Abstract
Allergen immunotherapy (AIT) has been practised since 1911 and remains the only therapy proven to modify the natural history of allergic diseases. Although efficacious in carefully selected individuals, the currently licensed whole allergen extracts retain the risk of IgE-mediated adverse events, including anaphylaxis and occasionally death. This together with the need for prolonged treatment regimens results in poor patient adherence. The central role of the T cell in orchestrating the immune response to allergen informs the choice of T cell targeted therapies for down-regulation of aberrant allergic responses. Carefully mapped short synthetic peptides that contain the dominant T cell epitopes of major allergens and bind to a diverse array of HLA class II alleles, can be delivered intradermally into non-inflamed skin to induce sustained clinical and immunological tolerance. The short peptides from allergenic proteins are unable to cross-link IgE and possess minimal inflammatory potential. Systematic progress has been made from in vitro human models of allergen T cell epitope-based peptide anergy in the early 1990s, through proof-of-concept murine allergy models and early human trials with longer peptides, to the current randomized, double-blind, placebo-controlled clinical trials with the potential new class of synthetic short immune-regulatory T cell epitope peptide therapies. Sustained efficacy with few adverse events is being reported for cat, house dust mite and grass pollen allergy after only a short course of treatment. Underlying immunological mechanisms remain to be fully delineated but anergy, deletion, immune deviation and Treg induction all seem contributory to successful outcomes, with changes in IgG4 apparently less important compared to conventional AIT. T cell epitope peptide therapy is promising a safe and effective new class of specific treatment for allergy, enabling wider application even for more severe allergic diseases.
Introduction
Allergic diseases constitute a global health problem affecting an estimated 20% of the population (up to 40% in some countries). There are many different triggers of allergic diseases and clinical patterns range from mild allergic rhinitis to potentially life-threatening asthma and anaphylaxis. Allergic diseases inflict a huge socio-economic burden, exaggerated by their typically chronic nature. Currently, there is no cure. Available pharmacotherapies, including antihistamines, bronchodilators, corticosteroids and the newer biologicals, aid symptom relief and adrenaline provides emergency treatment of anaphylaxis. To date, the only proven form of disease-modifying treatment is allergen immunotherapy (AIT). The goals of AIT are to induce sustained immunological and clinical tolerance to the allergen following cessation of treatment [1–3]. Current clinical regimens comprise repeated, often incremental, doses of whole allergen extracts via subcutaneous injection (SCIT), or sublingual drops or tablets (SLIT), often over several years.
Efficacy of AIT was first reported by Noon et al. [4] in the early 1900s in studies of grass pollen allergy. Since then, administration of whole allergen extracts for AIT has become accepted clinical practice for treatment of allergy to many aeroallergens and insect venoms (wasps, bees). Different forms and delivery routes of allergen have been trialled, but currently only whole allergen extracts are licensed for clinical practice, with SCIT, where indicated, remaining the most effective route [5,6].
Despite the success of AIT in appropriate individuals, there remain major concerns with safety, efficacy and adherence [7]. These result from the complexity of allergen extracts, prolonged treatment courses, and the risk of adverse events due to intact allergens with retained IgE reactivity. Several approaches to reduce allergenicity of whole allergen molecules, without affecting immunoregulatory activity, have been explored including allergoids, recombinant allergen derivatives and allergen fragments, some with evidence of clinical efficacy [8–13]. However, of particular interest and the focus of this review is the development of short T cell epitope-based peptides as a potential new class of pharmacotherapy for allergic diseases. Constituent peptides are designed to comprise immunodominant T cell epitopes with negligible IgE-binding and lacking inflammatory cell stimulatory capacity. Their presentation in a non-immunogenic form induces long-lasting allergen-specific T cell non-responsiveness after only a short course of treatment. Here, we retrace the origins of this therapy from the initial seminal reports of in vitro high-dose T cell epitope peptide-induced anergy in human allergen-specific T cells in the 1990s to proof-of-concept murine allergy models of anergy and early clinical studies. Finally, recent highly encouraging clinical trials of T cell epitope peptide therapies and associated data on immunological mechanisms are reviewed.
The rationale for T cell targeted therapy for allergic diseases
Refining effective immunological therapies for allergic diseases requires detailed understanding of the underlying immune response to allergens, especially factors that influence whether adverse reactions or tolerance ensues. Allergic reactions are caused by inflammatory mediators released from activated mast cells, basophils and eosinophils, processes driven by allergen cross-linking of cell-bound specific IgE and Th2 cell-derived cytokines: IL-4 and IL-13 switch allergen-stimulated B cells to produce IgE antibodies; IL-5 promotes eosinophil migration and activation in the skin and mucosae; IL-3 and GM-CSF promote eosinophil differentiation and, together with IL-4 and IL-9, the maturation and activation of mast cells and basophils [14–16]. Pathogenic allergen-specific Th2 cells can be further characterized by surface marker phenotype. Wambre et al. showed that CD27−CRTH2+ allergen-specific Th2 cells could be identified in grass pollen-allergic subjects, but not healthy controls and that this T cell population was preferentially lost following effective SCIT [17,18]. In contrast, allergen-specific Th1 and Treg (particularly IL-10 producing Tr1) subsets predominate in non-atopic subjects, or those with resolved clinical symptoms following conventional AIT [16–21]. High levels of IFN-γ and IL-10 are induced at sites of allergen exposure following successful AIT, augmenting Th1-reactivity whilst inhibiting Th2 cell proliferation. IFN-γ and IL-10 also promote B cell production of specific IgG1 and IgG4 antibodies that can inhibit formation of allergen-IgE complexes and prevent IgE-facilitated antigen presentation by B cells, further down-regulating adverse Th2-type inflammatory responses [22,23].
T cell epitope peptide therapy harnesses the fundamental immunological ability of peptides comprising dominant T cell epitopes to induce anergy and/or deletion of specific T cells. Specific anergy relies on the functional cytokine plasticity of Th cells in order to allow down-regulation of pathogenic effector T cell responses as well as inducing naive T cells to mount protective responses [24,25]. Other properties of allergen-specific T cells contribute to feasibility of this approach for treatment of allergy. Firstly, conserved repertoires of T cell epitopes of allergens were noted within a given individual when screened over 2 years [26] in contrast to changing T cell specificities over time for autoantigens [27]. Secondly, analysis of the human T cell repertoire reveals a bias in both the TCR-Vα and TCR-Vβ gene segment usage, as well as in vivo clonal dominance by long-lived house dust mite (HDM)-specific T cell clones [28]. Persistent grass pollen-specific T cell clones have also been demonstrated in vivo [29]. Importantly, T cells from the same clonal origin can ‘switch’ from dominant IL-4 production to dominant IL-10 or IFN-γ production during in vitro anergy induction or conventional AIT [29–31]. Taken together, these data strongly suggest that inactivation or elimination of dominant monoclonal populations of pathogenic allergen-reactive T cells would modify beneficially the immune response to allergen and observed clinical phenotype.
T cell epitope mapping of allergens and selection of peptides for a therapeutic
Identifying CD4+ T cell epitopes within allergens is mandatory for the design of T cell targeted therapeutics. T cell epitope mapping requires knowledge of the allergen sequence and isolation or identification of allergen-specific T cells from allergic donors, both of which have been greatly facilitated in recent years by the evolution of more sophisticated and/or high-throughput methodologies. Most major allergenic proteins have now been cloned and sequenced [see http://www.allergen.org, register of validated data maintained by the Allergen Nomenclature Sub-Committee of the World Health Organization and International Union of Immunological Societies (IUIS)], allowing synthesis of nested sets of peptides spanning the entire allergen sequence to determine sites of T cell reactivity, as described below. Due to low precursor frequencies of allergen-specific T cells in peripheral blood, analysis of T cell epitopes of allergens is facilitated by prior enrichment of allergen-specific T cells. Initially, this was achieved by limiting dilution of allergen-stimulated whole PBMC to obtain clonal T cell populations [32,33]. New methodologies for analysing T cell responses to allergen peptides include flow cytometry techniques with proliferation dyes such as carboxyfluorescein diacetate succinimidyl ester (CFSE) to detect proliferating cells by their reduced staining intensity [34,35], cytokine capture kits [36] and fluorochrome-conjugated HLA class II-peptide tetramers [29,37]. Carboxyfluorescein diacetate succinimidyl ester-based approaches provide a highly sensitive method for detecting T cell responses, particularly when used together with other activation markers, such as CD25, but bystander proliferation can decrease specificity [38] necessitating validation of potential T cell epitopes in large patient cohorts. ELISPOT-based approaches are useful for high-throughput screens of whole PBMC [36] and can also be used for core T cell epitope mapping using T cell lines or clones [39,40]. T cell epitope mapping using peptide-stimulated PBMC cultures (as opposed to T cell lines and clones) is feasible if the assay is rigorously designed and appropriate statistical methods are used. However, few such studies have been performed on allergens (e.g. [37]).
HLA-peptide tetrameric complexes facilitate the identification and characterization of allergen-specific T cells without the need for expression of particular functional activities, providing a sensitive and specific tool for analysis of peptide-specific T cells directly ex vivo [29,37]. However, generation of tetramers is expensive and currently only a small proportion of HLA class II molecules are available in this form. Importantly, tetramers cannot map precise core T cell epitopes for optimal T cell stimulation to inform selection of the shortest and safest peptide set for therapy (see below). In contrast to CFSE-approaches, tetramer-based approaches show very high specificity, but sometimes lack sensitivity [38]. Similarly, in silico algorithms can be used to predict CD4+ T cell epitopes by identifying theoretical HLA class II binding motifs within protein sequences based on analyses of thousands of known epitopes [41]. However, whilst such algorithms can provide preliminary guides cost-effectively, they are not comprehensive and predicted HLA-binding motifs require validation by analysis of peripheral blood T cell responses [38,42].
To identify all potential T cell epitopes, allergen-specific T cell lines and clones generated from a large patient cohort are screened for reactivity against overlapping synthetic peptides spanning the entire sequence of the allergen molecule, each usually 15–20 amino acids in length with overlaps ranging from five amino acids upwards. Following identification of T cell reactive peptides, precise core epitope sequences are mapped utilizing peptide sets truncated from the N- and C-termini, for example as demonstrated in early studies for a rye grass pollen allergen Lol p 5 T cell epitope [43]. Minimal core CD4+ T cell epitopes are typically eight or nine residues long, but lengths for optimal T cell stimulation may be longer and vary between subjects. This likely reflects varied requirements for flanking residues in stabilizing different HLA-peptide-TCR complexes and increasing persistence of the peptide at the APC surface [44–46]. Peptides selected for immunotherapy tend to range from 12 to 20 residues, consistent with naturally processed peptides eluted from HLA class II molecules [47,48].
T cell reactive sites have been mapped for many allergens and are catalogued in The Immune Epitope Database (http://www.iedb.org [49,50]). A meta-analysis of this database confirmed 1406 allergen-derived CD4+ T cell epitopes based on human T cell reactivity [51]. However, despite the large number, these represent less than 17% of all allergens in the IUIS allergen database (http://www.allergen.org). T cell epitopes are typically found throughout an allergen sequence, but responder frequency evaluations from large subject cohorts assign dominance [41], underpinning design of T cell targeted peptide therapeutics. Dominant T cell epitopes also typically have the strongest T cell stimulatory capacity, an important consideration for immunotherapy following the established immunological dogma that the strongest immunogens are the strongest tolerogens [52]. As specific allergic immune responsiveness in atopic individuals is not typically limited to a single dominant epitope, careful mapping of the critical minimal set of immunodominant T cell epitope peptides is essential. In addition to frequency of reactivity, peptide selection criteria can include patterns of reactivity, reproducibility of T cell response and, importantly, ability to induce a response in patient PBMC. In some cases, where two epitopes are in close proximity, the inclusion of both in a single peptide is desirable provided the final peptide length is kept below about 20 amino acid residues. For some closely related allergens, for example group 1 grass pollen allergens, cross-reactive T cell epitopes have been identified [53–57] which may be advantageous for obtaining broader acting therapeutics with applicability in different world regions.
For therapeutic production, some peptides require modification to ensure solubility and stability for ease of manufacture and administration. This may include modification of terminal residues and substitution of cysteine residues with alanine or other non-reactive residues such as serine to avoid potential peptide aggregation (e.g. [35]). In these cases, T cell reactivity of the modified peptide must be reconfirmed. Importantly, for safety of the therapeutic, all candidate T cell epitope peptides must finally be tested singly and in combination to ensure lack of ability to bind and cross-link inflammatory cell-bound IgE. A convenient and reliable assay to assess clinically relevant, functional IgE reactivity is the basophil activation test by flow cytometry or histamine release [58–61].
HLA class II restriction of T cell recognition of allergen peptides
A further important consideration when selecting candidate peptides for immunotherapy is whether the peptides can be presented to T cells by different HLA class II molecules and hence be suitable for targeting genetically diverse populations. CD4+ T cells recognize a specific epitope only when it is complexed with a particular HLA class II molecule encoded by one of three highly polymorphic loci, HLA-DR, HLA-DP or HLA-DQ. One strategy to inform HLA types likely to bind a known T cell epitope utilizes T cell epitope prediction algorithms. Previously, such algorithms could predict binding only to HLA-DR molecules, but recent advances now endorse HLA-DQ and HLA-DP predictions [41]. However, as with T cell epitope mapping, such predictions require experimental validation using isolated HLA molecules and/or transfected L cells or EBV-transformed B cell lines homozygous for defined HLA alleles [26,41,62–65]. Analysis of HLA-peptide binding can indicate clinically relevant specificity as well as avidity and/or affinity of the interaction [41,65,66].
Whilst algorithms can predict peptide binding to particular HLA types and some assays confirm binding, it is important to demonstrate the full repertoire of functional HLA-peptide complexes. Evidence of functionality requires assays of T cell proliferation or cytokine production for the given HLA-peptide complex. Initial broad determination of HLA-restriction specificity of T cell epitope recognition can be made using blocking monoclonal antibodies specific for HLA-DR, HLA-DP or HLA-DQ [26,35,62]. Tetramers provide another method for screening for T cell reactivity to a given HLA-epitope complex in samples such as blood analysed directly ex vivo [37]. Unfortunately, screens utilizing tetramers or homozygous cell lines require HLA-matched CD4+ T cells/patients which can be logistically challenging [67]. Furthermore, many HLA molecules are hard to isolate and use in tetramers, thus limiting the range of HLA types that can be tested.
Unlike some autoimmune conditions, allergic diseases generally are not closely linked with particular HLA types [68]. Reflecting this fact, allergen T cell epitopes often demonstrate extensive HLA-binding degeneracy and, in turn, allergen-specific CD4+ T cells may recognize a particular epitope complexed with several different HLA class II molecules [18,35,36,40,41,69–71]. Importantly, whilst nominal antigens are most commonly presented on HLA-DR molecules, many allergen T cell epitopes have been shown to also be presented on HLA-DQ and HLA-DP molecules [26,28,35,40,41,59,64,72,73]. This is highly advantageous for a therapeutic as HLA-DQ and HLA-DP subtypes are more conserved across populations than HLA-DR molecules; for example, HLA-DP*0401 and 0402 alleles are together present in ∼ 50% of the Caucasian population [74].
Experimental models of allergen T cell epitope peptide immune modulation
Functional inactivation of allergen-reactive human T cells in vitro
O'Hehir et al. [75] first reported T cell epitope peptide induction of anergy in allergen-specific T cells in the 1990s. Incubation of cloned HDM-specific T cells with supraoptimal concentrations of their specific ligand, resulted in decreased proliferation to a subsequent immunogenic challenge, decreased IL-4 and IL-5 synthesis but maintained IFN-γ and IL-10 production [30,75]. During the induction phase of anergy by allergen T cell epitope peptides, there was transient release of some chemokines and Th2 cytokines (IL-4 in particular) suggesting a period of hyperexcitation before the development of sustained anergy [30,31]. Cytolysis was not the mechanism in this model as T cells were responsive to exogenous IL-2 [75]. Loss of allergen-dependent proliferation and altered cytokine production was accompanied by down-regulation of TCR, and upregulation of CD2, CD25 and adhesion molecules such as LFA-1 [76,77]. There was also blunting of the typical upregulation of CD28 observed in activation [78]. Altered signalling pathways underlying defective TCR signalling were demonstrated by abrogated activity of p56lck and ZAP-70 tyrosine kinases in a bee venom allergen (PLA2)-specific CD4+ T cell model [79].
Functional inactivation of allergen-reactive T cells in vivo using murine models
Prior to clinical development of the T cell epitope peptide therapies, murine models of allergy were developed to validate the strategy and further explore mechanisms. Using a murine model of HDM allergy, inhalation of the immunodominant T cell epitope peptide of Der p 1 by naïve or sensitized mice inhibited the T cell response not only to the peptide but to whole Der p 1, termed linked suppression or infectious tolerance [80] (Fig.1). This is of particular importance when contemplating clinical potential in novel AIT [81]. At the same time, there was a report of peripheral T cell tolerance in naïve and primed mice following subcutaneous injections of T cell epitope peptides from Fel d 1, the major cat allergen [82]. Subsequently, similar findings confirming the robustness of anergy, linked suppression and changes in cytokine functional phenotype induced by dominant T cell epitope peptides were reported for murine models of allergy to birch pollen [83], Japanese cedar pollen [84,85], olive pollen [86], bee and hornet venom [87], bee venom [88], cat [89], egg-white [90] and Timothy grass pollen [91]. In some studies, anergy and regulatory activity were shown to coexist [80,92]. Using HLA-DR1 tetramers to track allergen-specific T cells in a murine model of cat allergy, Campbell et al. demonstrated Fel d 1 T cell epitope peptide-induced linked epitope suppression associated with IL-10+ T cells [89]. Mackenzie et al. [93] used adoptive transfer of Th2-polarized cells in a murine ovalbumin TCR transgenic model to explore the effects of peptide immunotherapy on antigen-experienced T cells. They showed preferential effects on cytokine production by CD62Llo cells (effector and effector memory T cells) rather than CD62LhiTh2 cells (associated with central memory T cells) in suppression of airway inflammation.
Fig. 1.
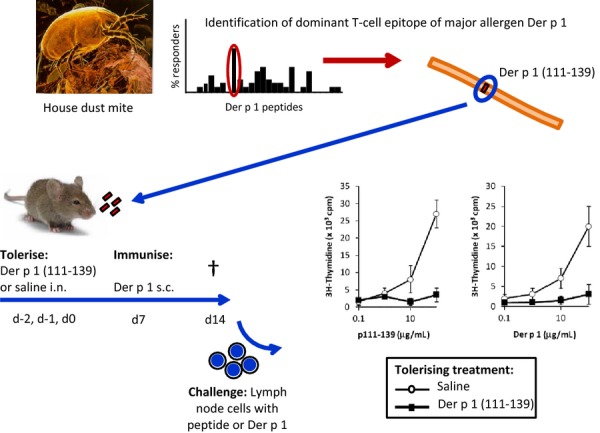
Linked epitope suppression by T cell peptide therapy in a murine model of allergy. Naïve mice were treated intranasally (i.n.) with the dominant T cell epitope peptide Der p 1(111–139) (tolerizing treatment), or with saline as a control, and then immunized with Der p 1 by subcutaneous (s.c.) injection. Immunogenic challenge of lymph node cells with peptide or Der p 1 in culture showed that inhalation of the dominant T cell peptide had induced T cell anergy/tolerance to the specific ligand as well as the intact house dust mite protein (adapted from [80]). This therapy was also effective in allergen-sensitized mice.
Clinical translation of T cell epitope-based peptide immunotherapy
Clinical trials of allergen-specific peptide immunotherapy have been conducted for allergy to bee venom and several aeroallergens, including recent phase IIb and III trials providing strong proof-of-concept and informing further development of this therapeutic strategy.
Bee venom allergy
In a pilot study of peptide immunotherapy for bee venom allergy, five bee venom-allergic patients were treated by subcutaneous injection with three T cell epitope peptides of the major bee venom allergen PLA2, each of 11–18 amino acid residues [94]. Consistent with linked suppression, clinical efficacy was achieved to a subsequent PLA2 challenge and live whole bee sting challenge. In follow-up studies, a semi-rush regimen of three long synthetic peptides encompassing the entire PLA2 sequence was followed by maintenance doses for up to 70 days [95]. Although increased Th1 cytokines and allergen-specific IgG4 were achieved during the study period, some subjects developed peptide-specific IgE and two subjects developed local erythema with occasional palmar pruritus. These findings emphasize the importance of using the shortest possible peptides comprising T cell epitopes to minimize the risk of IgE-mediated adverse events.
Cat allergy – first generation peptides
Early clinical trials of peptides for cat allergy showed variable efficacy, but large protein determinants were trialled rather than minimal epitopes. The early Fel d 1 peptides comprised an equimolar mixture of two long 27 amino acid sequences from the two chains of Fel d 1 and contained multiple T cell epitopes [96]. A double-blind placebo-controlled trial with 95 cat-allergic subjects was conducted using four subcutaneous injections of the peptide mixture (Allervax®CAT) or placebo [97]. Clinical benefit was demonstrable at 6 weeks but adverse events included nasal congestion, flushing, pruritus and chest tightness for minutes to hours after peptide delivery. The possibility of retained conformational structure within the long peptides and IgE-mediated reactivity likely explained the early adverse events. The asthmatic responses, in subjects with or without underlying asthma, were subsequently attributed to cytokine release from peptide-stimulated T cells [98], consistent with the initial T cell stimulation and cytokine flare observed early in the induction phase of allergen peptide-induced anergy in vitro [30,31], recognizing that IL-4 is a bronchoconstrictor. The delayed adverse effects diminished after repeated delivery. In another clinical trial using the same Fel d 1 peptides, several adverse events, including late asthma responses requiring adrenaline in three cases, were also observed [99]. These early studies with longer peptides given at very high concentrations subcutaneously were disappointing also in failing to achieve evidence of sustained clinical efficacy [99,100].
Synthetic peptide immuno-regulatory epitope therapy
Newer promising research pioneered by Larche and Kay in the late 1990s and early 2000s led to a second generation of T cell epitope-based peptides for allergy therapy, now designated as synthetic peptide immuno-regulatory epitopes (SPIRE) [98,101]. These comprise short peptide units, typically 13–17 amino acids in length, administered at lower concentrations (≤ 12 nmol; ∼ 75 vs. 750 μg) via the intradermal route into non-inflamed skin [60,101–105]. The first SPIRE, Cat-PAD (Circassia Ltd; Oxford, UK), comprises seven T cell epitope-based peptides (13–17 amino acids in length) derived from Fel d 1. It is produced as a lyophilizate and reconstituted in water for intradermal administration. A non-injectable device for transdermal delivery has been utilized in the most recent clinical trials which comprise four treatments at 4 week intervals before challenge testing and measurement of total rhino-conjunctivitis symptom score in an environmental exposure chamber (EEC). Early-phase studies demonstrated safety and clinical efficacy [60,104]. The shortness of the peptides avoids any potential for IgE-cross-linking or inflammatory cell activation and careful dose adjustment seems to avoid the late asthma response observed earlier with the longer peptides. In a recent phase III clinical field study, enduring clinical efficacy was demonstrated out to 2 years after one course of treatment with cat-PAD [106]. As seen with whole extract SCIT and SLIT studies [107,108], a substantial placebo effect was observed, but this was not sustained over the longer term and efficacy with the cat-PAD therapy was significantly higher. SPIRE therapies are currently being trialled with similar encouraging results from early-phase IIb studies for HDM [109,110], grass pollen [111] and ragweed pollen [112].
Mechanisms of T cell epitope-based peptide therapies from clinical studies
As clinical translation of T cell peptide therapy for allergy progresses, the underlying immunological mechanisms are being elucidated [113,114]. Although some mechanisms appear to overlap with conventional AIT, there seem to be distinct differences from current SCIT or SLIT (Fig.2) [16,115]. As for AIT with whole allergen, down-regulation of T cell proliferative and cytokine response to allergen is a consistent observation following peptide immunotherapy (e.g. [101,105]), but the precise mechanism underlying this altered response is not clear. In the early bee venom T cell peptide clinical study, the decreased PLA2-specific T cell proliferation and decreased IL-2, IL-4, IL-5, IL-13 and IFN-γ production were reversed by IL-2 and IL-15, suggesting anergy as the mechanism [94]. However, distinction between re-activation of anergized T cells and activation of naive T cells or indeed Treg could not be made due to the polyclonal nature of the cultures and limited phenotyping. Deletion of allergen-specific T cells is an alternative mechanism suggested from murine models of peptide-induced tolerance. A recent study used HLA class II tetramers to quantify allergen-specific clonal T cell populations ex vivo following conventional allergen SCIT for grass pollen allergy [18]. Preferential loss of clonal Th2-type T cells specific for dominant epitopes of major grass pollen allergens over T cells specific for less-dominant epitopes with a Th-1 or Tr1-phenotype was observed. A potential caveat of tetramer-based approaches is their reliance on detection of the TCR on the cell surface with the possible confounder of inability to distinguish between deletion and anergy given that there is down-modulation of TCR expression on anergic T cells. However, in this study, the pathogenic Th2 cells were further distinguished by lack of CD27 expression providing another marker to confirm selective loss of these cells. Furthermore, these cells also had decreased expression of the apoptotic inhibitor Bcl-2 over the cells that escaped deletion. Together these data suggest that dominant T cell epitope-based peptides of major allergens can cause targeted and desirable inactivation or deletion of the most pathogenic T cells in allergic subjects.
Fig. 2.
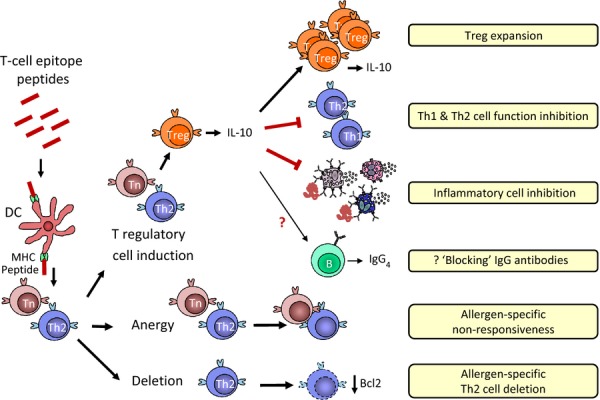
Immunological mechanisms of allergen T cell epitope-based peptide therapy. Murine and human studies suggest that down-regulation of the adverse Th2-polarized response to allergen by high-dose allergen T cell epitope peptide treatment is mediated by anergy of allergen-specific naïve CD4+ T cells (Tn) and Th2 cells, deletion of allergen-specific Th2 cells and/or induction of Treg with IL-10 production, further expanding Tregs and inhibiting Th1, Th2 and inflammatory cell function. CD4+ T cells show functional cytokine plasticity depending on the conditions of activation and cytokine milieu. The role of IgG4 blocking antibodies in T cell peptide-mediated clinical tolerance is unclear.
As mentioned earlier, the observed late asthmatic responses experienced with the first generation Allervax trials for cat allergy using high concentrations of peptides are likely due to the surge of Th2 cytokine release, specifically IL-4, early in the induction phase of anergy[30,31]. The lack of asthma on continued administration would be consistent with the lack of continued IL-4 secretion in established anergy. It may be that persistent antigen exposure is required to maintain the anergic state in vivo, either naturally in the environment as would be expected for cat, HDM and pollens or by booster antigen encounter.
Increased production of IL-10 and induction of Treg are the most frequently reported mechanisms underlying conventional AIT. Similarly, efficacy of allergen peptide immunotherapy, including early bee venom studies, early Allervax trials and SPIRE therapy, was associated with increased IL-10 production during therapy and a role for Treg is indicated [101,116]. In clinical studies on cat allergen peptide therapy, IL-10 was shown to be required for peptide-induced suppression of allergen-specific immune responses and linked epitope suppression [89], and induction of an antigen-specific CD4+ T cell population with regulatory function was demonstrated [117]. Analysis of skin from sites of allergen challenge showed an increased number of CD4+IFN-γ+ and CD25+ cells after peptide therapy suggesting roles for immune deviation and regulatory T cells [118]. It should be noted that interpretation of many studies of Treg subsets and function, especially using clinical samples, may be difficult due to overlap of surface marker expression between Treg and Teff, especially when activated. Further functional analysis and phenotyping of peripheral blood and tissue T cells are required to distinguish activated CD4+ T cells from natural or induced Treg [119].
There is less evidence for induction of specific IgG4 blocking antibody with successful peptide immunotherapy. Peptides used for AIT, in particular SPIRE, are short and screened for lack of IgE binding and inflammatory cell activating potential, so are unlikely to drive antibody production. However, subsequent exposure to the whole allergen in the context of an altered specific immune response could potentially result in production of specific IgG4 or IgA antibodies. In the first bee venom study, antibody responses were found to be unaltered during peptide therapy, but further subcutaneous whole allergen challenge caused an increase in specific IgG4 antibodies [94]. In contrast, in a subsequent study by Tarzi et al., the challenge-induced increase in specific IgG4 was marginal and transient [116]. Further follow-up studies after peptide therapy are required to assess the importance of allergen-specific IgG4 antibodies in establishing long-term clinical efficacy. Although blocking antibodies are considered important in allergen desensitization with whole allergen AIT, their requirement for durable tolerance induction is debated [113,120], and their desirability in the treatment of allergy to potent allergens such as peanut and shellfish is questioned.
Future prospects for T cell epitope-based peptide therapy
Although the underlying immunological mechanisms that underpin successful allergen T cell epitope-based peptide immunotherapy are still being elucidated, the positive outcomes of clinical trials using EEC coupled with absence of IgE-mediated adverse events augur well for future utility in the clinical setting. Accumulating data suggest a shared role with conventional AIT of Treg induction, IL-10 effects and immune deviation with a less convincing body of data around a role for IgG blocking antibody. The long-lasting clinical efficacy after only four intradermal doses, together with the early side-effect of late asthma with the first generation longer peptides, suggests to us that anergy may be a key mechanism. Co-administration of a beta agonist during the initial dosing to avoid any bronchoconstriction from T cell IL-4 release is worthy of consideration, as this side-effect was seen in both asthmatic and non-asthmatic patients. The point of change in functional phenotype of the anergized T cells following release of the Th2 cytokines is desired, and hence, blockage of any associated transient airway reactivity would be appropriate to allow the desired sustained therapeutic outcome.
Recent trials give confidence that delivery of the therapeutic by an intradermal route to non-inflamed skin is highly efficient in achieving the desired outcomes without the risk of anaphylaxis that frequently and unpredictably accompanies conventional whole allergen extract AIT and the newer forms of whole food extract oral immunotherapy. Final refinement of patient-friendly transdermal delivery devices, optimal concentration and dosing intervals of T cell peptide therapy for specific allergies is awaited with anticipation.
Conclusion
Taken together, the growing body of data from clinical trials in a range of allergic disorders supports the view that a new class of T cell peptide therapy for allergic diseases is imminent. Dominant T cell epitope-based allergen peptides seem to be particularly able to induce sustained immunological and clinical tolerance. Importantly, core epitope mapping informs selection of the critical short amino acid sequences of specific allergens that can provide the desired tolerance without the undesired effects of IgE-cross-linking and inflammatory cell activation. The well-established high level of degeneracy of binding of these allergen peptides to a range of HLA class II molecules further supports the ability to manufacture a population-based therapy, rather than requiring detailed patient endotyping and individualized medicines. Moreover, the demonstration of long-lasting sustained efficacy after only a short treatment course without adverse events raises optimism that the discipline of allergology is on the brink of a new era in allergen therapeutics.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Burks AW, Calderon MA, Casale T, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013;131:1288–96. doi: 10.1016/j.jaci.2013.01.049. e3. [DOI] [PubMed] [Google Scholar]
- 2.Canonica GW, Bousquet J, Casale T, et al. Sub-lingual immunotherapy: World Allergy Organization Position Paper 2009. Allergy. 2009;64(Suppl 91):1–59. doi: 10.1111/j.1398-9995.2009.02309.x. [DOI] [PubMed] [Google Scholar]
- 3.Pfaar O, Demoly P, Gerth van Wijk R, et al. Recommendations for the standardization of clinical outcomes used in allergen immunotherapy trials for allergic rhinoconjunctivitis: an EAACI Position Paper. Allergy. 2014;69:854–67. doi: 10.1111/all.12383. [DOI] [PubMed] [Google Scholar]
- 4.Noon L. Prophylactic inoculation against hay fever. Lancet. 1911;177:1555–626. [Google Scholar]
- 5.Bauer CS, Rank MA. Comparative efficacy and safety of subcutaneous versus sublingual immunotherapy. J Allergy Clin Immunol. 2014;134:765. doi: 10.1016/j.jaci.2014.07.024. e2. [DOI] [PubMed] [Google Scholar]
- 6.Chelladurai Y, Suarez-Cuervo C, Erekosima N, et al. Effectiveness of subcutaneous versus sublingual immunotherapy for the treatment of allergic rhinoconjunctivitis and asthma: a systematic review. J Allergy Clin Immunol Pract. 2013;1:361–9. doi: 10.1016/j.jaip.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Kiel MA, Roder E, Gerth van Wijk R, Al MJ, Hop WC, Rutten-van Molken MP. Real-life compliance and persistence among users of subcutaneous and sublingual allergen immunotherapy. J Allergy Clin Immunol. 2013;132:353–60. doi: 10.1016/j.jaci.2013.03.013. e2. [DOI] [PubMed] [Google Scholar]
- 8.Marsh DG, Norman PS, Roebber M, Lichtenstein LM. Studies on allergoids from naturally occurring allergens. III. Preparation of ragweed pollen allergoids by aldehyde modification in two steps. J Allergy Clin Immunol. 1981;68:449–59. doi: 10.1016/0091-6749(81)90199-8. [DOI] [PubMed] [Google Scholar]
- 9.Bousquet J, Maasch H, Martinot B, Hejjaoui A, Wahl R, Michel FB. Double-blind, placebo-controlled immunotherapy with mixed grass-pollen allergoids. II. Comparison between parameters assessing the efficacy of immunotherapy. J Allergy Clin Immunol. 1988;82:439–46. doi: 10.1016/0091-6749(88)90017-6. [DOI] [PubMed] [Google Scholar]
- 10.Negro JM, Wheeler AW, Hernandez J, et al. Comparison of the efficacy and safety of two preseasonal regimens of glutaraldehyde modified, tyrosine-adsorbed parietaria pollen extract over a period of three years in monosensitive patients. Allergol Immunopathol (Madr) 1999;27:153–64. [PubMed] [Google Scholar]
- 11.Pellaton C, Perrin Y, Boudousquie C, et al. Novel birch pollen specific immunotherapy formulation based on contiguous overlapping peptides. Clin Transl Allergy. 2013;3:17. doi: 10.1186/2045-7022-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spertini F, Perrin Y, Audran R, et al. Safety and immunogenicity of immunotherapy with Bet v 1-derived contiguous overlapping peptides. J Allergy Clin Immunol. 2014;134:239–40. doi: 10.1016/j.jaci.2014.04.001. e13. [DOI] [PubMed] [Google Scholar]
- 13.Valenta R, Niespodziana K, Focke-Tejkl M, et al. Recombinant allergens: what does the future hold? J Allergy Clin Immunol. 2011;127:860–4. doi: 10.1016/j.jaci.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Kay AB. Allergy and allergic diseases. Second of two parts. N Engl J Med. 2001;344:109–13. doi: 10.1056/NEJM200101113440206. [DOI] [PubMed] [Google Scholar]
- 15.Kay AB. Allergy and allergic diseases. First of two parts. N Engl J Med. 2001;344:30–7. doi: 10.1056/NEJM200101043440106. [DOI] [PubMed] [Google Scholar]
- 16.Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy: multiple suppressor factors at work in immune tolerance to allergens. J Allergy Clin Immunol. 2014;133:621–31. doi: 10.1016/j.jaci.2013.12.1088. [DOI] [PubMed] [Google Scholar]
- 17.Wambre E, DeLong JH, James EA, LaFond RE, Robinson D, Kwok WW. Differentiation stage determines pathologic and protective allergen-specific CD4+ T-cell outcomes during specific immunotherapy. J Allergy Clin Immunol. 2012;129:544–51. doi: 10.1016/j.jaci.2011.08.034. e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wambre E, DeLong JH, James EA, et al. Specific immunotherapy modifies allergen-specific CD4(+) T-cell responses in an epitope-dependent manner. J Allergy Clin Immunol. 2014;133:872–9. doi: 10.1016/j.jaci.2013.10.054. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bohle B, Kinaciyan T, Gerstmayr M, Radakovics A, Jahn-Schmid B, Ebner C. Sublingual immunotherapy induces IL-10-producing T regulatory cells, allergen-specific T-cell tolerance, and immune deviation. J Allergy Clin Immunol. 2007;120:707–13. doi: 10.1016/j.jaci.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 20.Han D, Wang C, Lou W, Gu Y, Wang Y, Zhang L. Allergen-specific IL-10-secreting type I T regulatory cells, but not CD4(+)CD25(+)Foxp3(+) T cells, are decreased in peripheral blood of patients with persistent allergic rhinitis. Clin Immunol. 2010;136:292–301. doi: 10.1016/j.clim.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Ajduk J, Marinic I, Aberle N, Rabatic S, Gagro A. Effect of house dust mite immunotherapy on transforming growth factor beta1-producing T cells in asthmatic children. Ann Allergy Asthma Immunol. 2008;100:314–22. doi: 10.1016/S1081-1206(10)60592-3. [DOI] [PubMed] [Google Scholar]
- 22.Scadding GW, Shamji MH, Jacobson MR, et al. Sublingual grass pollen immunotherapy is associated with increases in sublingual Foxp3-expressing cells and elevated allergen-specific immunoglobulin G4, immunoglobulin A and serum inhibitory activity for immunoglobulin E-facilitated allergen binding to B cells. Clin Exp Allergy. 2010;40:598–606. doi: 10.1111/j.1365-2222.2010.03462.x. [DOI] [PubMed] [Google Scholar]
- 23.van de Veen W, Stanic B, Yaman G, et al. IgG4 production is confined to human IL-10-producing regulatory B cells that suppress antigen-specific immune responses. J Allergy Clin Immunol. 2013;131:1204–12. doi: 10.1016/j.jaci.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 24.Murphy KM, Stockinger B. Effector T cell plasticity: flexibility in the face of changing circumstances. Nat Immunol. 2010;11:674–80. doi: 10.1038/ni.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu J, Paul WE. Heterogeneity and plasticity of T helper cells. Cell Res. 2010;20:4–12. doi: 10.1038/cr.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verhoef A, Higgins JA, Thorpe CJ, et al. Clonal analysis of the atopic immune response to the group 2 allergen of Dermatophagoides spp.: identification of HLA-DR and -DQ restricted T cell epitopes. Int Immunol. 1993;5:1589–97. doi: 10.1093/intimm/5.12.1589. [DOI] [PubMed] [Google Scholar]
- 27.Lehmann PV, Forsthuber T, Miller A, Sercarz EE. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992;358:155–7. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- 28.Wedderburn LR, O'Hehir RE, Hewitt CR, Lamb JR, Owen MJ. In vivo clonal dominance and limited T-cell receptor usage in human CD4+ T-cell recognition of house dust mite allergens. Proc Natl Acad Sci USA. 1993;90:8214–8. doi: 10.1073/pnas.90.17.8214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aslam A, Chan H, Warrell DA, Misbah S, Ogg GS. Tracking antigen-specific T-cells during clinical tolerance induction in humans. PLoS One. 2010;5:e11028. doi: 10.1371/journal.pone.0011028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Hehir RE, Lake RA, Schall TJ, Yssel H, Panagiotopoulou E, Lamb JR. Regulation of cytokine and chemokine transcription in a human TH2 type T-cell clone during the induction phase of anergy. Clin Exp Allergy. 1996;26:20–7. doi: 10.1111/j.1365-2222.1996.tb00052.x. [DOI] [PubMed] [Google Scholar]
- 31.Hoyne GF, Askonas BA, Hetzel C, Thomas WR, Lamb JR. Regulation of house dust mite responses by intranasally administered peptide: transient activation of CD4+ T cells precedes the development of tolerance in vivo. Int Immunol. 1996;8:335–42. doi: 10.1093/intimm/8.3.335. [DOI] [PubMed] [Google Scholar]
- 32.O'Hehir RE, Young DB, Kay AB, Lamb JR. Cloned human T lymphocytes reactive with Dermatophagoides farinae (house dust mite): a comparison of T- and B-cell antigen recognition. Immunology. 1987;62:635–40. [PMC free article] [PubMed] [Google Scholar]
- 33.O'Hehir RE, Askonas BA, Lamb JR. Albert WHW, Staines NA, editors. Cell culture: lymphocyte clones. Methods of immunological analysis 3. 1993. pp. 120–38. Springer-Verlag Heidelberg.
- 34.Mannering SI, Dromey JA, Morris JS, Thearle DJ, Jensen KP, Harrison LC. An efficient method for cloning human autoantigen-specific T cells. J Immunol Methods. 2005;298:83–92. doi: 10.1016/j.jim.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Prickett SR, Voskamp AL, Dacumos-Hill A, Symons K, Rolland JM, O'Hehir RE. Ara h 2 peptides containing dominant CD4+ T-cell epitopes: candidates for a peanut allergy therapeutic. J Allergy Clin Immunol. 2011;127:608–15. doi: 10.1016/j.jaci.2010.09.027. e1–5. [DOI] [PubMed] [Google Scholar]
- 36.Oseroff C, Sidney J, Kotturi MF, et al. Molecular determinants of T cell epitope recognition to the common Timothy grass allergen. J Immunol. 2010;185:943–55. doi: 10.4049/jimmunol.1000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwok WW, Roti M, Delong JH, et al. Direct ex vivo analysis of allergen-specific CD4+ T cells. J Allergy Clin Immunol. 2010;125:1407–9. doi: 10.1016/j.jaci.2010.03.037. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Hemelen D, Mahler V, Fischer G, et al. HLA class II peptide tetramers vs allergen-induced proliferation for identification of allergen-specific CD4 T cells. Allergy. 2015;70:49–58. doi: 10.1111/all.12524. [DOI] [PubMed] [Google Scholar]
- 39.Tye-Din JA, Stewart JA, Dromey JA, et al. Comprehensive, quantitative mapping of T cell epitopes in gluten in celiac disease. Sci Transl Med. 2010;2:41ra51. doi: 10.1126/scitranslmed.3001012. [DOI] [PubMed] [Google Scholar]
- 40.Bateman EA, Ardern-Jones MR, Ogg GS. Identification of an immunodominant region of Fel d 1 and characterization of constituent epitopes.[see comment] Clin Exp Allergy. 2008;38:1760–8. doi: 10.1111/j.1365-2222.2008.03098.x. [DOI] [PubMed] [Google Scholar]
- 41.Schulten V, Oseroff C, Alam R, et al. The identification of potentially pathogenic and therapeutic epitopes from common human allergens. Ann Allergy Asthma Immunol. 2013;110:7–10. doi: 10.1016/j.anai.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lundegaard C, Lund O, Nielsen M. Predictions versus high-throughput experiments in T-cell epitope discovery: competition or synergy? Expert Rev Vaccines. 2012;11:43–54. doi: 10.1586/erv.11.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burton MD, Blaher B, Suphioglu C, O'Hehir RE, Carbone FR, Rolland JM. T-cell receptor contact and MHC binding residues of a major rye grass pollen allergen T-cell epitope. J Allergy Clin Immunol. 1999;103:255–61. doi: 10.1016/s0091-6749(99)70499-9. [DOI] [PubMed] [Google Scholar]
- 44.Knapp B, Fischer G, Van Hemelen D, et al. Association of HLA-DR1 with the allergic response to the major mugwort pollen allergen: molecular background. BMC Immunol. 2012;13:43. doi: 10.1186/1471-2172-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Knapp B, Omasits U, Bohle B, et al. 3-Layer-based analysis of peptide-MHC interaction: in silico prediction, peptide binding affinity and T cell activation in a relevant allergen-specific model. Mol Immunol. 2009;46:1839–44. doi: 10.1016/j.molimm.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 46.Maillere B, Mourier G, Herve M, Menez A. Fine chemical modifications at N- and C-termini enhance peptide presentation to T cells by increasing the lifespan of both free and MHC-complexed peptides. Mol Immunol. 1995;32:1377–85. doi: 10.1016/0161-5890(95)00103-4. [DOI] [PubMed] [Google Scholar]
- 47.Mutschlechner S, Egger M, Briza P, et al. Naturally processed T cell-activating peptides of the major birch pollen allergen. J Allergy Clin Immunol. 2010;125:711–8. doi: 10.1016/j.jaci.2009.10.052. 8 e1–8 e2. [DOI] [PubMed] [Google Scholar]
- 48.Rudensky A, Preston-Hurlburt P, Hong SC, Barlow A, Janeway CA., Jr Sequence analysis of peptides bound to MHC class II molecules. Nature. 1991;353:622–7. doi: 10.1038/353622a0. [DOI] [PubMed] [Google Scholar]
- 49.Salimi N, Fleri W, Peters B, Sette A. The immune epitope database: a historical retrospective of the first decade. Immunology. 2012;137:117–23. doi: 10.1111/j.1365-2567.2012.03611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vita R, Zarebski L, Greenbaum JA, et al. The immune epitope database 2.0. Nucleic Acids Res. 2010;38:D854–62. doi: 10.1093/nar/gkp1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vaughan K, Greenbaum J, Kim Y, et al. Towards defining molecular determinants recognized by adaptive immunity in allergic disease: an inventory of the available data. J Allergy (Cairo) 2010;2010:628026. doi: 10.1155/2010/628026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gammon G, Sercarz E. How some T cells escape tolerance induction. Nature. 1989;342:183–5. doi: 10.1038/342183a0. [DOI] [PubMed] [Google Scholar]
- 53.Etto T, de Boer C, Prickett S, et al. Unique and cross-reactive T cell epitope peptides of the major Bahia grass pollen allergen, Pas n 1. Int Arch Allergy Immunol. 2012;159:355–66. doi: 10.1159/000338290. [DOI] [PubMed] [Google Scholar]
- 54.Glaspole IN, de Leon MP, Prickett SR, O'Hehir RE, Rolland JM. Clinical allergy to hazelnut and peanut: identification of T cell cross-reactive allergens. Int Arch Allergy Immunol. 2011;155:345–54. doi: 10.1159/000321268. [DOI] [PubMed] [Google Scholar]
- 55.Jahn-Schmid B, Radakovics A, Luttkopf D, et al. Bet v 1142-156 is the dominant T-cell epitope of the major birch pollen allergen and important for cross-reactivity with Bet v 1-related food allergens. J Allergy Clin Immunol. 2005;116:213–9. doi: 10.1016/j.jaci.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 56.Sone T, Dairiki K, Morikubo K, et al. Identification of human T cell epitopes in Japanese cypress pollen allergen, Cha o 1, elucidates the intrinsic mechanism of cross-allergenicity between Cha o 1 and Cry j 1, the major allergen of Japanese cedar pollen, at the T cell level. Clin Exp Allergy. 2005;35:664–71. doi: 10.1111/j.1365-2222.2005.02221.x. [DOI] [PubMed] [Google Scholar]
- 57.Schenk S, Breiteneder H, Susani M, et al. T-cell epitopes of Phl p 1, major pollen allergen of timothy grass (Phleum pratense): evidence for crossreacting and non-crossreacting T-cell epitopes within grass group I allergens. J Allergy Clin Immunol. 1995;96:986–96. doi: 10.1016/s0091-6749(95)70237-7. [DOI] [PubMed] [Google Scholar]
- 58.Drew AC, Eusebius NP, Kenins L, et al. Hypoallergenic variants of the major latex allergen Hev b 6.01 retaining human T lymphocyte reactivity. J Immunol. 2004;173:5872–9. doi: 10.4049/jimmunol.173.9.5872. [DOI] [PubMed] [Google Scholar]
- 59.Prickett SR, Voskamp AL, Phan T, et al. Ara h 1 CD4+ T-cell epitope-based peptides: candidates for a peanut allergy therapeutic. Clin Exp Allergy. 2013;43:684–97. doi: 10.1111/cea.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Worm M, Lee HH, Kleine-Tebbe J, et al. Development and preliminary clinical evaluation of a peptide immunotherapy vaccine for cat allergy. J Allergy Clin Immunol. 2011;127:89–97. doi: 10.1016/j.jaci.2010.11.029. e1–14. [DOI] [PubMed] [Google Scholar]
- 61.Santos AF, Douiri A, Becares N, et al. Basophil activation test discriminates between allergy and tolerance in peanut-sensitized children. J Allergy Clin Immunol. 2014;134:645–52. doi: 10.1016/j.jaci.2014.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O'Hehir RE, Eckels DD, Frew AJ, Kay AB, Lamb JR. MHC class II restriction specificity of cloned human T lymphocytes reactive with Dermatophagoides farinae (house dust mite) Immunology. 1988;64:627–31. [PMC free article] [PubMed] [Google Scholar]
- 63.O'Hehir RE, Mach B, Berte C, et al. Direct evidence for a functional role of HLA-DRB1 and -DRB3 gene products in the recognition of Dermatophagoides spp. (house dust mite) by helper T lymphocytes. Int Immunol. 1990;2:885–92. doi: 10.1093/intimm/2.9.885. [DOI] [PubMed] [Google Scholar]
- 64.Higgins JA, Lamb JR, Marsh SG, et al. Peptide-induced nonresponsiveness of HLA-DP restricted human T cells reactive with Dermatophagoides spp. (house dust mite) J Allergy Clin Immunol. 1992;90:749–56. doi: 10.1016/0091-6749(92)90098-m. [DOI] [PubMed] [Google Scholar]
- 65.McKinney DM, Southwood S, Hinz D, et al. A strategy to determine HLA class II restriction broadly covering the DR, DP, and DQ allelic variants most commonly expressed in the general population. Immunogenetics. 2013;65:357–70. doi: 10.1007/s00251-013-0684-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.ProImmune. ProImmune REVEAL® Class II Rapid Epitope Discovery System (Last accessed 24 April 2015). Available from: https://www.proimmune.com/ecommerce/page.php?page=reveal_class2.
- 67.Nepom GT. MHC class II tetramers. J Immunol. 2012;188:2477–82. doi: 10.4049/jimmunol.1102398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jahn-Schmid B, Pickl WF, Bohle B. Interaction of allergens, major histocompatibility complex molecules, and T cell receptors: a ‘menage a trois’ that opens new avenues for therapeutic intervention in type I allergy. Int Arch Allergy Immunol. 2011;156:27–42. doi: 10.1159/000321904. [DOI] [PubMed] [Google Scholar]
- 69.Crack LR, Chan HW, McPherson T, Ogg GS. Identification of an immunodominant region of the major house dust mite allergen Der p 2 presented by common human leucocyte antigen alleles. Clin Exp Dermatol. 2012;37:266–76. doi: 10.1111/j.1365-2230.2011.04227.x. [DOI] [PubMed] [Google Scholar]
- 70.Friedl-Hajek R, Spangfort MD, Schou C, Breiteneder H, Yssel H. Joost van Neerven RJ. Identification of a highly promiscuous and an HLA allele-specific T-cell epitope in the birch major allergen Bet v 1: HLA restriction, epitope mapping and TCR sequence comparisons. Clin Exp Allergy. 1999;29:478–87. doi: 10.1046/j.1365-2222.1999.00489.x. [DOI] [PubMed] [Google Scholar]
- 71.DeLong JH, Simpson KH, Wambre E, James EA, Robinson D, Kwok WW. Ara h 1-reactive T cells in individuals with peanut allergy. J Allergy Clin Immunol. 2011;127:1211–8. doi: 10.1016/j.jaci.2011.02.028. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van Neerven RJ, van de Pol MM, van Milligen FJ, Jansen HM, Aalberse RC, Kapsenberg ML. Characterization of cat dander-specific T lymphocytes from atopic patients. J Immunol. 1994;152:4203–10. [PubMed] [Google Scholar]
- 73.Yssel H, Johnson KE, Schneider PV, et al. T cell activation-inducing epitopes of the house dust mite allergen Der p I. Proliferation and lymphokine production patterns by Der p I-specific CD4+ T cell clones. J Immunol. 1992;148:738–45. [PubMed] [Google Scholar]
- 74.Castelli FA, Buhot C, Sanson A, et al. HLA-DP4, the most frequent HLA II molecule, defines a new supertype of peptide-binding specificity. J Immunol. 2002;169:6928–34. doi: 10.4049/jimmunol.169.12.6928. [DOI] [PubMed] [Google Scholar]
- 75.O'Hehir RE, Yssel H, Verma S, de Vries JE, Spits H, Lamb JR. Clonal analysis of differential lymphokine production in peptide and superantigen induced T cell anergy. Int Immunol. 1991;3:819–26. doi: 10.1093/intimm/3.8.819. [DOI] [PubMed] [Google Scholar]
- 76.O'Hehir RE, Aguilar BA, Schmidt TJ, Gollnick SO, Lamb JR. Functional inactivation of Dermatophagoides spp. (house dust mite) reactive human T-cell clones. Clin Exp Allergy. 1991;21:209–15. doi: 10.1111/j.1365-2222.1991.tb00832.x. [DOI] [PubMed] [Google Scholar]
- 77.Fasler S, Aversa G, Terr A, Thestrup-Pedersen K, de Vries JE, Yssel H. Peptide-induced anergy in allergen-specific human Th2 cells results in lack of cytokine production and B cell help for IgE synthesis. Reversal by IL-2, not by IL-4 or IL-13. J Immunol. 1995;155:4199–206. [PubMed] [Google Scholar]
- 78.Lake RA, O'Hehir RE, Verhoef A, Lamb JR. CD28 mRNA rapidly decays when activated T cells are functionally anergized with specific peptide. Int Immunol. 1993;5:461–6. doi: 10.1093/intimm/5.5.461. [DOI] [PubMed] [Google Scholar]
- 79.Faith A, Akdis CA, Akdis M, Simon HU, Blaser K. Defective TCR stimulation in anergized type 2 T helper cells correlates with abrogated p56(lck) and ZAP-70 tyrosine kinase activities. J Immunol. 1997;159:53–60. [PubMed] [Google Scholar]
- 80.Hoyne GF, O'Hehir RE, Wraith DC, Thomas WR, Lamb JR. Inhibition of T cell and antibody responses to house dust mite allergen by inhalation of the dominant T cell epitope in naive and sensitized mice. J Exp Med. 1993;178:1783–8. doi: 10.1084/jem.178.5.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.O'Hehir RE, Hoyne GF, Thomas WR, Lamb JR. House dust mite allergy: from T-cell epitopes to immunotherapy. Eur J Clin Invest. 1993;23:763–72. doi: 10.1111/j.1365-2362.1993.tb00729.x. [DOI] [PubMed] [Google Scholar]
- 82.Briner TJ, Kuo MC, Keating KM, Rogers BL, Greenstein JL. Peripheral T-cell tolerance induced in naive and primed mice by subcutaneous injection of peptides from the major cat allergen Fel d I. Proc Natl Acad Sci USA. 1993;90:7608–12. doi: 10.1073/pnas.90.16.7608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bauer L, Bohle B, Jahn-Schmid B, et al. Modulation of the allergic immune response in BALB/c mice by subcutaneous injection of high doses of the dominant T cell epitope from the major birch pollen allergen Bet v 1. Clin Exp Immunol. 1997;107:536–41. doi: 10.1046/j.1365-2249.1997.d01-953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yoshitomi T, Nakagami Y, Hirahara K, Taniguchi Y, Sakaguchi M, Yamashita M. Intraoral administration of a T-cell epitope peptide induces immunological tolerance in Cry j 2-sensitized mice. J Pept Sci. 2007;13:499–503. doi: 10.1002/psc.869. [DOI] [PubMed] [Google Scholar]
- 85.Hirahara K, Saito S, Serizawa N, et al. Oral administration of a dominant T-cell determinant peptide inhibits allergen-specific TH1 and TH2 cell responses in Cry j 2-primed mice. J Allergy Clin Immunol. 1998;102:961–7. doi: 10.1016/s0091-6749(98)70334-3. [DOI] [PubMed] [Google Scholar]
- 86.Marazuela EG, Rodriguez R, Fernandez-Garcia H, Garcia MS, Villalba M, Batanero E. Intranasal immunization with a dominant T-cell epitope peptide of a major allergen of olive pollen prevents mice from sensitization to the whole allergen. Mol Immunol. 2008;45:438–45. doi: 10.1016/j.molimm.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 87.King TP, Lu G, Agosto H. Antibody responses to bee melittin (Api m 4) and hornet antigen 5 (Dol m 5) in mice treated with the dominant T-cell epitope peptides. J Allergy Clin Immunol. 1998;101:397–403. doi: 10.1016/S0091-6749(98)70254-4. [DOI] [PubMed] [Google Scholar]
- 88.Astori M, von Garnier C, Kettner A, Dufour N, Corradin G, Spertini F. Inducing tolerance by intranasal administration of long peptides in naive and primed CBA/J mice. J Immunol. 2000;165:3497–505. doi: 10.4049/jimmunol.165.6.3497. [DOI] [PubMed] [Google Scholar]
- 89.Campbell JD, Buckland KF, McMillan SJ, et al. Peptide immunotherapy in allergic asthma generates IL-10-dependent immunological tolerance associated with linked epitope suppression. J Exp Med. 2009;206:1535–47. doi: 10.1084/jem.20082901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rupa P, Mine Y. Oral immunotherapy with immunodominant T-cell epitope peptides alleviates allergic reactions in a Balb/c mouse model of egg allergy. Allergy. 2012;67:74–82. doi: 10.1111/j.1398-9995.2011.02724.x. [DOI] [PubMed] [Google Scholar]
- 91.Till SJ, Raynsford EJ, Reynolds CJ, et al. Peptide-induced immune regulation by a promiscuous and immunodominant CD4T-cell epitope of Timothy grass pollen: a role of Cbl-b and Itch in regulation. Thorax. 2014;69:335–45. doi: 10.1136/thoraxjnl-2013-204324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hoyne GF, Jarnicki AG, Thomas WR, Lamb JR. Characterization of the specificity and duration of T cell tolerance to intranasally administered peptides in mice: a role for intramolecular epitope suppression. Int Immunol. 1997;9:1165–73. doi: 10.1093/intimm/9.8.1165. [DOI] [PubMed] [Google Scholar]
- 93.Mackenzie KJ, Fitch PM, Leech MD, et al. Combination peptide immunotherapy based on T-cell epitope mapping reduces allergen-specific IgE and eosinophilia in allergic airway inflammation. Immunology. 2013;138:258–68. doi: 10.1111/imm.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Muller U, Akdis CA, Fricker M, et al. Successful immunotherapy with T-cell epitope peptides of bee venom phospholipase A2 induces specific T-cell anergy in patients allergic to bee venom. J Allergy Clin Immunol. 1998;101:747–54. doi: 10.1016/S0091-6749(98)70402-6. [DOI] [PubMed] [Google Scholar]
- 95.Fellrath JM, Kettner A, Dufour N, et al. Allergen-specific T-cell tolerance induction with allergen-derived long synthetic peptides: results of a phase I trial. J Allergy Clin Immunol. 2003;111:854–61. doi: 10.1067/mai.2003.1337. [DOI] [PubMed] [Google Scholar]
- 96.Wallner BP, Gefter ML. Immunotherapy with T-cell-reactive peptides derived from allergens. Allergy. 1994;49:302–8. doi: 10.1111/j.1398-9995.1994.tb02272.x. [DOI] [PubMed] [Google Scholar]
- 97.Norman PS, Ohman JL, Jr, Long AA, et al. Treatment of cat allergy with T-cell reactive peptides. Am J Respir Crit Care Med. 1996;154:1623–8. doi: 10.1164/ajrccm.154.6.8970345. [DOI] [PubMed] [Google Scholar]
- 98.Haselden BM, Kay AB, Larche M. Immunoglobulin E-independent major histocompatibility complex-restricted T cell peptide epitope-induced late asthmatic reactions. J Exp Med. 1999;189:1885–94. doi: 10.1084/jem.189.12.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Maguire P, Nicodemus C, Robinson D, Aaronson D, Umetsu DT. The safety and efficacy of ALLERVAX CAT in cat allergic patients. Clin Immunol. 1999;93:222–31. doi: 10.1006/clim.1999.4795. [DOI] [PubMed] [Google Scholar]
- 100.Simons FE, Imada M, Li Y, Watson WT, HayGlass KT. Fel d 1 peptides: effect on skin tests and cytokine synthesis in cat-allergic human subjects. Int Immunol. 1996;8:1937–45. doi: 10.1093/intimm/8.12.1937. [DOI] [PubMed] [Google Scholar]
- 101.Oldfield WL, Larche M, Kay AB. Effect of T-cell peptides derived from Fel d 1 on allergic reactions and cytokine production in patients sensitive to cats: a randomised controlled trial. Lancet. 2002;360:47–53. doi: 10.1016/s0140-6736(02)09332-7. [DOI] [PubMed] [Google Scholar]
- 102.Larche M. Peptide immunotherapy for allergic diseases. Allergy. 2007;62:325–31. doi: 10.1111/j.1398-9995.2006.01309.x. [DOI] [PubMed] [Google Scholar]
- 103.Larche M. T cell epitope-based allergy vaccines. Curr Top Microbiol Immunol. 2011;352:107–19. doi: 10.1007/82_2011_131. [DOI] [PubMed] [Google Scholar]
- 104.Worm M, Patel D, Creticos PS. Cat peptide antigen desensitisation for treating cat allergic rhinoconjunctivitis. Expert Opin Investig Drugs. 2013;22:1347–57. doi: 10.1517/13543784.2013.827661. [DOI] [PubMed] [Google Scholar]
- 105.Smith TR, Alexander C, Kay AB, Larche M, Robinson DS. Cat allergen peptide immunotherapy reduces CD4(+) T cell responses to cat allergen but does not alter suppression by CD4(+) CD25(+) T cells: a double-blind placebo-controlled study. Allergy. 2004;59:1097–101. doi: 10.1111/j.1398-9995.2004.00601.x. [DOI] [PubMed] [Google Scholar]
- 106.Couroux P, Patel D, Armstrong K, Larche M, Hafner RP. Fel d 1-derived synthetic peptide immuno-regulatory epitopes show a long-term treatment effect in cat allergic subjects. Clin Exp Allergy. 2015;45:974–81. doi: 10.1111/cea.12488. [DOI] [PubMed] [Google Scholar]
- 107.Narkus A, Lehnigk U, Haefner D, Klinger R, Pfaar O, Worm M. The placebo effect in allergen-specific immunotherapy trials. Clin Transl Allergy. 2013;3:42. doi: 10.1186/2045-7022-3-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Durham SR. Sublingual immunotherapy: what have we learnt from the ‘big trials’? Curr Opin Allergy Clin Immunol. 2008;8:577–84. doi: 10.1097/ACI.0b013e3283196764. [DOI] [PubMed] [Google Scholar]
- 109.Larche M, Hickey P, Hebert J, Hafner R. Safety and Tolerability of Escalating Doses of House Dust Mite- Peptide Antigen Desensitization (HDM-PAD) J Allergy Clin Immunol. 2013;131:AB37. doi: 10.1016/j.jaci.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 110.Hafner RP, Couroux P, Armstrong K, Salapatek AM, Patel D, Larche M. Four doses of Der p derived synthetic peptide immuno-regulatory epitopes over 3 months results in a persistent treatment effect at 1 year on symptoms of House Dust Mite allergy. Allergy. 2014;69:31–2. [Google Scholar]
- 111.Ellis AK, Frankish CW, Armstrong K, et al. A short course of synthetic peptide immuno-regulatory epitopes derived from grass allergens leads to a reduction in grass allergy symptoms. Allergy. 2014;69:32. [Google Scholar]
- 112.Hafner R, Salapatek A, Patel D, Larche M, Laidler P. Validation of peptide immunotherapy as a new approach in the treatment of allergic rhinoconjunctivitis: the clinical benefits of treatment with Amb a 1-derived T cell epitopes. J Allergy Clin Immunol. 2012;129:AB368. [Google Scholar]
- 113.Larche M. Mechanisms of peptide immunotherapy in allergic airways disease. Ann Am Thorac Soc. 2014;11(Suppl 5):S292–6. doi: 10.1513/AnnalsATS.201402-090AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Larche M, Wraith DC. Peptide-based therapeutic vaccines for allergic and autoimmune diseases. Nat Med. 2005;11:S69–76. doi: 10.1038/nm1226. [DOI] [PubMed] [Google Scholar]
- 115.O'Hehir RE, Sandrini A, Anderson GP, Rolland JM. Sublingual allergen immunotherapy: immunological mechanisms and prospects for refined vaccine preparation. Curr Med Chem. 2007;14:2235–44. doi: 10.2174/092986707781696609. [DOI] [PubMed] [Google Scholar]
- 116.Tarzi M, Klunker S, Texier C, et al. Induction of interleukin-10 and suppressor of cytokine signalling-3 gene expression following peptide immunotherapy. Clin Exp Allergy. 2006;36:465–74. doi: 10.1111/j.1365-2222.2006.02469.x. [DOI] [PubMed] [Google Scholar]
- 117.Verhoef A, Alexander C, Kay AB, Larche M. T cell epitope immunotherapy induces a CD4+ T cell population with regulatory activity. PLoS Med. 2005;2:e78. doi: 10.1371/journal.pmed.0020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Alexander C, Ying S, Kay AB, Larche M. Fel d 1-derived T cell peptide therapy induces recruitment of CD4+ CD25+; CD4+ interferon-gamma+ T helper type 1 cells to sites of allergen-induced late-phase skin reactions in cat-allergic subjects. Clin Exp Allergy. 2005;35:52–8. doi: 10.1111/j.1365-2222.2005.02143.x. [DOI] [PubMed] [Google Scholar]
- 119.Rolland JM, Gardner LM, O'Hehir RE. Functional regulatory T cells and allergen immunotherapy. Curr Opin Allergy Clin Immunol. 2010;10:559–66. doi: 10.1097/ACI.0b013e32833ff2b2. [DOI] [PubMed] [Google Scholar]
- 120.Plaut M, Rotrosen D. Tolerance induced by allergen immunotherapy. Clin Allergy Immunol. 2004;18:681–702. [PubMed] [Google Scholar]


