Abstract
Hurler-Scheie syndrome is a rare lysosomal storage disease affecting the cardiovascular system. Besides the cardiac manifestations, it presents with complications from abnormal proteoglycan deposition in soft tissues in many locations, resulting in joint contractures, paraplegia, impaired vision, airway narrowing and restrictive lung function, to name a few. There are very few reports of surgical management of valvular heart disease due to mucopolysaccharidosis (MPS). We describe the successful management of a patient with an extremely challenging case of mitral valve stenosis and a giant left atrial appendage aneurysm due to MPS type 1 (Hurler-Scheie syndrome). The patient underwent mitral valve replacement and excision of the giant left atrial appendage aneurysm; a similar case has not been previously reported.
Background
Mucopolysaccharoidosis type 1 (MPS type 1 H-S), or Hurler-Scheie syndrome, is a subtype of a hereditary metabolic condition leading to accumulation of mucopolysaccharides in many tissues, including those of the joints and heart.1 Almost 76% of patients with Hurler-Scheie syndrome have cardiac abnormalities.2 It primarily affects the mitral valve, followed by aortic valve and coronary arteries, and, occasionally, results in dilation of the aorta. There are very few case reports on surgical management of valvular heart disease, due to the life-limiting nature of the underlying metabolic condition. There is some variability in the prognosis and presentation of the various subtypes of MPS type 1.
Case presentation
A 24-year-old woman, a law student, with Hurler-Scheie syndrome, on enzyme replacement therapy (laronidase), presented with orthopnoea, paroxysmal nocturnal dyspnoea and stridor. She weighed 22 kg and was 113 cm tall (body surface area 0.8 m2). Previous procedures included a right corneal graft, adenoidectomy and occipitocervical fusion with laminectomy at C1-C2. She had significant kyphoscoliosis, upper tracheal stenosis and pectus excavatum. She was diagnosed with mixed mitral valve disease that had progressively worsened with increasing pulmonary artery pressures despite conservative treatment optimisation.
Investigations
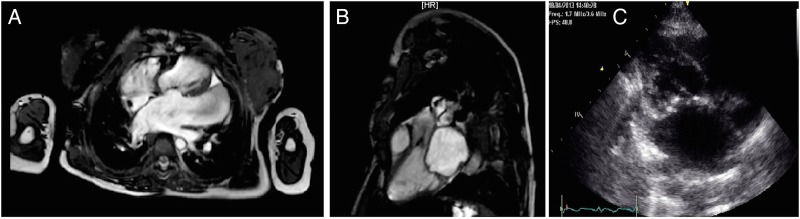
Transthoracic echocardiogram demonstrated severe mitral stenosis. The patient also had a large left atrial appendage aneurysm of 8 cm (video 1). The mitral valve area was calculated to be 0.9 cm2 with a mean pressure gradient, across the valve, of 14.52 mm Hg. The pulmonary artery pressure was 50 mm Hg, which was two-thirds systemic (80 mm Hg). Her forced expiratory volume in 1 s and forced vital capacity were 0.3 L (24%) and 0.33 L (22.4%), respectively, suggesting a severe restrictive lung defect, and chest CT demonstrated functional tracheomalacia. Her cervical spinal MRI did not reveal any spinal compression; the nasoendoscopy of her upper airway did reveal significant swelling of the supraglottic and glottic areas, with tracheomalacia distally. Cardiac MRI confirmed severe mitral stenosis, a large left atrial aneurysm and mild aortic stenosis (figure 1A, B). Coronary angiogram demonstrated normal coronary arteries.
Figure 1.
(A) Cardiac MRI demonstrating huge left atrium and appendage aneurysm. (B) Cardiac MRI showing left atrium and left ventricle (in diastole). (C) Transthoracic echocardiogram—modified parasternal long-axis view of the mitral valve.
Video 1.
Transthoracic echocardiogram—four-chamber view demonstrating the left atrium and the left atrial appendage aneurysm.
Differential diagnosis
At an adult congenital heart disease multidisciplinary team (MDT) meeting, it was felt that, in view of a life expectancy of a further 10–15 years, this patient's mitral stenosis was currently the life-limiting condition and hence she would benefit from corrective surgery.
Treatment
Shortly after the MDT meeting, the patient presented with a clot in her left atrial appendage aneurysm, despite being on therapeutic warfarin (international normalised ratio 2–3), and needed urgent surgery. She required fibreoptic intubation with a size 4.5 mm endotracheal tube. We placed electrodes to monitor the motor-evoked potentials of her limbs, in view of potential distant spinal ischaemia due to previous occipitocervical fusion. The mean arterial pressure was kept high throughout the surgery, to maintain adequate spinal perfusion. The mitral valve was approached through the left atrium. The mitral valve, subvalvar chordal apparatus and the tips of the two papillary muscles were resected. Owing to the extremely small mitral annulus, a size 17 mm aortic prosthesis (St Jude Regent aortic mechanical valve) was selected and reversely sutured into the mitral position. The left atrial appendage aneurysm was then resected. The patient was weaned off bypass and a tracheostomy was performed by the paediatric otolaryngologist. The patient was transferred to the intensive care unit, where a transthoracic echocardiogram with limited views was performed. This showed a well-seated mitral position valve with a small paravalvular leak but no signs of a significant pressure gradient across the valve. She was decannulated from her tracheostomy on the 15th postoperative day. She was discharged home on the 37th postoperative day. Prior to discharge, a repeat echocardiogram showed that the valve remained well seated with no evidence of regurgitation, with a mean pressure gradient of 5.29 mm Hg. The mitral valve E wave velocity was 1.86 m/s and A wave velocity was 1.04 m/s.
Outcome and follow-up
Now, 10 months following surgery, the patient is New York Heart Association class I, with much-improved quality of life and satisfactory findings on transthoracic echocardiogram (figure 1C).
Discussion
There are six subtypes of MPS based on genetic, clinical and biochemical evaluation.3 4 MPS type 1 is an autosomal recessive disorder due to the deficiency of α-l-iduronidase.
Cardiac involvement, especially valvular stenosis and/or insufficiency, is very common,2 but other cardiac pathology includes diastolic dysfunction preceded by hypertrophied myocardium and abnormal interstitial deposition of MPS in the myocardium.
Balloon mitral valvuloplasty was considered but, due to the perceived risk of rupture and lack of literature, not advised. Replacement of the cardiac valves can be challenging due to small size and rigidity of the annulus, as in our case. The tissue quality of the annulus can be poor, and use of pericardial patch reinforcement has been reported.5 Careful postoperative monitoring is essential due to diastolic dysfunction of the left ventricle. Extracardiac problems relate to skeletal deformities including fused spine, short neck, macroglossia, adenoids, narrow airway due to abnormal proteoglycan deposition in the trachea, interstitial fibrosis of the lungs and restrictive lung function from chest wall rigidity. Through careful multidisciplinary preoperative planning, our patient underwent a fibreoptic intubation and subsequent planned open tracheostomy at the end of the valve operation,5 on top of steroids to prevent laryngeal oedema.
Learning points.
Cardiac involvement is common in mucopolysaccharoidosis.
Surgery is challenging due to small body size, abnormal anatomy and poor tissues, so a thorough preoperative assessment is essential.
Although high risk, valve replacement surgery can give good outcome in patients who are thought to have life-limiting valvular disease.
Footnotes
Contributors: AB was the primary writer of the paper. RH provided surgical care to the patient and helped provide information needed to write the paper. PJ provided the medical care of the patient and helped provide the information needed to write the paper. AH provided surgical care of the patient and helped provide information needed to write the paper. He also amended and approved the final draft.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Yano S, Moseley K, Pavlova Z. Postmortem studies on a patient with mucopolysaccharidosis type I: histopathological findings after one year of enzyme replacement therapy. J Inherit Metab Dis 2009;32(Suppl 1):S53–7. 10.1007/s10545-009-1057-4 [DOI] [PubMed] [Google Scholar]
- 2.Rigante D, Segni G. Cardiac structural involvement in mucopolysaccharidoses. Cardiology 2002;98:18–20. doi:64674 [DOI] [PubMed] [Google Scholar]
- 3.Kitabayashi K, Matsumiya G, Ichikawa H et al. Surgical treatment for mitral stenosis in Scheie's syndrome: mucopolysaccharidosis type I-S. Ann Thorac Surg 2007;84:654–5. 10.1016/j.athoracsur.2007.03.042 [DOI] [PubMed] [Google Scholar]
- 4.Murashita T, Kobayashi J, Shimahara Y et al. Double-valve replacement for Scheie's syndrome subtype mucopolysaccaridosis type 1-S. Ann Thorac Surg 2011;92:1104–5. 10.1016/j.athoracsur.2011.03.051 [DOI] [PubMed] [Google Scholar]
- 5.Minakata K, Konishi Y, Matsumoto M et al. Surgical treatment for Scheie's syndrome (mucopolysaccharidosis type I-S): report of two cases. Jpn Circ J 1998;62:700–3. 10.1253/jcj.62.700 [DOI] [PubMed] [Google Scholar]