Abstract
Aims
Myeloperoxidase (MPO) catalyses the formation of a wide variety of oxidants, including hypochlorous acid (HOCl), and contributes to cardiovascular disease progression. We hypothesized that during its action MPO evokes substantial vasomotor responses.
Methods
Following exposure to MPO (1.92 mU mL−1) in the presence of increasing concentrations of hydrogen peroxide (H2O2), changes in arteriolar diameter of isolated gracilis skeletal muscle arterioles (SMAs) and coronary arterioles (CAs) and in the isometric force in basilar arteries (BAs) of the rat were monitored.
Results
Myeloperoxidase increased vascular tone to different degrees in CAs, SMAs and BAs. The mechanism of increased vasoconstriction was studied in detail in SMAs. MPO-evoked vasoconstrictions were prevented by the MPO inhibitor 4-aminobenzhydrazide (50 μm), by endothelium removal in the SMAs. Surprisingly, the HOCl scavenger L-methionine (100 μm), the thromboxane A2 (TXA2) antagonist SQ-29548 (1 μm) or the non-specific cyclooxygenase (COX) antagonist indomethacin (1 μm) converted the MPO-evoked vasoconstrictions to pronounced vasodilations in SMAs, not seen in the presence of H2O2. In contrast to noradrenaline-induced vasoconstrictions, the MPO-evoked vasoconstrictions were not accompanied by significant increases in arteriolar [Ca2+] levels in SMAs.
Conclusion
These data showed that H2O2-derived HOCl to be a potent vasoconstrictor upon MPO application. HOCl activated the COX pathway, causing the synthesis and release of a TXA2-like substance to increase the Ca2+ sensitivity of the contractile apparatus in vascular smooth muscle cells and thereby to augment H2O2-evoked vasoconstrictions. Nevertheless, inhibition of the HOCl–COX–TXA2 pathway unmasked the effects of additional MPO-derived radicals with a marked vasodilatory potential in SMAs.
Keywords: hydrogen peroxide, myeloperoxidase, smooth muscle calcium, thromboxane A2, vasoconstrictions
The effector enzyme myeloperoxidase (MPO) has a protective role in inflammatory processes. However, the activation of MPO may become deleterious and can also contribute to the development of cardiovascular diseases (Nicholls & Hazen 2005, Podrez et al. 2000, Klebanoff 2005). Accordingly, excessive levels of MPO in the plasma may be accompanied by an increased risk of subsequent cardiovascular events (Baldus et al. 2003, Zhang et al. 2001c, Vita et al. 2004, Brennan et al. 2003, Karakas & Koenig 2012, Kataoka et al. 2014), whereas individuals with an inherited MPO deficiency are at a reduced cardiovascular risk (Nikpoor et al. 2001, Hoy et al. 2001). There is currently no clear explanation of this situation.
Myeloperoxidase, a haem-containing, intensely green protein, was originally isolated from canine pus and from purulent fluids from patients with tuberculosis (Klebanoff 2005, Malle et al. 2007). The synthesis of MPO is initiated in the bone marrow during myeloid differentiation and is completed in the granulocytes (Lau & Baldus 2006, Hansson et al. 2006). MPO is stored primarily in the azurophil granules of the polymorphonuclear neutrophils and monocytes, but it has also been found in tissue macrophages (Daugherty et al. 1994, Lau & Baldus 2006, Hampton et al. 1998, Klebanoff 2005). To exert its antimicrobial effects, MPO primarily catalyses the reaction of hydrogen peroxide (H2O2) with chloride (Hampton et al. 1998), to form hypochlorous acid (HOCl) (Malle et al. 2007, Cook et al. 2012). The activation of MPO additionally gives rise to a number of other pro-oxidative radicals through its peroxidase activity. The biological effects of the MPO (e.g. vasomotor activity, permeability, apoptotic effect) system depend on the local concentration of H2O2 (Golubinskaya et al. 2014) of other substrates and/or antioxidant molecules (e.g. methionine (Met) (Podrez et al. 2000, Porszasz et al. 2002). Taken together, the involvement of MPO has been implicated in vascular inflammation in association with infection, diabetes and atherosclerosis (Malle et al. 2007, Cook et al. 2012, Zhang et al. 2004, Kataoka et al. 2014, Sugiyama et al. 2001, Sirpal 2009, Woods et al. 2003, Ford 2010).
It is not known at present how the persistent generation of MPO-derived oxidants evokes adverse effects in vascular tissues. MPO and its oxidative products are highly abundant in human atherosclerotic lesions (Daugherty et al. 1994, Hazen & Heinecke 1997, Hazen et al. 2000, Hazell et al. 1996). MPO is presumed to be involved in the oxidative modification of low-density lipoprotein, thereby converting it into a high-uptake form and hence promoting foamy cell formation (Podrez et al. 1999, Savenkova et al. 1994). Through its catalytic activity, MPO can consume nitrogen monoxide (NO), thereby limiting its bioavailability (Eiserich et al. 2002, Abu-Soud & Hazen 2000). MPO-derived HOCl reacts with L-arginine and produces NO synthesis inhibitors (Zhang et al. 2001b,a), and HOCl can impair endothelial NO bioactivity in a superoxide-dependent manner (Stocker et al. 2004). Furthermore, MPO and HOCl can activate matrix metalloproteinases and deactivate matrix metalloproteinase inhibitors, leading to weakening of the fibrous cap and the development of destabilized atherosclerotic plaque (Karakas & Koenig 2012, Fu et al. 2001). From a functional aspect, MPO treatment led to a decrease in myocardial perfusion in pigs and inhibited the acetylcholine-evoked relaxation in the internal mammary arteries (Rudolph et al. 2012). Vasorelaxation in response to acetylcholine was also found to be impaired in mice at relatively high plasma MPO levels (Zhang et al. 2013). Nevertheless, the mechanisms through which MPO modulates the vascular responses are not well understood. In this study, we made an effort to investigate the effects of MPO activation in vascular preparations in vitro. Moreover, we tried to characterize the possible mechanism of the vasomotor action of MPO in SMAs.
As the MPO substrate H2O2 was earlier identified as an important regulator of vascular diameter under both normal and pathological conditions, the vasoactive effects of MPO were contrasted to those of H2O2. H2O2 evokes a concentration-dependent biphasic effect in the skeletal muscle arterioles (SMAs) and mesenteric arteries in the rat, causing vasoconstriction at lower concentrations and vasodilation at higher concentrations (Gao et al. 2003, Cseko et al. 2004, Csato et al. 2014), whereas, H2O2 induces only vasodilation in the rat coronaries (Csato et al. 2014).
In this study, we investigated (i) the acute effects of MPO on the H2O2-evoked changes in diameter in isolated SMAs and coronary arterioles (CAs) and on the contractile force in the basilar arteries (BAs) of the rat, and (ii) the signal transduction pathways mediating the vascular effects of MPO-derived oxidative radicals.
Materials and methods
Animals, anaesthesia and tissue dissection
Male Wistar rats (weighing 250–350 g, 6–9 weeks old) obtained from Toxi-Coop Toxicological Research Center, Dunakeszi, Hungary, were fed a standard chow and drank tap water ad libitum. Anaesthesia was performed with an intraperitoneal injection of sodium pentobarbital (150 mg kg−1), and all efforts were made to minimize suffering of animals. The gracilis muscle, the heart and the brain were removed and placed into silicone-coated petri dishes containing 0–4 °C Krebs solution (composition in mm: 110 NaCl, 5.0 KCl, 2.5 CaCl2, 1.0 MgSO4, 1.0 KH2PO4, 5.0 glucose and 24.0 NaHCO3, obtained from Sigma-Aldrich, St. Louis, MO, USA) equilibrated with a gaseous mixture of 5% CO2, 10% O2 and 85% N2 at pH 7.4. All animal procedures used in this study were in full accordance with the rules of the Ethical Committee of the University of Debrecen and approved by the appropriate governmental body Directive 2010/63/EU of the European Parliament. The study conforms with Persson PB. Good Publication Practice in Physiology 2013 Guidelines for Acta Physiol (Oxf) (Persson 2013).
Materials and drugs
The TXA2 inhibitor SQ-29548 was purchased from BioMarker Kft. (Gödöllő, Hungary). MPO protein, MPO inhibitor and COX antibodies were obtained from Abcam (Cambridge, UK). Secondary antibodies were from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA, USA). All other chemicals were from Sigma-Aldrich and were kept under the conditions recommended by the manufacturer. All reported concentrations are cumulative concentrations in the organ chamber.
Measurement of arteriolar diameter
The rat SMAs and CAs were isolated and the changes in their diameters were measured as described earlier (Csato et al. 2014). Briefly, the isolated arterioles were transferred into an organ chamber and then were cannulated. The intraluminal pressure was set at 80 mmHg (pressure servo control system, Living Systems Instrumentation, St. Albans, VT, USA). The temperature was maintained at 37 °C by the built-in temperature controller in the tissue chamber (Living Systems Instrumentation). Changes in arteriolar diameter were recorded by a video microscope system (microscope: Nikon, Eclipse 80i; CCD camera: Topica Technology Co. Ltd., Taipei, Taiwan; video digitizer: National Institutes, Bethesda, USA). The isolated SMAs and CAs spontaneously developed a substantial myogenic tone (a decrease in diameter from 196 ± 6 μm to 160 ± 6 μm, n = 45, and from 234 ± 14 μm to 178 ± 14 μm, n = 9 respectively) in response to an intraluminal pressure of 80 mmHg.
Measurement of arteriolar contractions under isometric conditions
Basilar arteries were prepared from rat brains with microsurgical tools, and approx. 4-mm-long rings were then mounted in an isometric contraction measurement system (DMT-510, Danish Myotechnology, Aarhus, Denmark). Before exposure to test solutions, vessel tone was normalized. To this end, preparations were stretched at a force by increasing 1.5 mN every 15 s until the calculated intraluminal pressure reached 13.4 kPa. The experiments were then performed at this stretch level (isometric contractions).
Experimental protocols
The endothelial function was tested with acetylcholine (1 nm–10 μm) and the smooth muscle function with noradrenaline (1 nm–10 μm, in SMAs), serotonin (1 nm–10 μm in CAs) or potassium chloride (10–60 mm, in BAs).
Myeloperoxidase activity was measured via detection of the chemiluminescence produced upon the oxidation of luminol. H2O2 working solutions were prepared from the stabilized 30% stock solution (Sigma-Aldrich) immediately before the experiments and were stored on ice. The arterioles were first treated with MPO (1.92 mU mL−1, 300 s treatment duration, diameter measured every 10 s) to record the effects of MPO alone. This was followed by the addition of H2O2 (1 μm–10 mm) and the responses to MPO+H2O2 were then determined. In the BAs, the effects of MPO and H2O2 were tested after pre-contractions were evoked with 60 mm potassium chloride.
The mechanism of MPO-evoked vasomotor responses was explored in detail in SMAs. In some experiments, the endothelium was removed by the perfusion of air bubbles through the arterioles (denudation). Successful endothelium denudation was verified by the loss of dilation in response to acetylcholine (10 μm, 96 ± 4% dilation before and −6 ± 4% after endothelium removal, n = 5), while a maintained smooth muscle function was confirmed with noradrenaline (71 ± 1% constriction before and 64 ± 2% after endothelium removal). The effects of MPO and H2O2 were also measured in the presence of an MPO inhibitor (50 μm 4-aminobenzhydrazide), a TXA2 receptor inhibitor (1 μm SQ-29548) and a COX antagonist (10 μm indomethacin) in the SMAs. The effects of MPO were tested after incubation of the vessels with the HOCl scavenger L-Met (20, 40 and 100 μm) in all three vessel types. At the end of the experiments, the maximal (passive) arteriolar diameter was determined in the absence of extracellular Ca2+.
Simultaneous measurement of vascular diameter and intracellular Ca2+ concentrations
Simultaneous measurements of intracellular Ca2+ and arteriolar diameter were performed as described previously (Csato et al. 2014, Czikora et al. 2012, Kandasamy et al. 2013). Briefly, SMAs were isolated and cannulated as mentioned above, except that the tissue bath was supplemented with 1% bovine serum albumin (Sigma-Aldrich) and 5 μm Fura-2AM a ratiometric fluorescent Ca2+ indicator dye (Molecular Probes, Eugene, OR, USA) until a spontaneous myogenic tone developed. Intracellular Ca2+ concentrations were measured with an Incyte IM system (Intracellular Imaging Inc, Cincinnati, OH, USA). Fura-2 fluorescence (recorded every 2–5 s) was excited alternately by 340- and 380-nm light, and the emitted fluorescence was detected above 510 nm. The outer arteriolar diameter was determined in each recorded image. Arteriolar Ca2+ concentration was determined as the Fura-2 fluorescence ratio (F340/380).
Immunohistochemistry
The gracilis muscle was removed from the rat and embedded in Tissue-Tek OCT compound (Electron Microscopy Sciences; Hatfield, PA, USA). Cryostat sections (10 μm thick, Electron Microscopy Sciences; Hatfield, PA, USA) were prepared, fixed in acetone for 5–10 min and blocked with normal goat sera for 20 min (1.5% in PBS, Sigma-Aldrich). COX enzymes were stained with COX-1 (rabbit anti-COX-1: ab109025, dilution: 1:50) and COX-2-specific antibodies (rabbit anti-COX-2: ab15191, dilution: 1:50). Antibodies were visualized through the use of fluorescent secondary antibodies (goat anti-rabbit biotin, dilution: 1:100; goat anti-mouse FITZ, dilution: 1:300). Gracilis muscle was co-stained with antismooth muscle actin (NCL-SMA, dilution, 1:20; Novocastra Laboratories, Newcastle, UK) and DAPI (Vector Laboratories, Burlingame, California, USA). Pictures were processed by ImageJ software (NIH, Bethesda, MD, USA).
Measurement of inhibitory effect of L-Met on the chlorinating activity of MPO
Myeloperoxidase-evoked chlorinating activity was measured with a commercial assay kit (Cayman Chemical Company, Ann Arbor, MI, USA) in accordance with the manufacturer's instructions. The measurement is based on the cleavage of non-fluorescent 2-[6-(4-aminophenoxy)-3-oxo-3H-xanthen-9-yl] benzoic acid (APF) to fluorescein by MPO-generated hypochlorite (-OCl). The reaction mixtures contained 45 μm APF, 30 μm H2O2, 3 U L−1 MPO and 200–0.39 mm L-Met (serially diluted). The measurements were performed in phosphate-buffered saline (PBS, pH = 7.4) independently from the in vitro vascular experiments. Changes in fluorescence intensity (λex = 485 nm and λem = 520 nm) were measured at 30-s intervals for 5 min with a plate reader (NovoStar plate reader, BMG Labtech). Fluorescence intensity values were plotted as a function of time and fitted by linear regression (before saturation). The slope of this relation was used to calculate MPO activities.
Data analysis and statistical procedures
The internal diameters of arterioles are shown as means ± SEM. Arteriolar constriction was expressed as the change in diameter as a percentage of the initial diameter (before addition of the vasoactive agents) measured at an intraluminal pressure of 80 mmHg. Arteriolar dilation was calculated as the percentage of the maximal (passive) diameter determined in the absence of extracellular Ca2+ at the end of the experiments. The contractile force was indicated in absolute values, as the difference from the initial force in the case of isometric measurements. Statistical analyses were performed with Microsoft Office Excel software by the Student's t-test. P < 0.05 was considered statistically significant.
Results
MPO promotes H2O2-evoked vasoconstriction
Myeloperoxidase (1.92 mU mL−1) increased the vascular tone and promoted the development of vasoconstriction in the presence of H2O2 in vascular beds of different origin. In the SMAs, a robust MPO-dependent vasoconstrictive effect was observed, that is from a 50 ± 21% level of vasodilation (at 1 mm H2O2) to 47 ± 11% vasoconstriction following the addition of MPO (P = 0.004; Fig.1a). In the CAs, where H2O2 evoked only vasodilation, MPO administration resulted in significant vasoconstriction in a wide range of H2O2 concentrations, for example 13 ± 4% dilation at 100 μm H2O2, but 6 ± 3% constriction following the addition of MPO (P = 0.006; Fig.1b). In the BAs, the MPO-dependent vasoconstriction was relatively less pronounced, for example 1.1 ± 0.5 mN dilation at 100 μm H2O2 and 1.6 ± 0.7 mN constriction following the addition of MPO (P < 0.05; Fig.1c). Vascular diameters measured under various test conditions are to be seen in Tables 1 and 2.
Figure 1.
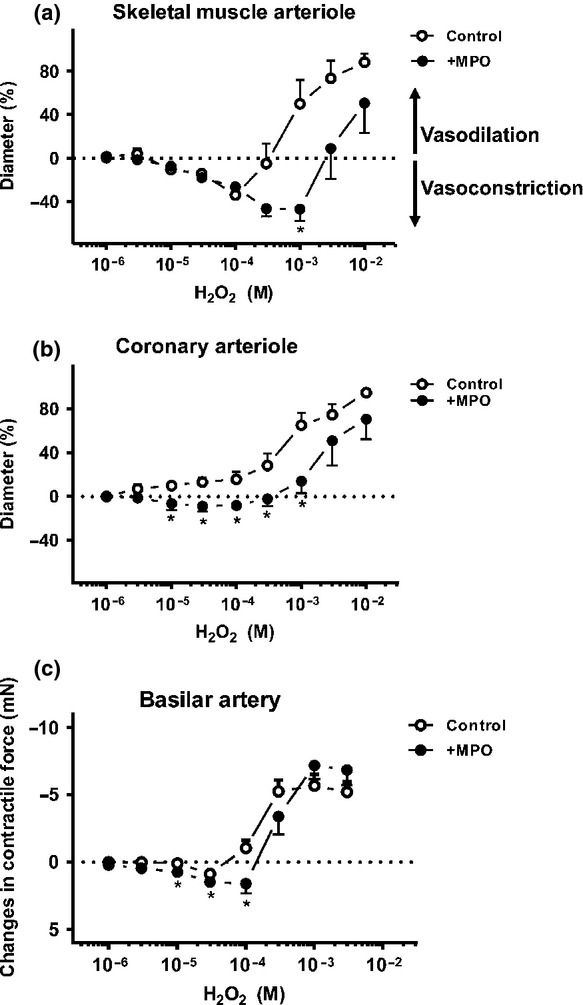
MPO promotes H2O2-evoked vasoconstriction in different vascular beds. After pre-incubation with MPO (activity: 1.92 mU mL−1, 600 s), isolated, cannulated SMAs (initial diameter (id): 182 ± 12 μm, n = 5 arterioles from four different animals; panel (a) or CAs (id: 180 ± 17 μm, n = 5 arterioles from five different animals; panel (b) with intact endothelium were treated with increasing concentrations (1 μm–10 mm) of H2O2. In SMAs, H2O2 alone (10 μm, 30 μm and 100 μm) evoked significant vasoconstriction compared to the zero line (P < 0.02). In the presence of MPO, H2O2 caused significant vasoconstriction from 10 μm–1 mm H2O2 compared to the control and the zero line (P < 0.05, panel a). In CAs, H2O2 (30 μm and 100 μm) and MPO-evoked significant vasoconstriction comparing to the control (P < 0.05) which was not significant compared to the baseline (panel b). The arteriolar diameter was recorded and cumulative concentration–response relationships were determined. Changes in relative arteriolar diameter are shown. Values during vasodilations are expressed as percentages of the difference between the maximal passive diameter (maximal dilation (100%) in the absence of extracellular Ca2+) and the initial diameter, while constriction is expressed as a percentage of the initial diameter (illustrated at 0% on the y scale). Similarly, isolated BAs (n = 5 arterioles from five different animals) pre-contracted with KCl were incubated in the presence of MPO (activity: 1.92 mU mL−1, 600 s). Arteries were exposed to the increasing concentrations of H2O2 (1 μm–3 mm, panel c). H2O2-evoked vasoconstriction was significant at 30 μm, whereas in the presence of MPO, the vasoconstriction was significant at 10 μm, 30 μm and 100 μm H2O2 compared to the baseline. MPO and H2O2 caused significant vasoconstriction compared to the control (10, 30 and 100 μm H2O2, panel c). The contractile forces are indicated in absolute values, as differences from the initial baseline force. Asterisks denote significant differences from the control (H2O2 without MPO).
Table 1.
Effects of different inhibitors and endothelium removal on the MPO- and H2O2-induced arteriolar responses
| Type of arteriole | Coronary arterioles |
Skeletal muscle arterioles |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | None/Control | MPO+ L-Met | None/Control | MPO+ SQ-29548 | MPO+ endothelium denudation | MPO+ indomethacin | MPO+ 100 μm L-Met | 100 μm L-Met | MPO+ 40 μm L-met | MPO+ 20 μm L-Met | MPO+4-aminobenzhydrazide |
| No. of experiments | 5 | 4 | 5 | 5 | 5 | 5 | 5 | 5 | 4 | 6 | 5 |
| Initial diameter | 180 ± 17 | 85 ± 15 | 182 ± 12 | 136 ± 15 | 171 ± 7 | 178 ± 8 | 115 ± 23 | 123 ± 8 | 151 ± 9 | 183 ± 25 | 188 ± 7 |
| Diameter after inhibitor | – | 76 ± 12 | – | 141 ± 14 | – | 166 ± 7 | 112 ± 20 | – | 143 ± 12 | 176 ± 25 | – |
| Diameter after MPO | 190 ± 16 | 73 ± 9 | 182 ± 12 | 142 ± 13 | 172 ± 7 | 168 ± 8 | 115 ± 19 | 120 ± 14 | 143 ± 13 | 175 ± 24 | 181 ± 8 |
| Diameter after 1 mm H2O2 | 191 ± 12 | 105 ± 15 | 93 ± 17 | 171 ± 19 | 179 ± 6 | 193 ± 8 | 175 ± 22 | 168 ± 13 | 184 ± 18 | 191 ± 26 | 143 ± 28 |
| Passive diameter | 234 ± 12 | 123 ± 10 | 233 ± 11 | 182 ± 13 | 190 ± 4 | 199 ± 8 | 179 ± 18 | 184 ± 6 | 193 ± 15 | 208 ± 26 | 225 ± 3 |
Tissue sources of arteriolar beds are indicated (CAs or SMAs). Diameters are shown as means ± SEM in absolute values (μm). The number of experiments performed is also indicated. Arteriolar diameters are given at the beginning of the experiments (initial diameter) and after treatment with 100 μm (the maximum constrictor dose in the control) or 10 mm (the maximum dilator dose in the control) H2O2. The effects of pre-incubations with inhibitors (diameter after the inhibitor) and the maximum diameter of the vessels (the passive diameter) are also indicated.
Table 2.
Effects of different treatments on the MPO- and H2O2-induced changes in isometric contractile force in the BAs
| Treatment | None/Control | MPO+ 100 μm L-Met |
|---|---|---|
| No. of experiments | 5 | 5 |
| Initial force | 5.5 ± 1.70 | 0.55 ± 0.65 |
| Force after 10 mm KCl | 1 ± 0.47 | 0.52 ± 0.41 |
| Force after 60 mm KCl | 9.97 ± 1.41 | 7.16 ± 1.41 |
| Force after MPO | 9.97 ± 1.41 | 8.02 ± 1.59 |
| Force after 1 mm H2O2 | 2.77 ± 0.46 | 2.35 ± 0.80 |
Force values are given as means ± SEM in absolute values (mN). The number of experiments performed is also indicated. Contractile forces refer to the beginnings of the experiments (initial force), after pre-contraction with KCl (10 mm or 60 mm) and after treatment with MPO and 1 mm H2O2.
Myeloperoxidase alone (without the addition of its substrate H2O2) did not affect the diameters of the SMAs or the CAs or the contractile force in the BAs (data not shown).
HOCl mediates the MPO-induced vasoconstriction in the SMAs
The mechanical effects of the chlorinating activity of MPO were assessed comparing the vascular responses in the presence of the HOCl scavenger L-Met (100 μm) with those in the presence of the MPO-specific inhibitor 4-aminobenzhydrazide (50 μm) (Fig.2a and b). The extracellular concentration of H2O2 can reach as high as 300 μmin vivo, and our studies were therefore highlighted at this H2O2 concentration. The MPO-specific inhibitor prevented the development of MPO-dependent vasoconstriction (maximal vasoconstriction at 300 μm H2O2+MPO: 47 ± 7% vs. 16 ± 6% vasoconstriction, P < 0.0001) as expected. In the presence of L-Met, however, the MPO-induced vasoconstrictions were converted to robust vasodilations (e.g. 73 ± 11% dilation at 300 μm H2O2, P < 0.0001 vs. MPO+H2O2) suggesting an MPO-evoked, but HOCl-independent vasodilation mechanism. L-Met (100 μm) alone did not affect the H2O2-evoked vasoconstriction in the absence of MPO (Fig.2c). In a parallel in vitro enzyme assay, 100 μm L-Met fully opposed the chlorinating activity of MPO (Fig.2d).
Figure 2.
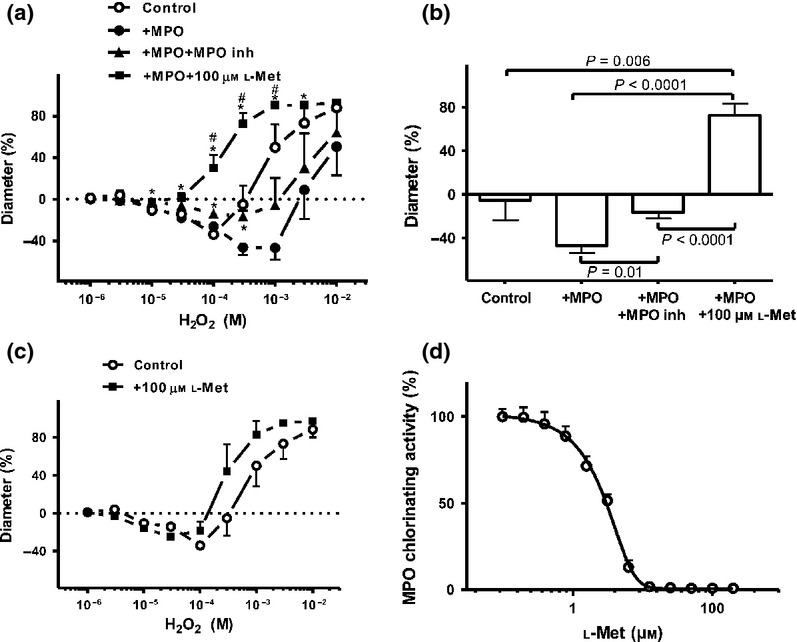
HOCl mediates the vasoconstriction evoked by MPO in the SMAs. MPO-induced vasoconstriction was inhibited with the MPO inhibitor 4-aminobenzhydrazide (50 μm) (id: 182 ± 8 μm, n = 5 arterioles from four different animals; closed triangles); however, significant vasoconstriction was still observed at 100 μm and 300 μm (P < 0.05) compared to the baseline, panel a. 100 μm L-Met converted the MPO-induced vasoconstriction to vasodilation (id: 115 ± 19 μm, n = 5 arterioles from five different animals; closed squares). Open circles represent the effects of H2O2 alone, while closed circles illustrate the effects of H2O2 in the presence of MPO. Asterisks denote significant differences from the MPO, and # denote significant differences between MPO+MPO inhibitor and MPO+L-Met. The effects of MPO alone and in combination with the MPO inhibitor or L-Met in the presence of 300 μm H2O2 (control) on the vascular diameter in the SMAs (panel b). The H2O2-induced biphasic response did not change in the presence of 100 μm L-Met (id: 120 ± 14 μm, n = 5 arterioles from five different animals; closed squares, but it caused significant vasoconstriction relative to the zero line at 10 μm and 30 μm H2O2; panel (c). Increasing concentrations of L-Met inhibited the chlorinating activity of MPO in a concentration-dependent manner (100%: maximal activity without L-Met, panel (d).
Divergent effects of L-Met treatments on MPO-evoked vasodilations in different vessel types
The MPO-stimulated HOCl-independent vasodilating mechanism was screened in different vascular beds (Fig.3). In the SMAs, the above mechanism exhibited an apparent L-Met concentration dependence (maximal vasoconstriction at 300 μm H2O2 47 ± 7% vs. vasodilations of 8 ± 19, 35 ± 23 and 73 ± 11% in the presence of 20, 40 and 100 μm L-Met respectively; Fig.3a and b). In the CA, the maximal L-Met concentration (100 μm) also provoked vasodilation at a high (1 mm) H2O2 concentration, whereas at 300 μm H2O2, L-Met did not modulate the vascular tone (i.e. 3 ± 9 vs. 13 ± 7% vasodilation; P = 0.44, Fig.3c and d). Finally, 100 μm L-Met treatment did not significantly influence the MPO-evoked vascular responses in the BAs (e.g. 3.3 ± 1 mN vasoconstriction at 300 μm H2O2 vs. 4.0 ± 1 mN vasoconstriction, P = 0.61; Fig.3e and f).
Figure 3.
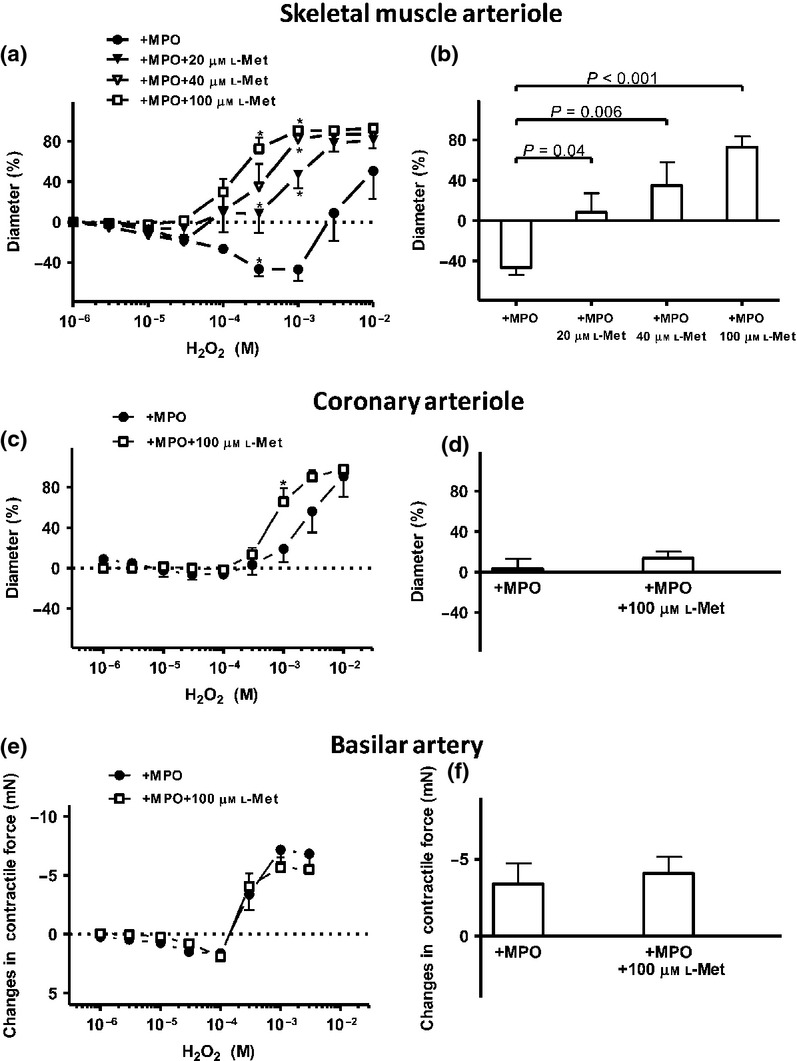
Effects of L-Met on the MPO-mediated vascular effects in different arteriolar beds. Increasing concentrations of L-Met (20, 40 or 100 μm) inhibited the MPO-mediated vasoconstriction in the SMAs in concentration-dependent manner (Id: 175 ± 24 μm, n = 6 arterioles from four different animals, with 20 μm L-Met, (closed triangles); id: 143 ± 13 μm, n = 4 arterioles from four different animals, with 40 μm L-methionine, (open triangles), id: 115 ± 19 μm, n = 5 arterioles from five different animals, with 100 μm L-Met (open squares). MPO and 20 μm L-methionine evoked significant vasoconstriction at 10 μm H2O2 compared to the baseline; panel a). The effects of MPO alone and in combination with increasing L-Met concentrations in the presence of 300 μm H2O2 (control) on the vascular diameter in the SMAs (panel b). In the CAs, L-Met (100 μm; open squares) inhibited the MPO-evoked vasoconstriction only at a higher concentration of H2O2 (id: 73 ± 10 μm, n = 4 arterioles from four different animals). Asterisks denote significant differences from MPO (panel c). The effects of MPO alone and in combination with 100 μm L-Met in the presence of 300 μm H2O2 (control) on the vascular diameter in the CAs (panel d). L-Met (100 μm; open squares) did not significantly influence the MPO-evoked changes in the isometric force in the BAs compared to the control (n = 6 arterioles from three different animals, panel e), but comparing to the zero line, MPO together with L-met caused significant vasoconstriction at 30 μm and 100 μm H2O2 (P ≤ 0.05). The effects of MPO alone and in combination with 100 μm L-Met in the presence of 300 μm H2O2 (control) on the vascular diameter in the BAs (panel f).
The signalling mechanism of MPO-evoked vasoconstriction in the SMAs
Endothelium removal inhibited the MPO-evoked vasoconstriction in the SMAs (e.g. 47 ± 7% vasoconstriction at 300 μm H2O2+MPO with intact endothelium vs. 13 ± 15% vasoconstriction + MPO without endothelium, P = 0.07; Fig.4a).
Figure 4.
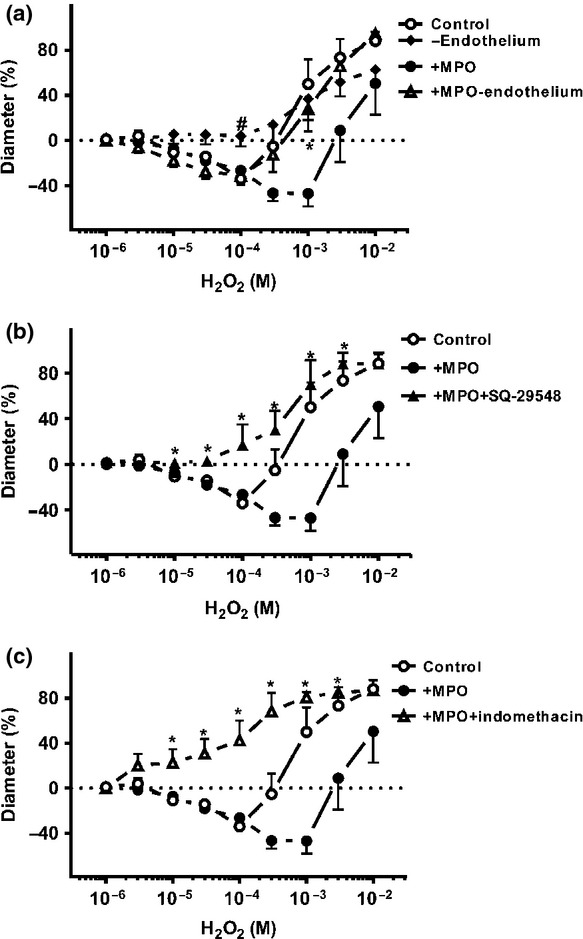
The mechanism of MPO-induced vasoconstriction in the SMAs. H2O2-evoked vasoconstriction (open circles, control) was abolished after endothelium denudation (id: 138 ± 10 μm, n = 4 arterioles from four different animals; closed diamonds, panel a). However, in the presence of MPO, and at relatively low H2O2 concentrations, vasoconstrictions (significant vasoconstriction at 10 μm–100 μm H2O2 compared to the baseline; P < 0.05) were still observed in the absence of endothelium (id: 172 ± 7 μm, n = 5 arterioles from four different arterioles; open triangles). Closed circles illustrate the effects of MPO. Asterisks denote significant differences from the action of MPO in the presence and absence of endothelium, and # indicate significant differences between the endothelium removal and the control. The MPO- and H2O2-induced vasoconstriction was tested in the presence of the TXA2 receptor antagonist (id: 142 ± 13 μm, n = 5 arterioles from four different animals; closed triangles, panel b) and in the presence of the COX inhibitor (id: 168 ± 8 μm, n = 5 arterioles from three different animals; open triangles, panel c). Asterisks denote significant differences from MPO.
Next, the involvement of the TXA2 receptors in the MPO-evoked vasoconstrictive effects was tested. Inhibition of the TXA2 receptors by 1 μm SQ-29548 converted the MPO-evoked vasoconstrictions to vasodilations (e.g. 47 ± 7% vasoconstriction at 300μm H2O2+MPO vs. 30 ± 17% dilation at 300 μm H2O2+MPO+TXA2 receptor inhibitor; P = 0.002, Fig.4b).
The role of COXs in the MPO-evoked vascular responses was also examined using the non-specific COX inhibitor indomethacin (1 μm); similarly to TXA2 inhibition, this not only prevented the MPO-evoked vasoconstriction, but converted it to vasodilation (47 ± 7% vasoconstriction at 300 μm H2O2 vs. 69 ± 16% vasodilation; P = 0.002; Fig.4c).
Vascular expression of COXs in the SMAs
The expression of COX isoenzymes in SMAs was tested by immunohistochemistry. Both the vascular smooth muscle layer and the endothelial cells were stained positively with the anti-COX-1 antibody, whereas the anti-COX-2 antibody did not produce a COX-specific staining pattern (Fig.5).
Figure 5.
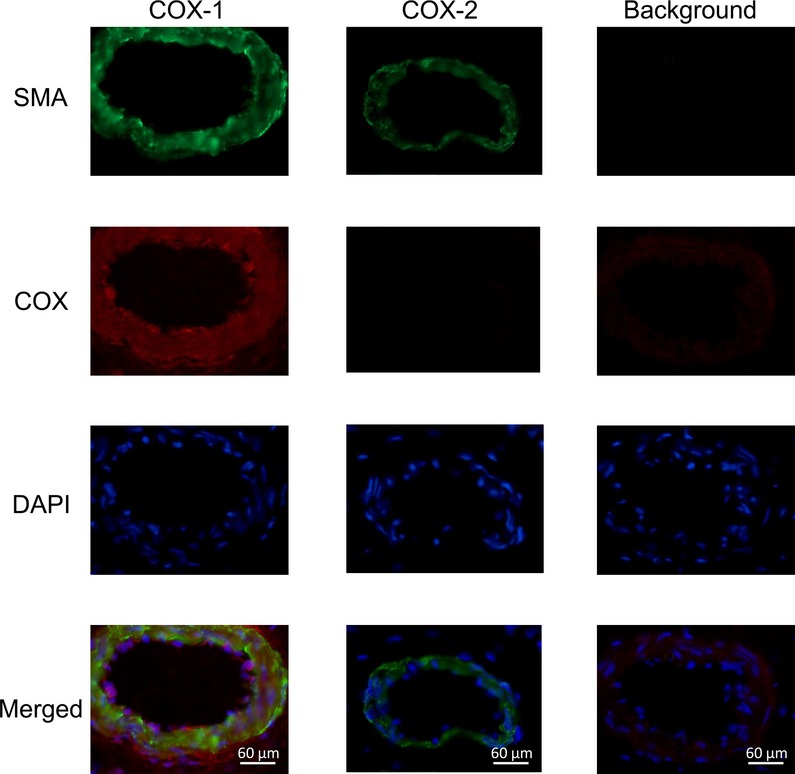
COX-1 isoenzyme is present in the vascular endothelial and smooth muscle cells in the SMAs. The presence of COX-1 isoenzyme in the vascular smooth muscle cells and in the vascular endothelium was confirmed by immunohistochemistry. Smooth muscle actin is labelled in green, COX in red, and nuclei in blue (from top to bottom). Control images (without primary antibodies) are indicated in the right-hand column.
MPO-induced vasoconstriction develops in the absence of significant intracellular Ca2+ concentration changes
Measurements of the intracellular Ca2+ concentration and the arteriolar diameter changes were performed in parallel in the SMAs. MPO-evoked vasoconstriction (29 ± 3% vasoconstriction at 1 mm H2O2; P = 0.04 vs. the baseline) developed without significant changes in the F340/380 ratio signal in the range of H2O2 concentrations between 1 μm and 1 mm (Fig.6a). In contrast, the noradrenaline-evoked (1 nm–10 μm) vasoconstrictions with comparable magnitudes (44 ± 4% constriction at 10 μm noradrenaline; P = 0.0005 vs. the baseline) were accompanied by significant increases in the F340/380 ratio (from 0.85 ± 0.03 to 1.15 ± 0.09; Fig.6b). MPO alone did not have any effect on the arteriolar diameter or on the F340/380 signal (not shown).
Figure 6.
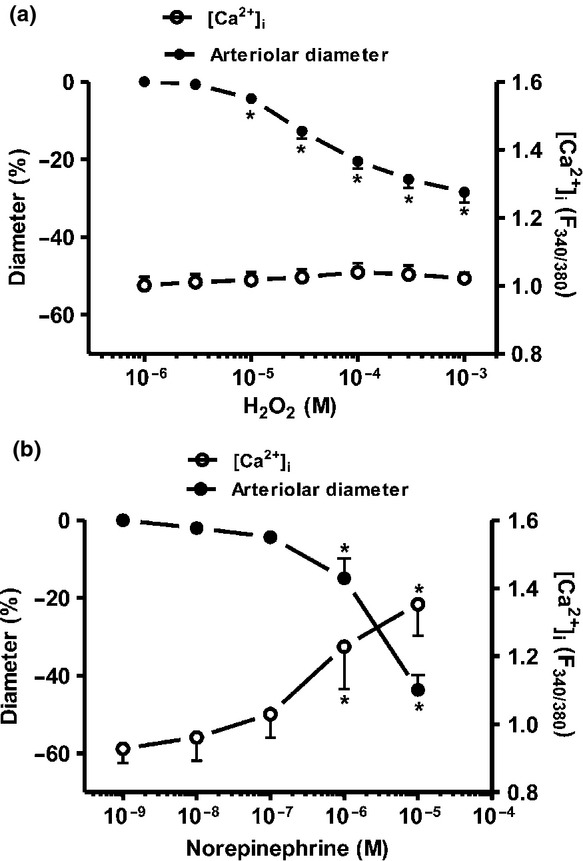
MPO increases the Ca2+ sensitivity of force production in the vascular smooth muscle cells. The changes in intracellular Ca2+ levels (F340/380 signals) and external arteriolar diameters were studied in SMAs under control conditions (id: 297 ± 9 μm, n = 7 arterioles from six different animals; panel a), or after treatment with noradrenaline (id: 314 ± 16 μm, n = 7 arterioles from six different animals; panel b). Asterisks denote significant differences from the initial values.
Discussion
Vascular inflammation during endothelial dysfunction (Zhang et al. 2001a), atherosclerosis (Sugiyama et al. 2001, Sirpal 2009, Woods et al. 2003, Ford 2010), diabetes mellitus (Zhang et al. 2004, Kataoka et al. 2014) and coronary artery disease (Cavusoglu et al. 2007, Mayyas et al. 2014) is characterized by increased levels of production and local release of both H2O2 and MPO. Moreover, the increased generation of MPO was observed in neurodegenerative disorders (Reynolds et al. 1999, Pennathur et al. 1999), arthritis (Bender et al. 1986) and some cancers (Reynolds et al. 1997). We hypothesized that MPO evokes substantial vasomotor responses in the presence of H2O2. This process may have immediate (acute) effects on the vascular diameter, which was tested here under in vitro conditions. The details of intracellular mechanisms responsible for the MPO elicited vasomotor responses were studied in SMAs. The most important findings of this study are that (1) MPO has the potential to promote vasoconstriction in H2O2-treated SMAs, CAs or BAs of the rat; (2) in the SMAs, MPO facilitates the H2O2-dependent activation of COX-1 and the TXA2 receptors, resulting in an increase in the Ca2+ sensitivity of force production in the smooth muscle cells; and (3) L-Met inhibits the chlorinating activity of MPO and converts MPO-evoked vasoconstrictions to vasodilations in the SMAs.
The question arises as to whether the observed decreased vasodilation in the presence of MPO originates from H2O2 consumption by MPO, thereby requiring a higher nominal H2O2 concentration to produce comparable vasodilations. At lower concentrations of H2O2, the level of vasoconstriction was similar in the absence and in the presence of MPO, while at higher concentrations of H2O2, MPO led to higher maximal vasoconstriction levels, thereby suggesting that MPO did not simply shift the apparent H2O2 concentration dependences of the vascular responses. We therefore postulate alternative mechanisms for the explanation of the MPO-dependent vascular effects.
One of the major products of the MPO-mediated conversion of H2O2 is HOCl. Our in vitro vascular measurements were performed in Ca2+ containing Krebs solution which provided the chloride ions for the MPO to generate HOCl. The mechanisms through which HOCl can affect vascular tissues have been examined by a number of research groups. HOCl initiates the halogenation, nitration and oxidative cross-linking of amino acids, lipids and nucleotides (Prutz 1996, Albrich et al. 1981). Less is known about the molecular pathways involved in the HOCl-evoked changes in vascular dynamics. One such possibility relates to a decrease in NO bioavailability, as suggested by observations on HOCl-dependent impairments in endothelial function (Yang et al. 2006, Stocker et al. 2004, Xu et al. 2006). Similarly to our findings, HOCl was found to cause vasoconstriction in bovine pulmonary arteries, but the exact mechanism of this effect remained unclear (Turan et al. 2000). The present investigation revealed increases in vasoconstriction in the SMAs, CAs and BAs, thereby extending the range of vascular beds affected in this way by MPO. We additionally made an effort to identify the molecular mechanisms contributing to these vasoconstrictive effects, besides to the decreased NO bioavailability reported earlier. One of the major observations was that the widely accepted HOCl scavenger L-Met (Okabe et al. 1993, Zhang et al. 2003, 2004) not only inhibited the vasoconstriction evoked by MPO, but also unmasked a robust vasodilatory effect in the SMAs. The employed MPO-specific inhibitor, 4-aminobenzhydrazide, blocked both the chlorinating and the peroxidase activities of the MPO (Malle et al. 2007, Kettle et al. 1995, 1997) and prevented the vasoconstriction evoked by MPO. In the presence of 4-aminobenzhydrazide and MPO, however, the vascular responses to H2O2 did not differ significantly from those in the absence of MPO. Collectively, the above data suggested that MPO-mediated chlorination has a major role in the activation of a signalling pathway leading to vasoconstriction. L-Met not only antagonized this effect, but revealed an additional MPO-dependent mechanism leading to vasodilation. This latter effect was probably related to the peroxidase activity of MPO that was not inhibited by L-Met. It is worthy of consideration that in the CAs and BAs, where MPO-evoked vasoconstrictions were less pronounced than those in the SMAs, L-Met did not result in significant vasodilations, which is suggestive of differential expressions of the MPO-responsive vasodilatory pathways in the different vascular beds.
Effector structures responding to MPO-derived radicals were first tested by removal of the endothelium in SMAs, which eliminated the endothelium-derived effects, including decreased NO bioavailability (Stocker et al. 2004, Xu et al. 2006, Turan et al. 2000). Importantly, H2O2-evoked vasoconstrictions were found in a previous study to be completely endothelium dependent (Csato et al. 2014). However, the vasoconstriction evoked by H2O2 in the presence of MPO was only partially opposed by endothelium removal (Fig.4a), suggesting that the MPO-evoked vasoconstriction was only partially endothelium dependent. These observations, together with those in the presence of the COX inhibitor indomethacin and the TXA2 inhibitor SQ-29548, implicate that MPO causes the generation of a vasoconstrictive prostanoid derivate (potentially TXA2) not only in the endothelial cells, but also in the vascular smooth muscle cells, through the activation of COXs. To confirm this possibility, the expression of COXs enzymes was explored by means of immunohistochemistry, and COX-1-specific staining was indeed confirmed both in the endothelial layer and in the smooth muscle cells of the SMAs. Interestingly, not only was the MPO-mediated vasoconstriction prevented by either TXA2 receptor inhibition or COX inhibition, but similarly as when L-Met was applied, it was converted to vasodilation. A role for TXA2 was implicated by its pharmacological inhibitor; nevertheless, we did not examine TXA2 production upon MPO exposures. Taken together, we postulate that the MPO-evoked vasoconstriction is mediated by a vasoconstrictive prostanoid derivative through TXA2 receptor activation. Hence, the above findings point to a HOCl–COX1–TXA2 pathway as being decisive in the prevention of MPO-dependent vasodilation in the SMAs (Fig.7).
Figure 7.
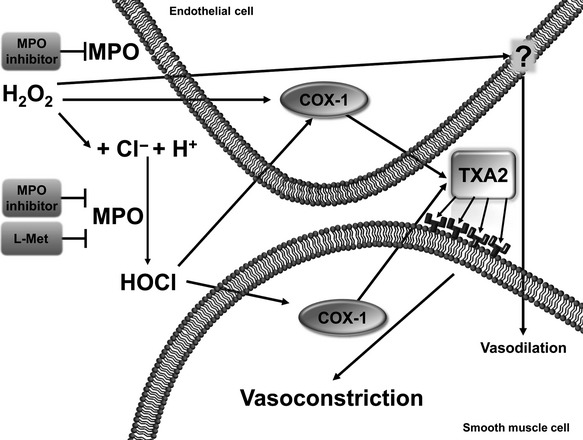
A proposed mechanism for the vascular effects of MPO in the SMA. During its anti-inflammatory activity, MPO modulates the vascular action of H2O2. The release of MPO causes the production of hypochlorous acid (HOCl), which increases the generation of thromboxane A2 (TXA2) both in endothelial cells and in vascular smooth muscle cells, leading to vasoconstriction through a Ca2+-sensitizing mechanism in vascular smooth muscle cells. An MPO inhibitor prevents both the peroxidation and the chlorinating activity, while L-Met inhibits only the chlorinating activity of the enzyme. In the presence of L-Met, the peroxidation pathway is still functional and vasodilation is observed, probably due to the generation of a vasodilatative peroxidation product (marked by a question mark).
Numerous previous studies have furnished evidence that H2O2 is an important regulator of the vascular diameter (Matoba et al. 2000, Yada et al. 2003, Matoba et al. 2003, Koller & Bagi 2004, Miura et al. 2003, Gao & Lee 2005, Gao et al. 2003, Gao & Lee 2001). It is difficult to specify the physiologic concentration of H2O2 in vascular tissues in vivo. Nevertheless, it has been found that under pathological conditions, it may increase up to 0.3 mm. In our study, H2O2 was used in a wide concentration range (1 μm–10 mm), thus covering also pharmacological levels. This approach allowed us to reveal the mechanisms of MPO-derived vascular effects developing on top of the biphasic H2O2-dependent responses (Liu & Zweier 2001, Root & Metcalf 1977, Cseko et al. 2004). In higher concentrations, H2O2 may cause vasodilation. The possible mechanism of the H2O2-evoked vasodilation has been investigated by a number of groups in different vessel types (Iida & Katusic 2000, Thengchaisri & Kuo 2003, Zhang et al. 2012). Our previous results implicated the involvement of the NO/cyclic guanosine monophosphate pathway and the activation of K+ channels in SMAs (Cseko et al. 2004).
Under pathological conditions associated with inflammation, such as acute infections (Hampton et al. 1998, Pullar et al. 2000, Hirche et al. 2005), diabetes (Zhang et al. 2004, Kataoka et al. 2014), atherosclerosis (Sugiyama et al. 2001, Sirpal 2009, Woods et al. 2003, Ford 2010), arthritis (Bender et al. 1986), Alzheimer disease (Reynolds et al. 1999) and Parkinson's disease (Pennathur et al. 1999), MPO is released together with H2O2. In vivo conditions, MPO is released together with H2O2. Under these circumstances, L-Met may prevent H2O2-evoked vasoconstriction or even convert it into vasodilation, because L-Met in its presumed physiological concentration range (i.e. 20–40 μm) (Mayo Medical Laboratories 2015) also largely prevents the vasoconstrictions evoked by MPO in the SMAs. Hence, the ultimate effect on the vascular tone and thereby on local microcirculation will be a function of the availability of a range of local regulators (e.g. H2O2, MPO, L-Met) which are of high potency in vasoregulation (Cseko et al. 2004).
The MPO-induced vasoconstrictions were not accompanied by significant increases in the intracellular Ca2+ concentration in the H2O2 concentration range of between 100 μm and 1 mm. In contrast, noradrenaline treatment evoked vasoconstrictions to similar degrees, together with significant increases in the intracellular Ca2+ concentration, suggesting that MPO (similarly to the thromboxane A2 receptor agonist U46619) activated a Ca2+-sensitizing mechanism, causing vasoconstriction rather than increasing the intracellular Ca2+ concentration (Csato et al. 2014). The mechanism of MPO-mediated vasodilation was beyond the scope of this study.
Overall, our present results suggest that MPO-derived HOCl can enhance the production of a TXA2-like vasoconstrictive molecule both in the endothelium and in the vascular smooth muscle cells of SMAs, thereby increasing the sensitivity of the contractile protein machinery in the vascular smooth muscle cells to produce vasoconstriction. Nevertheless, in the absence of a functional HOCl–COX1–TXA2 pathway, an MPO-dependent vasodilatory mechanism may prevail in the SMAs of the rat during tissue inflammation associated with neutrophil degranulation.
Study limitations
In this study, we aimed to explore the effects of MPO and H2O2 in vascular preparations with different origins. Due to differences in vascular diameters for SMAs, CAs and BAs (i.e. approx. 160 μm, approx. 180 μm and approx. 250 μm respectively), the same experimental set-up could not be employed for all vascular beds. Prior to test incubations, spontaneous myogenic tone developed in isotonic preparations (SMAs and CAs), while during isometric measurements (BAs), agonist-induced constrictions were applied. Consequently, the extent of the observed vascular responses may reflect differences in experimental arrangements. Nevertheless, the direction of vascular responses (vasodilation vs. vasoconstriction) could be determined convincingly because results were contrasted to controls under the same experimental conditions.
Significance
Cardiovascular diseases are associated with inflammation and increased oxidative stress. An understanding of the physiological responses as concerns pro-oxidant mechanisms may contribute to the development of new and more effective drugs in the fight against cardiovascular diseases. The most important message of this paper is that L-Met not only has the potential to prevent the vasoconstrictive responses due to activation of the HOCl–COX1–TXA2 pathway, but can evoke pronounced vasodilations in the presence of the proinflammatory enzyme MPO.
Acknowledgments
The work was funded by grants from the Hungarian Scientific Research Fund (OTKA): K 84300 (to AT) and K 109083 (to ZP), and the Hungarian Social Renewal Operational Program TÁMOP-4.2.A-11/1KONV-2012-0045.
Conflicts of interest
None declared.
References
- Abu-Soud HM. Hazen SL. Nitric oxide is a physiological substrate for mammalian peroxidases. J Biol Chem. 2000;275:37524–37532. doi: 10.1074/jbc.275.48.37524. [DOI] [PubMed] [Google Scholar]
- Albrich JM, McCarthy CA. Hurst JK. Biological reactivity of hypochlorous acid: implications for microbicidal mechanisms of leukocyte myeloperoxidase. Proc Natl Acad Sci USA. 1981;78:210–214. doi: 10.1073/pnas.78.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldus S, Heeschen C, Meinertz T, Zeiher AM, Eiserich JP, Munzel T, Simoons ML. Hamm CW. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation. 2003;108:1440–1445. doi: 10.1161/01.CIR.0000090690.67322.51. [DOI] [PubMed] [Google Scholar]
- Bender JG, Van Epps DE, Searles R. Williams RC., Jr Altered function of synovial fluid granulocytes in patients with acute inflammatory arthritis: evidence for activation of neutrophils and its mediation by a factor present in synovial fluid. Inflammation. 1986;10:443–453. doi: 10.1007/BF00915828. [DOI] [PubMed] [Google Scholar]
- Brennan ML, Penn MS, Van Lente F, Nambi V, Shishehbor MH, Aviles RJ, Goormastic M, Pepoy ML, McErlean ES, Topol EJ, Nissen SE. Hazen SL. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med. 2003;349:1595–1604. doi: 10.1056/NEJMoa035003. [DOI] [PubMed] [Google Scholar]
- Cavusoglu E, Ruwende C, Eng C, Chopra V, Yanamadala S, Clark LT, Pinsky DJ. Marmur JD. Usefulness of baseline plasma myeloperoxidase levels as an independent predictor of myocardial infarction at two years in patients presenting with acute coronary syndrome. Am J Cardiol. 2007;99:1364–1368. doi: 10.1016/j.amjcard.2006.12.060. [DOI] [PubMed] [Google Scholar]
- Cook NL, Viola HM, Sharov VS, Hool LC, Schoneich C. Davies MJ. Myeloperoxidase-derived oxidants inhibit sarco/endoplasmic reticulum Ca2+-ATPase activity and perturb Ca2+ homeostasis in human coronary artery endothelial cells. Free Radic Biol Med. 2012;52:951–961. doi: 10.1016/j.freeradbiomed.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csato V, Peto A, Koller A, Edes I, Toth A. Papp Z. Hydrogen peroxide elicits constriction of skeletal muscle arterioles by activating the arachidonic acid pathway. PLoS ONE. 2014;9:e103858. doi: 10.1371/journal.pone.0103858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cseko C, Bagi Z. Koller A. Biphasic effect of hydrogen peroxide on skeletal muscle arteriolar tone via activation of endothelial and smooth muscle signaling pathways. J Appl Physiol (1985) 2004;97:1130–1137. doi: 10.1152/japplphysiol.00106.2004. [DOI] [PubMed] [Google Scholar]
- Czikora A, Lizanecz E, Bako P, Rutkai I, Ruzsnavszky F, Magyar J, Porszasz R, Kark T, Facsko A, Papp Z, Edes I. Toth A. Structure-activity relationships of vanilloid receptor agonists for arteriolar TRPV1. Br J Pharmacol. 2012;165:1801–1812. doi: 10.1111/j.1476-5381.2011.01645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty A, Dunn JL, Rateri DL. Heinecke JW. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest. 1994;94:437–444. doi: 10.1172/JCI117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiserich JP, Baldus S, Brennan ML, Ma W, Zhang C, Tousson A, Castro L, Lusis AJ, Nauseef WM, White CR. Freeman BA. Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Science. 2002;296:2391–2394. doi: 10.1126/science.1106830. [DOI] [PubMed] [Google Scholar]
- Ford DA. Lipid oxidation by hypochlorous acid: chlorinated lipids in atherosclerosis and myocardial ischemia. Clin Lipidol. 2010;5:835–852. doi: 10.2217/clp.10.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Kassim SY, Parks WC. Heinecke JW. Hypochlorous acid oxygenates the cysteine switch domain of pro-matrilysin (MMP-7). A mechanism for matrix metalloproteinase activation and atherosclerotic plaque rupture by myeloperoxidase. J Biol Chem. 2001;276:41279–41287. doi: 10.1074/jbc.M106958200. [DOI] [PubMed] [Google Scholar]
- Gao YJ. Lee RM. Hydrogen peroxide induces a greater contraction in mesenteric arteries of spontaneously hypertensive rats through thromboxane A(2) production. Br J Pharmacol. 2001;134:1639–1646. doi: 10.1038/sj.bjp.0704420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ. Lee RM. Hydrogen peroxide is an endothelium-dependent contracting factor in rat renal artery. Br J Pharmacol. 2005;146:1061–1068. doi: 10.1038/sj.bjp.0706423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ, Hirota S, Zhang DW, Janssen LJ. Lee RM. Mechanisms of hydrogen-peroxide-induced biphasic response in rat mesenteric artery. Br J Pharmacol. 2003;138:1085–1092. doi: 10.1038/sj.bjp.0705147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golubinskaya V, Brandt-Eliasson U, Gan LM, Kjerrulf M. Nilsson H. Endothelial function in a mouse model of myeloperoxidase deficiency. Biomed Res Int. 2014;2014:128046. doi: 10.1155/2014/128046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton MB, Kettle AJ. Winterbourn CC. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood. 1998;92:3007–3017. [PubMed] [Google Scholar]
- Hansson M, Olsson I. Nauseef WM. Biosynthesis, processing, and sorting of human myeloperoxidase. Arch Biochem Biophys. 2006;445:214–224. doi: 10.1016/j.abb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Hazell LJ, Arnold L, Flowers D, Waeg G, Malle E. Stocker R. Presence of hypochlorite-modified proteins in human atherosclerotic lesions. J Clin Invest. 1996;97:1535–1544. doi: 10.1172/JCI118576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazen SL. Heinecke JW. 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J Clin Invest. 1997;99:2075–2081. doi: 10.1172/JCI119379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazen SL, Gaut JP, Crowley JR, Hsu FF. Heinecke JW. Elevated levels of protein-bound p-hydroxyphenylacetaldehyde, an amino-acid-derived aldehyde generated by myeloperoxidase, are present in human fatty streaks, intermediate lesions and advanced atherosclerotic lesions. Biochem J. 2000;352(Pt 3):693–699. [PMC free article] [PubMed] [Google Scholar]
- Hirche TO, Gaut JP, Heinecke JW. Belaaouaj A. Myeloperoxidase plays critical roles in killing Klebsiella pneumoniae and inactivating neutrophil elastase: effects on host defense. J Immunol. 2005;174:1557–1565. doi: 10.4049/jimmunol.174.3.1557. [DOI] [PubMed] [Google Scholar]
- Hoy A, Tregouet D, Leininger-Muller B, Poirier O, Maurice M, Sass C, Siest G, Tiret L. Visvikis S. Serum myeloperoxidase concentration in a healthy population: biological variations, familial resemblance and new genetic polymorphisms. Eur J Hum Genet. 2001;9:780–786. doi: 10.1038/sj.ejhg.5200702. [DOI] [PubMed] [Google Scholar]
- Iida Y. Katusic ZS. Mechanisms of cerebral arterial relaxations to hydrogen peroxide. Stroke. 2000;31:2224–2230. doi: 10.1161/01.str.31.9.2224. [DOI] [PubMed] [Google Scholar]
- Kandasamy K, Bezavada L, Escue RB. Parthasarathi K. Lipopolysaccharide induces endoplasmic store Ca2+-dependent inflammatory responses in lung microvessels. PLoS ONE. 2013;8:e63465. doi: 10.1371/journal.pone.0063465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakas M. Koenig W. Myeloperoxidase production by macrophage and risk of atherosclerosis. Curr Atheroscler Rep. 2012;14:277–283. doi: 10.1007/s11883-012-0242-3. [DOI] [PubMed] [Google Scholar]
- Kataoka Y, Shao M, Wolski K, Uno K, Puri R, Murat Tuzcu E, Hazen SL, Nissen SE. Nicholls SJ. Myeloperoxidase levels predict accelerated progression of coronary atherosclerosis in diabetic patients: insights from intravascular ultrasound. Atherosclerosis. 2014;232:377–383. doi: 10.1016/j.atherosclerosis.2013.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettle AJ, Gedye CA, Hampton MB. Winterbourn CC. Inhibition of myeloperoxidase by benzoic acid hydrazides. Biochem J. 1995;308(Pt 2):559–563. doi: 10.1042/bj3080559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettle AJ, Gedye CA. Winterbourn CC. Mechanism of inactivation of myeloperoxidase by 4-aminobenzoic acid hydrazide. Biochem J. 1997;321(Pt 2):503–508. doi: 10.1042/bj3210503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- Koller A. Bagi Z. Nitric oxide and H2O2 contribute to reactive dilation of isolated coronary arterioles. Am J Physiol Heart Circ Physiol. 2004;287:H2461–H2467. doi: 10.1152/ajpheart.00295.2004. [DOI] [PubMed] [Google Scholar]
- Lau D. Baldus S. Myeloperoxidase and its contributory role in inflammatory vascular disease. Pharmacol Ther. 2006;111:16–26. doi: 10.1016/j.pharmthera.2005.06.023. [DOI] [PubMed] [Google Scholar]
- Liu X. Zweier JL. A real-time electrochemical technique for measurement of cellular hydrogen peroxide generation and consumption: evaluation in human polymorphonuclear leukocytes. Free Radic Biol Med. 2001;31:894–901. doi: 10.1016/s0891-5849(01)00665-7. [DOI] [PubMed] [Google Scholar]
- Malle E, Furtmuller PG, Sattler W. Obinger C. Myeloperoxidase: a target for new drug development? Br J Pharmacol. 2007;152:838–854. doi: 10.1038/sj.bjp.0707358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, Kanaide H. Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest. 2000;106:1521–1530. doi: 10.1172/JCI10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoba T, Shimokawa H, Morikawa K, Kubota H, Kunihiro I, Urakami-Harasawa L, Mukai Y, Hirakawa Y, Akaike T. Takeshita A. Electron spin resonance detection of hydrogen peroxide as an endothelium-derived hyperpolarizing factor in porcine coronary microvessels. Arterioscler Thromb Vasc Biol. 2003;23:1224–1230. doi: 10.1161/01.ATV.0000078601.79536.6C. [DOI] [PubMed] [Google Scholar]
- Mayo Medical Laboratories. 2015. Test ID: AAQP Amino Acids, Quantitative, Plasma (www document) http://www.mayomedicallaboratories.com/test-catalog/Clinical+and+Interpretive/9265.
- Mayyas FA, Al-Jarrah MI, Ibrahim KS. Alzoubi KH. Level and significance of plasma myeloperoxidase and the neutrophil to lymphocyte ratio in patients with coronary artery disease. Exp Ther Med. 2014;8:1951–1957. doi: 10.3892/etm.2014.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura H, Bosnjak JJ, Ning G, Saito T, Miura M. Gutterman DD. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ Res. 2003;92:e31–e40. doi: 10.1161/01.res.0000054200.44505.ab. [DOI] [PubMed] [Google Scholar]
- Nicholls SJ. Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2005;25:1102–1111. doi: 10.1161/01.ATV.0000163262.83456.6d. [DOI] [PubMed] [Google Scholar]
- Nikpoor B, Turecki G, Fournier C, Theroux P. Rouleau GA. A functional myeloperoxidase polymorphic variant is associated with coronary artery disease in French-Canadians. Am Heart J. 2001;142:336–339. doi: 10.1067/mhj.2001.116769. [DOI] [PubMed] [Google Scholar]
- Okabe E, Takahashi S, Norisue M, Manson NH, Kukreja RC, Hess ML. Ito H. The effect of hypochlorous acid and hydrogen peroxide on coronary flow and arrhythmogenesis in myocardial ischemia and reperfusion. Eur J Pharmacol. 1993;248:33–39. doi: 10.1016/0926-6917(93)90022-i. [DOI] [PubMed] [Google Scholar]
- Pennathur S, Jackson-Lewis V, Przedborski S. Heinecke JW. Mass spectrometric quantification of 3-nitrotyrosine, ortho-tyrosine, and o, o’-dityrosine in brain tissue of 1-methyl-4-phenyl-1,2,3, 6-tetrahydropyridine-treated mice, a model of oxidative stress in Parkinson's disease. J Biol Chem. 1999;274:34621–34628. doi: 10.1074/jbc.274.49.34621. [DOI] [PubMed] [Google Scholar]
- Persson PB. Good publication practice in physiology: guidelines for Acta Physiol. Acta Physiol. 2013;209:250–253. [Google Scholar]
- Podrez EA, Schmitt D, Hoff HF. Hazen SL. Myeloperoxidase-generated reactive nitrogen species convert LDL into an atherogenic form in vitro. J Clin Invest. 1999;103:1547–1560. doi: 10.1172/JCI5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podrez EA, Abu-Soud HM. Hazen SL. Myeloperoxidase-generated oxidants and atherosclerosis. Free Radic Biol Med. 2000;28:1717–1725. doi: 10.1016/s0891-5849(00)00229-x. [DOI] [PubMed] [Google Scholar]
- Porszasz R, Porkolab A, Ferencz A, Pataki T, Szilvassy Z. Szolcsanyi J. Capsaicin-induced nonneural vasoconstriction in canine mesenteric arteries. Eur J Pharmacol. 2002;441:173–175. doi: 10.1016/s0014-2999(01)01596-5. [DOI] [PubMed] [Google Scholar]
- Prutz WA. Hypochlorous acid interactions with thiols, nucleotides, DNA, and other biological substrates. Arch Biochem Biophys. 1996;332:110–120. doi: 10.1006/abbi.1996.0322. [DOI] [PubMed] [Google Scholar]
- Pullar JM, Vissers MC. Winterbourn CC. Living with a killer: the effects of hypochlorous acid on mammalian cells. IUBMB Life. 2000;50:259–266. doi: 10.1080/713803731. [DOI] [PubMed] [Google Scholar]
- Reynolds WF, Chang E, Douer D, Ball ED. Kanda V. An allelic association implicates myeloperoxidase in the etiology of acute promyelocytic leukemia. Blood. 1997;90:2730–2737. [PubMed] [Google Scholar]
- Reynolds WF, Rhees J, Maciejewski D, Paladino T, Sieburg H, Maki RA. Masliah E. Myeloperoxidase polymorphism is associated with gender specific risk for Alzheimer's disease. Exp Neurol. 1999;155:31–41. doi: 10.1006/exnr.1998.6977. [DOI] [PubMed] [Google Scholar]
- Root RK. Metcalf JA. H2O2 release from human granulocytes during phagocytosis. Relationship to superoxide anion formation and cellular catabolism of H2O2: studies with normal and cytochalasin B-treated cells. J Clin Invest. 1977;60:1266–1279. doi: 10.1172/JCI108886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph TK, Wipper S, Reiter B, Rudolph V, Coym A, Detter C, Lau D, Klinke A, Friedrichs K, Rau T, et al. Myeloperoxidase deficiency preserves vasomotor function in humans. Eur Heart J. 2012;33:1625–1634. doi: 10.1093/eurheartj/ehr193. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savenkova ML, Mueller DM. Heinecke JW. Tyrosyl radical generated by myeloperoxidase is a physiological catalyst for the initiation of lipid peroxidation in low density lipoprotein. J Biol Chem. 1994;269:20394–20400. [PubMed] [Google Scholar]
- Sirpal S. Myeloperoxidase-mediated lipoprotein carbamylation as a mechanistic pathway for atherosclerotic vascular disease. Clin Sci (Lond) 2009;116:681–695. doi: 10.1042/CS20080322. [DOI] [PubMed] [Google Scholar]
- Stocker R, Huang A, Jeranian E, Hou JY, Wu TT, Thomas SR. Keaney JF., Jr Hypochlorous acid impairs endothelium-derived nitric oxide bioactivity through a superoxide-dependent mechanism. Arterioscler Thromb Vasc Biol. 2004;24:2028–2033. doi: 10.1161/01.ATV.0000143388.20994.fa. [DOI] [PubMed] [Google Scholar]
- Sugiyama S, Okada Y, Sukhova GK, Virmani R, Heinecke JW. Libby P. Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am J Pathol. 2001;158:879–891. doi: 10.1016/S0002-9440(10)64036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thengchaisri N. Kuo L. Hydrogen peroxide induces endothelium-dependent and -independent coronary arteriolar dilation: role of cyclooxygenase and potassium channels. Am J Physiol Heart Circ Physiol. 2003;285:H2255–H2263. doi: 10.1152/ajpheart.00487.2003. [DOI] [PubMed] [Google Scholar]
- Turan NN, Demiryurek AT. Kanzik I. Hypochlorous acid-induced responses in sheep isolated pulmonary artery rings. Pharmacol Res. 2000;41:589–596. doi: 10.1006/phrs.1999.0628. [DOI] [PubMed] [Google Scholar]
- Vita JA, Brennan ML, Gokce N, Mann SA, Goormastic M, Shishehbor MH, Penn MS, Keaney JF., Jr Hazen SL. Serum myeloperoxidase levels independently predict endothelial dysfunction in humans. Circulation. 2004;110:1134–1139. doi: 10.1161/01.CIR.0000140262.20831.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods AA, Linton SM. Davies MJ. Detection of HOCl-mediated protein oxidation products in the extracellular matrix of human atherosclerotic plaques. Biochem J. 2003;370:729–735. doi: 10.1042/BJ20021710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Xie Z, Reece R, Pimental D. Zou MH. Uncoupling of endothelial nitric oxidase synthase by hypochlorous acid: role of NAD(P)H oxidase-derived superoxide and peroxynitrite. Arterioscler Thromb Vasc Biol. 2006;26:2688–2695. doi: 10.1161/01.ATV.0000249394.94588.82. [DOI] [PubMed] [Google Scholar]
- Yada T, Shimokawa H, Hiramatsu O, Kajita T, Shigeto F, Goto M, Ogasawara Y. Kajiya F. Hydrogen peroxide, an endogenous endothelium-derived hyperpolarizing factor, plays an important role in coronary autoregulation in vivo. Circulation. 2003;107:1040–1045. doi: 10.1161/01.cir.0000050145.25589.65. [DOI] [PubMed] [Google Scholar]
- Yang J, Ji R, Cheng Y, Sun JZ, Jennings LK. Zhang C. L-arginine chlorination results in the formation of a nonselective nitric-oxide synthase inhibitor. J Pharmacol Exp Ther. 2006;318:1044–1049. doi: 10.1124/jpet.106.104422. [DOI] [PubMed] [Google Scholar]
- Zhang C, Patel R, Eiserich JP, Zhou F, Kelpke S, Ma W, Parks DA, Darley-Usmar V. White CR. Endothelial dysfunction is induced by proinflammatory oxidant hypochlorous acid. Am J Physiol Heart Circ Physiol. 2001a;281:H1469–H1475. doi: 10.1152/ajpheart.2001.281.4.H1469. [DOI] [PubMed] [Google Scholar]
- Zhang C, Reiter C, Eiserich JP, Boersma B, Parks DA, Beckman JS, Barnes S, Kirk M, Baldus S, Darley-Usmar VM. White CR. L-arginine chlorination products inhibit endothelial nitric oxide production. J Biol Chem. 2001b;276:27159–27165. doi: 10.1074/jbc.M100191200. [DOI] [PubMed] [Google Scholar]
- Zhang R, Brennan ML, Fu X, Aviles RJ, Pearce GL, Penn MS, Topol EJ, Sprecher DL. Hazen SL. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA. 2001c;286:2136–2142. doi: 10.1001/jama.286.17.2136. [DOI] [PubMed] [Google Scholar]
- Zhang C, Yang J, Jacobs JD. Jennings LK. Interaction of myeloperoxidase with vascular NAD(P)H oxidase-derived reactive oxygen species in vasculature: implications for vascular diseases. Am J Physiol Heart Circ Physiol. 2003;285:H2563–H2572. doi: 10.1152/ajpheart.00435.2003. [DOI] [PubMed] [Google Scholar]
- Zhang C, Yang J. Jennings LK. Leukocyte-derived myeloperoxidase amplifies high-glucose–induced endothelial dysfunction through interaction with high-glucose–stimulated, vascular non–leukocyte-derived reactive oxygen species. Diabetes. 2004;53:2950–2959. doi: 10.2337/diabetes.53.11.2950. [DOI] [PubMed] [Google Scholar]
- Zhang DX, Borbouse L, Gebremedhin D, Mendoza SA, Zinkevich NS, Li R. Gutterman DD. H2O2-induced dilation in human coronary arterioles: role of protein kinase G dimerization and large-conductance Ca2+-activated K+ channel activation. Circ Res. 2012;110:471–480. doi: 10.1161/CIRCRESAHA.111.258871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Xu H, Weihrauch D, Jones DW, Jing X, Shi Y, Gourlay D, Oldham KT, Hillery CA. Pritchard KA., Jr Inhibition of myeloperoxidase decreases vascular oxidative stress and increases vasodilatation in sickle cell disease mice. J Lipid Res. 2013;54:3009–3015. doi: 10.1194/jlr.M038281. [DOI] [PMC free article] [PubMed] [Google Scholar]


