Abstract
Background
A strategy for accelerating liver regeneration after hepatectomy would offer great benefits in preventing postoperative liver failure and improving surgical outcomes. Transforming growth factor (TGF) β is a potent inhibitor of hepatocyte proliferation. Recently, thrombospondin (TSP) 1 has been identified as a negative regulator of liver regeneration by activation of local TGF-β signals. This study aimed to clarify whether the LSKL (leucine–serine–lysine–leucine) peptide, which inhibits TSP-1-mediated TGF-β activation, promotes liver regeneration after hepatectomy in mice.
Methods
Mice were operated on with a 70 per cent hepatectomy or sham procedure. Operated mice received either LSKL peptide or normal saline intraperitoneally at abdominal closure and 6 h after hepatectomy. Perioperative plasma TSP-1 levels were measured by enzyme-linked immunosorbent assay in patients undergoing hepatectomy.
Results
Administration of LSKL peptide attenuated Smad2 phosphorylation at 6 h. S-phase entry of hepatocytes was accelerated at 24 and 48 h by LSKL peptide, which resulted in faster recovery of the residual liver and bodyweight. Haematoxylin and eosin tissue staining and blood biochemical examinations revealed no significant adverse effects following the two LSKL peptide administrations. In the clinical setting, plasma TSP-1 levels were lowest on the first day after hepatectomy. However, plasma TSP-1 levels at this stage were significantly higher in patients with subsequent liver dysfunction compared with levels in those without liver dysfunction following hepatectomy.
Conclusion
Only two doses of LSKL peptide during the early period after hepatectomy can promote liver regeneration. The transient inhibition of TSP-1/TGF-β signal activation using LSKL peptide soon after hepatectomy may be a promising strategy to promote subsequent liver regeneration.
Surgical relevance
Although the mechanisms of liver regeneration after hepatectomy have been explored intensively in vivo, no therapeutic tools are thus far available to accelerate liver regeneration after hepatectomy in the clinical setting. Recently, the matricellular protein thrombospondin (TSP) 1, a major activator of latent transforming growth factor (TGF) β1, has been identified as a negative regulator of liver regeneration after hepatectomy.
In this study, the inhibition of TSP-1-mediated TGF-β signal activation by LSKL (leucine–serine–lysine–leucine) peptide in the early period after hepatectomy accelerated liver regeneration without any adverse effects. In addition, continuous high plasma TSP-1 levels after hepatectomywere associated with liver damage in humans.
The transient inhibition of TSP-1/TGF-β signal activation using LSKL peptide in the early period after hepatectomy could be a novel therapeutic strategy to accelerate liver regeneration after hepatectomy.
Introduction
The liver is the largest internal organ in the body, with multiple functions such as protein synthesis, detoxification, glycogen storage and production of various enzymes. The liver is uniquely characterized by its ability to regenerate itself in response to injury1,2. Hepatectomy, which induces loss of liver volume and the corresponding liver functions, has been performed as the most common curative procedure for liver cancer based on the expectation of successful liver regeneration following removal of liver tissue3,4. However, postoperative liver failure is not eliminated totally, despite advancements in surgical techniques and perioperative care, and morbidity and mortality rates after major hepatectomy remain high, ranging from 12·9 to 47·1 per cent5–11 and from 2·6 to 7·4 per cent5–10 respectively. Therefore, a delay in liver regeneration following hepatectomy can result in liver dysfunction and, in extreme cases, hepatic failure12. However, in the clinical setting, no established therapeutic strategy is yet available to accelerate liver regeneration after hepatic resection.
The process of liver regeneration following hepatectomy is coordinated by both proliferative and antiproliferative factors2,13. Transforming growth factor (TGF) β1 is a potent inhibitor of mitogen-stimulated DNA synthesis in cultured hepatocytes. As TGF-β is synthesized and secreted as a latent complex, an important step in regulating its biological activity is conversion of the latent form into the active one14. Recently, the matricellular protein thrombospondin (TSP) 1, a major activator of latent TGF-β1, has been identified as a negative regulator of liver regeneration following hepatectomy in an experimental model15. After partial hepatectomy in mice, TSP-1 protein expression in the regenerating liver was induced with a peak at approximately 6 h, returning to basal levels by 24 h15. Corresponding with this induction of TSP-1 expression, the TGF-β–Smad signalling pathway was activated through phosphorylation of Smad215. Furthermore, TSP-1 deficiency resulted in a significant reduction in local TGF-β–Smad signal activation and accelerated liver regeneration in mice15. In patients with liver metastasis from colorectal cancer, high levels of plasma TSP-1 at 24 h after hepatectomy have been associated with postoperative liver dysfunction and severe morbidity16. These findings indicate the possibility of a novel therapeutic strategy for accelerating liver regeneration by targeting TSP-1-mediated TGF-β1 activation in the early postoperative period after partial hepatectomy.
TGF-β is stored in the extracellular matrix by binding to latency-associated peptide (LAP)14,17. TSP-1 can convert latent TGF-β to its active form by binding to LAP, thereby releasing the active TGF-β from LAP. Subsequently, active TGF-β1 can be released from the latent TGF-β1 complex and bind to the TGF-β receptor, thus inducing signal transduction. The LSKL (leucine–serine–lysine–leucine) peptide can inhibit TSP-1 binding to LAP. The present study was designed to examine whether administration of the LSKL peptide could successfully accelerate liver regeneration after partial hepatectomy in mice by inhibiting local TGF-β–Smad signal activation through its effect on TSP-1.
Methods
Partial hepatectomy in mice
Wild-type 8–12-week-old male mice (C57BL/6) were used for all experiments. The two anterior hepatic lobes (median and left lateral lobes), which comprise 70 per cent of the liver weight, were resected, while the caudate and right lobes were left intact, as described previously15. Animals were divided into three groups: sham-operated, normal saline and LSKL peptide groups (Fig. 1). For each group, mice were killed at 6, 24, 48, 72 and 168 h after the operation, and the residual liver weight was measured. Postoperative bodyweight was measured 168 h after the 70 per cent hepatectomy and the bodyweight recovery rate was calculated as: (postoperative bodyweight − preoperative bodyweight) × 100/preoperative bodyweight. Six mice per time point were used for each group, with a total of 90 mice. The study was approved by the Kumamoto University Animal Care and Use Committee, and followed the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.
Fig. 1.
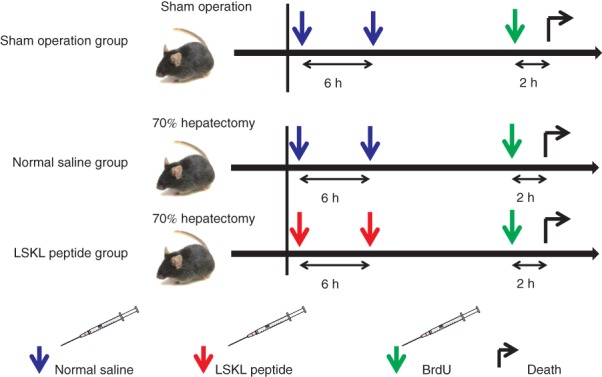
Schedule of LSKL (leucine–serine–lysine–leucine) peptide administration. C57BL/6 male mice were operated on with a 70 per cent hepatectomy or sham procedure. In the LSKL peptide group, LSKL peptide (30 mg/kg) was administered intraperitoneally before abdominal wall closure and 6 h after the hepatectomy. In the normal saline group, saline (6 ml/kg) was administered intraperitoneally at the same time points. In the sham-operated group, laparotomy was performed and the incision was sutured following intraperitoneal administration of normal saline (6 ml/kg). Mice received an intraperitoneal injection of 5-bromo-2-deoxyuridine (BrdU) 2 h before being killed
Intraperitoneal LSKL peptide administration
LSKL TSP-1 inhibitory peptide (AnaSpec, Freemont, California, USA) was diluted to 5 mg/ml with normal saline. In the LSKL peptide group, LSKL peptide (30 mg/kg bodyweight in 6 ml/kg) was administered intraperitoneally before abdominal wall closure and at 6 h after the 70 per cent hepatectomy. In the normal saline group, saline (6 ml/kg) was given intraperitoneally at the same time points. In the sham-operated group, laparotomy was performed and the incision was sutured after normal saline (6 ml/kg) had been administered intraperitoneally.
Histology and immunohistochemistry
For histological analyses, liver samples (the same lobe from each mouse) were fixed overnight in 10 per cent neutral buffered formalin, dehydrated in a graded alcohol series, and embedded in paraffin; 4-µm sections were prepared. Epitope retrieval was performed by autoclave pretreatment in Histofine® antigen retrieval solution (pH 9) (Nichirei, Tokyo, Japan). Endogenous peroxidase activity was blocked with 3 per cent hydrogen peroxide, and the sections were incubated with diluted 1 : 15 antibromodeoxyuridine peroxidase Fab fragments overnight at 4°C. The sections were also incubated with 1 : 100 Phospho-Smad2 (138D4; Cell Signaling Technology, Danvers, Massachusetts, USA) and 1 : 100 TSP-1 (A6.1; Thermo Scientific, Waltham, Massachusetts, USA) antibodies. Diaminobenzidine solution was used as chromogen, followed by counterstaining with Mayer's haematoxylin.
Western blotting
The same lobe from each mouse was used for protein isolation and subsequent analyses. A 50-mg piece was resected from liver tissue and homogenized in 500 µl radioimmunoprecipitation assay buffer containing a protease and phosphatase inhibitor cocktail (Thermo Scientific). For immunoblotting analyses, after electrophoresis, samples were transferred on to Immun-Blot® PVDF membrane (Bio-Rad Laboratories, Hercules, California, USA) using Mini Trans-Blot® cell (Bio-Rad Laboratories) and probed with primary antibodies as rabbit monoclonal antibody (mAb) against Phospho-Smad2 (138D4) and mouse mAb against TSP-1 (D4.6; Thermo Scientific). Image J 1·40 software (National Institutes of Health, Bethesda, Maryland, USA) was used for densitometric analysis.
Real-time PCR
A 25-mg piece of liver tissue was homogenized in 1500 µl TRIzol® Reagent (Thermo Scientific). Total RNA was extracted according to the manufacturer's instructions. Total RNA was converted to cDNA by reverse transcription–PCR. To measure mRNA expression, real-time PCR assay was performed using the LightCycler® 480 system (Roche Diagnostics, Indianapolis, Indiana, USA). Probes and primers were designed using Roche probe library system for each gene expression; 18S rRNA was used as reference. The probes and primers are summarized in Table S1 (supporting information).
Assessment of 5-bromo-2-deoxyuridine incorporation
Mice received an intraperitoneal injection of 100 mg/kg 5-bromo-2-deoxyuridine (BrdU; Roche Applied Science, Penzberg, Germany) 2 h before killing. Five random high-power visual fields (0·64 mm2 per field) per mouse were evaluated to determine the mean number of BrdU-positive hepatocyte nuclei per field.
Mouse blood sample collection and analyses
Blood samples were collected at the time of mouse death with the addition of heparin. Plasma was extracted from the blood samples after centrifugation at 3000 r.p.m. for 15 min at 4°C and stored at −80°C until analysis. All blood biochemical examinations were performed using BioMajesty™ JCA-BM6050 analyser (JEOL Products, Tokyo, Japan).
Human plasma collection and analyses
Plasma EDTA samples were collected from 30 patients who underwent hepatectomy for liver tumours. Samples were taken before and immediately after hepatectomy, as well as on postoperative day (POD) 1, 3 and 7. Samples were stored at −80 °C until analysis. Plasma TSP-1 levels were measured using a commercially available enzyme-linked immunosorbent assay kit (Quantikine®; R&D Systems, Minneapolis, Minnesota, USA) according to the manufacturer's instructions.
Statistical analysis
All experiments were performed in triplicate, and data are shown as representative results. Data are expressed as mean(s.d.) values. Data analysis was performed with JMP® 10.0.2 software (SAS Institute, Cary, North Carolina, USA). Statistical analyses were carried out using Student's t test or ANOVA. Student's t test was performed if equal variance was ascertained in two groups by an F test. P < 0·050 was considered significant.
Results
LSKL peptide suppresses TSP-1-mediated TGF-β–Smad signal activation after hepatectomy
At 6 h after surgery, expression of phosphorylated Smad2-positive nuclei in hepatocytes in the LSKL peptide group was decreased in comparison with expression in the saline group (Fig. 2a). On western blot analysis, induction of Smad2 phosphorylation was observed at 6 h in saline and sham groups. Administration of the LSKL peptide significantly inhibited hepatectomy-induced Smad2 phosphorylation (P = 0·004) (Fig. 2b,c).
Fig. 2.
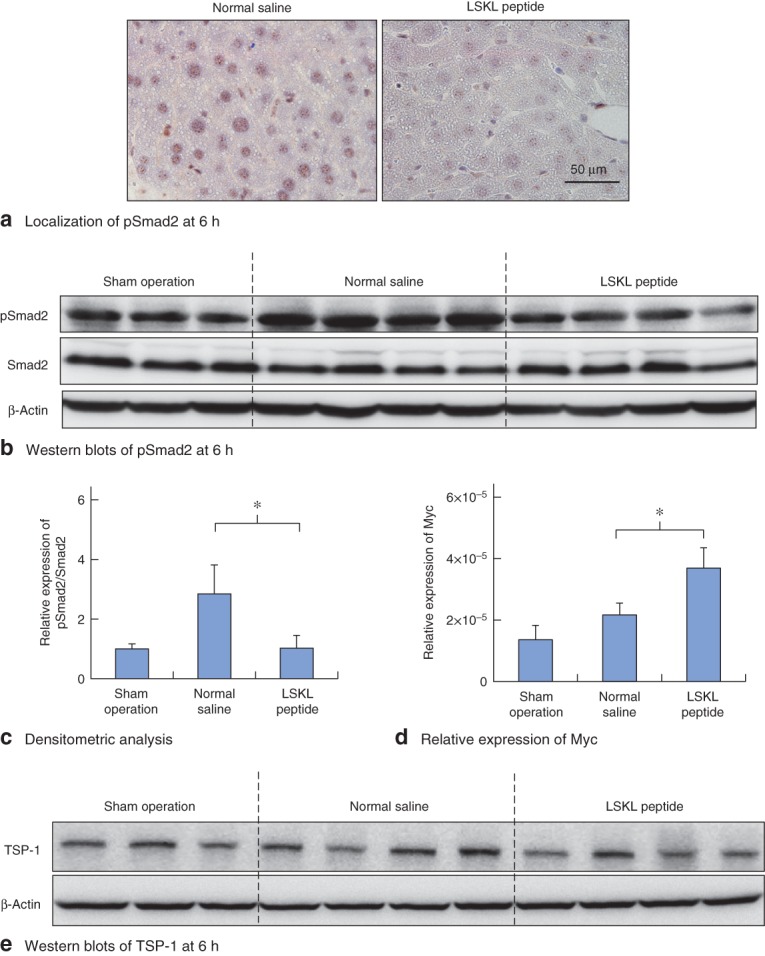
LSKL (leucine–serine–lysine–leucine) peptide successfully inhibits transforming growth factor (TGF) β–Smad signal activation induced by partial hepatectomy. a Assessment of phosphorylated Smad2 (pSmad2) nuclear localization. Immunohistochemical staining for pSmad2 at 6 h in mouse liver from normal saline and LSKL peptide groups. b Effects of LSKL peptide on pSmad2 expression in the regenerating liver at 6 h. Western blot analysis of pSmad2 in mouse liver from sham-operated, normal saline and LSKL peptide groups. β-Actin served as a loading control. c Densitometric analysis for western blots of pSmad2 in mouse liver for the three groups. d Real-time PCR analysis of relative expression of Myc mRNA in mouse liver for the three groups. c,d Values are mean(s.d.). *P < 0·010 (Student's t test). e Western blotting of thrombospondin (TSP) 1 protein at 6 h after hepatectomy. Expression of TSP-1 was similar in normal saline and LSKL peptide groups
Myc is a downstream target that is negatively regulated by the TGF-β–Smad pathway18. Myc mRNA expression was analysed at 6 h by real-time PCR, and was significantly higher in the LSKL peptide group than in the normal saline group (P < 0·001) (Fig. 2d). These results show that LSKL peptide successfully upregulated Myc expression via suppression of Smad2 phosphorylation. Thus, a single administration of LSKL peptide during abdominal wall closure successfully inhibited partial hepatectomy-induced Smad2 phosphorylation at 6 h. As the TGF-β–Smad signalling pathway is known to be activated between 6 and 12 h after hepatectomy, the optimal schedule of LSKL peptide administration was determined by giving a further dose of the peptide 6 h after the first dose in order to obtain the continuous suppressive effects (Fig. 1).
No difference in TSP-1 expression between LSKL peptide and saline groups
The effect of the LSKL peptide on TSP-1 expression levels following hepatectomy was analysed by western blot analysis of liver tissue. At 6 h after hepatectomy, levels of TSP-1 in the LSKL peptide group were similar to those in the normal saline group (Fig. 2e). On immunohistochemical staining, TSP-1 protein was confirmed to be expressed in sinusoidal endothelial cells in both the LSKL peptide and normal saline groups (Fig. S1, supporting information) at 6 h after partial hepatectomy, as reported previously15. These findings suggest that LSKL peptide successfully attenuates TGF-β–Smad signal activation by antagonizing TSP-1, but not by reducing TSP-1 protein expression.
Accelerated liver regeneration after LSKL peptide treatment
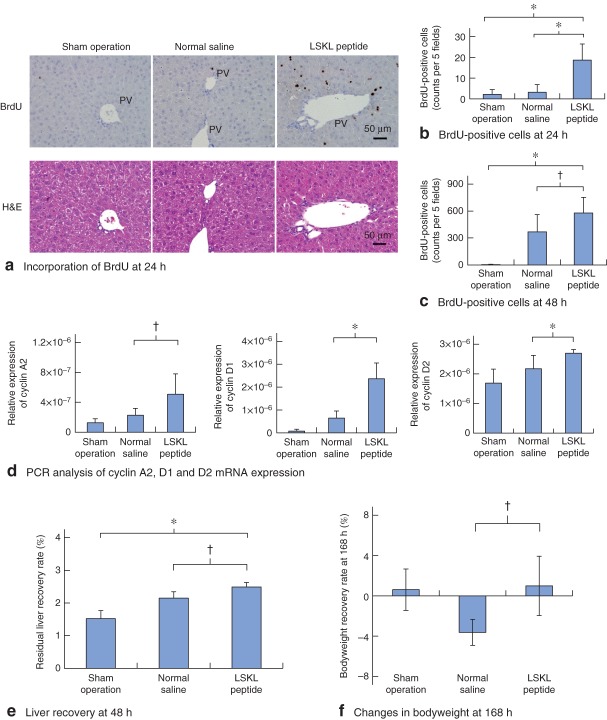
To address whether the suppressed TGF-β–Smad signal activation by LSKL peptide leads to accelerated liver regeneration, hepatocyte proliferation was evaluated using a BrdU incorporation assay at 24 and 48 h after hepatectomy. Only a few BrdU-positive hepatocytes was detectable at 24 h in the sham and normal saline groups. In contrast, in the LSKL peptide group a significantly increased number of BrdU-positive hepatocytes was observed, particularly around the portal vein (P < 0·001) (Fig. 3a,b). At 48 h after hepatectomy, a significant difference in BrdU-positive hepatocytes was still observed between the LSKL peptide group and the normal saline group (P = 0·028) (Fig. 3c). Furthermore, the expression of cyclin A2 mRNA, a cell cycle regulator in S-phase progression, was significantly upregulated in the LSKL peptide group compared with that in the saline group at 24 h (P = 0·012) (Fig. 3d). Cyclin D family accumulation is needed for passage through the restriction point in G1 phase, just before entry into S phase19. Cyclin D1 mRNA expression was also increased in the LSKL peptide group, compared with that in the normal saline group (P < 0·001) (Fig. 3d). Furthermore, expression of cyclin D2 mRNA, which is downstream of Myc20,21, was also upregulated in the LSKL peptide group in comparison with the normal saline group (P = 0·009) (Fig. 3d).
Fig. 3.
Administration of LSKL (leucine–serine–lysine–leucine) peptide accelerates hepatocyte proliferation after hepatectomy. a Immunohistochemical assessment of 5-bromo-2-deoxyuridine (BrdU) incorporation in the regenerating liver (upper panel) at 24 h after hepatectomy in mouse livers from sham operation, normal saline and LSKL peptide groups. PV, portal vein. Lower panel: Haematoxylin and eosin (H&E) staining. b Analysis of BrdU-positive cells at 24 h after hepatectomy. c Analysis of BrdU-positive cells at 48 h after hepatectomy. b,c The number of BrdU-positive hepatocytes per five high-power fields is shown (n = 6 per time point for each group). d Real-time PCR analysis of relative expression of cyclin A2, cyclin D1 and cyclin D2 mRNA in mouse liver for the three groups. e Residual liver recovery rate at 48 h after hepatectomy. f Changes in bodyweight at 168 h after hepatectomy. Bodyweight recovery rate (%) was calculated as (postoperative bodyweight − preoperative bodyweight) × 100/preoperative bodyweight. b–f Values are mean(s.d.). *P < 0·010, †P < 0·050 (Student's t test)
These results indicate that the administration of LSKL peptide promoted hepatocyte proliferation by accelerating S-phase entry after hepatectomy. In addition to the observed acceleration of hepatocyte proliferation, LSKL peptide also significantly promoted recovery of the residual liver weight at 48 h after hepatectomy compared with that in the normal saline group (P = 0·015) (Fig. 3e), a difference that was not significant at 72 h. Furthermore, at 168 h after hepatectomy, the residual liver weight in the LSKL peptide group was similar to that in the normal saline group, suggesting that LSKL peptide administration did not affect the termination of liver regeneration after hepatectomy. Taken together, these results indicate that LSKL peptide promoted liver regeneration by accelerating S-phase entry of hepatocytes after hepatectomy, especially during the early postoperative period.
LSKL peptide accelerated the recovery of bodyweight after partial hepatectomy
As shown above, administration of LSKL peptide accelerated the hepatic regenerative response after partial hepatectomy, particularly during the early postoperative period from 24 to 48 h. However, the residual liver weight at 168 h after hepatectomy (at the termination phase of liver regeneration) was not significantly different in the LSKL peptide and normal saline groups. Whether the accelerated liver regenerative response during the early period following LSKL peptide administration led to any improvement in the total body nutrition was analysed. The changes in bodyweight at 168 h after hepatectomy with respect to preoperative bodyweight were +0·61, −3·62 and +0·99 per cent in the sham operation, normal saline and LSKL peptide groups respectively (Fig. 3f). Thus, at 168 h after hepatectomy, although bodyweight in the normal saline group had not recovered to the preoperative weight, those in the LSKL peptide group displayed significantly faster recovery (P = 0·018). These results suggest that LSKL peptide administration promoted the recovery in bodyweight following hepatectomy.
No adverse effects following LSKL peptide administration
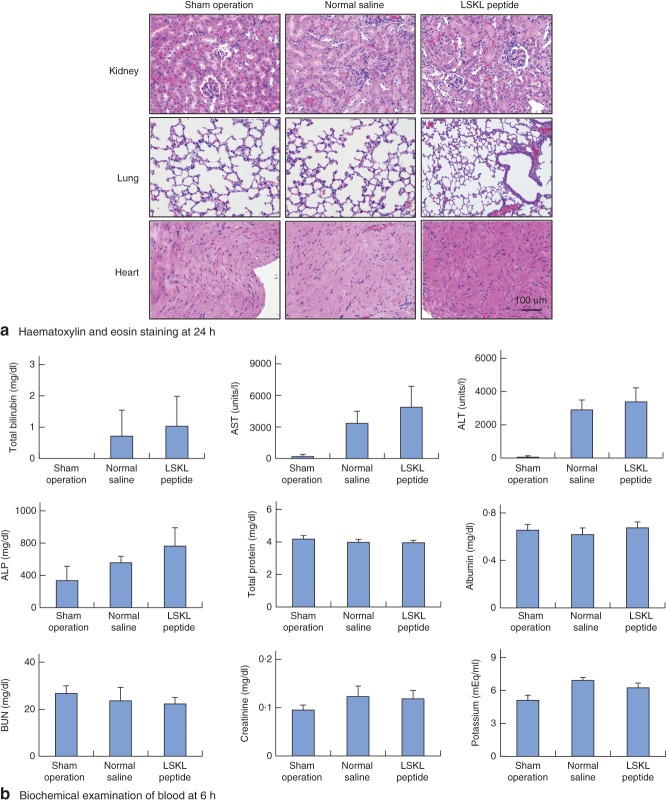
Although LSKL peptide successfully promoted liver regeneration and recovery of bodyweight after hepatectomy, it was important to elucidate whether LSKL peptide administration induced any adverse effects in other organs. Haematoxylin and eosin staining of heart, lung and kidney at 6 h showed no adverse effects such as inflammatory or necrotic changes following LSKL peptide administration (Fig. 4a). To examine further whether there were any adverse effects of LSKL peptide administration, biochemical examination of blood at 6 and 24 h after hepatectomy were performed (Fig. 4b and Fig. S2, supporting information, respectively); no significant adverse effects were noted. These results suggest that only two doses of LSKL peptide in the early period could promote liver regeneration and recovery of bodyweight after hepatectomy without any significant adverse effects on the function of other organs.
Fig. 4.
No adverse effects following LSKL (leucine–serine–lysine–leucine) peptide administration. a Haematoxylin and eosin staining of lung, heart and kidney at 24 h after hepatectomy. b Biochemical examination of blood at 6 h. Values are mean(s.d.). AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; BUN; blood urea nitrogen
Plasma TSP-1 level in patients after hepatectomy
A previous study15 showed that hepatectomy-associated stress induces immediate TSP-1 expression in the regenerating liver. Furthermore, the findings of the present study indicate that decreased TSP-1-mediated TGF-β–Smad signalling by the LSKL peptide promotes hepatocyte proliferation after hepatectomy. The perioperative changes in plasma TSP-1 levels before and after hepatectomy were analysed in patients, whose clinical characteristics are shown in Table S2 (supporting information). The plasma TSP-1 level significantly decreased to the lowest level on POD 1 compared with the pretreatment value (P < 0·001) (Fig. 5a), suggesting that a reduced plasma TSP-1 level is required for a regenerative response in the liver after hepatectomy. The plasma TSP-1 level returned to 55.4 per cent of the preoperative level on POD 3. In addition, the clinical significance of the decreased plasma TSP-1 concentration between pretreatment and POD 1 (ΔTSP-1 = TSP-1Day1 − TSP-1Pre) was examined. ΔTSP-1 was positively and significantly correlated with the levels of total bilirubin (R2 = 0·286, P = 0·003) and alanine aminotransferase (ALT) (R2 = 0·164, P = 0·029) on POD 3, but not with aspartate aminotransferase (AST) (R2 = 0·105, P = 0·086) (Fig. 5b). ΔTSP-1 was also positively and significantly correlated with the level of total bilirubin on POD 1 and of ALT on POD 5 (data not shown). Furthermore, ΔTSP-1 in high total bilirubin (at least 2·0 mg/dl) or high ALT (at least 600 units/l) groups on POD 3 was significantly higher than in low total bilirubin (less than 2·0 mg/dl) or low ALT (less than 600 units/l) groups (P = 0·027 and P = 0·048 respectively) (Fig. 5c). These results suggest that a high plasma TSP-1 level on POD 1 after hepatectomy reflects the liver damage induced by hepatectomy, and that measurements of ΔTSP-1 could be useful to predict further liver damage, such as high levels of total bilirubin and ALT on POD 3.
Fig. 5.
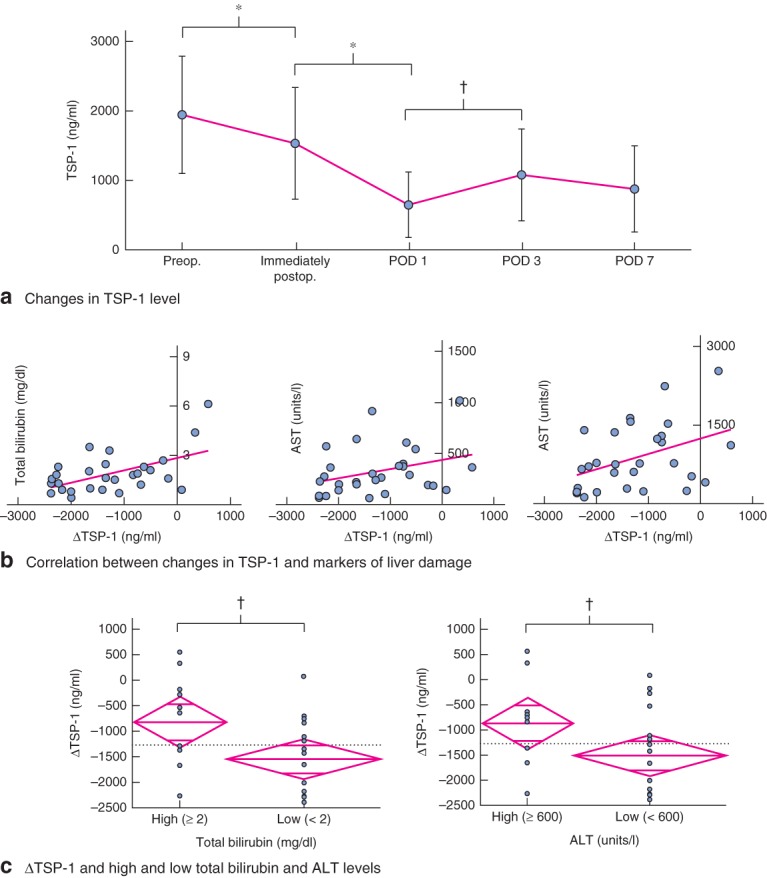
Plasma thrombospondin (TSP) 1 levels before and after hepatectomy. a Sequential changes in mean(s.d.) plasma TSP-1 concentration before and after hepatectomy (n = 30). POD, postoperative day. b Correlation between changes in plasma TSP-1 level, ΔTSP-1 (TSP-1Day1 − TSP-1Pre), and markers of liver damage including total bilirubin, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels on POD 3. c Comparison of ΔTSP-1 in groups with high and low total bilirubin levels (left), and high and low ALT levels (right) on POD 3. The dotted horizontal line represents the mean of ΔTSP-1 for the two groups. The central line of the diamond indicates mean ΔTSP-1 in that group. The top and bottom points of the diamond represent 95 per cent c.i. calculated with error variance. The width of the diamond indicates the mean size of the group. The lines near the top and bottom of the diamond represent the overlap mark; if the interval between these lines for one group does not overlap the interval between these lines for another group, the group means are significantly different. a–c *P < 0·010, †P < 0·050 (Student's t test )
Discussion
This study demonstrates that administration of the LSKL peptide, which inhibits TSP-1-mediated local TGF-β activation, successfully accelerates liver regeneration after hepatectomy in mice. The development of a novel strategy with the aim of accelerating liver regeneration following hepatectomy could provide significant benefits in preventing postoperative liver failure and improving surgical outcomes. Although the mechanisms of liver regeneration after hepatectomy have been explored intensively in vivo1,2,13,22, no therapeutic tools are thus far available to accelerate liver regeneration after hepatectomy in the clinical setting. Although several previous studies have investigated the accelerators of hepatic regeneration after hepatectomy, such as epidermal growth factor (EGF)23–25, hepatocyte growth factor (HGF)26–28 and interleukin (IL) 629–31, the focus here was on TGF-β1, a negative regulator of liver regeneration. As TGF-β1 is synthesized by many cell types and plays an important role in tissue homeostasis, complete and long-term inhibition at the level of its synthesis has been shown to lead to severe adverse events, such as robust inflammatory reactions in TGF-β1 knockout mice32,33. Therefore, the inhibition of local TGF-β1 activation is considered a more promising strategy for accelerating liver regeneration after hepatectomy. Indeed, the systemic depletion of TSP-1, one of the major activators of local TGF-β1 signalling, is known to accelerate liver regeneration after hepatectomy15. Previously, the local TGF-β–Smad pathway in the regenerating liver was shown to be activated 6–12 h after partial hepatectomy, corresponding with transient TSP-1 expression, whereas local TGF-β–Smad signalling in TSP-1-null mice was attenuated in the early period (approximately 6–12 h)15. These findings suggest that transient inhibition of TSP-1-mediated local TGF-β signal activation in the early period after hepatectomy could promote liver regeneration, although TSP-1 expression was not affected by LSKL peptide administration.
For the clinical application of the novel therapeutic approach, an evaluation of adverse effects of the administered agents is crucial. Indeed, the clinical use of recombinant IL-6, an accelerator of liver regeneration in vivo, is severely limited by its proinflammatory properties, which induce influenza-like symptoms including hypotension, fatigue and myalgia34. In the present study, the two administrations of LSKL peptide in the early period (at 0 and 6 h) did not induce any major adverse effects with respect to the histological structure of heart, kidney or lung tissue. Biochemical examination of blood samples at 6 and 24 h also revealed no adverse effects. Moreover, administration of LSKL peptide led to earlier recovery in bodyweight following hepatectomy. Furthermore, in other disease models in vivo35–38 (Table 1), daily administration of LSKL peptide for 4 weeks prevented liver fibrosis induced by dimethylnitrosamine, with no adverse events35. LSKL peptide has also been reported36,37 to reduce renal injury after unilateral ureteral obstruction and unilateral nephrectomy by suppression of TGF-β signal activation, again without any adverse events. These findings suggest that the two doses of LSKL peptide administered in the early period following hepatectomy would be an effective and tolerable strategy for accelerating liver regeneration.
Table 1.
Efficacy of LSKL peptide in vivo
| Target model | Animal | Dosage | Efficacy | TGF–Smad signal |
|---|---|---|---|---|
| DMN-induced liver fibrosis35 | Rat | 100 µg/animal (once daily for 4 weeks) | Liver fibrosis ↓ | pSmad2 ↓ |
| UUO-induced renal fibrosis36 | Rat | 4 mg/kg (once daily for 2 weeks) | Renal fibrosis ↓ | pSmad2 ↓ |
| Uninephrectomy-induced nephropathy37 | Mouse | 30 mg/kg (three times weekly for 15 weeks) | Diabetic nephropathy ↓ | pSmad2 ↓ |
| Streptozocin-induced diabetic cardiomyopathy38 | Rat | 4 mg/kg (three times weekly for 6 weeks) | Cardiac fibrosis ↓ | pSmad2 ↓ |
| Xenograft model of A43139 | Nude mouse | 500 µg/animal (once daily for 12 days) | Tumour growth ↓ | n.a. |
| Partial hepatectomy-induced liver regeneration (present study) | Mouse | 30 mg/kg (0 and 6 h after hepatectomy) | Liver regeneration ↑ | pSmad2 ↓ |
LSKL, leucine–serine–lysine–leucine; TGF, transforming growth factor; DMN, dimethylnitrosamine; pSmad2, phosphorylated Smad2; UUO, unilateral ureteral obstruction; A431, squamous cell carcinoma cell line; n.a., not applicable.
A limitation of this study is the unclear mechanism of promoted bodyweight recovery by LSKL peptide. There were no significant differences in final liver volume between the LSKL peptide and normal saline groups at 168 h after hepatectomy, suggesting that LSKL peptide did not affect the termination of liver regeneration after hepatectomy. On the other hand, LSKL peptide promoted the recovery in bodyweight following hepatectomy. It is possible that LSKL peptide accelerated functional liver regeneration. To see whether the accelerated growth was accompanied by improved function, further biochemical examinations of blood were performed at 72 and 168 h after hepatectomy. Unexpectedly, there were no significant differences in markers of liver damage (total bilirubin, AST and ALT) or nutrition (total protein and albumin) (data not shown). Prealbumin is more sensitive to changes in protein synthesis status than albumin, and its concentration closely reflects recent nutrition rather than overall nutritional status40. The rapid-turnover proteins (retinol-binding protein, prealbumin and transferrin) may be more suitable for assessing the functional analysis within 168 h after hepatectomy in mice, although the measurement of rapid-turnover proteins in mice is still difficult. A recent in vivo study41 provided the interesting finding that functional liver regeneration after hepatectomy is impaired in the early phase of liver regeneration compared with volumetric regeneration in rats. Such a discrepancy between liver volume and the corresponding liver function can be detectable by single-photon emission CT (SPECT)10. Thus, further studies using a SPECT system in vivo might be useful for analysing the relation between accelerated liver regeneration and functional changes induced by LSKL peptide.
Partial hepatectomy is usually performed in patients with liver tumours. However, curative resection of all cancer cells is not always achieved by hepatectomy. Where remnant cancer cells persist in the liver, it is not clear how the administration of LSKL peptide would affect the behaviour of these remnant cells. As TGF-β plays the dual role of a tumour suppressor, by inhibiting cell proliferation42, and a tumour promoter, by inducing epithelial mesenchymal transition43, in various cancers, there is a possibility that blocking of the TGF-β signalling pathway by LSKL peptide may lead to either the progression or the suppression of cancer cells remaining in the liver after hepatectomy. Similar problems relate to the use of recombinant human HGF and EGF, known accelerators of liver regeneration after hepatectomy, in the clinical setting, as there is experimental evidence that HGF and EGF promote cancer cell regrowth, tumour recurrence and tumour metastasis in vivo44–47. In contrast, administration of LSKL peptide for 12 days has been reported to inhibit tumour growth in squamous cell carcinoma in a xenograft model39. Although a limited (two doses) administration of LSKL peptide in the early period is likely to influence remnant cancer behaviour minimally in comparison with complete and continuous blocking of the TGF-β signalling pathway, further studies are needed to elucidate the LSKL peptide-derived influence on remnant cancer behaviour following hepatectomy.
To assess further how TSP-1 affects the liver regenerative response in the clinical setting, perioperative plasma TSP-1 levels in patients after partial hepatectomy were analysed. A high plasma TSP-1 level on day 1 after hepatectomy reflected the liver damage induced by hepatectomy, and changes in TSP-1 concentration (ΔTSP-1) could potentially be useful to predict further liver damage, such as high levels of total bilirubin and ALT at later stages of recovery. Increased plasma total bilirubin and ALT levels have been reported48 to be associated with liver failure after hepatectomy represented by low Ki-67 expression in residual hepatocytes. These findings suggest that a continuously high plasma TSP-1 concentration after hepatectomy is harmful for a normal regenerative response. There is a possibility that LSKL peptide can promote liver regeneration after hepatectomy in the clinical setting too.
Administration of LSKL peptide in the early period after hepatectomy successfully attenuated TGF-β signal activation, leading to accelerated liver regeneration with no major adverse effects. The transient inhibition of TGF-β signal activation in the early period after hepatectomy is sufficient to accelerate cell cycle progression for faster liver regeneration, and could be a novel therapeutic strategy for improved surgical outcomes and liver function following hepatectomy.
Acknowledgments
This work was supported by a Grant-in-Aid for Young Scientists (B), the Ministry of Education, Culture, Sports, Science and Technology of Japan (no. 24791434) (to H.H.), Yokoyama Rinsho Yakuri Foundation (to H.H.) and Takeda Science Foundation, Japan (to H.H.).
Disclosure: The authors declare no conflict of interest.
Supporting Information
Additional supporting information may be found in the online version of this article:
Table S1 Probes and primers used for real-time PCR (Word document)
Table S2 Clinical characteristics of patients undergoing hepatectomy (Word document)
Fig. S1 Immunohistochemistry of thrombospondin 1 protein at 0, 6 and 24 h after hepatectomy (pdf file)
Fig. S2 Biochemical examination of blood at 24 h after hepatectomy (pdf file)
References
- 1.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 2.Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836–847. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- 3.Manizate F, Hiotis SP, Labow D, Roayaie S, Schwartz M. Liver functional reserve estimation: state of the art and relevance to local treatments. Oncology. 2010;78(Suppl 1):131–134. doi: 10.1159/000315241. [DOI] [PubMed] [Google Scholar]
- 4.Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356:1545–1559. doi: 10.1056/NEJMra065156. [DOI] [PubMed] [Google Scholar]
- 5.Palavecino M, Kishi Y, Chun YS, Brown DL, Gottumukkala VN, Lichtiger B, et al. Two-surgeon technique of parenchymal transection contributes to reduced transfusion rate in patients undergoing major hepatectomy: analysis of 1557 consecutive liver resections. Surgery. 2010;147:40–48. doi: 10.1016/j.surg.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 6.Reddy SK, Barbas AS, Turley RS, Steel JL, Tsung A, Marsh JW, et al. A standard definition of major hepatectomy: resection of four or more liver segments. HPB (Oxford) 2011;13:494–502. doi: 10.1111/j.1477-2574.2011.00330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddy SK, Barbas AS, Turley RS, Gamblin TC, Geller DA, Marsh JW, et al. Major liver resection in elderly patients: a multi-institutional analysis. J Am Coll Surg. 2011;212:787–795. doi: 10.1016/j.jamcollsurg.2010.12.048. [DOI] [PubMed] [Google Scholar]
- 8.Yang T, Zhang J, Lu JH, Yang GS, Wu MC, Yu WF. Risk factors influencing postoperative outcomes of major hepatic resection of hepatocellular carcinoma for patients with underlying liver diseases. World J Surg. 2011;35:2073–2082. doi: 10.1007/s00268-011-1161-0. [DOI] [PubMed] [Google Scholar]
- 9.Fan ST, Mau Lo C, Poon RT, Yeung C, Leung Liu C, Yuen WK, et al. Continuous improvement of survival outcomes of resection of hepatocellular carcinoma: a 20-year experience. Ann Surg. 2011;253:745–758. doi: 10.1097/SLA.0b013e3182111195. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi H, Beppu T, Okabe H, Kuroki H, Nakagawa S, Imai K, et al. Functional assessment versus conventional volumetric assessment in the prediction of operative outcomes after major hepatectomy. Surgery. 2015;157:20–26. doi: 10.1016/j.surg.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Andreou A, Vauthey JN, Cherqui D, Zimmitti G, Ribero D, Truty MJ, et al. International Cooperative Study Group on Hepatocellular Carcinoma. Improved long-term survival after major resection for hepatocellular carcinoma: a multicenter analysis based on a new definition of major hepatectomy. J Gastrointest Surg. 2013;17:66–77. doi: 10.1007/s11605-012-2005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcea G, Maddern GJ. Liver failure after major hepatic resection. J Hepatobiliary Pancreat Surg. 2009;16:145–155. doi: 10.1007/s00534-008-0017-y. [DOI] [PubMed] [Google Scholar]
- 13.Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayashi H, Sakai T. Biological significance of local TGF-beta activation in liver diseases. Front Physiol. 2012;3:12. doi: 10.3389/fphys.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayashi H, Sakai K, Baba H, Sakai T. Thrombospondin-1 is a novel negative regulator of liver regeneration after partial hepatectomy through transforming growth factor-beta1 activation in mice. Hepatology. 2012;55:1562–1573. doi: 10.1002/hep.24800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Starlinger P, Schauer D, Alidzanovic L, Zikeli S, Gebhardt K, Luf F, et al. Clinical evidence for thrombospondin-1 as a relevant suppressor of liver regeneration. J Hepatol. 2013;58:1053–1054. doi: 10.1016/j.jhep.2012.11.037. [DOI] [PubMed] [Google Scholar]
- 17.Sweetwyne MT, Murphy-Ullrich JE. Thrombospondin 1 in tissue repair and fibrosis: TGF-beta-dependent and independent mechanisms. Matrix Biol. 2012;31:178–186. doi: 10.1016/j.matbio.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JW, Kim HS, Kim IK, Kim MR, Cho EY, Kim HK, et al. Transforming growth factor-beta 1 induces apoptosis through down-regulation of c-myc gene and overexpression of p27Kip1 protein in cervical carcinoma. Gynecol Oncol. 1998;69:230–236. doi: 10.1006/gyno.1998.5003. [DOI] [PubMed] [Google Scholar]
- 19.Quelle DE, Ashmun RA, Shurtleff SA, Kato JY, Bar-Sagi D, Roussel MF, et al. Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev. 1993;7:1559–1571. doi: 10.1101/gad.7.8.1559. [DOI] [PubMed] [Google Scholar]
- 20.Bouchard C, Thieke K, Maier A, Saffrich R, Hanley-Hyde J, Ansorge W, et al. Direct induction of cyclin D2 by Myc contributes to cell cycle progression and sequestration of p27. EMBO J. 1999;18:5321–5333. doi: 10.1093/emboj/18.19.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bouchard C, Dittrich O, Kiermaier A, Dohmann K, Menkel A, Eilers M, et al. Regulation of cyclin D2 gene expression by the Myc/Max/Mad network: Myc-dependent TRRAP recruitment and histone acetylation at the cyclin D2 promoter. Genes Dev. 2001;15:2042–2047. doi: 10.1101/gad.907901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fausto N. Liver regeneration. J Hepatol. 2000;32(Suppl):19–31. doi: 10.1016/s0168-8278(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 23.Glanemann M, Shi B, El-Zidy N, Gaebelein G, Kronbach Z, Neuhaus P, et al. Subcutaneous administration of epidermal growth factor: a true treatment option in case of postoperative liver failure? Int J Surg. 2009;7:200–205. doi: 10.1016/j.ijsu.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Jones DE, Jr, Tran-Patterson R, Cui DM, Davin D, Estell KP, Miller DM. Epidermal growth factor secreted from the salivary gland is necessary for liver regeneration. Am J Physiol. 1995;268(Pt 1):G872–G878. doi: 10.1152/ajpgi.1995.268.5.G872. [DOI] [PubMed] [Google Scholar]
- 25.Skov Olsen P, Boesby S, Kirkegaard P, Therkelsen K, Almdal T, Poulsen SS, et al. Influence of epidermal growth factor on liver regeneration after partial hepatectomy in rats. Hepatology. 1988;8:992–996. doi: 10.1002/hep.1840080503. [DOI] [PubMed] [Google Scholar]
- 26.Kaibori M, Kwon AH, Nakagawa M, Wei T, Uetsuji S, Kamiyama Y, et al. Stimulation of liver regeneration and function after partial hepatectomy in cirrhotic rats by continuous infusion of recombinant human hepatocyte growth factor. J Hepatol. 1997;27:381–390. doi: 10.1016/s0168-8278(97)80185-7. [DOI] [PubMed] [Google Scholar]
- 27.Yanagida H, Kaibori M, Hijikawa T, Kwon AH, Kamiyama Y, Okumura T. Administration of rhHGF-activator via portal vein stimulates the regeneration of cirrhotic liver after partial hepatectomy in rats. J Surg Res. 2006;130:38–44. doi: 10.1016/j.jss.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Yoshikawa A, Kaido T, Seto S, Yamaoka S, Sato M, Ishii T, et al. Hepatocyte growth factor promotes liver regeneration with prompt improvement of hyperbilirubinemia in hepatectomized cholestatic rats. J Surg Res. 1998;78:54–59. doi: 10.1006/jsre.1998.5350. [DOI] [PubMed] [Google Scholar]
- 29.Nechemia-Arbely Y, Shriki A, Denz U, Drucker C, Scheller J, Raub J, et al. Early hepatocyte DNA synthetic response posthepatectomy is modulated by IL-6 trans-signaling and PI3K/AKT activation. J Hepatol. 2011;54:922–929. doi: 10.1016/j.jhep.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 30.Tiberio GA, Tiberio L, Benetti A, Cervi E, Montani N, Dreano M, et al. IL-6 promotes compensatory liver regeneration in cirrhotic rat after partial hepatectomy. Cytokine. 2008;42:372–378. doi: 10.1016/j.cyto.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Peters M, Blinn G, Jostock T, Schirmacher P, Meyer zum Büschenfelde KH, Galle PR, et al. Combined interleukin 6 and soluble interleukin 6 receptor accelerates murine liver regeneration. Gastroenterology. 2000;119:1663–1671. doi: 10.1053/gast.2000.20236. [DOI] [PubMed] [Google Scholar]
- 32.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, et al. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Gameren MM, Willemse PH, Mulder NH, Limburg PC, Groen HJ, Vellenga E, et al. Effects of recombinant human interleukin-6 in cancer patients: a phase I–II study. Blood. 1994;84:1434–1441. [PubMed] [Google Scholar]
- 35.Kondou H, Mushiake S, Etani Y, Miyoshi Y, Michigami T, Ozono K. A blocking peptide for transforming growth factor-beta1 activation prevents hepatic fibrosis in vivo. J Hepatol. 2003;39:742–748. doi: 10.1016/s0168-8278(03)00377-5. [DOI] [PubMed] [Google Scholar]
- 36.Xie XS, Li FY, Liu HC, Deng Y, Li Z, Fan JM. LSKL, a peptide antagonist of thrombospondin-1, attenuates renal interstitial fibrosis in rats with unilateral ureteral obstruction. Arch Pharm Res. 2010;33:275–284. doi: 10.1007/s12272-010-0213-6. [DOI] [PubMed] [Google Scholar]
- 37.Lu A, Miao M, Schoeb TR, Agarwal A, Murphy-Ullrich JE. Blockade of TSP1-dependent TGF-beta activity reduces renal injury and proteinuria in a murine model of diabetic nephropathy. Am J Pathol. 2011;178:2573–2586. doi: 10.1016/j.ajpath.2011.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belmadani S, Bernal J, Wei CC, Pallero MA, Dell'italia L, Murphy-Ullrich JE, et al. A thrombospondin-1 antagonist of transforming growth factor-beta activation blocks cardiomyopathy in rats with diabetes and elevated angiotensin II. Am J Pathol. 2007;171:777–789. doi: 10.2353/ajpath.2007.070056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yee KO, Streit M, Hawighorst T, Detmar M, Lawler J. Expression of the type-1 repeats of thrombospondin-1 inhibits tumor growth through activation of transforming growth factor-beta. Am J Pathol. 2004;165:541–552. doi: 10.1016/s0002-9440(10)63319-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ingenbleek Y, Young VR. Significance of transthyretin in protein metabolism. Clin Chem Lab Med. 2002;40:1281–1291. doi: 10.1515/CCLM.2002.222. [DOI] [PubMed] [Google Scholar]
- 41.de Graaf W, Bennink RJ, Heger M, Maas A, de Bruin K, van Gulik TM. Quantitative assessment of hepatic function during liver regeneration in a standardized rat model. J Nucl Med. 2011;52:294–302. doi: 10.2967/jnumed.110.078360. [DOI] [PubMed] [Google Scholar]
- 42.Jahn SC, Law ME, Corsino PE, Law BK. TGF-beta antiproliferative effects in tumor suppression. Front Biosci (Schol Ed) 2012;4:749–766. doi: 10.2741/s297. [DOI] [PubMed] [Google Scholar]
- 43.Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goyal L, Muzumdar MD, Zhu AX. Targeting the HGF/c-MET pathway in hepatocellular carcinoma. Clin Cancer Res. 2013;19:2310–2318. doi: 10.1158/1078-0432.CCR-12-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hecht M, Papoutsi M, Tran HD, Wilting J, Schweigerer L. Hepatocyte growth factor/c-Met signaling promotes the progression of experimental human neuroblastomas. Cancer Res. 2004;64:6109–6118. doi: 10.1158/0008-5472.CAN-04-1014. [DOI] [PubMed] [Google Scholar]
- 46.Hoelting T, Siperstein AE, Clark OH, Duh QY. Epidermal growth factor enhances proliferation, migration, and invasion of follicular and papillary thyroid cancer in vitro and in vivo. J Clin Endocrinol Metab. 1994;79:401–408. doi: 10.1210/jcem.79.2.8045955. [DOI] [PubMed] [Google Scholar]
- 47.Huang SM, Li J, Harari PM. Molecular inhibition of angiogenesis and metastatic potential in human squamous cell carcinomas after epidermal growth factor receptor blockade. Mol Cancer Ther. 2002;1:507–514. [PubMed] [Google Scholar]
- 48.Makino H, Togo S, Kubota T, Morioka D, Morita T, Kobayashi T, et al. A good model of hepatic failure after excessive hepatectomy in mice. J Surg Res. 2005;127:171–176. doi: 10.1016/j.jss.2005.04.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Probes and primers used for real-time PCR (Word document)
Table S2 Clinical characteristics of patients undergoing hepatectomy (Word document)
Fig. S1 Immunohistochemistry of thrombospondin 1 protein at 0, 6 and 24 h after hepatectomy (pdf file)
Fig. S2 Biochemical examination of blood at 24 h after hepatectomy (pdf file)