Abstract
Aim
One way to assess foetal health of smokers is to ask mothers to count perceived movements, an unreliable method hiding differences in prenatal development. The aim of this pilot study was to assess subtle foetal movements in ultrasound scans and establish whether they differ in foetuses of mothers who smoked and nonsmoking mothers.
Methods
This longitudinal pilot study recruited twenty mothers (16 nonsmoking; 4 smoking) scanned four times from 24 to 36 weeks gestation (80 ultrasound scans). Two types of fine-grained movements were coded offline and analysed using a Poisson log-linear mixed model.
Results
Foetuses of smoking mothers showed a significantly higher rate of mouth movements compared to foetuses of nonsmoking mothers (p = 0.02), after controlling for maternal stress and depression. As pregnancy progressed, these differences between the smoking and nonsmoking groups widened. Differences between the two groups in the rate of foetal facial self-touch remained constant as pregnancy progressed and were borderline significant (p = 0.07).
Conclusion
Rates of foetal mouth movement and facial self-touch differ significantly between smokers and nonsmokers. A larger study is needed to confirm these results and to investigate specific effects, including the interaction of maternal stress and smoking. Additionally, the feasibility of this technique for clinical practice should be assessed.
Keywords: Fine-grained foetal movements, Foetal 4D ultrasound, Maternal stress, Smoking
Introduction
Cigarette smoking has proven harmful effects on foetal development (1). Despite antismoking advice to pregnant women having intensified over the past two decades, rates of maternal smoking have remained relatively constant, as shown in a Cochrane systematic review carried out by Lumley, Oliver, Chamberlain and Oakley (2). A more recent review (3) cites differences in smoking prevalence between affluent classes and socially disadvantaged women. Although smoking rates have significantly declined for women of higher social classes, pregnant women from lower social classes still continued smoking with the same rate. The most recent statistics indicate that there is variability of smoking at delivery in the UK as a whole (12%) with the much higher percentage of 20.9% observed in the Durham, Darlington and Tees Health authorities (UK; (4)).
Decreased oxygenation of foetal blood because of foetal exposure to cigarette smoke affects the neurological development of the foetus in general (5) with atypical autonomous regulation at the cellular and organ levels (6). More specific effects of smoking during pregnancy include differential heart rate variability (7) and breathing movements (1). Hofhuis et al. (1) reported that exposure to tobacco smoke reduced the frequency of foetal breathing movements and resulted in delayed maturation of the lungs, evident following birth.
Exposure to smoking is associated with anatomical differences. Lampl et al. (8), comparing foetuses of 10 smokers and 24 nonsmokers at 23, 27 and 32 weeks, found exposure was significantly associated with early growth acceleration in head and abdominal diameters, altered head shape, longer arms and shorter legs as well as a reduced tibia/femur ratio. These foetal body growth patterns were attributed to the foetus of smokers being in a state of chronic hypoxia (8). Habek (9) found, when comparing brisk to sluggish foetal movements, that foetuses of nonsmoking mothers showed significantly more brisk movements. Foetuses of heavy smokers made significantly fewer isolated head and arm movements. In contrast, foetuses of relatively light smokers (around 10 cigarettes or fewer per day) and nonsmokers did not differ significantly in their rate of spontaneous isolated head and arm movements. However, these results might have to do with the fact that the movements coded were relatively gross and an analysis of finer movements might reveal more specific data. The more fine-grained analysis is especially important in the light of findings indicating that even relatively small quantities of nicotine affect brain development. Specifically, Garvey and Longo (10) found that even low-level exposure to carbon monoxide affects the growth and development of the foetus. Hence, the importance of comparing the development of fine-grained foetal movements which might not be perceived by the mother of foetuses exposed to cigarettes and those not exposed.
Key notes
This longitudinal pilot study investigated foetal subtle movements using 4D ultrasound scans of smoking and nonsmoking mothers
Results showed that foetal facial movements and self-touch appear to differentiate between foetuses of smoking and nonsmoking mothers, with more movement in the foetuses of smoking mothers.
The results of the proof-of-concept study warrant replication with a larger sample.
Although women are generally advised to stop smoking during pregnancy (11), one study found that as a result of stress a sizable number of pregnant women smoke with increased frequency (12). Accordingly, there may be an interaction between smoking, stress and foetal movements. Stress has been found to influence neurobehavioural development (13). Indeed, one study of maternal stress and foetal movements (smooth and jerky upper limb movements) found that elevated stress in foetuses resulted in jerkier arm movements (14). High levels of prenatal maternal stress may be a risk factor for developmental disorders postnatally, as stress alters the biochemical equilibrium in the uterus (15). To summarise, maternal stress during pregnancy results in increased levels of maternal cortisol and is a potential mechanism for perturbing foetal brain development which might lead to altered cortical laterality in the offspring (15). Similarly to stress, maternal depression has been implicated as a factor in foetal development and movement, with foetuses of depressed mothers being significantly more active in the fifth, sixth and seventh months of gestation (16).
The proposed new method of coding foetal movements might highlight subtle differences which are missed with conventional methods investigating the effects of smoking. Although maternal perception of foetal movements has been used to establish whether foetuses are affected by maternal behaviours including smoking (17), these perceptions are not specific enough to compare movements of foetuses exposed to cigarette smoke (18). Indeed, a review of foetal movement counting, which asked mothers to quantify foetal movements they perceived during pregnancy, was inconclusive (17). This was because maternal counting of movements was not related in any studies to health outcome at birth. Furthermore, even when comparing Doppler measures of foetal movements with maternal perception of foetal movements, mothers were only able to report 16% of the movements which had been detected with Doppler measurements (18). In this pilot study, we suggest that the examination of fine-grained movements using 4D scans will provide a more objective measure of prenatal movement especially when particular areas (such as the face) of the foetus are examined. Frequency counts of specific types of facial movement can be determined and coded reliably.
Findings regarding foetal movements indicate that the development of neurological functions can be inferred from the development of normal foetal movement, which include general movement, as well as more specific facial touch and foetal facial movement (19). The current longitudinal preliminary cohort study examines the effects of smoking upon two types of fine-grained foetal movement, namely foetal mouth movements and foetal facial self-touch, during the second and third trimesters of pregnancy. As stress and depression are identified in the literature as additional factors, these were recorded at each scan and are adjusted for in the analysis.
Methods
Participants
Mothers who had completed normal 20-week anomaly scans were invited to participate in this study. Twenty mothers were recruited, including four mothers smoking during pregnancy (2 boys and 2 girls) and sixteen nonsmoking mothers (8 girls and 8 boys). All mothers who agreed to participate completed the study and attended all four scans. Participants were recruited through the sonographers of the antenatal unit of the James Cook University Hospital, Middleborough, UK, following approved ethical procedures. During consent and before each procedure, mothers were made aware that additional scans were for research purposes and were not routine medical scans. No information was collected on those who did not respond to the invitation to participate.
Procedure
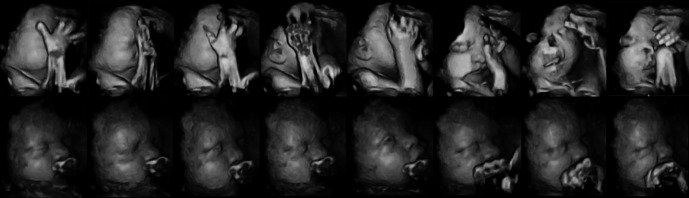
All participating mothers received four additional scans at 24, 28, 32 and 36 weeks gestational age, with foetuses being scanned in the morning for approximately 15–20 minutes. The scans took place in the radiography department, where mothers had previously undergone their routine 12 and 20 week medical scans, with the mothers lying in a darkened room on their back or on their side, depending on the position of the foetus and how comfortable mothers were. The foetal face and upper torso were visualised resulting in full frontal or facial profile of the foetus by means of 4D colour ultrasound recordings. Additionally, the sequences of traditional monochrome images were recorded in 2D. 4D scans provide a detailed frame by frame view of a precise area of a foetus, specifically the face. Both were recorded for off line analysis with a GE Voluson E8 Expert Ultrasound System using a GE RAB4–8L Macro 4D Convex Array Transducer, with a frame rate of 2 Hz, field of view of 70o and transducer frequency of 2–8 MHz. Frames are delivered every 0.5 of a second. Figure1 provides selected images of a showing movements of a foetus of a smoking mother and a foetus of a nonsmoking mother over a ten-second period, which illustrates the quality of the images permitting the reliable coding of mouth movements and facial touch behaviour. Foetuses did not change uterine position during the length of the scan, but some foetuses changed uterine position from one gestational age to the next. Mothers were provided with a DVD copy of their scans.
Figure 1.
Illustrative 4D scan frames of a 32-week-old foetus of a smoking mother (top line) and a 32-week-old foetus of a nonsmoking mother (bottom line) over a 10-second period of observation.
Measures
Scan recordings were used to code mouth movements and facial touch behaviours using an adaptation of the Facial Action Coding System (20) found to be reliable in previous research (19,21). Following established procedures, 11 types of mouth movements were identified: upper lip raiser, lip pull, lip corner depressor, lower lip depressor, lip pucker, tongue show, lip stretch, lip pressor, lips parting, mouth stretch and lip suck (21). Because of variations in these movements, with some occurring rarely we did not distinguish between mouth movements but analysed them as generic mouth movements. The number of face touches with either hand of the foetus was also recorded.
Mothers completed the Perceived Stress Scale (PSS) questionnaire, assessing stress levels at each scan (22). The PSS is a widely used valid and reliable 10 item five-point Likert-based scale (ranging from 0 = ‘no stress’ experienced during the last month to 4 = ‘very often’ stressed), measuring the degree to which mothers perceive their life as stressful. The PSS score ranges from 0 (minimum) to 40 (maximum). Additionally, mothers completed the Hospital Anxiety and Depression Scale (HADS) (23). The scale has seven items and ranges from 0 (minimum) to 21 (maximum). Following established methods, smoking status at each scan was assessed by maternal report and verified in the hospital notes.
Ethics
Ethical permission for the study was obtained from the County Durham and Tees Valley 2 Research Ethics Committee (REC Ref: 08/H0908/31 and REC Ref: 11/NE/03/61) and the research and development department of James Cook University Hospital, as well as the Durham University (Department of Psychology ethics committee). All mothers gave informed written consent.
Reliability
Reliability of scan coding was assessed using Cohen's Kappa (κ), with 44% of scans of nonsmoking mothers and 33% of scans of smoking mothers assessed independently by a trained coder. Reliability estimates for mouth movements were very high for nonsmokers κ = 0.90, (range 0.70–1) and for smokers κ = 0.87, (range 0.70–1). Similarly, reliability estimates for facial touch behaviours were very high for nonsmokers κ = 0.88, (range 0.70–1) and for smokers κ = 0.90 (range 0.85–1).
Statistical analysis
Reflecting the longitudinal, repeated structure of the measurements, a Poisson log-linear mixed effects analysis (24) was used to assess differential changes in development, expressed as the rate of movement over gestational age between the smoking and nonsmoking groups. The analysis models the number of events – of foetal mouth movement events or facial self-touch events – as a count variable adjusted by the length of scan as an exposure variable, with covariates of gestational age, sex, maternal age, stress and depression, together with an interaction between gestational age and smoking status to allow for differential development and a random individual foetus effect. The individual random foetus effect essentially allows for individual variability between foetuses in their propensity to the event and is assumed to be normally distributed.
Formally, we can write the model as
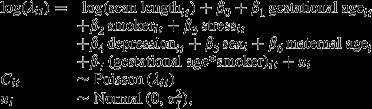 |
where Cit are the event counts for foetus i at gestational age t, λit is the underlying Poisson rate, β0 to β7 are unknown regression coefficients, and 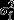 is the individual foetus variance. The indices to the individual covariates show which of them vary over time in our model. Thus, gestational age, stress and depression are measured at each scan, whereas the others are measured once.
is the individual foetus variance. The indices to the individual covariates show which of them vary over time in our model. Thus, gestational age, stress and depression are measured at each scan, whereas the others are measured once.
To fit the model, we centre the gestational age variable at the midpoint of 30 weeks, so that the effect of smoking which is measured by the parameter β2 is assessed at the midpoint of the study. The parameter β7 measures the extra change in slope in gestational age of the participant being a smoker.
Mixed effects modelling was carried out using the glmer function (25) in the lme4 package of the statistical package R. Testing between models was carried out using likelihood ratio tests, allowing differences in deviance to be compared to a chi-squared distribution on the appropriate number of degrees of freedom. Parameter estimates were interpreted by exponentiating them, with the resultant values interpreted as multiplicative effects on the event rates. Confidence intervals for 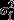 are typically nonsymmetric, and so a 95% confidence interval is reported for this parameter rather than a standard error.
are typically nonsymmetric, and so a 95% confidence interval is reported for this parameter rather than a standard error.
Results
Demographics
Nonsmoking participants had a mean age of 29.4 years (range 19–40 years), and smoking participants had a mean age of 27.0 years (range 20–36 years), with no significant difference between the groups (t = 0.67; df = 18, p = 0.51). Smoking status was measured at each scan, but there was no change over time in this variable – all smokers remained smokers, and all nonsmokers remained nonsmokers. Smoking mothers smoked a mean of 14.25 cigarettes a day over the four scans; this is similar to the reported mean of 12 cigarettes a day for women in 2012 in Great Britain reported by Action on Smoking and Health (26). The first research scan of smoking mothers was performed at a mean age of 24.2 weeks (range 23.4–25.2 weeks); the second at 28.0 weeks (range 27.4–28.6 weeks); the third at 32.0 weeks (range 31.2–33.2); the fourth at 35.9 weeks (range 35.2–36.7 weeks). For nonsmoking mothers, research scans were performed at nearly identical times; the first at a mean age of 24.1 weeks (range 23.5–25.6 weeks); the second at a mean age of 28.1 weeks (range 27.5–28.5 weeks); the third at a mean age of 32.0 weeks (range 31.1–32.5 weeks); the fourth at a mean age of 36.1 weeks (range 35.6–36.5 weeks). In total, 77 scans were analysed over 20 foetuses (the position of the foetus in three scans did not allow data to be extracted).
All foetuses were clinically assessed and declared to be healthy after birth. Foetuses of nonsmokers were born at a mean of 39.6 weeks (range 37–42 weeks) gestational age, similar to nonsmokers, with a mean of 39.9 (t = 0.29 on 18 df, p = 0.18). Apgar scores were measured at 1 and 5 minutes were 9 in both smokers and nonsmokers. There was no significant difference in the mean birthweight of foetuses of nonsmokers (3313 grams; SD 488 grams) and smokers (3255 grams; SD 512 grams) (t = 0.21 on 18 df, p = 0.83). There was also no significant difference in the head-circumference (foetuses of smokers (mean = 33.5 cm) and nonsmokers (mean = 34.9 cm); t = 0.922 on 17 df; p = 0.37).
Analysis of mouth movements
In total, 4528 mouth events were recorded. Table 1 shows the estimates of the parameter estimates for the Poisson mixed effects analysis for the mouth movement events. Model 1 shows the estimates when all covariates are included in the model. All parameter estimates are significant except for the sex of the foetus and maternal age. Model 2 shows the adjusted model excluding sex and maternal age. A likelihood ratio test comparing Model 2 and Model 1 gave a change in −2 log likelihood of 2.54 on 2 df, which was not significant when compared to a chi-squared distribution (p = 0.28).
Table 1.
Analysis of mouth movement rate showing developmental age effects of smoking, stress and depression
| Model 1 −2 log L = 1177.63 |
Model 2 (excluding sex and maternal age) −2 log L = 1180.17 |
||||||
|---|---|---|---|---|---|---|---|
| Variable | Estimate | SE | p-value | Estimate | SE | p-value | Exp (estimate) |
| Intercept (β0) | −1.8878 | 0.3886 | −2.3425 | 0.1002 | |||
| Foetal age | −0.0306 | 0.0043 | <0.001 | −0.0305 | 0.0043 | <0.001 | 0.970 |
| Smoker(yes) | 0.4292 | 0.2033 | 0.035 | 0.4596 | 0.1984 | 0.021 | 1.583 |
| Stress | 0.0089 | 0.0033 | 0.008 | 0.0087 | 0.0033 | 0.009 | 1.009 |
| Depression | −0.0465 | 0.0104 | <0.001 | −0.0458 | 0.0103 | <0.001 | 0.955 |
| Sex (male) | −0.0128 | 0.1715 | 0.456 | ||||
| Maternal age | −0.0133 | 0.0137 | 0.331 | ||||
| Foetal age by smoker interaction | 0.0160 | 0.0081 | 0.048 | 0.0160 | 0.0081 | 0.048 | 1.016 |
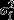 and 95% confidence interval and 95% confidence interval |
0.1164 (0.0578, 0.2815) | 0.1030 (0.0602, 0.2367) | |||||
The final model (Model 2) can be interpreted as follows. The estimate for gestational age is significant and negative, showing a declining rate of mouth movements for the nonsmoking group (the reference group) as the foetus matures. The multiplicative effect for gestational age is 0.970, showing a 3.0% decline for each extra week of age. The estimate for the smoker variable is positive, and the multiplicative effect is 1.583, showing that at 30 weeks, foetuses of smoking mothers have 58% more mouth movements than nonsmokers (p = 0.021). The interaction between foetal age and smoker is also significant and estimates the change in foetal age slope between smokers and nonsmokers. Foetuses of smoking mothers decline more slowly in their rate of mouth movements than foetuses of nonsmokers – the multiplicative effect for foetuses of smokers is estimated by (0.970*1.1016 = 0.985) or a 1.5% decline for each extra week of foetal age – about half the decline of foetuses of nonsmokers. Finally, the effect of stress is positive, suggesting increasing rate of mouth movements with increasing stress. The multiplicative effect is 1.009, suggesting around a 1% increase for a unit increase in stress score, or a 9% increase for a change of ten stress units. In contrast, the depression estimate is negative, indicating that increasing depression is associated with a decreasing rate of mouth movement.
Reparametrisation of Model 2 using different centring values for foetal age can be used to assess the difference between foetuses of smoking and nonsmoking mothers at different foetal ages. Centring at 24 weeks, the difference in slope is nonsignificant (p = 0.072); at 28 weeks, it becomes significant (p = 0.030) and at 36 weeks is highly significant (p = 0.006). The differences in mouth movement rate between foetuses of smokers and those of nonsmokers, therefore, becomes wider as the foetus matures and becomes significant at around 28 weeks. Figure2 shows the fitted developmental model for fixed values of stress (12.0) and depression (5.0) for smoking and nonsmoking participants on the log scale. The increasing separation of the two trajectories is evident.
Figure 2.
Final fitted model for rate of foetal facial mouth movements by maternal smoking status and gestational age.
Analysis of facial self-touch movements
A similar analysis was carried out on the number of facial self-touch events. In total, 1114 facial touch events were recorded. Table 2 shows the estimates of the parameter estimates for the Poisson mixed effects analysis. Model 3 shows the estimates when all covariates are included in the model. In this analysis, the estimates for the smoking by age interaction and the main effects of depression, gender and maternal age are not significant. The effect of smoking is borderline significant. Model 4 shows the estimates once the above four nonsignificant terms have been excluded from the model, with smoking status retained in the model. The effect of excluding the three variables and the interaction was tested using a likelihood ratio test. The change in −2 log L (649.74–648.97) is 0.77 on 4 degrees of freedom which is not significant (p = 0.94).
Table 2.
Analysis of facial self-touch rate showing developmental effects of smoking and stress
| Model 3 −2 log L = 648.97 |
Model 4 (excluding sex, depression, maternal age, age by smoker interaction) −2 log L = 649.74 |
||||||
|---|---|---|---|---|---|---|---|
| Variable | Estimate | SE | p-value | Estimate | SE | p-value | exp (estimate) |
| Intercept (β0) | −4.3937 | 0.5978 | −4.3407 | 0.1546 | |||
| Foetal age | −0.0407 | 0.0085 | <0.001 | −0.0421 | 0.0071 | <0.001 | 0.959 |
| Smoker(yes) | 0.5942 | 0.3063 | 0.052 | 0.5238 | 0.2899 | 0.070 | 1.688 |
| Stress | 0.0281 | 0.0065 | <0.001 | 0.0273 | 0.0064 | <0.001 | 1.028 |
| Depression | −0.0126 | 0.0193 | 0.516 | ||||
| Sex(male) | −0.1503 | 0.2556 | 0.556 | ||||
| Maternal age | 0.0057 | 0.0210 | 0.787 | ||||
| Foetal age by smoker interaction | −0.0003 | 0.0159 | 0.985 | ||||
 and 95% confidence interval and 95% confidence interval |
0.2434 (0.1290, 0.5111) | 0.2485 (0.1317, 0.5211) | |||||
The facial self-touch statistical model is essentially similar to the model for the analysis of mouth movements. The estimate for age is significant and negative, showing (with the absence of an interaction effect) an identical declining rate of mouth movements for both the nonsmoking and smoking groups as the foetus matures. The multiplicative effect for age is 0.959, showing around a 4% decline for each extra week of age. The estimate of maternal smoking, although just failing to reach formal statistical significance, is similar in effect to the previous analysis; the multiplicative effect is in fact slightly larger at 1.688, suggesting that the facial self-touch rate for foetuses of smoking mothers is raised by around 69% compared to nonsmokers. Finally, the effect of stress is again positive, suggesting increasing rate of touch movements with increasing stress. The multiplicative effect of 1.028 suggests a 2.8% increase in touch movement for a unit increase in stress score, or a 31% increase for a larger change of ten stress units. Figure3 shows the estimated fitted developmental model (Model 4) on the log scale for a fixed value of stress of 12.0 for smoking and nonsmoking participants, showing the common declining rate for both groups.
Figure 3.
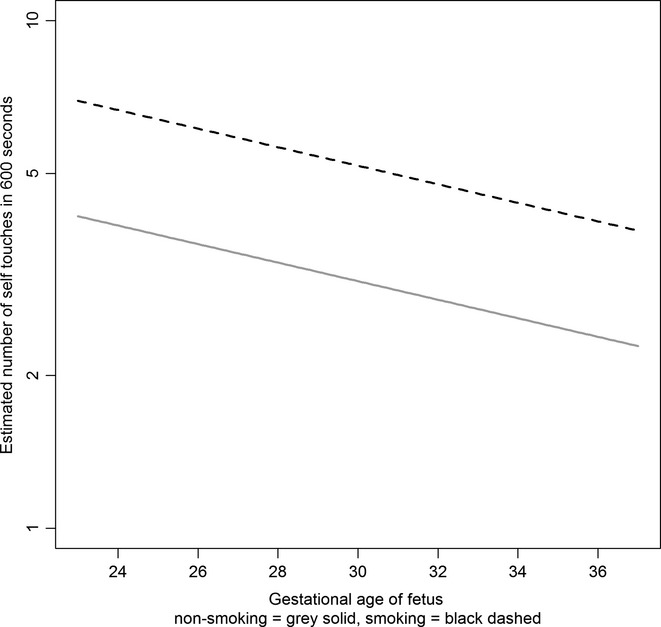
Final fitted model for rate of foetal facial self-touches by maternal smoking status and gestational age.
Discussion
This is the first study which compares longitudinally two types of movements, namely facial movements and touch behaviours of foetuses of mothers smoking during pregnancy and foetuses of mothers who did not smoke during pregnancy. Consistent with the results of other studies analysing general body movements (17), mothers who reported higher levels of perceived stress had foetuses who showed more of these subtle movements.
Maternal smoking is a crucial risk factor for poor psychological, behavioural and physical outcomes in infant development (1), with increasing evidence of the harmful effect of smoking on foetal development. This study found that the rate of foetal facial movements was associated with maternal smoking: within a general pattern of declining movement with gestational age, foetuses of smoking mothers showed more movements at 30 weeks compared with nonsmoking mothers, after controlling for maternal stress and depression. Additionally, the rate of decline in movements was significantly slower for foetuses of smoking mothers. An analysis of facial self-touch showed similar effects. The difference between the rate of movement of foetuses of smoking and nonsmoking mothers was larger in magnitude, and was marginally significant (p = 0.07). There was a common decline in the rate of movement by gestational age in both the smoking and nonsmoking groups.
Although antismoking advice to pregnant women has intensified over the past two decades (11), quit rates have remained relatively low. Ebert and Fahi (11) found that smokers perceive health risks associated with smoking as abstract constructs too far removed from their understanding, when set against the immediate gratification from smoking. Hence, more concrete measures of observable movements need to be introduced. Our pattern of foetal fine-grained movements in relation to smoking and gestational age indicate some differences to other studies analysing more gross foetal body movements (9,27). The first of these studies (27) found that foetuses (<37 weeks gestation) of mothers who smoked did not differ in their general movements when compared with similarly aged foetuses of nonsmoking mothers. A second study (9) reported that foetuses of smoking mothers showed less frequent gross movements. These results relate to general foetal movement and derive from cross-sectional rather than longitudinal data. In contrast, the present study examined the frequency of two types of specific subtle foetal movements observable in ultrasound scans, namely mouth movements and facial self-touch behaviours. We argue that these fine-grained movement observations provide a more sensitive measure for studies of foetal movements. In addition, the type of movement needs to be considered. Self-touch has been found to be used by young infants to soothe themselves during stressful situations (28); thus, stressed foetuses may use self-touch more frequently compared with nonstressed foetuses. Our finding of maternal stress and frequency of foetal facial touch supports this hypothesis.
Previous research has identified a smoking dose-dependent difference in foetal general movements. Habek (9) found a significant difference between nonsmokers and mothers smoking over 20 cigarettes per day, but not 10 cigarettes per day. In the present study, pregnant women smoked about 14 cigarettes per day, and an increase of subtle foetal movements was recorded. Similarly to our findings, Dieter et al. (16) found increased movements of foetuses exposed to smoking before 8 months gestational age compared to foetuses of nonsmokers. It needs to be noted that stress also had a positive impact on frequency of movements supporting findings of other research cited above. The effect of a ten unit increase in stress for mouth and touch movements of 9% and 31% increases respectively, was however less than the changes for being a smoker (58% and 69% increases respectively). Smoking appears to be more important than stress in our pilot analysis.
Thus, over and above the effect of stress, smoking status had a significant effect on frequency of foetal mouth movements and a marginally significant effect on facial self-touch. We can suggest one possible mechanism. The difference in fine-grained foetal movement analysis might reflect the fact that the foetal central nervous system maturation is affected by maternal smoking (29), and hence, foetuses of smokers declined more slowly in their rate of mouth movements than foetuses of nonsmokers. Specifically, foetuses of smokers showed a significant delay compared with the normal declining rate of movements. Such delay has been reported for example in relation to speech processing abilities in infants exposed to smoking during pregnancy (30). Key et al. (30) examined event-related potentials (ERPs) 34 hours after birth in response to six consonant–vowel syllables in healthy newborn infants of smokers and nonsmokers and found that not only did infants of smokers discriminate between fewer syllables but also that they were slower in their reaction times. Huizink (29) discusses a number of mechanisms which bind nicotine to nicotinic acetylcholine receptors in the brain leading to premature cell differentiation and ultimately to premature brain cell death. Such mechanisms might play a role in the delay of declining movements in the current sample which indicates that foetuses of smoking mothers show less mature behavioural patterns compared with foetuses of nonsmoking mothers (29).
In sum, this exploratory pilot study indicates that foetal facial movement patterns differ significantly between foetuses of smoking compared with nonsmoking mothers with foetuses of smoking mothers showing a raised rate of movement compared to nonsmoking mothers, and a slower rate of decline. There is also some evidence that similar effects exist for facial self-touch although failing to reach significance. Our findings concur with others that stress and depression have a significant impact on foetal movements (12,27) and need to be controlled for, but additionally these results point to the fact that nicotine exposure per se has an effect on foetal development over and above the effects of stress and depression.
Finally, we conclude with some caveats to our results. Firstly, this is a pilot study, and larger studies are required to confirm reported associations and further understand the relationship between maternal smoking, stress, depression and foetal development. The lack of significance in the foetal facial self-touch results may be caused by low power. Secondly, given the computational limitations of the Voluson E8 which delivers a new image every ½ second, we might miss some very fast movements. However, given that one of the most common movements observed (mouth stretch) lasts on average 3.1 seconds (range 1.6–4.1) the frame rate would not significantly affect our ability to observe fine-grained mouth movements. Thirdly, we have not attempted to control for social class in our analysis, and it is possible that differences in social class between the smoking and nonsmoking mothers may account for some of the differences in foetal movement between the two groups. Similarly, we did not include information on paternal effects, including paternal smoking behaviour. In the statistical analysis, we were unable to consider additional interaction effects beyond the interaction of smoking with gestational age due to small numbers of cases. Future studies should consider both the inclusion of these additional controlling factors and the inclusion of further interactions in the statistical model to fully understand the relationship between maternal smoking and foetal fine-grained movement.
Acknowledgments
We thank all mothers for taking part in this study. We thank Karen Lincoln for her support and independent coders for coding and reliability analysis of the data and to the anonymous referees for valuable comments. We also thank Joe Austen and Louisa Buttanshaw for their technical assistance, and especially Kendra Exley for her skills and expertise in ultrasound.
Author contributions
NR conceived and designed the experiments. NR and KK wrote protocol and were responsible for ethics. NR performed the experiments. BF designed the statistical analysis and analysed the data. NR, BF, JM and KK wrote the paper.
References
- 1.Hofhuis W, de Jongste JC, Merkus PJM. Adverse health effects of prenatal and postnatal tobacco smoke exposure on children. Arch Dis Child. 2003;88:1086–90. doi: 10.1136/adc.88.12.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lumley J, Oliver SS, Chamberlain C, Oakley L. Interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2004;18(4):CD001055. doi: 10.1002/14651858.CD001055.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Chamberlain C, O'Mara-Eves A, Oliver S, Caird JR, Perlen SM, Eades SJ, et al. Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database Syst Rev. 2013;(10) doi: 10.1002/14651858.CD001055.pub4. Art. No.: CD001055. DOI: 10.1002/14651858.CD001055.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. The Health and Social Care Information Centre, Statistical release. Statistics on Women's Smoking Status at time of delivery: England. Quarter 3, 2013/14, 2014. Retrieved 22 May 2014 http://www.hscic.gov.uk/catalogue/PUB13675.
- 5.Cowperthwaite B, Hains SM, Kisilevsky B. Fetal behaviour in smoking compared to non-smoking pregnant women. Infant Behav Dev. 2007;30:422–30. doi: 10.1016/j.infbeh.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Zeskind PS, Gingras JL. Maternal cigarette-smoking during pregnancy disrupts rhythms in fetal heart rate. J Pediatr Psychol. 2006;31:5–14. doi: 10.1093/jpepsy/jsj031. [DOI] [PubMed] [Google Scholar]
- 7.Kisilevsky BS, Low JA. Human fetal behaviour: 100 years of study. Dev Rev. 1998;18:1–29. [Google Scholar]
- 8.Lampl M, Kuzawa C, Jeanty P. Growth patterns of the heart and kidney suggest interorgan collaboration in facultative fetal growth. Am J Hum Biol. 2005;17:178–94. doi: 10.1002/ajhb.20109. [DOI] [PubMed] [Google Scholar]
- 9.Habek D. Effects of smoking and fetal hypokinesia in early pregnancy. Arch Med Res. 2007;38:864–7. doi: 10.1016/j.arcmed.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Garvey DJ, Longo LD. Chronic low level maternal carbon monoxide exposure and fetal growth and development. Biol Reprod. 1978;19:8–14. doi: 10.1095/biolreprod19.1.8. [DOI] [PubMed] [Google Scholar]
- 11.Ebert LM, Fahi K. Why do women continue to smoke in pregnancy? Women Birth. 2007;20:161–8. doi: 10.1016/j.wombi.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 12.McCurry N, Thompson K, Parahoo K, O'Doherty E, Doherty A. Pregnant women's perception of the implementation of smoking cessation advice. Health Educ J. 2002;61:20–31. [Google Scholar]
- 13.DiPietro JA, Hilton SC, Hawkins M, Costigan KA, Pressman EK. Maternal stress and affect influence fetal neurobehavioral development. Dev Psychol. 2002;38:659–68. [PubMed] [Google Scholar]
- 14.Reissland N, Francis B. The quality of fetal arm movements as indicators of fetal stress. Early Hum Dev. 2010;86:813–6. doi: 10.1016/j.earlhumdev.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Van den Bergh BR, Mulder EJ, Mennes M, Glover V. Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: links and possible mechanisms. A review. Neurosci Biobehav Rev. 2005;29:237–58. doi: 10.1016/j.neubiorev.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Dieter JN, Field T, Hernandez-Reif M, Jones NA, Lecanuet JP, Salman FA, et al. Maternal depression and increased fetal activity. J Obstet Gynaecol. 2001;5:468–73. doi: 10.1080/01443610120072009. [DOI] [PubMed] [Google Scholar]
- 17.Mangesi L, Hofmeyr GJ, Smith V. Fetal movement counting for assessment of fetal wellbeing. Cochrane Database Syst Rev. 2007;(1) doi: 10.1002/14651858.CD004909.pub2. Art. No.: CD004909. DOI: 10.1002/14651858.CD004909.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Johnson TR, Jordan ET, Paine LL. Doppler recordings of fetal movement: II. Comparison with maternal perception. J Obstet Gynaecol. 1990;76:42–3. [PubMed] [Google Scholar]
- 19.Reissland N, Francis B, Aydin E, Mason J, Schaal B. The development of anticipation in the fetus. A longitudinal account of human fetal mouth movements in reaction to and anticipation of touch. Dev Psychobiol. 2013;56:955–63. doi: 10.1002/dev.21172. [DOI] [PubMed] [Google Scholar]
- 20.Ekman P, Friesen WV. Facial action coding system: A technique for the measurement of facial movement. Palo Alto, CA: Consulting Psychologists Press; 1978. [Google Scholar]
- 21.Reissland N, Francis B, Mason J, Lincoln K. Do facial expressions develop before birth? PLoS ONE. 2011;6:e24081. doi: 10.1371/journal.pone.0024081. doi: 10.1371/journal.pone.0024081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen S, Kamarck T, Mermelstein RA. global measure of perceived stress. J Health Soc Behav. 1983;24:386–96. [PubMed] [Google Scholar]
- 23.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 24.Pinheiro JC, Bates DM. Mixed effects models in S and S-plus. New York: Springer; 2000. [Google Scholar]
- 25.Bates D, Maechler M, Bolker B, Walker S. Linear mixed-effects models using Eigen and S4. R package version 1.0-5 http://CRAN.R-project.org/package=lme4, 2013. Accessed 30 April, 2014.
- 26.Action on Smoking and Health. Smoking statistics - who smokes and how much, Available at: http://ash.org.uk/files/documents/ASH_106.pdf. Accessed 18 September, 2014.
- 27.Hains SMJ, Kisilvesky B. Fetal behavior in smoking compared to non-smoking pregnant women. Infant Behav Dev. 2007;30:422–30. doi: 10.1016/j.infbeh.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Jean ADL, Stack DM. Full-term and very-low-birth-weight preterm infants' self-regulating behaviors during a Still-Face interaction: influences of maternal touch. Infant Behav Dev. 2012;35:779–91. doi: 10.1016/j.infbeh.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 29.Huizink AC. Moderate use of alcohol, tobacco and cannabis during pregnancy: new approaches and update on research findings. Reprod Toxicol. 2009;28:143–51. doi: 10.1016/j.reprotox.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 30.Key PFA, Ferguson M, Molfese DL, Peach K, Lehman C, Molfese VL. Smoking during Pregnancy Affects Speech-Processing Ability in Newborn Infants. Environ Health Perspect. 2007;115:623–9. doi: 10.1289/ehp.9521. [DOI] [PMC free article] [PubMed] [Google Scholar]