Abstract
Sex differences in philopatry and dispersal have important consequences on the genetic structure of populations, social groups, and social relationships within groups. Among mammals, male dispersal and female philopatry are most common and closely related taxa typically exhibit similar dispersal patterns. However, among four well-studied species of baboons, only hamadryas baboons exhibit female dispersal, thus differing from their congenerics, which show female philopatry and close-knit female social relationships. Until recently, knowledge of the Guinea baboon social system and dispersal pattern remained sparse. Previous observations suggested that the high degree of tolerance observed among male Guinea baboons could be due to kinship. This led us to hypothesize that this species exhibits male philopatry and female dispersal, conforming to the hamadryas pattern. We genotyped 165 individuals from five localities in the Niokolo-Koba National Park, Senegal, at 14 autosomal microsatellite loci and sequenced a fragment of the mitochondrial hypervariable region I (HVRI) of 55 individuals. We found evidence for higher population structuring in males than in females, as expected if males are the more philopatric sex. A comparison of relatedness between male–male and female–female dyads within and among communities did not yield conclusive results. HVRI diversity within communities was high and did not differ between the sexes, also suggesting female gene flow. Our study is the first comprehensive analysis of the genetic population structure in Guinea baboons and provides evidence for female-biased dispersal in this species. In conjunction with their multilevel social organization, this finding parallels the observations for human hunter-gatherers and strengthens baboons as an intriguing model to elucidate the processes that shaped the highly cooperative societies of Homo. Am. J. Primatol. 77:878–889, 2015. © 2015 The Authors. American Journal of Primatology Published by Wiley Periodicals Inc.
Keywords: social system, male philopatry, microsatellites, population structure, hypervariable region I
INTRODUCTION
Dispersal, an organism's movement away from its original site or group [Pusey & Packer, 1987], has major implications for both the dynamics and the genetic makeup of populations [Bohonak, 1999; Prugnolle & de Meeus, 2002] and social groups [Archie et al., 2008; Di Fiore, 2012; Hoelzer et al., 2004; Hughes, 1998], and hence, on kinship-related social relationships within groups [Lukas & Clutton-Brock, 2011]. Many taxa exhibit sex-biased dispersal, i.e., one sex shows a greater tendency to leave its natal area or to move further away than the other [Greenwood, 1980; Pusey, 1987]. Male dispersal and female philopatry is predominant in mammals [Greenwood 1980], but exceptions can be found, e.g., in some non-human primates, equids, and some bats [Lukas & Clutton-Brock, 2011], and presumably in the majority of human societies [Lawson Handley & Perrin, 2007; Marks et al., 2012; Seielstad et al., 1998; Wilkins & Marlowe, 2006].
In many social mammals, the aggregation of individuals and their social relationships are determined by kinship [Smith, 2014] and, as a consequence of sex-biased dispersal, more social affiliation, tolerance, and cooperation are expected among the philopatric sex, due to kin selection [Clutton-Brock & Lukas, 2012; Di Fiore, 2012; Greenwood, 1980; Gouzoules, 1984; Hamilton, 1964a,1964b; Moore, 1992]. Hence, in many mammalian species, philopatric and therefore related females form matrilines and gain fitness benefits from close social ties with their kin [Broad et al., 2006; Chesser, 1998; Gompper et al., 1997; Lambin & Yoccoz, 1998; Moses & Millar, 1994; Silk et al., 2006a,2006b; Silk, 2007]. This paradigm has been most thoroughly studied in primates [Langergraber, 2012; Silk, 2002, 2007; Sterck et al., 1997], with baboons, genus Papio, being one of the prime examples for female kin-based bonding in matrilocal multimale–multifemale groups [Kapsalis, 2004; Seyfarth et al., 2014; Silk et al., 2006a,2006b; Sterck et al., 1997]. Baboons are distributed over most of sub-Saharan Africa, and comprise six commonly recognized species: chacma (Papio ursinus), Kinda (P. kindae), yellow (P. cynocephalus), olive (P. anubis), hamadryas (P. hamadryas), and Guinea baboons (P. papio) [Anandam et al., 2013]. In contrast to the general female-bonded pattern, hamadryas baboons are prominent for exhibiting a multi-level society [Abegglen, 1984; Grueter et al., 2012; Kummer, 1968, 1995; Schreier & Swedell, 2009; Zinner et al., 2001], with male philopatry and female-biased dispersal [Hammond et al., 2006; Hapke et al., 2001; Kopp et al., 2014; Sigg et al., 1982; Städele et al., 2015]. While female dispersal in hamadryas baboons is behaviourally not analogous to female dispersal in other taxa [Swedell et al., 2011], the genetic effects are the same [Hammond et al., 2006; Kopp et al., 2014; Städele et al., 2015]. In spite of the fact that baboons are among the most intensively studied primates [Barrett & Henzi, 2008], Guinea baboons are vastly understudied and our knowledge about their social system is still limited [Barton, 2000; Galat-Luong et al., 2006; Henzi & Barrett, 2003; Maciej et al., 2013a; Maestripieri et al., 2007; Patzelt et al., 2011; Patzelt et al., 2014]. Compared to other baboon species, they have a rather small distribution in West Africa, but occupy diverse habitats and climate zones, ranging from humid Guinean high forests in the South to arid Sahelian savannah in the North, occupying even isolated mountain ranges in the desert of Mauretania [Anandam et al., 2013; Galat-Luong et al., 2006; Oates et al., 2008; Oates, 2011]. They live in a multi-male–multi-female society, which is organized in a multi-layered way [Galat-Luong et al., 2006; Maciej et al., 2013a; Patzelt et al., 2011; Patzelt et al., 2014; Sharman, 1981]. Three to five adult males with several females and young form a party, which is assumed to be equivalent to the clan level in hamadryas baboons [Patzelt et al., 2014]. Parties regularly associate in a gang of approximately 60 individuals (hamadryas band), and several gangs share a home range and aggregate in a community of more than 350 individuals [Maciej et al., 2013a; Patzelt et al., 2014]. Subgrouping seems to be flexible both on a daily and a seasonal scale [Patzelt et al., 2011] and male Guinea baboons show a peculiar high degree of tolerance toward each other compared to other baboon taxa [Maciej et al., 2013b; Patzelt et al., 2014; Sharman, 1981]. This could be a consequence of male philopatry, and therefore high relatedness among males within groups. A recent study on mitochondrial DNA (mtDNA) variation over the whole range of Guinea baboons found a high level of female-mediated gene flow, suggesting female-biased dispersal [Kopp et al., 2014].
In our study, we investigated the genetic structure of a Guinea baboon population in south-eastern Senegal to further elucidate their social system. We examined the genetic relatedness within one community and among several communities at different spatial scales using non-invasive genotyping of individuals. More specifically, we compared the relatedness between males and females, respectively, within and among communities, as well as population structuring of autosomal markers over a broader spatial range. Differences could reveal sex-biased dispersal and philopatry, both important determinants of the social system of a species. Through the analysis of sequence information of the maternally transmitted mtDNA, we aim to unveil matrilineal structures. Additionally, we used a genetic capture–recapture approach [Arandjelovic et al., 2011; Lukacs & Burnham, 2005] to assess the stability of subgrouping on a short temporal scale, in order to evaluate whether this methodolgy can be used to distinguish between structured multi-level societies and more flexible fission–fusion societies based on genetic samples only.
We hypothesized that Guinea baboons exhibit male philopatry and, as a consequence of inbreeding avoidance, female dispersal and therefore predicted to find (i) higher population structuring of males compared to females; (ii) higher relatedness among males within communities than among males of different communities and the reversed pattern for females; and (iii) a generally high diversity of mtDNA haplotypes within communities and no difference in mtDNA variation between males and females.
METHODS
Field Work
The study was conducted at the Centre de Recherche de Primatologie (CRP) Simenti in the Niokolo Koba National Park (PNNK) in south-eastern Senegal (N13.03° W13.29°). Since 2007, a community of more than 350 Guinea baboons is under investigation.
We collected 452 fecal samples of the Simenti community between May and July 2009 during morning (0630–1130) and evening (1700–1900) follows. At that time, identification of individual baboons was not possible. Furthermore, we collected additional samples at four localities inside the national park: potential neighboring communities are represented by Gue Damantan (n = 62) and Camp du Lion (n = 54) with a distance to Simenti of 3 km and 6 km, respectively. Lingue Kountou (n = 53; 23 km) and Niokolo (n = 52; 62 km) were chosen to enable comparisons over larger geographic scales (Fig. 1).
Fig. 1.
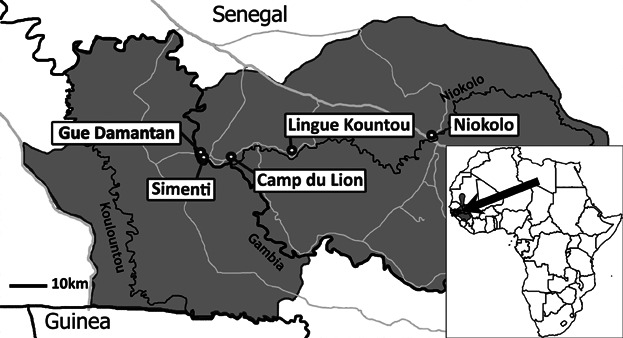
Sampling sites of Guinea baboon communities in the Niokolo Koba National Park, Senegal.
Fecal samples were collected and stored following the two-step protocol [Nsubuga et al., 2004; Roeder et al., 2004]. For each sample, consecutive number, date, time, and GPS coordinates were recorded. For the Simenti samples, we listed which samples were collected from the same gang. Due to a large flight distance and poor visibility of the animals, we were not able to assign sex and age classes to the samples, hence post- and pre-dispersal individuals cannot be distinguished in the statistical analyses. All samples were stored in the field at ambient temperature for up to 3 months and at −20 °C in the laboratory.
This project complied with the protocols approved by the German Primate Center, Göttingen, Germany, the animal care regulations and principles of the American Society of Primatologists for the ethical treatment of nonhuman primates, and the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES). Permits for research and sample export were obtained from the Senegalese authorities and research adhered to the legal requirements of both Senegal and Germany.
Genetic Analysis
DNA was extracted using the QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany) according to the protocol for isolation of DNA from stool for human DNA analysis with slight modifications [Haus et al., 2013]. To determine the sex of individuals, we used a PCR-based gonosomal sexing system (Roos, unpublished data).
We genotyped all samples for which we reliably determined the sex at 15 autosomal microsatellite loci (Table SI) developed in humans and reported to also amplify in baboons [Ferreira da Silva et al., 2014; Roeder et al., 2009; Rogers et al., 2000]. Microsatellites were amplified in five multiplex reactions, containing two to four different primer pairs (Table SII). Details on screening of microsatellites and laboratory procedures can be found in the supporting information. To assure accuracy, genotyping was repeated several times leading to a consensus genotype (multiple tubes approach [Morin et al., 2001; Navidi et al., 1992; Taberlet et al., 1996]).
For 55 samples, we amplified and sequenced a fragment of the hypervariable region I (HVRI) of the mitochondrial genome comprising 339 base pairs (bp) following established protocols [Kopp et al., 2014]. MtDNA sequences were uploaded to GenBank and can be accessed through the following accession numbers: KF692784–788, 790–800, 811–814, 816, 818, 847–852, 856, 879–884, 886, 894, 895, 897–908, 910, 911, and 913–915.
Statistical Analyses
Obtaining accurate microsatellite genotypes from fecal samples can be difficult due to low DNA quality and quantity or poor extract quality (PCR inhibitors) [Taberlet et al., 1999]. We, therefore, rigorously evaluated genotyping errors and only included samples that passed our quality control (further details can be found in the supporting information). Genotype matching was performed using Gimlet 1.3.3 [Valiere et al., 2002] allowing one mismatch. Every duplicate genotype was excluded from the final dataset. The probability that a single genotype actually represents one single individual was calculated with the probability of identity P(ID) [Paetkau & Strobeck, 1994] and the more conservative estimator Probability of Identity between sibs P(ID) sib [Evett & Weir, 1998; Taberlet & Luikart, 1999] as implemented in Gimlet. The final dataset was converted to the specific input file formats of each software program using Create 1.3 [Coombs et al., 2008].
Departures from Hardy–Weinberg equilibrium (HWE) were tested with exact tests using the program Genepop 4.0.11 (default settings: dememorization number: 10,000; number of batches: 20; iterations per batch: 5,000) [Rousset, 2008; Raymond & Rousset, 1995]. Expected heterozygosity HE and observed heterozygosity HO were calculated in Arlequin 3.5.1.2 [Excoffier & Lischer, 2010]. Allelic richness and FIS were calculated in Fstat 2.9.3.2 [Goudet, 1995].
Population genetic parameters were calculated to investigate whether there is any population structuring despite the fact that there are no obvious barriers for gene flow between the sampling sites. First, the program Structure 2.3.3 [Pritchard et al., 2000] was used, which is based on a Bayesian approach. It identifies the most likely number of populations (K) in a data set and the likelihood of an individual to belong to this population. Program settings were set to a total run length of 1,000,000 iterations, a burnin of 100,000, and values of K from 1 through 6. The analysis was repeated 10 times to assure the consistency of the results. We chose the admixture model as ancestry model and the correlated frequency model as allele frequency model [Falush et al., 2003]. Furthermore, we used the Locprior model that takes into account the sampling location of individuals as a prior information to assist the clustering if the signal is relatively weak [Hubisz et al., 2009]. All other settings were left at their default value. To evaluate the most probable number of clusters, we employed the method suggested by Evanno et al. [2005] as implemented in Structure Harvester Web v0.6.92 [Earl & VonHoldt, 2011]. To further investigate population structuring, Weir & Cockerham's fixation index FST [Weir & Cockerham, 1984] among the sampling sites was calculated in Fstat and the relationship between geographic and genetic distances among sampling sites (isolation by distance; IBD) was tested with a Mantel test in Genepop using 1,000 permutations.
We tested for sex-bias in dispersal by comparing several parameters between males and females. To begin with, population structure and IBD of females and males were examined with the same settings as in the analysis of the total population. To quantify the degree of population structuring, FST values were calculated for each sex separately and tested two-sided predicting males being philopatric with 1,000 permutations using Fstat. Sampling sites Gue Damantan, Simenti, Camp Du Lion, and Lingue Kountou were grouped together as one cluster and Niokolo constituted a second cluster following the results from the population structure analysis. Allelic frequencies of the dispersing sex should be more homogeneous, and therefore FST should be lower for the dispersing than for the philopatric sex. We refrained from testing other parameters available in the sex-biased dispersal test in Fstat, on the one hand, to avoid multiple testing and on the other hand, because these parameters have been shown to perform poorly under certain conditions, whereas the FST statistic is the most powerful measure to detect sex-bias in dispersal, regardless of sampling scheme and magnitude [Goudet et al., 2002]. Sex-biased dispersal should also influence the distribution of relatedness in a population. Pairwise relatedness coefficients R were calculated using the regression estimator derived by Queller & Goodnight [Queller & Goodnight, 1989] as implemented in Coancestry 1.0 [Wang, 2011]. The average relatedness of males and females within a gang, among gangs, and among communities, respectively, was compared (for within gang comparisons only dyads in the Simenti community were included). We tested for significance using a permutation test as implemented in the R package coin [Hothorn et al., 2008] in R 3.1.1 [R Development Core Team, 2014] with 99,999 Monte Carlo resamplings. A set of 14 microsatellites does not suffice to infer kinship reliably without any additional information and putative misclassification would lead to erroneous conclusions [Van Horn et al., 2008]. With the absence of pedigree (e.g. known mother-offspring pairs) and demographic information [Arora et al., 2012; Harris et al., 2009], we therefore refrained from analyzing dyadic relatedness.
To visualize the genetic distances and frequencies of HVRI haplotypes, we generated a haplotype network in HapStar 0.6 [Teacher & Griffiths, 2011] based on pairwise distances output from Arlequin. In order to assess the diversity of HVRI haplotypes, we calculated the levels of nucleotide and haplotype diversity for males and females, respectively, using DNaSP version 5.10.1 [Librado & Rozas, 2009], both for the whole study population and for every community separately, as well as for females and males, respectively. We tested for significance using the difference test in Statistica (StatSoft® (Europe) GmbH, Hamburg, German).
To investigate the temporal stability of gangs, we examined whether individuals that were sampled multiple times on different days were repeatedly sampled with the same individuals in the same gang.
RESULTS
From a total of 339 extracted and sexed samples, 149 were determined as males and 113 as females, the rest was excluded because of no visible amplification product, ambiguous results, or suspected contamination. The 211 successfully genotyped samples of the final data set yielded 165 different individuals (68 females and 97 males), that were typed at a minimum of 13 loci with a mean of 13.9 loci (Table SIV). Loci had a good power to discriminate between individuals with a total P(ID) sib of 5.984 × 10−5 (P(ID) = 2.080 × 10−10). The quality of samples and estimated genotyping error rates (Table SIII) fall in the normal range for non-invasive samples [Arandjelovic et al., 2009; Bayes et al., 2000; Lathuillière et al., 2001; Miquel et al., 2006; Smith et al., 2000] and allow population genetic analysis. While it cannot be ruled out that some multilocus genotypes contain errors, they are sufficiently rare and should be distributed randomly throughout the dataset, thus not biasing the analysis of sex-biases.
All loci were polymorphic, with number of alleles ranging from three to seven (mean = 5.36 ± SD 1.22) and a mean allelic richness of 3.76 (± SD 0.95). Loci showed no significant deviations from HWE. Expected and observed heterozygosity were similar (HE =0.60 ± 0.13; HO =0.63 ± 0.14), FIS values ranged around zero with a mean of −0.068. Both nucleotide diversity and expected heterozygosity are lower in Guinea baboons than in their congenerics (Table SV).
The Structure analysis revealed population structuring, with K = 2 being the most probable (Fig. 2). Individuals from Niokolo were found to differ from all other communities. There was a significant positive correlation between geographic and genetic distance, indicating IBD (r2 = 0.600; P = 0.039) (Fig. 3).
Fig. 2.
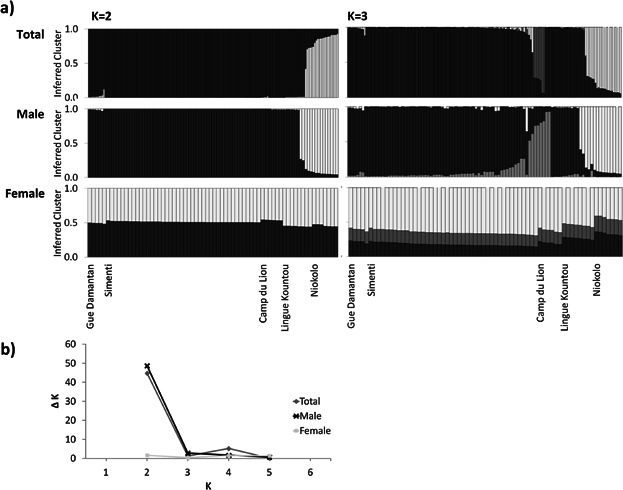
(a) Genetic population structure of male and female Guinea baboons, as well as the total sample set using the software Structure and clustering of K = 2 and K = 3. (b) Inference of the most probable number of clusters (K) for the three data sets (male, female, total) using the ad-hoc statistic ΔK [Evanno et al., 2005] returns K = 2 as the most probable solution for both males and the total population but K = 1 for females.
Fig. 3.
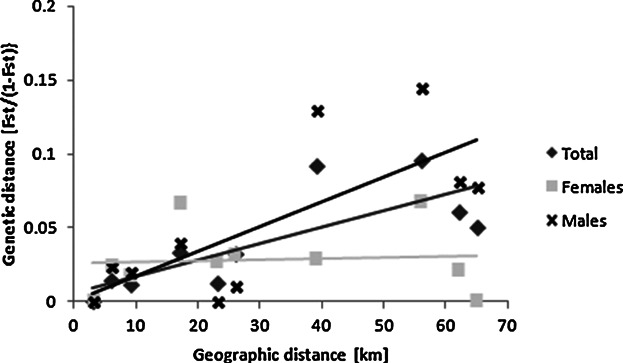
Correlations between genetic differentiation, as measured by FST, and geographic distance between sampling sites suggest that the total population shows evidence for isolation-by-distance (r2 = 0.600, P = 0.039), there is a trend for IBD in males (r2 = 0.559, P = 0.127) but not in females (r2 = 0.015, P = 0.348).
Sex-Biased Dispersal
The Structure analysis revealed differences in population structuring between males and females, respectively. For males K = 2 was found to be the most probable, whereas females did not show any structuring (Fig. 2), indicating that male gene flow is more restricted, as expected for the philopatric sex. We also found a slight trend for IBD in males (r2 = 0.559, P =0.127) but not in females (r2 = 0.015, P = 0.348) (Fig. 3). The comparison of FST values between the sexes showed significantly higher values for males than for females, also suggesting a stronger population structure in males (FST♂ = 0.08, FST♀ = 0.02, P = 0.018).
The second approach to examine sex-biased dispersal was to analyze the effects of distance and sex on relatedness. Mean pairwise relatedness was significantly higher among females than among males, both within and among communities. (N♀ = 68, N♂ = 97; R♀within = 0.0357 ± SD 0.2005, R♂within = 0.0092 ± SD 0.2143, Z = 3.5618, P < 0.001; R♀among = −0.0203 ± SD 0.1891, R♂among = −0.0446 ± SD 0.1982, Z = 3.3397, P < 0.001) and both males and females were less related among than within communities (females: Z = −6.7837, P < 0.001; males: Z = −8.6657, P < 0.001). For both male, female, and mixed-sex dyads, mean pairwise relatedness decreased considerably from the gang to the community to the population level (Fig. 4a). Looking at the well-sampled Simenti community more closely, we found a small, but significant difference in the relatedness coefficients of male versus female dyads (N♀Simenti = 42, N♂Simenti = 66; R♀Simenti = 0.0344 ± SD 0.1952, R♂Simenti = −0.0006 ± SD 0.2111; Z = 4.1453, P < 0.001; Fig. 4b).
Fig. 4.
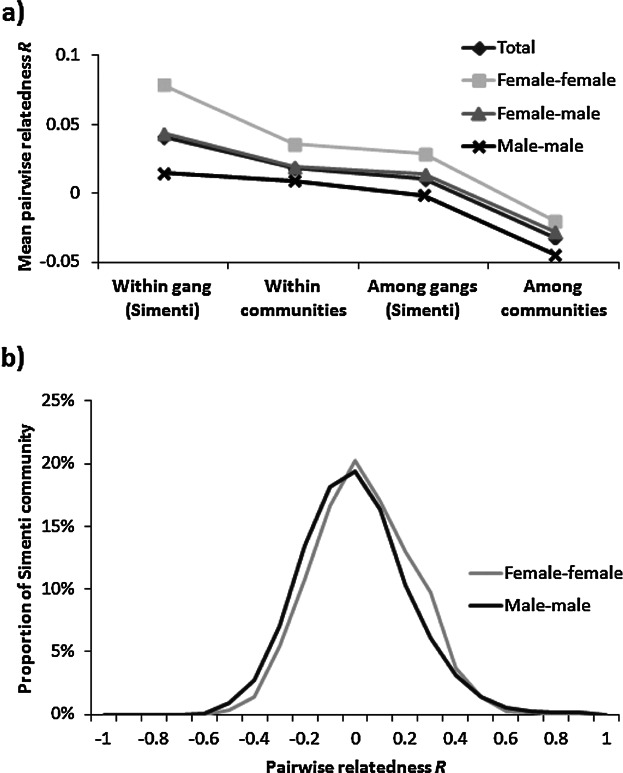
(a) Mean pairwise relatedness as inferred from autosomal microsatellites among male and female dyads within gangs of the Simenti community, within communities, among gangs of the Simenti community, and among communities. (Number of dyads: female–female/within gangs = 101; female–female/within community = 1145; female–female/among gangs = 760; female–female/among communities = 1133; female–male/within gangs = 236; female–male/within community = 3559; female–male/among gangs = 2536; female–male/among communities = 3037; male–male/within gangs = 170; male–male/within community = 2681; male–male/among gangs = 1975; male–male/among communities = 1975; total/within gangs = 507; total/within community = 7385; total/among gangs = 5271; total/among communities = 6145); (b) Distribution of relatedness coefficients of male and female dyads in the Simenti community.
MtDNA Diversity
The PNNK study population comprised 13 HVRI haplotypes with an overall haplotype diversity Hd of 0.798 (± SD 0.047) and nucleotide diversity π of 0.01030 (± SD 0.00134). The haplotype network revealed two coarse haplotype clusters divided by four mutational steps, albeit without any clear geographical signal (Fig. 5). One haplotype was very common (N=23) and was discovered in every community except Lingue Kountou, while several other haplotypes were only observed once. Within communities, we found a median number of 3 haplotypes (range 2–6), mean Hd of 0.6334 (± 0.116), and mean π of 0.008032 (± 0.00473). On average, there was no considerable difference in within-community Hd between the sexes (Hd♀ = 0.5788 ± 0.3595, N♀ = 25; Hd♂ = 0.5974 ± 0.1203, N♂ = 30, P < 0.7909), but π was nearly twice as high for females within communities than for males (π♀ = 0.0101 ± 0.0061, N♀ = 25, π♂ = 0.0055 ± 0.0054, N♂ = 30, P < 0.0036).
Fig. 5.
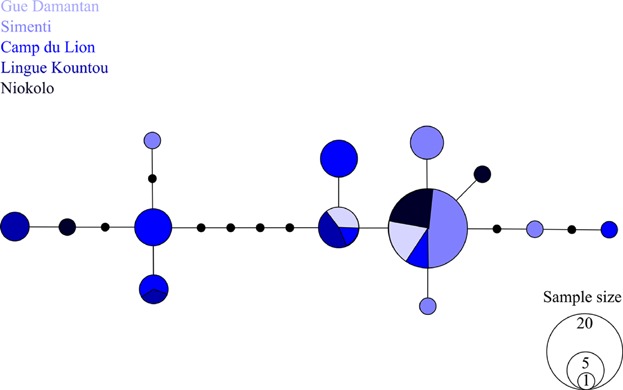
Network of HVRI haplotypes found in the Niokolo Koba National Park. Different haplotypes are colored according to the sampling sites where they were found.
Stability of Gangs
Nineteen individuals were sampled multiple times on 2 to 3 different days. Of these individuals, 14 were sampled repeatedly together with the same other individual(s), resulting in six dyads and one triad (Fig. 6). These mostly consisted of individuals of the same sex, but one dyad and the triad also contained both a male and one or two females. Time span between repeated sampling varied between 1 and 48 days (mean: 11.6 days).
Fig. 6.
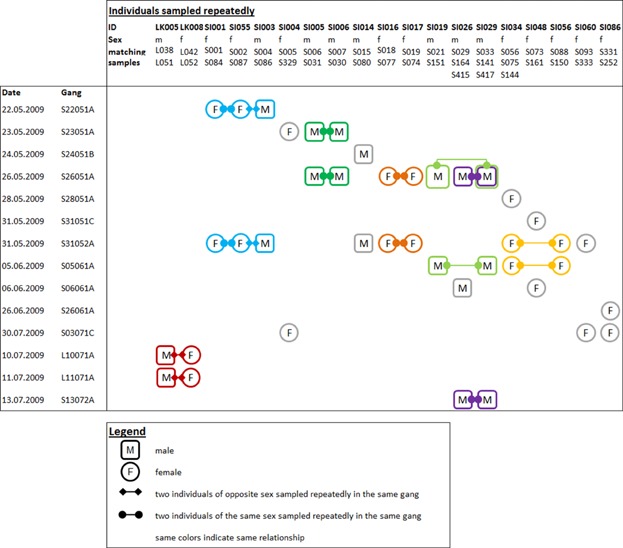
Fourteen individuals were repeatedly sampled with a least one other particular individual on different days.
DISCUSSION
We investigated the genetic structure of a Guinea baboon population to gain a better understanding of their social system, specifically their dispersal pattern. We found differences in population structure between males and females, with significantly higher FST values for males. This structuring is probably attributable to a stronger IBD effect in males than in females, implying that male gene flow is more restricted than female gene flow, which is consistent with male philopatry and female-biased dispersal. The assessment of mean pairwise relatedness coefficients to infer sex-bias in dispersal, however, did not yield conclusive results: The finding that females are more closely related than males within communities is against our predictions for female dispersal, whereas the higher relatedness among females from different communities than among males from different communities is consistent with our predictions. It needs to be highlighted that the magnitude of differences in average relatedness is rather small and presumably arose out of the presence of a moderately larger number of related dyads among females. The PNNK population of Guinea baboons was characterized by a high mitochondrial haplotype diversity within communities, as expected for species with female dispersal [Städele et al., 2015], which leads to the accumulation of several haplotypes in single localities. Additionally, the fact that males and females show a similar haplotype diversity strongly supports the hypothesis of female dispersal.
One problem regarding the detection of sex-bias in dispersal and philopatry from genetic data was that we were not able to assign age-classes to the sampled individuals. Especially the sampling of mothers together with their dependent offspring is a potential source of error. Firstly, this inflates the relatedness within communities, thus hampering the detection of differences in relatedness between males and females. This shortcoming of our study design might be a reason why our relatedness analyses failed to give conclusive results. Secondly, the sampling of mothers with their offspring complicates the examination of male dispersal via mtDNA variation, because pre-dispersal males, carrying the mtDNA variant of their resident mother, would weaken the predicted effect of higher male mtDNA variation. Hence, the inclusion of pre-dispersal individuals introduces a considerable amount of noise that may silence differences that are expected between males and females if sex-bias in dispersal exists [Prugnolle & de Meeus, 2002]. Accordingly, sex differences may actually be stronger than they are reported here. Furthermore, home range overlap among the communities was unknown. Possibly, individuals that were treated as belonging to different communities actually belonged to the same. This applies specifically to animals of neighboring localities, such as Simenti and Gue Damantan.
The fact that individuals were repeatedly sampled together indicates that the composition of gangs is stable over a substantial period, a finding that is now supported by behavioral observations [Patzelt et al., 2014]. This fact and the finding that the average relatedness is higher in gangs as compared to the whole community corroborate the view that in Guinea baboons, the gang constitutes an important social unit [Maciej et al., 2013b]. A decrease in relatedness through the different levels of hierarchically structured societies has also been described in hamadryas baboons [Städele et al., 2015], female geladas (Theropithecus gelada [Snyder-Mackler et al., 2014]), and elephants (Loxondonta africana, [Wittemyer et al., 2009]). In both geladas and elephants, relatedness was found to be a predictor of group fission and fusion [Archie et al., 2006; Snyder-Mackler et al., 2014]. Future studies will elucidate in detail the socio-genetic structure of the complex Guinea baboon society.
Overall, the relatedness of individuals within the Simenti community is extremely low, regardless of sex, and comparable to the values described for hamadryas baboons [Städele et al., 2015]. This result is concordant with other studies, which showed that in large groups mean pairwise relatedness is not necessarily higher in the philopatric sex, because many unrelated dyads may dilute the effects of few highly related dyads [Lukas et al., 2005]. Relatedness values are also affected by reproductive skew [Lukas et al., 2005]. If one or a few males are able to monopolize reproduction over a long time, the amount of paternal half-siblings in the group is high. In contrast, if reproductive skew is low because multiple males are able to reproduce, within group relatedness is expected to be relatively low. Long-term behavioral observations and paternity analyses will be needed to clarify the mating system of Guinea baboons.
The low relatedness among males within the community suggests that male tolerance is not conditional on kinship in this species, which is supported by Patzelt et al. [2014], who found that relatedness did not predict the quality of male–male bonds in Guinea baboons. Similarly, in chimpanzees, cooperative behavior is not solely determined by kinship [Langergraber et al., 2007]. Still, male philopatry has the potential to facilitate the establishment of strong male bonds [Langergraber et al., 2007; Mitani et al., 2002] through the early formation of peer groups that, in the absence of male dispersal, can persist from early childhood into adulthood [Boese, 1975]. Moreover, this system obliges females to counterbalance the negative effects of dispersal, especially the unavailability of kin [Silk, 2002]. In some species, unrelated females form strong bonds, which provide direct fitness benefits through social integration [Cameron et al., 2009; Lehmann & Boesch, 2009], while in other species, females regularly disperse together with or into groups with relatives to maintain kin associations [Bradley et al., 2007; Starin, 1994].
Our finding of female-biased dispersal in this Guinea baboon population confirms and refines the results of a previous study, which, based on patterns of mtDNA variation, recovered female gene flow in both Guinea and hamadryas baboons species-wide [Kopp et al., 2014]. We cannot draw a conclusion about the magnitude of the sex difference in dispersal and the social level at which this bias manifests, and are not rejecting that male philopatry might be weak. These questions, however, can only be ascertained by analyzing Y-chromosomal haplotypes in the future [Petit et al., 2002] and by incorporating detailed data on the multiple levels of the community [Städele et al., 2015]. Unfortunately, we failed to find informative, polymorphic loci when screening several Y-chromosomal markers upon initiation of this study. An extremely low level of diversity on the Y-chromosome has also been described in hamadryas baboons [Lawson Handley et al., 2006; Städele et al., 2015] and is a common problem in mammalian non-model organisms [Greminger et al., 2010]. Still, on average, females appear to migrate more often and/or further away than males in this population of Guinea baboons. Research on different populations throughout the range of Guinea baboons covering most of the habitats they occupy could help evaluate how climatic and ecological variation, as well as anthropogenic disturbances, may alter dispersal behavior. Guinea baboons occupy a vast variety of habitats and climate zones [Anandam et al., 2013; Galat-Luong et al., 2006; Oates et al., 2008; Oates, 2011], and poaching and habitat destruction is a major threat in certain regions of their range [Ferreira da Silva et al., 2014]. A comparison of different populations would provide the data necessary to evaluate how flexibly this species can respond to ecological variables [Wikberg et al., 2012] and how strongly it is influenced by evolutionary constraints.
Unfortunately, ecological and behavioral data on Guinea baboons that are required to investigate evolutionary causes of their dispersal pattern are still scarce. It remains unknown how costs and benefits of dispersal and philopatry are distributed among the sexes and how, for instance, the avoidance of local resource competition and inbreeding [Clutton-Brock & Lukas, 2012; Lukas & Clutton-Brock, 2011] shaped this pattern. It is also premature to speculate on the analogy of female dispersal behavior in Guinea and hamadryas baboons. Still, given that female philopatry and male dispersal are most likely the ancestral state in the Papionini [Di Fiore & Rendall, 1994; Lukas & Clutton-Brock, 2011], it would be interesting to examine possible evolutionary causes for a sex reversal in dispersal in hamadryas and Guinea baboons. Jolly [2009] proposed that demographic factors in expanding frontier populations rather than ecological conditions led to male philopatry both in Guinea and hamadryas baboons, because neighboring olive baboons occupy the same habitats and this species usually exhibits male-biased dispersal [Packer, 1975, 1979; Vinson et al., 2005]. Other scholars have also questioned the direct effects of ecological factors on the evolution of female dispersal [Lukas & Clutton-Brock, 2011]. To test this hypothesis, a well-resolved phylogeny of baboons, especially of the northern clade, including Guinea, hamadryas, and olive baboons [Boissinot et al., 2014], is needed [Pozzi et al., 2014]. This will enable us to investigate whether Guinea and hamadryas baboons evolved female-biased dispersal independently or whether it was inherited from their common ancestor, and whether phylogeographic processes, such as range expansions [Jolly, 2009], could have had an influence.
CONCLUSION
Our results corroborate that Guinea baboons are one of the few mammalian taxa characterized by female-biased dispersal. While the causes of this exceptional pattern remain unclear, it reinforces the view that the social system of this species should receive more attention in the future, in particular, possible demographic and ecological factors influencing dispersal behavior. Their dispersal pattern in combination with their multilevel social organization and strong male–male bonds parallels the social system of humans and strengthens the case for the use of baboons as models to elucidate the processes that shaped the highly cooperative societies of Homo.
Acknowledgments
We thank the Diréction des Parcs Nationaux and Ministère de l′Environnement et de la Protéction de la Nature de la République du Sénégal for permission to work in the Niokolo Koba National Park and exporting samples. We particularly thank the then Conservator of the park Cdt. Samuel Diemé for his support and cooperation. We gratefully acknowledge the help of Anja Ebenau, Jacky Bassene, Samba Ciss, and Issakha N'Diaye in collecting samples. Many thanks to Joana Maria Ferreira da Silva for sharing her laboratory protocol and fruitful discussions, as well as to Cliff Jolly for stimulating ideas about sex-biased dispersal in baboons. We acknowledge the help of Christof Neumann, Adeelia Goffe, Christopher Young, and Kurt Hammerschmidt in the statistical analysis. We are grateful to Linda Vigilant and three anonymous reviewers whose comments greatly improved this manuscript.
Supporting Information
Additional supporting information may be found in the online version of this article at the publisher's web-site.
Supporting Information.
REFERENCES
- Abegglen JJ. On socialization in hamadryas baboons: A field study. Cranbury, NJ: Associated University Press; 1984. p. 207. [Google Scholar]
- Anandam MV, Bennett EL, Davenport TRB. Species accounts of Cercopithecidae. In: Mittermeier RA, Rylands AB, Wilson DE, et al., editors. Handbook of the mammals of the world Vol. 3 primates. Barcelona: Lynx Edicions; 2013. pp. 628–753. [Google Scholar]
- Arandjelovic M, Guschanski K, Schubert G, et al. Two-step multiplex polymerase chain reaction improves the speed and accuracy of genotyping using DNA from noninvasive and museum samples. Molecular Ecology Resources. 2009;9:28–36. doi: 10.1111/j.1755-0998.2008.02387.x. [DOI] [PubMed] [Google Scholar]
- Arandjelovic M, Head J, Rabanal LI, et al. Non-invasive genetic monitoring of wild central chimpanzees. PLoS ONE. 2011;6:e14761. doi: 10.1371/journal.pone.0014761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archie EA, Moss CJ, Alberts SC. The ties that bind: genetic relatedness predicts the fission and fusion of social groups in wild African elephants. Proceedings of the Royal Society B: Biological Sciences. 2006;273:513–522. doi: 10.1098/rspb.2005.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archie EA, Maldonado JE, Hollister-Smith JA, et al. Fine-scale population genetic structure in a fission-fusion society. Molecular Ecology. 2008;17:2666–2679. doi: 10.1111/j.1365-294X.2008.03797.x. [DOI] [PubMed] [Google Scholar]
- Arora N, Van Noordwijk Ma, Ackermann C, et al. Parentage-based pedigree reconstruction reveals female matrilineal clusters and male-biased dispersal in nongregarious Asian great apes, the Bornean orang-utans (Pongo pygmaeus. Molecular Ecology. 2012;21:3352–3362. doi: 10.1111/j.1365-294X.2012.05608.x. [DOI] [PubMed] [Google Scholar]
- Barrett L, Henzi SP. Baboons. Current Biology. 2008;18:R404–R406. doi: 10.1016/j.cub.2008.02.074. [DOI] [PubMed] [Google Scholar]
- Barton RA. Socioecology of baboons: the interaction of male and female strategies. In: Kappeler PM, editor. Primate males: causes and consequences of variation in group composition. Cambridge: Cambridge University Press; 2000. pp. 97–107. [Google Scholar]
- Bayes MK, Smith KL, Alberts SC, Altmann J, Bruford MW. Testing the reliability of microsatellite typing from faecal DNA in the savannah baboon. Conservation Genetics. 2000;1:173–176. [Google Scholar]
- Boese GK. Social behavior and ecological considerations of West African baboons (Papio papio. In: Tuttle R, editor. Socioecology and psychology of primates. The Hague: Mouton Publication; 1975. pp. 205–230. [Google Scholar]
- Bohonak AJ. Dispersal, gene flow, and population structure. Quarterly Review of Biology. 1999;74:21–45. doi: 10.1086/392950. [DOI] [PubMed] [Google Scholar]
- Boissinot S, Alvarez L, Giraldo-Ramirez J, Tollis M. Neutral nuclear variation in baboons (genus Papio) provides insights into their evolutionary and demographic histories. American Journal of Physical Anthropology. 2014;155:621–634. doi: 10.1002/ajpa.22618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley BJ, Doran-Sheehy DM, Vigilant L. Potential for female kin associations in wild western gorillas despite female dispersal. Proceedings of the Royal Society B: Biological Sciences. 2007;274:2179–2185. doi: 10.1098/rspb.2007.0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broad KD, Curley JP, Keverne EB. Mother-infant bonding and the evolution of mammalian social relationships. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2006;361:2199–2214. doi: 10.1098/rstb.2006.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron EZ, Setsaas TH, Linklater WL. Social bonds between unrelated females increase reproductive success in feral horses. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13850–13853. doi: 10.1073/pnas.0900639106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesser RK. Relativity of behavioral interactions in socially structured populations. Journal of Mammalogy. 1998;79:713–724. [Google Scholar]
- Clutton-Brock T, Lukas D. The evolution of social philopatry and dispersal in female mammals. Molecular Ecology. 2012;21:472–492. doi: 10.1111/j.1365-294X.2011.05232.x. [DOI] [PubMed] [Google Scholar]
- Coombs JA, Letcher BH, Nislow KH. Create: a software to create input files from diploid genotypic data for 52 genetic software programs. Molecular Ecology Resources. 2008;8:578–580. doi: 10.1111/j.1471-8286.2007.02036.x. [DOI] [PubMed] [Google Scholar]
- Di Fiore A. The evolution of primate societies. In: Mitani JC, Call J, Kappeler PM, Palombit RA, Silk JB, editors. Genetic consequences of primate social organization. Chicago: The University of Chicago Press; 2012. pp. 269–292. [Google Scholar]
- Di Fiore A, Rendall D. Evolution of social organization: a reappraisal for primates by using phylogenetic methods. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:9941–9945. doi: 10.1073/pnas.91.21.9941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl DA, VonHoldt BM. STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conservation Genetics Resources. 2011;4:359–361. [Google Scholar]
- Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Molecular Ecology. 2005;14:2611–2620. doi: 10.1111/j.1365-294X.2005.02553.x. [DOI] [PubMed] [Google Scholar]
- Evett I, Weir BS. Interpreting DNA evidence: statistical genetics for forensic scientists. Sunderland, MA: Sinauer Associates; 1998. p. 278. [Google Scholar]
- Excoffier L, Lischer HEL. Arlequin suite ver 3.5: a new series of programs to perform population genetic analyses under Linux and Windows. Molecular Ecology Resources. 2010;10:564–567. doi: 10.1111/j.1755-0998.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics. 2003;164:1567–1587. doi: 10.1093/genetics/164.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira da Silva MJ, Godinho R, Casanova C, et al. Assessing the impact of hunting pressure on population structure of Guinea baboons (Papio papio) in Guinea-Bissau. Conservation Genetics. 2014;15:1339–1355. [Google Scholar]
- Galat-Luong A, Galat G, Hagall S. The social and ecological flexibility of Guinea baboons: implications for Guinea baboons social organization and male strategies. In: Swedell L, Leigh S, editors. Reproduction and fitness in Baboons. Behavioral, ecological, and life history perspectives. New York: Springer; 2006. pp. 105–121. [Google Scholar]
- Gompper ME, Gittleman JL, Wayne RK. Genetic relatedness, coalitions and social behaviour of white-nosed coatis. Nasua narica. Animal Behaviour. 1997;53:781–797. [Google Scholar]
- Goudet J. FSTAT (Version 1.2): a computer program to calculate F-statistics. Journal of Heredity. 1995;86:485–486. [Google Scholar]
- Goudet J, Perrin N, Waser PM. Tests for sex-biased dispersal using bi-parentally inherited genetic markers. Molecular Ecology. 2002;11:1103–1114. doi: 10.1046/j.1365-294x.2002.01496.x. [DOI] [PubMed] [Google Scholar]
- Gouzoules S. Primate mating systems, kin associations, and cooperative behavior: evidence for kin recognition? Yearbook of Physical Anthropology. 1984;27:99–134. [Google Scholar]
- Greenwood PJ. Mating systems, philopatry and dispersal in birds and mammals. Animal Behaviour. 1980;28:1140–1162. [Google Scholar]
- Greminger MP, Krützen M, Schelling C, Pienkowska-Schelling A, Wandeler P. The quest for Y-chromosomal markers—methodological strategies for mammalian non-model organisms. Molecular Ecology Resources. 2010;10:409–420. doi: 10.1111/j.1755-0998.2009.02798.x. [DOI] [PubMed] [Google Scholar]
- Grueter CC, Chapais B, Zinner D. Evolution of multilevel social systems in nonhuman primates and humans. International Journal of Primatology. 2012;33:1002–1037. doi: 10.1007/s10764-012-9618-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton WD. The genetical evolution of social behaviour. I. Journal of Theoretical Biology. 1964a;7:1–16. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- Hamilton WD. The genetical evolution of social behaviour. II. Journal of Theoretical Biology. 1964b;7:17–52. doi: 10.1016/0022-5193(64)90039-6. [DOI] [PubMed] [Google Scholar]
- Hammond RL, Lawson Handley LJ, Winney BJ, Bruford MW, Perrin N. Genetic evidence for female-biased dispersal and gene flow in a polygynous primate. Proceedings of the Royal Society B: Biological Sciences. 2006;273:479–484. doi: 10.1098/rspb.2005.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hapke A, Zinner D, Zischler H. Mitochondrial DNA variation in Eritrean hamadryas baboons (Papio hamadryas hamadryas): life history influences population genetic structure. Behavioral Ecology and Sociobiology. 2001;50:483–492. [Google Scholar]
- Harris TR, Caillaud D, Chapman CA, Vigilant L. Neither genetic nor observational data alone are sufficient for understanding sex-biased dispersal in a social-group-living species. Molecular Ecology. 2009;18:1777–1790. doi: 10.1111/j.1365-294X.2009.04139.x. [DOI] [PubMed] [Google Scholar]
- Haus T, Akom E, Agwanda B, et al. Mitochondrial diversity and distribution of African green monkeys (Chlorocebus Gray, 1870) American Journal of Primatology. 2013;75:350–360. doi: 10.1002/ajp.22113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henzi P, Barrett L. Evolutionary ecology, sexual conflict, and behavioral differentiation among baboon populations. Evolutionary Anthropology. 2003;12:217–230. [Google Scholar]
- Hoelzer GA, Morales JC, Melnick DJ. Dispersal and the population genetics of primate species. In: Chapais B, Berman CM, editors. Kinship and behavior in primates. New York: Oxford University Press; 2004. pp. 109–131. [Google Scholar]
- Hothorn T, Hornik K, van de Wiel MA, Zeileis A. Implementing a class of permutation tests: the coin package. Journal of Statistical Software. 2008;28:1–23. [Google Scholar]
- Hubisz MJ, Falush D, Stephens M, Pritchard JK. Inferring weak population structure with the assistance of sample group information. Molecular Ecology Resources. 2009;9:1322–1332. doi: 10.1111/j.1755-0998.2009.02591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C. Integrating molecular techniques with field methods in studies of social behavior: a revolution results. Ecology. 1998;79:383–399. [Google Scholar]
- Jolly CJ. Fifty years of looking at human evolution: backward, forward, and sideways. Current Anthropology. 2009;50:187–199. doi: 10.1086/597196. [DOI] [PubMed] [Google Scholar]
- Kapsalis E. Matrilineal kinship and primate behavior. In: Chapais B, Berman CM, editors. Kinship and behavior in primates. New York: Oxford University Press; 2004. pp. 153–176. [Google Scholar]
- Kopp GH, Ferreira da Silva MJ, Fischer J, et al. The influence of social systems on patterns of mitochondrial DNA variation in baboons. International Journal of Primatology. 2014;35:210–225. doi: 10.1007/s10764-013-9725-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer H. Social organization of hamadryas baboons. A field study. Chicago: The University of Chicago Press; 1968. p. 189. [Google Scholar]
- Kummer H. In quest of the sacred baboon: A scientist's journey. Princeton: Princeton University Press; 1995. p. 337. [Google Scholar]
- Lambin X, Yoccoz NG. The impact of population kin-structure on nestling survival in Townsend's voles, Microtus townsendii. Journal of Animal Ecology. 1998;67:1–16. [Google Scholar]
- Langergraber KE. Cooperation among kin. In: Mitani JC, Call J, Kappeler PM, Palombit RA, Silk JB, editors. The evolution of primate societies. Chicago: The University of Chicago Press; 2012. pp. 491–513. [Google Scholar]
- Langergraber KE, Mitani JC, Vigilant L. The limited impact of kinship on cooperation in wild chimpanzees. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:7786–7790. doi: 10.1073/pnas.0611449104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathuillière M, Ménard N, Gautier-Hion A, Crouau-Roy B. Testing the reliability of noninvasive genetic sampling by comparing analyses of blood and fecal samples in Barbary macaques (Macaca sylvanus. American Journal of Primatology. 2001;55:151–158. doi: 10.1002/ajp.1048. [DOI] [PubMed] [Google Scholar]
- Lawson Handley LJ, Perrin N. Advances in our understanding of mammalian sex-biased dispersal. Molecular Ecology. 2007;16:1559–1578. doi: 10.1111/j.1365-294X.2006.03152.x. [DOI] [PubMed] [Google Scholar]
- Lawson Handley LJ, Hammond RL, Emaresi G, Reber A, Perrin N. Low Y chromosome variation in Saudi-Arabian hamadryas baboons (Papio hamadryas hamadryas. Heredity. 2006;96:298–303. doi: 10.1038/sj.hdy.6800803. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Boesch C. Sociality of the dispersing sex: the nature of social bonds in West African female chimpanzees. Pan troglodytes. Animal Behaviour. 2009;77:377–387. [Google Scholar]
- Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- Lukacs PM, Burnham KP. Review of capture-recapture methods applicable to noninvasive genetic sampling. Molecular Ecology. 2005;14:3909–3919. doi: 10.1111/j.1365-294X.2005.02717.x. [DOI] [PubMed] [Google Scholar]
- Lukas D, Clutton-Brock T. Group structure, kinship, inbreeding risk and habitual female dispersal in plural-breeding mammals. Journal of Evolutionary Biology. 2011;24:2624–2630. doi: 10.1111/j.1420-9101.2011.02385.x. [DOI] [PubMed] [Google Scholar]
- Lukas D, Reynolds V, Boesch C, Vigilant L. To what extent does living in a group mean living with kin? Molecular Ecology. 2005;14:2181–2196. doi: 10.1111/j.1365-294X.2005.02560.x. [DOI] [PubMed] [Google Scholar]
- Maciej P, Ndao I, Hammerschmidt K, Fischer J. Vocal communication in a complex multi-level society: constrained acoustic structure and flexible call usage in Guinea baboons. Frontiers in Zoology. 2013a;10:58. doi: 10.1186/1742-9994-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciej P, Patzelt A, Ndao I, Hammerschmidt K, Fischer J. Social monitoring in a multilevel society: a playback study with male Guinea baboons. Behavioral Ecology and Sociobiology. 2013b;67:61–68. doi: 10.1007/s00265-012-1425-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestripieri DJ, Mayhew J, Carlson CL, Radtke JM. One-male harems and female social dynamics in Guinea baboons. Folia Primatologica. 2007;78:56–68. doi: 10.1159/000095686. [DOI] [PubMed] [Google Scholar]
- Marks SJ, Levy H, Martinez-Cadenas C, Montinaro F, Capelli C. Migration distance rather than migration rate explains genetic diversity in human patrilocal groups. Molecular Ecology. 2012;21:4958–4969. doi: 10.1111/j.1365-294X.2012.05689.x. [DOI] [PubMed] [Google Scholar]
- Miquel C, Bellemain E, Poillot C, et al. Quality indexes to assess the reliability of genotypes in studies using noninvasive sampling and multiple-tube approach. Molecular Ecology Notes. 2006;6:985–988. [Google Scholar]
- Mitani JC, Watts DP, Pepper JW, Merriwether DA. Demographic and social constraints on male chimpanzee behaviour. Animal Behaviour. 2002;64:727–737. [Google Scholar]
- Moore J. Dispersal, nepotism, and primate social behavior. International Journal of Primatology. 1992;13:361–378. [Google Scholar]
- Morin PA, Chambers KE, Boesch C, Vigilant L. Quantitative polymerase chain reaction analysis of DNA from noninvasive samples for accurate microsatellite genotyping of wild chimpanzees (Pan troglodytes verus. Molecular Ecology. 2001;10:1835–1844. doi: 10.1046/j.0962-1083.2001.01308.x. [DOI] [PubMed] [Google Scholar]
- Moses RA, Millar JS. Philopatry and mother-daughter associations in bushy-tailed woodrats: space use and reproductive success. Behavioral Ecology and Sociobiology. 1994;35:131–140. [Google Scholar]
- Navidi W, Arnheim N, Waterman MS. A multiple-tubes approach for accurate genotyping of very small DNA samples by using PCR—statistical considerations. American Journal of Human Genetics. 1992;50:347–359. [PMC free article] [PubMed] [Google Scholar]
- Nsubuga AM, Robbins MM, Roeder AD, et al. Factors affecting the amount of genomic DNA extracted from ape faeces and the identification of an improved sample storage method. Molecular Ecology. 2004;13:2089–2094. doi: 10.1111/j.1365-294X.2004.02207.x. [DOI] [PubMed] [Google Scholar]
- Oates JF. Primates of West Africa. A field guide and natural history. Arlington, VA: Conservation International; 2011. p. 555. [Google Scholar]
- Oates JF, Gippoliti S, Groves CP. 2008. Papio papio. IUCN 2010. IUCN red list of threatened species. Version 2010. 1.
- Packer C. Male transfer in olive baboons. Nature. 1975;255:219–220. [Google Scholar]
- Packer C. Inter-troop transfer and inbreeding avoidance in Papio anubis. Animal Behaviour. 1979;27:1–36. doi: 10.1016/0003-3472(79)90127-1. [DOI] [PubMed] [Google Scholar]
- Paetkau D, Strobeck C. Microsatellite analysis of genetic variation in black bear populations. Molecular Ecology. 1994;3:489–495. doi: 10.1111/j.1365-294x.1994.tb00127.x. [DOI] [PubMed] [Google Scholar]
- Patzelt A, Zinner D, Fickenscher G, et al. Group composition of Guinea baboons (Papio papio) at a water place suggests a fluid social organization. International Journal of Primatology. 2011;32:652–668. doi: 10.1007/s10764-011-9493-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzelt A, Maciej P, Kopp GH, et al. Male tolerance and male-male bonds in a multi-level primate society. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:14740–14745. doi: 10.1073/pnas.1405811111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit EJ, Balloux F, Excoffier L. Mammalian population genetics: why not Y? Trends in Ecology and Evolution. 2002;17:28–33. [Google Scholar]
- Pozzi L, Bergey CM, Burrell AS. The use (and misuse) of phylogenetic trees in comparative behavioral analyses. International Journal of Primatology. 2014;35:32–54. [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prugnolle F, de Meeus T. Inferring sex-biased dispersal from population genetic tools: a review. Heredity. 2002;88:161–165. doi: 10.1038/sj.hdy.6800060. [DOI] [PubMed] [Google Scholar]
- Pusey AE. Sex-biased dispersal and inbreeding avoidance in birds and mammals. Trends in Ecology and Evolution. 1987;2:295–299. doi: 10.1016/0169-5347(87)90081-4. [DOI] [PubMed] [Google Scholar]
- Pusey AE, Packer C. Dispersal and philopatry. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate societies. Chicago: The University of Chicago Press; 1987. pp. 250–266. [Google Scholar]
- Queller CR, Goodnight KF. Estimating relatedness using genetic markers. Evolution. 1989;43:258–275. doi: 10.1111/j.1558-5646.1989.tb04226.x. [DOI] [PubMed] [Google Scholar]
- R Development Core Team 2014. R: A language and environment for statistical computing. Vienna/Austria: The R Foundation for Statistical Computing. ISBN 3-900051-07-0, URL http://www.R-project.org.
- Raymond M, Rousset F. Genepop (Version-1.2)—Population-genetics software for exact tests and ecumenicism. Journal of Heredity. 1995;86:248–249. [Google Scholar]
- Roeder AD, Archer FI, Poiner HN, Morin PA. A novel method for collection and preservation of faeces for genetic studies. Molecular Ecology Notes. 2004;4:761–764. [Google Scholar]
- Roeder AD, Bonhomme M, Heijmans C, et al. A large panel of microsatellite markers for genetic studies in the infra-order Catarrhini. Folia Primatologica. 2009;80:63–69. doi: 10.1159/000211121. [DOI] [PubMed] [Google Scholar]
- Rogers J, Mahaney MC, Witte SM, et al. A genetic linkage map of the baboon (Papio hamadryas) genome based on human microsatellite polymorphisms. Genomics. 2000;67:237–247. doi: 10.1006/geno.2000.6245. [DOI] [PubMed] [Google Scholar]
- Rousset F. GENEPOP ' 007: a complete re-implementation of the GENEPOP software for Windows and Linux. Molecular Ecology Resources. 2008;8:103–106. doi: 10.1111/j.1471-8286.2007.01931.x. [DOI] [PubMed] [Google Scholar]
- Schreier AL, Swedell L. The fourth level of social structure in a multi-level society: ecological and social functions of clans in hamadryas baboons. American Journal of Primatology. 2009;71:948–955. doi: 10.1002/ajp.20736. [DOI] [PubMed] [Google Scholar]
- Seielstad MT, Minch E, Cavalli-Sforza LL. Genetic evidence for a higher female migration rate in humans. Nature Genetics. 1998;20:278–280. doi: 10.1038/3088. [DOI] [PubMed] [Google Scholar]
- Seyfarth RM, Silk JB, Cheney DL. Social bonds in female baboons: the interaction between personality, kinship and rank. Animal Behaviour. 2014;87:23–29. [Google Scholar]
- Sharman MJ. 1981. Feeding, ranging and social organisation of the Guinea baboon. [dissertation]. St. Andrews: University of St. Andrews.
- Sigg H, Stolba A, Abegglen JJ, Dasser V. Life history of hamadryas baboons: physical development, infant mortality, reproductive parameters and family relationships. Primates. 1982;23:473–487. [Google Scholar]
- Silk JB. Kin selection in primate groups. International Journal of Primatology. 2002;23:849–875. [Google Scholar]
- Silk JB. The adaptive value of sociality in mammalian groups. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2007;362:539–559. doi: 10.1098/rstb.2006.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JB, Alberts SC, Altmann J. Social relationships among adult female baboons (Papio cynocephalus) II. Variation in the quality and stability of social bonds. Behavioral Ecology and Sociobiology. 2006a;61:197–204. [Google Scholar]
- Silk JB, Altmann J, Alberts SC. Social relationships among adult female baboons (Papio cynocephalus) I. Variation in the strength of social bonds. Behavioral Ecology and Sociobiology. 2006b;61:183–195. [Google Scholar]
- Smith JE. Hamilton's legacy: kinship, cooperation and social tolerance in mammalian groups. Animal Behaviour. 2014;92:291–304. [Google Scholar]
- Smith KL, Alberts SC, Bayes MK, et al. Cross-species amplification, non-invasive genotyping, and non-Mendelian inheritance of human STRPs in Savannah baboons. American Journal of Primatology. 2000;51:219–227. doi: 10.1002/1098-2345(200008)51:4<219::AID-AJP1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Snyder-Mackler N, Alberts SC, Bergman TJ. The socio-genetics of a complex society: female gelada relatedness patterns mirror association patterns in a multilevel society. Molecular Ecology. 2014;23:6179–6191. doi: 10.1111/mec.12987. [DOI] [PubMed] [Google Scholar]
- Städele V, van Doren V, Pines M, Swedell L, Vigilant L. Fine-scale genetic assessment of sex-specific dispersal patterns in a multilevel primate society. Journal of Human Evolution. 2015;78:103–113. doi: 10.1016/j.jhevol.2014.10.019. [DOI] [PubMed] [Google Scholar]
- Starin ED. Philopatry and affiliation among red colobus. Behaviour. 1994;130:253–270. [Google Scholar]
- Sterck EHM, Watts DP, van Schaik CP. The evolution of female social relationships in nonhuman primates. Behavioral Ecology and Sociobiology. 1997;41:291–309. [Google Scholar]
- Swedell L, Saunders J, Schreier A, et al. Female “dispersal” in hamadryas baboons: transfer among social units in a multilevel society. American Journal of Physical Anthropology. 2011;145:360–370. doi: 10.1002/ajpa.21504. [DOI] [PubMed] [Google Scholar]
- Taberlet P, Luikart G. Non-invasive genetic sampling and individual identification. Biological Journal of the Linnean Society. 1999;68:41–55. [Google Scholar]
- Taberlet P, Griffin S, Benoît G, et al. Reliable genotyping of samples with very low DNA quantities using PCR. Nucleic Acids Research. 1996;24:3189–3194. doi: 10.1093/nar/24.16.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taberlet P, Waits LP, Luikart G. Noninvasive genetic sampling: look before you leap. Trends in Ecology and Evolution. 1999;14:323–327. doi: 10.1016/s0169-5347(99)01637-7. [DOI] [PubMed] [Google Scholar]
- Teacher AGF, Griffiths DJ. HapStar: automated haplotype network layout and visualization. Molecular Ecology Resources. 2011;11:151–153. doi: 10.1111/j.1755-0998.2010.02890.x. [DOI] [PubMed] [Google Scholar]
- Valiere N, Berthier P, Mouchiroud D, Pontier D, Valière N. Gemini: software for testing the effects of genotyping errors and multitubes approach for individual identification. Molecular Ecology Notes. 2002;2:83–86. [Google Scholar]
- Van Horn RC, Altmann J, Alberts SC. Can't get there from here: inferring kinship from pairwise genetic relatedness. Animal Behaviour. 2008;75:1173–1180. [Google Scholar]
- Vinson A, Packer C, Rogers J. Patterns of relatedness and the population genetic effects of male-biased dispersal in savannah baboons at Gombe National Park and Mikumi National Park, Tanzania. American Journal of Physical Anthropology. 2005;S40:214. [Google Scholar]
- Wang J. Coancestry: a program for simulating, estimating and analysing relatedness and inbreeding coefficients. Molecular Ecology Resources. 2011;11:141–145. doi: 10.1111/j.1755-0998.2010.02885.x. [DOI] [PubMed] [Google Scholar]
- Weir BS, Cockerham CC. Estimating F-statistics for the analysis of population-structure. Evolution. 1984;38:1358–1370. doi: 10.1111/j.1558-5646.1984.tb05657.x. [DOI] [PubMed] [Google Scholar]
- Wikberg EC, Sicotte P, Campos FA, Ting N. Between-group variation in female dispersal, kin composition of groups, and proximity patterns in a black-and-white colobus monkey (Colobus vellerosus. PLoS ONE. 2012;7:e48740. doi: 10.1371/journal.pone.0048740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins JF, Marlowe FW. Sex-biased migration in humans: what should we expect from genetic data? BioEssays. 2006;28:290–300. doi: 10.1002/bies.20378. [DOI] [PubMed] [Google Scholar]
- Wittemyer G, Okello JBA, Rasmussen HB, et al. Where sociality and relatedness diverge: the genetic basis for hierarchical social organization in African elephants. Proceedings of the Royal Society B: Biological Sciences. 2009;276:3513–3521. doi: 10.1098/rspb.2009.0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinner D, Peláez F, Torkler F. Group composition and adult sex-ratio of hamadryas baboons (Papio hamadryas hamadryas) in Central Eritrea. International Journal of Primatology. 2001;22:415–430. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information.


