: Child injury, Pharmaceutical poisoning, Poison prevention, Medication packaging, Medication safety
The Poison Prevention Packaging Act (PPPA) of 1970 requires child-resistant packaging for most medicines in the United States.1 Since then, it has been estimated that child-resistant packaging has contributed to the prevention of hundreds, if not thousands, of pediatric deaths from unsupervised medication ingestions.2,3 Nevertheless, each year a half million calls are made to poison centers after young children find and ingest medicines.4 The number of emergency department (ED) visits for unsupervised medication ingestions is rising, with over 60,000 visits by young children annually.5,6 National data on the dose form of medicines involved in unsupervised ingestions are limited; however, approximately 80% of ED visits for ingestion of cough and cold medicines and 37% of visits for ingestion of acetaminophen products involved liquid medicines.7,8 A study of poison center calls found that liquid antibiotics prescribed for the child or a sibling were the most frequently ingested prescription medicines.9
Most ED visits for unsupervised medicine ingestions involve children younger than 5 years, with a peak incidence in 2-year-olds.10,11 While research on the circumstances surrounding these ingestions is limited, previous studies have shown that most occur in home environments,9,12-13 during a brief moment when the caregiver is not watching,13 and when medicines are not in their usual storage location.9,12-13 Children also gain access to medicines when caregivers do not use child-resistant packaging correctly (e.g., when caps are left off or are incompletely secured).9,14
The bottle-and-cap system commonly used in the United States for medication packaging requires the adult user to correctly re-engage the child-resistant closure each time the bottle is opened; otherwise the entire contents may be accessible. Flow restrictors, adapters added to the neck of a bottle to limit the release of liquid, have been suggested as a means to limit the amount of liquid medicine a young child could access even if the child-resistant closure is breached.5,15 Manufacturers began adding flow restrictors to over-the-counter (OTC) infants’ acetaminophen in 2011;16 however, the efficacy of flow restrictors in limiting accessibility of medicines to young children has not been assessed.
We sought to determine whether adding flow restrictors affects the proportion of preschool-aged children who can access bottle contents, the amount accessed, and the time required for children to empty bottles compared with traditional bottles without flow restrictors.
METHODS
Study Design
The standard child test protocol for re-closeable packages outlined in the Poison Prevention Packaging Act (hereafter PPPA protocol)17 was modified to assess the efficacy of flow restrictors in limiting children's access to liquid medicines. The study was approved by the institutional review board (IRB) of the Centers for Disease Control and Prevention with concurrence of the IRB of Emory University School of Medicine and the Research Oversight Committee of Grady Health System. Legal guardians provided written permission.
In the standard PPPA protocol, children participate in pairs and are asked to open a bottle with a child-resistant closure. In this study, children participated individually to ensure statistical independence between participants. Each child participated in two consecutive trials. In both trials, children were asked to “get everything out” of a bottle filled with a test liquid. To isolate the effect of flow restrictors and simulate improper child-resistant closure use, children were given an uncapped bottle with a flow restrictor (hereafter FR-bottle) for one trial. For the other trial, children were given a traditional bottle without a cap (open control) or with an incompletely-closed child-resistant cap (incompletely-closed control). To simulate what they might find at home, children were given the specific dosing device packaged with each bottle (dosing syringes with FR-bottles; dosing cups with control bottles).
Testers instructed children using a script based on the PPPA protocol. A second investigator recorded observations and timed the trials. If the child did not empty the bottle after 5 minutes, the tester demonstrated removal of liquid to simulate what a child might observe at home. As in the PPPA protocol, the tester then reminded the child that teeth could be used and gave the child 5 additional minutes to remove liquid. Once both trials were complete, children were given age-appropriate messages about medicine safety.
Participants and Setting
The study was conducted in a convenience sample of 5 preschools in the Atlanta metropolitan area in April and May 2012. Although the PPPA protocol includes children aged 42 to 51 months, to facilitate enrollment, permission forms and information materials were distributed to guardians of children in classrooms with students aged 36 to 59 months. As in the PPPA protocol, children with overt illnesses, injuries, or physical or mental disabilities (assessed by guardians) were excluded. Guardians also confirmed that their children were English speakers and had no dietary restrictions or allergies to test liquid ingredients.
Test Products
Flow restrictors, bottles, and dosing devices that were currently in use or in production for use with oral OTC liquid medicines in the United States were provided by three manufacturers (referred to as designs A, B, or C). One flow restrictor was a rubber septum which reseals after syringe removal. Another design contained a small orifice engineered to match a corresponding syringe. The third design incorporated a “lock-and-key” mechanism which requires alignment of a specific syringe and a flow restrictor with a self-closing valve (Figure 1).
Figure 1. Flow Restrictor Designs.
Flow restrictor designs viewed from an angle. These adapters are added to the neck of a standard liquid medicine bottle to limit the release of liquid. The flow restrictor depicted (*) is no longer on the marketed product.18 It has been enhanced to minimize the risk of the flow restrictor being pushed into the bottle when inserting the syringe.
Bottles were filled to their intended volume (two 30 mL bottles; one 120 mL bottle) with a test liquid with similar fluid characteristics to medicines for which the flow restrictors were intended (NesQuik Strawberry Syrup). Incompletely-closed control bottles were prepared at the preschools immediately before testing by aligning the threading on bottles and caps and rotating the “push-down-and-turn” caps 270 degrees clockwise, closing the bottle but not engaging the child-resistant locking mechanism.
Sample Size
The study was powered to detect a difference in the proportion of children who removed ≥5 mL of test liquid from their FR-bottle compared to their control bottle. The authors predicted that ≥5 mL of liquid would be removed from 90% of open control bottles, 50% of incompletely-closed control bottles, and 15% of FR-bottles. We calculated that 30 incompletely-closed / FR-bottle trials and 9 open control / FR-bottle trials for each of the 3 FR-designs (A, B, and C) would be required to achieve 80% power at 5% significance level.
Sample Allocation
Children were assigned bottles for testing by randomizing a fixed block size of 12 pairs to attain: even distribution of the 3 FR-bottle designs (A, B, or C) at each site; even distribution of FR-bottle designs throughout the duration of the testing period at each site; and 3:1 allocation of incompletely-closed (3) or open (1) control bottles across each FR-bottle design and the duration of the testing period at each site. Each assigned pairing of FR-bottle and control bottle was tested by two children. To ensure even distribution of testing order, one child tested an FR-bottle first and the other child tested a control bottle first. Prior to initiation of testing, each of the 5 sites was assigned a randomly selected block from 120 possible block permutations. If a block assignment was not completed at one testing site, the untested bottle assignments were completed at a subsequent site where additional participants were available.
Outcome Measures
Data included observational measures. The primary outcome measures were the proportions of children who emptied bottles, removed ≥25 mL (≥5 typical doses), and removed ≥5 mL (≥1 typical dose) of test liquid from FR-bottles compared with incompletely-closed control bottles or open control bottles. To determine the amount of liquid removed, bottles were weighed before the trials, after 5 minutes, after the full 10-minute testing period, or when emptied. Weights were converted to mL for analysis.
Secondary outcome measures included time required to empty the bottles and proportion of liquid removed. For FR-bottles, amounts of liquid removed by age, sex, and site and approaches used to remove liquid from bottles were also assessed. Although our primary objective was to assess the 3 flow restrictor designs in combination, we also assessed the amount of test liquid removed for each FR-bottle design.
Statistical Analysis
For the primary outcomes, a McNemar test for paired proportions was used to assess differences in the proportion of children who removed specific amounts of test liquid from FR-bottles compared with each type of control bottle. The sign test was used to assess differences in the proportion of liquid that was removed from FR-bottles compared with control bottles. To determine whether age, sex, or site were associated with removal of specified amounts of liquid from FR-bottles, χ2 or Fisher exact tests were used. Two-sided P values less than .05 were considered statistically significant. Data were analyzed using SAS version 9.2 (SAS Institute Inc).
RESULTS
Across the 5 sites, guardians of 120 children who met study inclusion criteria provided permission and 110 children (92%) participated; 5 were absent on testing days and 5 (all 3-year-olds) refused to participate. Participants’ mean age was 49 months (range, 36-59 months); 57% were boys (Table 1). Assignment to specific FR-bottle designs (A, B, or C) was similar by age and sex of participants and by site.
Table 1.
Participant Demographics
| Characteristic | No. (%) of Participants |
|---|---|
| Age (months) | |
| 36 - 41 | 14 (13) |
| 42 - 47 | 37 (34) |
| 48 - 53 | 23 (21) |
| 54 - 59 | 36 (33) |
| Sex | |
| Female | 47 (43) |
| Male | 63 (57) |
| Site | |
| 1 | 22 (20) |
| 2 | 22 (20) |
| 3 | 26 (24) |
| 4 | 18 (16) |
| 5 | 22 (20) |
| Total | 110 (100) |
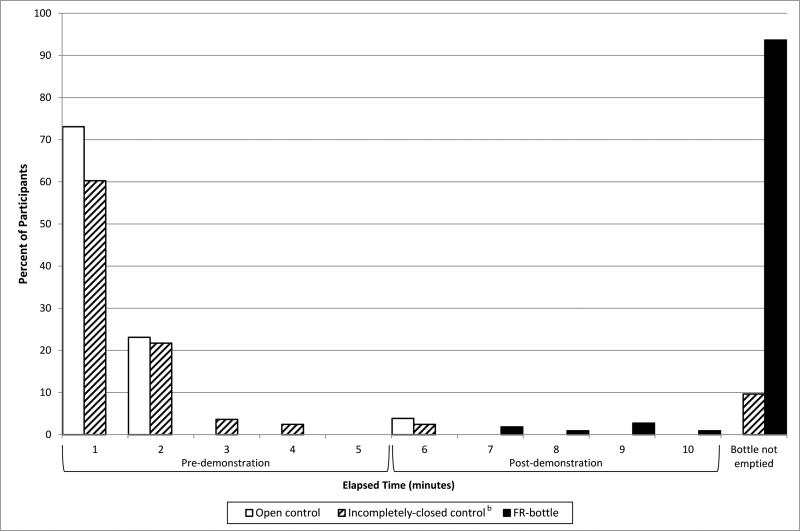
Children emptied incompletely-closed control bottles almost as frequently as they emptied open control bottles. Within 2 minutes, 96% of open controls (25/26) and 82% of incompletely-closed controls (68/83) were emptied (Figure 2). In contrast, none of the FR-bottles were emptied before 6 minutes. Only 7 children (6%) emptied an FR-bottle within the full 10-minute testing period.
Figure 2.
Time Required for Children to Empty Open Control Bottles, Incompletely-closed Control Bottles, and Bottles with Flow Restrictorsa
Abbreviations: FR-bottle, uncapped bottle with a flow restrictor
a Child testing of control bottles was ended and the bottles were considered empty when the tester noted pauses ≥1 second between drops of test liquid when fully inverted. Weighing of control bottles confirmed removal of ≥88% of test liquid in all cases. Flow restrictor bottles were manually inspected when a child who had been successfully removing test liquid appeared to be unable to remove additional amounts. If a bottle appeared to be empty by manual inspection, testing was ended. Weighing of flow restrictor bottles confirmed removal of ≥88% of test liquid in all cases.
b Time not recorded for 1 trial with an incompletely-closed control bottle.
The proportions of children who removed specified amounts of test liquid were lower for each FR-bottle design compared with control bottles. Among children who tested FR-designs A or B, 17% (6/36) removed ≥25 mL of liquid from each design (P < .001, compared to paired incompletely-closed controls). Twenty-two percent of children who tested design A (8/36) and one-third who tested design B (12/36) removed ≥5 mL of liquid (P < .001, and P = .001, respectively, compared to paired incompletely-closed controls). Only 1 child removed ≥5 mL of liquid (5.7 mL) from FR-design C (P <.001, compared to paired incompletely-closed controls).
Considering the 3 flow restrictor designs together, children removed less liquid from FR-bottles than from open control or incompletely-closed control bottles (P < .001 by sign test). All children assigned open controls (26/26) and 90% assigned incompletely-closed controls (76/84) removed ≥25 mL of liquid, almost always during the first 5-minute test period (Table 2). Overall, 12 children (11%) removed ≥25 mL of liquid from FR-bottles, but only 1 child did so during the first 5 minutes. Twenty-one children (19%) removed ≥5 mL of liquid from FR-bottles, but only 4 (4%) removed ≥5 mL within 5 minutes. Pairwise comparisons of removal of ≥25 mL liquid and ≥5 mL liquid from FR-bottles compared with each type of control bottle were statistically significant (P < .001).
Table 2.
Proportion of Children Who Removed ≥ 25 mL or ≥ 5 mL Test Liquid by Bottle Type
| ≥ 25 mL Removed | |||||||
|---|---|---|---|---|---|---|---|
| Bottle Pairing | Total | Pre-demonstration | Overall | ||||
| N | n | % | P Value | n | % | P Value | |
| Open control | 26 | 25 | 96 | <0.001 | 26 | 100 | <0.001 |
| FR-bottle | 0 | 0 | 1 | 4 | |||
| Incompletely-closed control | 84 | 74 | 88 | <0.001 | 76 | 90 | <0.001 |
| FR-bottle | 1 | 1 | 11 | 13 | |||
| ≥ 5 mL Removed | |||||||
|---|---|---|---|---|---|---|---|
| Bottle Pairing | Total | Pre-demonstration | Overall | ||||
| N | n | % | P Value | n | % | P Value | |
| Open control | 26 | 25 | 96 | <0.001 | 26 | 100 | <0.001 |
| FR-bottle | 0 | 0 | 2 | 8 | |||
| Incompletely-closed control | 84 | 76 | 90 | <0.001 | 77 | 92 | <0.001 |
| FR-bottle | 4 | 5 | 19 | 23 | |||
Abbreviations: FR-bottle, uncapped bottle with a flow restrictor.
Older children were more successful than younger children at removing ≥25 mL (P = .002) and ≥5 mL (P = .02) of liquid from FR-bottles. Of the 12 children who removed ≥25 mL of liquid, 10 were from the oldest age group (54 - 59 months). None of the youngest children (36 – 41 months) removed even 5 mL of liquid. No significant differences were detected in ability to remove ≥25 mL or ≥5 mL of liquid by sex or study site.
Children attempted a variety of strategies to remove liquid from FR-bottles, including using the dosing syringe (102/110; 93%); pouring, shaking, or squeezing from the bottle (66/110; 60%); using teeth or attempting to manually remove the flow restrictor (50/110; 45%); and drinking from the bottle (4/110; 4%). All children who removed ≥25 mL used the syringe. One child also used teeth to remove the flow restrictor and subsequently emptied the bottle.
DISCUSSION
Designing safety packaging that limits the amount of medication a child can remove even if a child-resistant closure is breached is a new approach to addressing unsupervised medication ingestions. To our knowledge, this is the first study to assess the efficacy of flow restrictors in limiting young children's access to liquid medicines. Compared with open bottles and bottles with incompletely-closed child-resistant caps, flow restrictors decreased the proportion of children who accessed liquid, and, for those who accessed liquid, flow restrictors decreased the amount of liquid that children accessed and increased the amount of time required to empty a bottle. Our findings suggest that adding flow restrictors to bottles with child-resistant closures could provide a complementary dose-limiting barrier for liquid medicines.
Standard child-resistant packaging is designed to prevent, or at least delay, young children from opening bottles for a “reasonable time” to increase the likelihood that caregivers may intervene.19 Two limitations of current re-closeable child-resistant packaging are reliance on caregivers to correctly re-secure the cap after every use and accessibility of the entire bottle contents once the cap is removed. Although data are limited, imperfect practices have been implicated in unsupervised ingestions,9,14 and in at least one study, 80% of ingestions occurred within 5 minutes.13 In this trial, when the “push-down-and-turn”-style child-resistant cap was not completely re-secured, 82% of children emptied their bottles within 2 minutes. The addition of flow restrictors delayed children from accessing bottle contents, even when there were no child-resistant caps on bottles. None of the children emptied an FR-bottle until over 6 minutes had elapsed (and after demonstration of liquid removal); only 6% emptied their FR-bottles within the full 10-minute testing period. The added time required to access contents from FR-bottles may provide an opportunity for caregiver intervention before substantial amounts are removed. Furthermore, study participants were asked to remove all liquid from their bottles and, as specified in the PPPA protocol, they were gently but repeatedly encouraged to keep trying. In a home environment, at least some young children might stop trying without such encouragement.
Flow restrictors also limited the dose that preschool-aged children accessed. For a 2-3 year-old child, the recommended dose of infants’ acetaminophen is 5 mL. Given an uncapped bottle, 10 minutes, and gentle encouragement, flow restrictors prevented 81% of participants from removing even a single 5 mL dose and 89% from removing 5 or more doses (≥25 mL). In contrast, an incompletely-closed control bottle prevented only 10% of participants from removing 5 or more doses.
Expanding the use of flow restrictors beyond infants’ acetaminophen could reduce the severity of ingestions and the number of children referred for costly emergency evaluation and treatment. The addition of flow restrictors on infants’ acetaminophen bottles may reduce parental distress and unnecessary emergency visits, but the reformulated concentration (160 mg/5 mL) and small bottle size (30 mL) limit the maximum available dose to non-toxic levels for most children. However, if a child younger than 5 years ingested of a full 120 mL bottle of liquid children's acetaminophen, he or she would likely be referred for emergency evaluation.20 The threshold dose for emergency evaluation is lower for other children's OTC medicines. Children younger than 5 years would likely be referred to an ED for suspected ingestion of half of a 120 mL bottle of children's diphenhydramine 21 and for a fifth of a 120 mL bottle of some dextromethorphan products.22
This study focused on assessing innovative safety packaging used with OTC pediatric liquid medicines because these medicines are intended for the children who are most at risk for unsupervised ingestions. Of course, children get into medicines other than OTC pediatric liquids. A study by Bailey et al suggests that current child-resistant closures may not provide sufficient protection for some medications, particularly opioids, that can be lethal to a young child at a single dose.23 Prescription medicines that are harmful to young children at low doses,24 such as opioid-containing liquid medicines, may be good candidates for incorporating flow restrictors in addition to child-resistant closures.
Our study has several limitations. First, to isolate the effect of flow restrictors and replicate the circumstances of improper use of child-resistant closures, we tested flow restrictors alone, without child-resistant caps. In practice, medicine bottles with flow restrictors are packaged with child-resistant closures, so we likely underestimated the efficacy for concurrent use of both safety barriers. Second, we did not assess the usability or acceptability of FR-bottles and accompanying dosing syringes with adults. While studies have shown that adults measure doses more accurately using oral syringes than with other devices,25,26 usability and acceptability of each design should be assessed. As with some early child-resistant closures, if adults cannot use them with relative ease, they circumvent them.27 Third, our results may not be generalizable to all FR-designs or all medication formulations and viscosities. Because we assessed products where a specific flow restrictor is mated to a bottle of a specific size, the effect of bottle size cannot be separated from FR-design. Specific flow restrictors, bottles, and their intended contents should be compatible and design-contents combinations should have efficacy demonstrated. Fourth, this study was not designed to assess differences in performance among FR-designs; however, findings from this study and feedback from user experience may inform future design refinements. Fifth, preschools were located in urban and suburban settings and served children from a range of socio-economic groups, but we did not assess the knowledge or experience of individual children with child-resistant closures or flow restrictors prior to study participation. Lastly, the experimental study design may also affect generalizability to all children and home settings. We were surprised that only 4 children tried to drink from FR-bottles, but we suspect that observation by adults may have discouraged them. The apparent reluctance to drink directly from bottles may also have been related to the children's age. In general, study participants were slightly older than the ages specified in the PPPA protocol (42-51 months). Younger children may have been more likely to put the bottles in their mouths, but younger children have not participated reliably in previous studies.28,29 Nonetheless, because none of the youngest children in our study (36-41 months) removed even 5 mL of liquid and most ED visits for unsupervised medicine ingestion are by still younger children (1- and 2-year-olds),10,11 we likely underestimated the efficacy of flow restrictors for the children who are at greatest risk.
Conclusions
Child-resistant caps are efficacious in delaying children from accessing medicines only when completely re-secured after every use. Our findings suggest that flow restrictors may limit the amount of liquid medicine that a young child can access even when the child-resistant closure is not fully secured; future studies might focus on application of similar passive engineering and dose-limiting features to solid-dose medicine packaging. Importantly, flow restrictors are designed as a secondary barrier and caregivers should not rely on flow restrictors alone. While improved packaging can limit ingestions, educational interventions should continue to highlight the importance of locking child-resistant caps after every use and storing medicines up and away and out of sight of young children.30
Acknowledgements
We thank the children, and school administrators, staff, and teachers who participated in and assisted with this study. We also thank the participating pharmaceutical and packaging manufacturing companies for providing flow restrictors, bottles, and dosing devices for evaluation and for advising on issues related to product specifications (Mark Plezia, Associate Director Research and Development, McNeil Consumer Healthcare and Ed Kuffner, Vice President Medical Affairs and Clinical Research, McNeil Consumer Healthcare; David A. Manera, Innovations Manager, Comar, Inc.; and Amit Shah, Technical Advisor, Accudial Pharmaceutical, Inc., and Mark D. Kairalla, Project Manager, Accudial Pharmaceutical, Inc.). We also thank David Kleinbaum, PhD and Jonathan Edwards, MStat of CDC for statistical consultation and Andrew Geller, MD of CDC for thoughtful review of the manuscript. Packaging improvements to prevent unsupervised medicine ingestions have been a key activity of the CDC-led public-private PROTECT Initiative (http://www.cdc.gov/medicationsafety/protect/protect_initiative.html) and we thank PROTECT members for valuable discussions that helped shape this study.
Abbreviations and Acronyms
- ED
Emergency Department
- FR-bottle
Uncapped bottle with a flow restrictor
- IRB
Institutional Review Board
- OTC
Over-the-Counter
- PPPA
Poison Prevention Packaging Act
Footnotes
Conflict of Interest Disclosures: None reported
Manuscript Authorship: The first draft of the manuscript was written primarily by Maribeth C. Lovegrove and Daniel S. Budnitz. Only the listed authors contributed to writing the manuscript.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Poison Prevention Packaging Act, 15 USC §. 1970:1471–1476. [Google Scholar]
- 2.Rodgers GB. The safety effects of child-resistant packaging for oral prescription drugs. Two decades of experience. JAMA. 1996;275:1661–1665. [PubMed] [Google Scholar]
- 3.US Consumer Product Safety Commission . Poison prevention packaging: a guide for healthcare professionals. US Consumer Product Safety Commission; Washington DC: 2005. [Google Scholar]
- 4.Bronstein AC, Spyker DA, Cantilena LR, Jr, Green JL, Rumack BH, Dart RC. 2010 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 28th annual report. Clin Toxicol (Phila) 2011;49:910–941. doi: 10.3109/15563650.2011.635149. [DOI] [PubMed] [Google Scholar]
- 5.Budnitz DS, Salis S. Preventing medication overdoses in young children: an opportunity for harm elimination. Pediatrics. 2011;127:e1597–1599. doi: 10.1542/peds.2011-0926. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC) National Poison Prevention Week: 50th anniversary – March 18-24, 2012. MMWR Morb Mortal Wkly Rep. 2012;61:177. [Google Scholar]
- 7.Shehab N, Schaefer MK, Kegler SR, Budnitz DS. Adverse events from cough and cold medications after a market withdrawal of products labeled for infants. Pediatrics. 2010;126:1100–1107. doi: 10.1542/peds.2010-1839. [DOI] [PubMed] [Google Scholar]
- 8.Budnitz DS, Lovegrove MC, Crosby AE. Emergency department visits for overdoses of acetaminophen-containing products. Am J Prev Med. 2011;40:585–592. doi: 10.1016/j.amepre.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 9.Jacobson BJ, Rock AR, Cohn MS, Litovitz T. Accidental ingestions of oral prescription drugs: a multicenter survey. Am J Public Health. 1989;79:853–856. doi: 10.2105/ajph.79.7.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schillie SF, Shehab N, Thomas KE, Budnitz DS. Medication overdoses leading to emergency department visits among children. Am J Prev Med. 2009;37:181–187. doi: 10.1016/j.amepre.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 11.Chien C, Marriott JL, Ashby K, Ozanne-Smith J. Unintentional ingestion of over the counter medications in children less than 5 years old. J Paediatr Child Health. 2003;39:264–269. doi: 10.1046/j.1440-1754.2003.00148.x. [DOI] [PubMed] [Google Scholar]
- 12.Wiseman HM, Guest K, Murray VS, Volans GN. Accidental poisoning in childhood: a multicentre survey. 2. The role of packaging in accidents involving medications. Hum Toxicol. 1987;6:303–314. doi: 10.1177/096032718700600407. [DOI] [PubMed] [Google Scholar]
- 13.Ozanne-Smith J, Day L, Parsons B, Tibballs J, Dobbin M. Childhood poisoning: access and prevention. J Paediatr Child Health. 2001;37:262–265. doi: 10.1046/j.1440-1754.2001.00654.x. [DOI] [PubMed] [Google Scholar]
- 14.Lembersky RB, Nichols MH, King WD. Effectiveness of child-resistant packaging on toxin procurement in young poisoning victims. Vet Hum Toxicol. 1996;38:380–383. [PubMed] [Google Scholar]
- 15.Bond GR, Woodward RW, Ho M. The growing impact of pediatric pharmaceutical poisoning. J Pediatr. 2012;160:265–270. doi: 10.1016/j.jpeds.2011.07.042. [DOI] [PubMed] [Google Scholar]
- 16.OTC industry announces voluntary transition to one concentration of single-ingredient pediatric liquid acetaminophen medicines [news release] Consumer Healthcare Products Association; Washington, D.C.: May 4, 2011. [August 27, 2012]. http://www.chpa-info.org/05_05_11_PedAceConv.aspx. [Google Scholar]
- 17.Code of Federal Regulations, 16 CFR §. :1700.20. [Google Scholar]
- 18.Grape Due to Dosing System Complaints [news release] McNeil Consumer Healthcare Division of McNEIL-PPC, Inc.; Fort Washington, PA: Feb 17, 2012. [August 27, 2012]. McNeil Consumer Healthcare Announces Voluntary Nationwide Recall of Infants' TYLENOL® Oral Suspension, 1 oz. http://www.tylenol.com/page2.jhtml?id=tylenol/news/subp_tylenol_recall_8.inc. [Google Scholar]
- 19.Code of Federal Regulations, 16 CFR §. :1700.1. [Google Scholar]
- 20.Dart RC, Erdman AR, Olson KR, Christianson G, Manoguerra AS, Chyka PA, et al. Acetaminophen poisoning: an evidence-based consensus guideline for out-of-hospital management. Clin Toxicol (Phila) 2006;44:1–18. doi: 10.1080/15563650500394571. [DOI] [PubMed] [Google Scholar]
- 21.Scharman EJ, Erdman AR, Wax PM, Chyka PA, Caravati EM, Nelson LS, et al. Diphenhydramine and dimenhydrinate poisoning: an evidence-based consensus guideline for out-of-hospital management. Clin Toxicol (Phila) 2006;44:205–223. doi: 10.1080/15563650600585920. [DOI] [PubMed] [Google Scholar]
- 22.Chyka PA, Erdman AR, Manoguerra AS, Christianson G, Booze LL, Nelson LS, et al. Dextromethorphan poisoning: an evidence-based consensus guideline for out-of-hospital management. Clin Toxicol (Phila) 2007;45:662–677. doi: 10.1080/15563650701606443. [DOI] [PubMed] [Google Scholar]
- 23.Bailey JE, Campagna E, Dart RC. RADARS System Poison Center Investigators. The underrecognized toll of prescription opioid abuse on young children. Ann Emerg Med. 2009;53:419–424. doi: 10.1016/j.annemergmed.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 24.Michael JB, Sztajnkrycer MD. Deadly pediatric poisons: nine common agents that kill at low doses. Emerg Med Clin North Am. 2004;22:1019–1050. doi: 10.1016/j.emc.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Yin HS, Mendelsohn AL, Wolf MS, Parker RM, Fierman A, van Schaick L, et al. Parents' medication administration errors: role of dosing instruments and health literacy. Arch Pediatr Adolesc Med. 2010;164:181–6. doi: 10.1001/archpediatrics.2009.269. [DOI] [PubMed] [Google Scholar]
- 26.Sobhani P, Christopherson J, Ambrose PJ, Corelli RL. Accuracy of oral liquid measuring devices: comparison of dosing cup and oral dosing syringe. Ann Pharmacother. 2008;42:46–52. doi: 10.1345/aph.1K420. [DOI] [PubMed] [Google Scholar]
- 27.Requirements for the special packaging of household substances: final rule. Fed Regist. 1995;60:37710–37744. Codified at 16 CFR § 1700. [Google Scholar]
- 28.Done AK, Jung AL, Wood MC, Klauber MR. Evaluations of safety packaging for the protection of children. Pediatrics. 1971;48:613–628. [PubMed] [Google Scholar]
- 29.Thien WMA, Rogmans WHJ. Testing child resistant packaging for access by infants and the elderly. Accid Anal Prev. 1984;16:185–190. [Google Scholar]
- 30.UpAndAway.org. [August 31, 2012];Put your medicines up and away and out of sight. http://www.upandaway.org/.