Abstract
Background
TAK-438 (vonoprazan) is a potassium-competitive acid blocker that reversibly inhibits gastric H+, K+-ATPase.
Aim
To evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of TAK-438 in healthy Japanese and non-Japanese men.
Methods
In two Phase I, randomised, double-blind, placebo-controlled studies, healthy men (Japan N = 60; UK N = 48) received TAK-438 10–40 mg once daily at a fixed dose level for 7 consecutive days. Assessments included safety, tolerability, pharmacokinetics and pharmacodynamics (intragastric pH).
Results
Plasma concentration–time profiles of TAK-438 at all dose levels showed rapid absorption (median Tmax ≤2 h). Mean elimination half-life was up to 9 h. Exposure was slightly greater than dose proportional, with no apparent time-dependent inhibition of metabolism. There was no important difference between the two studies in AUC0-tau on Day 7. TAK-438 caused dose-dependent acid suppression. On Day 7, mean 24-h intragastric pH>4 holding time ratio (HTR) with 40 mg TAK-438 was 100% (Japan) and 93.2% (UK), and mean night-time pH>4 HTR was 100% (Japan) and 90.4% (UK). TAK-438 was well tolerated. The frequency of adverse events was similar at all dose levels and there were no serious adverse events. There were no important increases in serum alanine transaminase activity. Serum gastrin and pepsinogen I and II concentrations increased with TAK-438 dose.
Conclusions
TAK-438 in multiple rising oral dose levels of 10–40 mg once daily for 7 days was safe and well tolerated in healthy men and caused rapid, profound and sustained suppression of gastric acid secretion throughout each 24-h dosing interval. Clinicaltrials.gov identifiers: NCT02123953 and NCT02141711.
Introduction
Acid-related diseases, such as gastro-oesophageal reflux disease (GERD) and peptic ulcer disease, are important healthcare problems because of their high prevalence and chronic nature.1 They result from distinct, but overlapping, pathogenic mechanisms that typically involve the effects of acid on compromised mucosal defences in the gastrointestinal tract.2 Drug-induced gastric acid suppression is a key component of the management of acid-related disease.3
In acid-related diseases, treatment options and their outcome improved markedly with the advent of proton pump inhibitor (PPI) drugs, which remain the standard of care.1 However, acid-suppressive therapy with PPIs has limitations. For instance, up to 40% of GERD patients respond poorly to standard doses of PPI.4 Even in those who respond well, maximum efficacy is typically reached only after 3–5 days of standard dosing, and acid suppression remains incomplete.1,4,5 In patients with erosive oesophagitis, healing is often not achieved after 8 weeks of PPI therapy and relapses are common in healed patients on PPI maintenance regimens.6,7 Furthermore, PPIs may not control night-time intragastric acidity adequately in all GERD patients, and efficacy varies because of the influence of hepatic cytochrome P450 polymorphism.1,8–11 Finally, owing to a combination of their short half-lives and requirement for activation by acid, PPIs must be dosed in the fasting state, before meals, to be fully effective.11
Potassium-competitive acid blockers (P-CABs), a new class of acid-suppressing agents, are a potential alternative to PPIs for the treatment of acid-related diseases.5,11 Like PPIs, the P-CABs inhibit gastric H+, K+-adenosine triphosphatase (ATPase), an enzyme that catalyses the critical final step in gastric acid secretion.5,11 However, unlike PPIs, they inhibit the enzyme by reversible K+-competitive ionic binding (rather than irreversible covalent binding) and do not require acid activation within the parietal cell secretory canaliculus.5,11
TAK-438 (vonoprazan) is a novel, orally active P-CAB (synthesised by Takeda Pharmaceutical Company Ltd, Tokyo, Japan), which is currently in development as a treatment for acid-related diseases. Its structure lacks the imidazopyridine ring (common to some other P-CABs) that could be linked to liver enzyme elevation.12–14 In pre-clinical studies, TAK-438 caused more rapid, more profound and longer lasting acid suppression compared with PPIs or other prototype P-CABs.15–18 These characteristics appear to be related to accumulation in gastric glands.15–18
In Phase I, single-dose studies in healthy male volunteers, TAK-438 was well tolerated at all doses studied (1–120 mg) and produced rapid, profound and sustained suppression of gastric acid secretion in the 24 h after single doses in the range 20–120 mg.19 TAK-438 was also shown to have an elimination half-life of up to 9 h and its pharmacokinetics were unaffected by CYP2C19 genotype.19
In this article, we describe the results of two Phase I studies investigating the safety, tolerability, pharmacokinetics and pharmacodynamics of TAK-438 after multiple rising oral dose levels ranging from 10 to 40 mg once daily for 7 days in healthy adult male subjects in Japan and the UK. The studies were designed to aid the selection of doses of TAK-438 for investigation in Phase II studies in patients with acid-related diseases.
Subjects and methods
Study design
The two trials were prospective, randomised, double-blind, single-centre, ascending-dose, placebo-controlled Phase I studies to evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of TAK-438 given in repeated oral doses of 10, 15 (Japan only), 20, 30 and 40 mg once daily in healthy male subjects in Japan (five cohorts) and the UK (four cohorts). The Japanese study was conducted between 21 October 2008 and 16 March 2009, and the UK study between 31 October 2008 and 27 February 2009.
The doses in the studies were chosen on the basis of pharmacokinetic and pharmacodynamic data from two preceding single-dose escalation studies conducted in Japan and the UK.19
In both studies, each cohort comprised 12 subjects. Eligible subjects were sequentially block randomised to TAK-438 (n = 9) or matching placebo (n = 3) according to the randomisation schedule that had been generated by the study Sponsor. All randomisation information was stored in a secure area, accessible only by authorised personnel. Investigators and subjects were blinded to each subject's medication, and blinding was maintained throughout the studies. The pharmacokinetic analysis data, to be used for dose escalation, were blinded by the bioanalytical laboratory for provision to the study team. After randomisation, the subjects in each cohort were treated at a fixed dose level for 7 consecutive days (Days 1–7).
After a ≥10-h overnight fast, subjects took the dose with 150 mL (Japan) or 240 mL (UK) water at about 09:00 hours (Japan) or 08:00 hours (UK). Meals were given at 4, 10 and 13 h post-dose (Japan), and 4, 9 and 12 h post-dose (UK) throughout the treatment period. The starting dose was 10 mg TAK-438 and the decision to proceed to the next dose in the next cohort was made after review of blinded safety and pharmacokinetic data from the preceding cohort. The next cohort was dosed only if the preceding dose level had been well tolerated (both studies) and if the predicted mean exposure at the next dose level did not exceed the no-observed-adverse-effect level area under the plasma concentration–time curve (AUC) in dogs (UK criterion only). Placebo was administered using the same number of tablets required to achieve the TAK-438 dose within each cohort. The subjects were discharged on Day 8 (Japan) and Day 9 (UK), and returned for follow-up on Day 15 (Japan) and Day 16 (UK).
Both studies were reviewed and approved by their respective ethics committees and were conducted in accordance with the Good Clinical Practice guideline and all applicable local regulations. All participants gave written informed consent, in accordance with the 1996 Declaration of Helsinki, before their study participation. The studies are registered with https://www.clinicaltrials.gov, numbers NCT02123953 and NCT02141711.
Participants
Participants had to be healthy adult male Japanese subjects aged 20–45 years old (Japan) or Western non-Japanese subjects aged 18–45 years (UK), with a body mass index (BMI) 18.5–25.0 kg/m2 (Japan) or 18.0–30 kg/m2 (UK) and body weight ≥50 kg (Japan) at screening. Participants were excluded if they were smokers (UK criterion only); had acid-related disorders or a history of any such diseases, including reflux oesophagitis, gastric or duodenal ulcer, non-erosive GERD, Barrett's oesophagus and Zollinger–Ellison syndrome; had undergone upper gastrointestinal tract surgery or vagotomy in the past (Japan criterion only); had undergone Helicobacter (H.) pylori eradication within 6 months before first dosing of TAK-438 (Japan criterion) or had a positive H. pylori test result at screening (UK criterion); or showed hypoacidity or anacidity (pH ≥ 5.5) at baseline pH measurement (Japan criterion only).
During preliminary screening assessment, all participants were assessed for eligibility by inclusion/exclusion criteria, medical history (including tobacco, alcohol and caffeine use), medical examination (including height, body weight and BMI), urine drug toxicology and alcohol screening, 12-lead electrocardiogram (ECG) and H. pylori status using a serum antibody test in Japan or a urea breath test in the UK. Eligible volunteers proceeded to baseline screening (Japan from Day −3 to Day −1; UK from Day −2 to Day −1) and their eligibility was reassessed.
Pharmacokinetic measurements
Serial venous blood samples were collected in heparinised tubes. Plasma was separated by centrifugation at approximately 1500 g at 4 °C for 10 min, then frozen at −20 °C or lower until analysis. Total urine collections were made on Days 1 and 7 (Japan) or on Days 1–7 (UK). Urine aliquots were frozen at −20 °C or lower until analysis.
Plasma and urine concentrations of TAK-438 were determined using a validated liquid chromatography tandem mass spectrometry assay. The TAK-438 lower limit of quantification (LLOQ) was 0.1 ng/mL for plasma and 1 ng/mL for urine.
The main pharmacokinetic parameters included the maximum observed concentration (Cmax), time to reach Cmax (Tmax), area under the plasma concentration–time curve (AUC) from time 0 to time tau, where tau equals 24 h (AUC0–tau), AUC from time 0 to infinity (AUC0–inf), terminal elimination half-life (T½), apparent oral clearance (CL/F), accumulation factor R(AUC) and the accumulation index AI(AUC).
The main urinary pharmacokinetic parameter of TAK-438 was the fraction of drug excreted per 24 h (%Fe).
Pharmacodynamic measurements
The subjects underwent 24-h intragastric pH monitoring at baseline and during the treatment phase [Japan: Days −1, 1 and 7; UK: Days −1, 1, 4 and 7 (48 h)]. Intragastric pH was measured continuously with a pH probe (CM-181; Chemical Instruments, Tokyo, Japan in the Japanese study, or Zinetics Medical, Salt Lake City, UT, USA in the UK study) inserted into the stomach and its position confirmed by X-ray (Japan), or a sharp rise or fall in pH identified the point at which the sensor crossed the sphincter (UK).
Intragastric pH was recorded with a one-channel pH meter (101ZG; Chemical Instruments) or with a Flexilog 2020 ambulatory pH monitor (Oakfield Instruments, Oxfordshire, UK). The primary pharmacodynamic endpoint was the percentage of total time that pH was >4 [pH >4 holding time ratio (HTR)] and pH was >5 (pH >5 HTR), calculated from the intragastric pH in the 24 h after dosing. The night-time pH HTR was defined as the percentage of time pH >4 and pH >5 during the period 12–24 h post-dose (Japan) or 20:00–08:00 hours (UK).
Safety assessments
Spontaneous reports of adverse events were collected throughout the studies from initial screening until follow-up and were assessed for severity and relationship with the study drug. Clinical laboratory tests [serum chemistry, including serum alanine transaminase (ALT) and aspartate transaminase (AST), haematology and urinalysis], medical examination (including body weight) and vital signs were also performed from initial screening to follow-up.
Triplicate 12-lead ECGs were recorded daily up to Day 8 (Japan) and Day 9 (UK) and also at follow-up on Day 15 (Japan) and Day 16 (UK).
In each study, serial blood samples for serum gastrin and pepsinogen I and II assay were taken on Days 1–7 (Japan) and Days 1–9 (UK) and at follow-up (Japan only). Serum gastrin concentrations were measured using a gastrin kit TFB Co., Ltd. at Medichem Business Division, Tokyo, Japan, or L2KGA2 (Siemens) on a Siemens Immulite 2000 analyzer at HMR Analytical Laboratory, London, UK. Serum pepsinogen I and II concentrations were measured using ARCHITECT Pepsinogen I (Abbott Japan Co., Ltd., Tokyo, Japan) and ARCHITECT Pepsinogen II (Abbott Japan Co., Ltd.) on an ARCHITECT analyzer i2000 at Medichem Business Division, Tokyo, Japan or Pepsinogen I (601020.01; Biohit Healthcare, Helsinki, Finland) and Pepsinogen II (601020.02; Biohit Healthcare) ELISA kits on a Grifols Triturus analyzer at HMR Analytical Laboratory, London, UK. In the Japanese study, blood was also collected at 3 h after first dosing for evaluation of CYP2C19 genotype using an Invader assay to detect G681A (*2) and G636A (*3) of CYP2C19 (conducted by Mitsubishi Chemical Medience Corporation, Tokyo, Japan).
Statistical analysis
No formal sample-size calculation was performed. The planned sample sizes (N = 60 in the Japanese study; N = 48 in the UK study) were based on safety considerations and study drug exposure, and were considered adequate for the evaluation of the planned pharmacokinetic and pharmacodynamic endpoints.
The safety analysis set comprised all subjects who received at least one dose of the study drug on Day 1. The pharmacokinetic analysis set comprised all subjects who received the study drug and who had sufficient plasma concentration data to calculate at least one pharmacokinetic parameter. The pharmacodynamic analysis set comprised all subjects who received the study drug on Day 1 and who had sufficient pharmacodynamic data to derive at least one pharmacodynamic parameter.
In the Japanese study, intragastric pH data were recorded every 10 s from 08:30 to 09:10 hours of the following day, then the electrode was removed and its calibration checked using standard pH 4 and pH 7 solutions. The arithmetic mean intragastric pH was calculated at each time point during each 24-h period. In the UK study, data were collected by the pH monitor every 6 s. Post-dose measurements were calculated from the time of dosing. The average pH value was calculated by subject from the data collected over the 24- or 48-h monitoring period. For each 15-min interval, the median pH, mean pH and the standard deviation (s.d.) of the mean pH during the 15-min interval were calculated.
Linear and semilogarithmic graphs of the mean and individual plasma and urine concentration–time curves on Days 1 and 7 were plotted. Pharmacokinetic parameters were derived by noncompartmental analysis (WinNonlin V 5.2; Pharsight Corporation, Cary, NY, USA).
A power model20 with a fixed effect for regional effect (Japanese vs. non-Japanese) was used to investigate dose proportionality and assess potential regional differences on Day 7.
The dose–response relationships for pH >4 HTR, pH >5 HTR and mean pH were assessed by analysis of covariance (ancova)/analysis of variance (anova), with the baseline-adjusted pharmacodynamic parameters as dependent covariables/variables and dose group as a fixed effect. Least squares (LS) means were calculated for each TAK-438 dose level and the differences between dose levels on each day. The residual variance from the anova was used to calculate 95% confidence intervals (CI) for the mean differences between the dose levels, which were then back-transformed to provide geometric LS means, point estimates and 95% CI for the ratio of dose levels.
Descriptive statistics were used to summarise relevant pharmacokinetic parameters, pH HTRs, mean pH and serum concentrations of gastrin and pepsinogen I and II in each treatment group.
Safety data were summarised by descriptive statistics and by figures or scatter plots. Descriptive statistics were also used to summarise vital signs, clinical laboratory test results and 12-lead ECG findings in each treatment group.
All statistical analyses were performed by using the sas system, version 8.2 or higher (SAS Institute Inc., Cary, NC, USA).
Results
Study population
A total of 60 subjects received treatment and completed the Japanese study [mean age 27 ± 6.3 years; mean body weight 62 ± 7.0 kg; mean BMI 21 ± 1.5 kg/m2 (range 19–24 kg/m2)] and 48 subjects [41 Caucasians, 5 Asians (excluding Japanese) and 2 Black or African Americans] received treatment and completed the UK study [mean age 28 ± 7.0 years; mean body weight 75 ± 7.6 kg; mean BMI 24 ± 2.4 kg/m2 (range 19–29 kg/m2)]. In both studies, the safety and pharmacodynamic analyses sets included all subjects and the pharmacokinetic analysis set included all subjects who received TAK-438.
The demographic and baseline characteristics of the subjects in both studies are shown in Table S1. As expected, the subjects in the UK study had higher mean body weight than those in the Japanese study.
All subjects in the UK study, and those in the placebo and TAK-438 30 mg treatment groups in the Japanese study, were H. pylori antibody negative. In the Japanese study, H. pylori antibodies were positive in one subject each in the 10, 15 and 20 mg treatment groups and in three subjects in 40 mg treatment group. No subject had previously had any serious gastrointestinal illness or surgery, nor did any subject have gastric hypoacidity.
Pharmacokinetic data
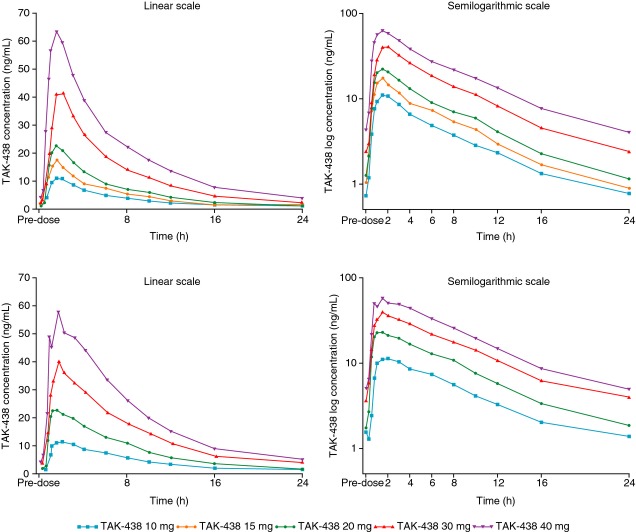
At all dose levels, the plasma TAK-438 concentration–time profiles showed rapid absorption, with median Tmax ≤2 h under fasting conditions. Mean elimination T½ ranged from 5.7 h on Day 1 to 7.0 h on Day 7 (Japan) and from 6.1 h on Day 1 to 8.8 h on Day 7 (UK; Figure1; Table 1). T½ and Tmax for TAK-438 were independent of dose. Pharmacokinetic parameters for each dose were similar on Days 1 and 7 (Table 1).
Figure 1.
Time course of mean plasma TAK-438 concentrations in the Phase I, randomised, double-blind, placebo-controlled, repeated-dose Japanese (top panel) and UK (bottom panel) studies in healthy male subjects receiving TAK-438 10–40 mg once daily at a fixed dose level for 7 consecutive days (Pharmacokinetic analysis set: Japan N = 60; UK N = 48).
Table 1.
Pharmacokinetic parameters of plasma TAK-438 on Days 1 and 7 of dosing with TAK-438 10–40 mg once daily at a fixed dose level for 7 consecutive days in healthy male subjects in Japanese and UK studies (Pharmacokinetic analysis set: Japan N = 60; UK N = 48)
| Parameter | 10 mg (n = 9) | 15 mg (n = 9) | 20 mg (n = 9) | 30 mg (n = 9) | 40 mg (n = 9) |
|---|---|---|---|---|---|
| Japanese study | |||||
| Cmax (ng/mL) | |||||
| Day 1 | 10.1 ± 2.0 | 16.1 ± 4.8 | 19.5 ± 6.1 | 38.8 ± 16.7 | 62.0 ± 24.9 |
| Day 7 | 12.0 ± 1.8 | 18.1 ± 5.8 | 23.3 ± 6.6 | 48.6 ± 17.4 | 75.2 ± 25.3 |
| Tmax (h) | |||||
| Day 1 | 1.50 (0.75–3.00) | 2.00 (1.00–3.00) | 1.50 (1.50–3.00) | 1.50 (1.00–3.00) | 1.50 (0.75–2.00) |
| Day 7 | 1.50 (0.75–3.00) | 1.50 (0.75–2.00) | 1.50 (0.75–3.00) | 1.50 (1.00–2.00) | 1.50 (0.75–3.00) |
| AUC0–tau (ng·h/mL) | |||||
| Day 1 | 61.6 ± 13.5 | 97.5 ± 33.5 | 121.6 ± 32.8 | 231.3 ± 72.6 | 391.6 ± 176.1 |
| Day 7 | 79.5 ± 16.1 | 112.4 ± 35.6 | 151.6 ± 40.3 | 291.2 ± 101.2 | 458.5 ± 151.7 |
| AUC0–inf (ng·h/mL) | |||||
| Day 1 | 67.4 ± 14.7 | 103.3 ± 36.3 | 129.3 ± 34.9 | 247.4 ± 79.1 | 429.8 ± 205.3 |
| T½ (h) | |||||
| Day 1 | 7.0 ± 1.9 | 5.8 ± 0.7 | 5.8 ± 1.0 | 5.7 ± 0.7 | 6.7 ± 1.8 |
| Day 7 | 7.0 ± 1.6 | 6.0 ± 0.9 | 6.1 ± 1.2 | 5.8 ± 0.6 | 6.1 ± 1.1 |
| CL/F (L/h) | |||||
| Day 1 | 156.4 ± 42.3 | 162.3 ± 56.8 | 166.0 ± 48.2 | 131.0 ± 35.8 | 113.6 ± 52.7 |
| Day 7 | 131.3 ± 32.1 | 145.6 ± 44.6 | 140.5 ± 36.8 | 113.8 ± 36.4 | 96.0 ± 32.2 |
| R(AUC) | |||||
| Day 7 | 1.3 ± 0.1 | 1.2 ± 0.2 | 1.3 ± 0.2 | 1.3 ± 0.2 | 1.2 ± 0.2 |
| AI(AUC) | |||||
| Day 7 | 1.2 ± 0.1 | 1.1 ± 0.1 | 1.2 ± 0.2 | 1.2 ± 0.2 | 1.1 ± 0.2 |
| UK study | |||||
| Cmax (ng/mL) | |||||
| Day 1 | 10.9 ± 5.7 | 26.2 ± 14.8 | 37.2 ± 11.9 | 58.5 ± 10.9 | |
| Day 7 | 12.2 ± 4.0 | 26.2 ± 10.7 | 41.6 ± 11.0 | 59.9 ± 15.4 | |
| Tmax (h) | |||||
| Day 1 | 1.50 (0.75–3.00) | 1.50 (0.75–2.00) | 1.50 (0.75–4.00) | 1.50 (0.75–2.02) | |
| Day 7 | 1.50 (1.00–2.00) | 1.10 (0.75–2.00) | 1.50 (1.10–4.00) | 1.50 (0.75–4.00) | |
| AUC0–tau (ng·h/mL) | |||||
| Day 1 | 81.4 ± 31.4 | 177.1 ± 96.1 | 255.5 ± 53.2 | 420.8 ± 107.5 | |
| Day 7 | 104.9 ± 41.5 | 195.7 ± 66.0 | 338.5 ± 83.8 | 488.4 ± 130.9 | |
| AUC0–inf (ng·h/mL) | |||||
| Day 1 | 93.4 ± 38.9 | 195.3 ± 107.1 | 277.4 ± 58.5 | 458.5 ± 120.7 | |
| T½ (h) | |||||
| Day 1 | 7.5 ± 1.3 | 6.9 ± 1.6 | 6.1 ± 0.4 | 6.3 ± 0.6 | |
| Day 7 | 8.8 ± 3.0 | 8.6 ± 1.9 | 8.8 ± 1.2 | 8.2 ± 0.8 | |
| CL/F (L/h) | |||||
| Day 1 | 128.0 ± 61.3 | 127.0 ± 56.4 | 112.8 ± 26.1 | 93.6 ± 28.3 | |
| Day 7 | 113.0 ± 52.5 | 113.1 ± 38.9 | 95.6 ± 33.1 | 87.7 ± 25.1 | |
| R(AUC) | |||||
| Day 7 | 1.3 ± 0.2 | 1.2 ± 0.3 | 1.3 ± 0.2 | 1.2 ± 0.1 | |
| AI(AUC) | |||||
| Day 7 | 1.1 ± 0.2 | 1.1 ± 0.3 | 1.2 ± 0.2 | 1.1 ± 0.1 | |
All data are represented as mean ± s.d., except for Tmax values (median ± range).
AI, accumulation index; AUC, area under the plasma concentration–time curve; AUC0–inf, AUC from time 0 to infinity; AUC0–tau, AUC from time 0 to time tau, where tau equals 24 h; Cmax, maximum observed concentration; CL/F, apparent oral clearance; R, accumulation factor R; s.d., standard deviation; T½, terminal elimination half-life; Tmax, time to reach Cmax.
Mean AUC0–inf and Cmax on Day 7 increased slightly more than dose proportionally over the range 10–40 mg. Dose proportionality of Cmax and AUC0–tau was compared between the Japanese and UK subjects using a power model. There were no significant differences between Japanese and non-Japanese patients, as the two-sided 95% CI of region effect for both slope and y-intercept after logarithmic transformation included zero in the case of both Cmax [−0.422 to 0.123 (slope) and −0.351 to 0.340 (y-intercept)] and AUC0–tau [−0.383 to 0.142 (slope) and −0.058 to 0.609 (y-intercept)] (Figure S1).
Mean AI(AUC) was 1.1–1.2 in both studies, so only minor accumulation of TAK-438 occurred during repeated dosing (Table 1).
An exploratory analysis of the Japanese data compared dose-normalised AUC0–tau on Day 7 among the different CYP2C19 genotypes (Figure S2). No correlation was found between CYP2C19 genotype (*1/*1, *1/*2, *1/*3, *2/*2, *2/*3, *3/*3; Figure S2) and AUC0–tau.
Urinary excretion of unchanged TAK-438 in the 24 h after dosing on Day 7 was low after all doses. The fraction of dose excreted (%Fe) was 4.0–6.3% in Japan and 4.0–4.4% in the UK.
Pharmacodynamic data
The pharmacodynamics results were similar in the Japanese and UK studies.
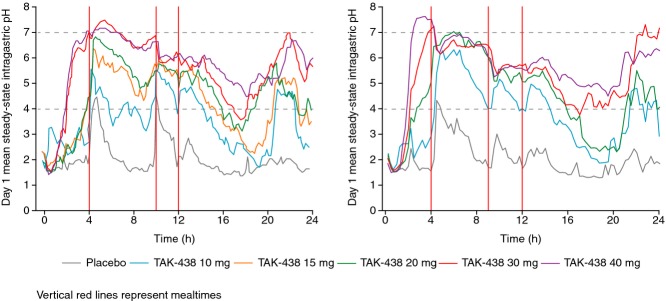
On Day 1, there was dose-dependent elevation in mean intragastric pH (Figure2). The onset of the increase in pH was rapid – at all dose levels, mean intragastric pH was >4.0 by 4 h after the first dose. The rapidity of onset was dose dependent, with the higher doses clearly having an earlier effect on pH than the lower doses did. The acid suppressant effect of TAK-438 persisted throughout the 24-h interval before the second dose on Day 2, whereas, pre-dose pH on Day 1 was approximately 2.0, the corresponding pH values on Day 2 showed dose-dependent increases compared with placebo. In both studies, the normal tendency for intragastric pH to fall during sleep was attenuated by TAK-438 in a strongly dose-dependent fashion.
Figure 2.
Mean intragastric pH on Day 1 in healthy male subjects after a single dose of TAK-438 10–40 mg after overnight fasting in the Japanese (left panel) and UK (right panel) studies (Pharmacodynamic analysis set: Japan N = 60; UK N = 48).
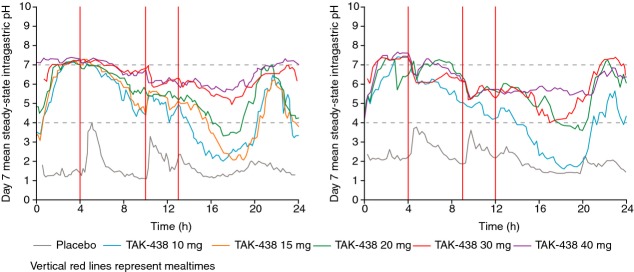
The mean intragastric pH–time profiles on Day 7 of repeated dosing with TAK-438 showed that pH before dosing and in the first 4 h afterwards were higher than at the corresponding time points on Day 1 – the increase in pH was strongly dose dependent (Figures2 and 3). After the 20, 30 and 40 mg doses of TAK-438, intragastric pH during the 12-h night-time period tended to be higher on Day 7 than on Day 1, particularly in the Japanese study; after the 10 and 15 mg dose (Japanese study only), intragastric pH during the night-time period on Day 7 remained as high as it had been on Day 1, but did not increase further (Figures4; Table 2).
Figure 3.
Mean steady-state intragastric pH vs. time profiles after TAK-438 on Day 7 in the Japanese (left) and UK studies (right) in healthy male subjects receiving TAK-438 10–40 mg once daily after overnight fasting at a fixed dose level for 7 consecutive days (Pharmacodynamic analysis set: Japan N = 60; UK N = 48).
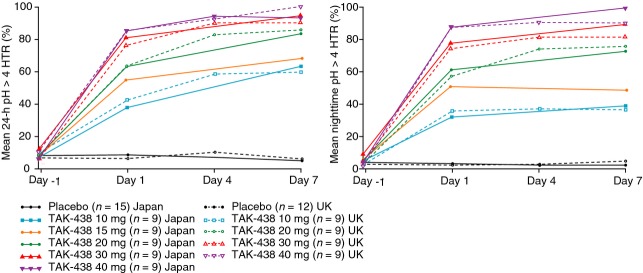
Figure 4.
Mean 24-h (left panel) and night-time (12–24 h post dose in the Japanese study, 20:00–08:00 hours in the UK study; right panel) pH> 4 HTR in healthy male subjects receiving TAK-438 10–40 mg once daily at a fixed dose level for 7 consecutive days (Pharmacodynamic analysis set: Japan N = 60; UK N = 48).
Table 2.
Dose–response relationship for mean 24-h intragastric pH >4 and >5 holding time ratio [HTR (%)] during the 24-h dosing interval and during the night-time period in healthy male subjects who received TAK-438 10–40 mg once daily at a fixed dose level for 7 consecutive days in Japanese and UK studies (Pharmacodynamic analysis set: Japan N = 60; UK N = 48)
| TAK-438 |
||||||
|---|---|---|---|---|---|---|
| Placebo | 10 mg | 15 mg | 20 mg | 30 mg | 40 mg | |
| Japanese study | ||||||
| n | 15 | 9 | 9 | 9 | 9 | 9 |
| 24-h pH >4 HTR (%) | ||||||
| Day 1 | 8.3 ± 4.0 | 38.4 ± 22.3 | 55.4 ± 13.2 | 63.3 ± 17.9 | 80.8 ± 14.0 | 85.3 ± 8.3 |
| Day 7 | 5.6 ± 3.5 | 63.3 ± 8.7 | 68.5 ± 16.1 | 83.4 ± 16.7 | 95.2 ± 10.1 | 100.0 ± 0.0 |
| 24-h pH >5 HTR (%) | ||||||
| Day 1 | 3.7 ± 2.4 | 25.1 ± 19.0 | 40.3 ± 16.8 | 53.5 ± 21.5 | 73.1 ± 16.1 | 78.3 ± 10.6 |
| Day 7 | 1.5 ± 1.5 | 52.6 ± 10.7 | 60.2 ± 16.8 | 73.2 ± 18.9 | 92.0 ± 12.5 | 98.6 ± 2.0 |
| Night-time pH >4 HTR (12–24 h post-dose) (%) | ||||||
| Day 1 | 3.0 ± 3.8 | 32.4 ± 18.7 | 50.5 ± 18.6 | 61.1 ± 29.3 | 78.1 ± 24.8 | 86.5 ± 15.5 |
| Day 7 | 2.0 ± 2.4 | 39.0 ± 13.1 | 48.8 ± 22.2 | 73.0 ± 26.5 | 90.4 ± 20.1 | 100 ± 0.1 |
| Night-time pH >5 HTR (12–24 h post-dose) (%) | ||||||
| Day 1 | 1.0 ± 1.6 | 18.6 ± 14.3 | 30.4 ± 19.2 | 47.9 ± 31.8 | 64.5 ± 29.3 | 73.6 ± 19.6 |
| Day 7 | 0.3 ± 0.6 | 27.1 ± 12.9 | 37.2 ± 19.4 | 55.9 ± 27.5 | 84.5 ± 24.2 | 97.2 ± 4.0 |
| UK study | ||||||
| n | 12 | 9 | 9 | 9 | 9 | |
| 24-h pH >4 HTR (%) | ||||||
| Day 1 | 6.2 ± 3.2 | 43.1 ± 21.2 | 62.7 ± 16.8 | 76.8 ± 9.9 | 85.6 ± 7.4 | |
| Day 4 | 10.5 ± 10.6 | 58.9 ± 20.9 | 82.9 ± 14.7 | 90.2 ± 9.2 | 94.0 ± 9.1 | |
| Day 7 | 6.5 ± 4.5 | 60.2 ± 19.1 | 85.2 ± 12.3 | 90.1 ± 7.9 | 93.2 ± 10.5 | |
| 24-h pH >5 HTR (%) | ||||||
| Day 1 | 2.6 ± 2.0 | 31.5 ± 20.8 | 49.2 ± 19.9 | 64.8 ± 15.4 | 73.1 ± 11.0 | |
| Day 4 | 6.7 ± 10.6 | 43.4 ± 18.5 | 75.3 ± 19.3 | 81.5 ± 11.6 | 88.6 ± 11.9 | |
| Day 7 | 3.2 ± 3.2 | 49.5 ± 15.8 | 78.6 ± 14.0 | 79.9 ± 13.7 | 85.0 ± 21.7 | |
| Night-time pH >4 HTR (20:00–08:00 hours) (%) | ||||||
| Day 1 | 2.1 ± 2.1 | 36.3 ± 21.2 | 57.3 ± 23.2 | 74.4 ± 17.3 | 87.7 ± 11.4 | |
| Day 4 | 2.8 ± 3.9 | 37.5 ± 19.2 | 73.8 ± 22.4 | 81.2 ± 18.2 | 91.0 ± 14.5 | |
| Day 7 | 4.8 ± 5.3 | 36.9 ± 16.4 | 75.4 ± 20.6 | 81.3 ± 15.2 | 90.4 ± 14.8 | |
| Night-time pH >5 HTR (20:00–08:00 hours) (%) | ||||||
| Day 1 | 1.1 ± 1.3 | 24.2 ± 20.8 | 37.8 ± 27.4 | 55.2 ± 24.5 | 65.6 ± 18.3 | |
| Day 4 | 1.0 ± 2.0 | 17.9 ± 10.8 | 61.6 ± 29.3 | 68.4 ± 21.2 | 84.1 ± 18.2 | |
| Day 7 | 2.2 ± 3.4 | 22.8 ± 10.9 | 66.9 ± 22.2 | 65.9 ± 26.5 | 77.5 ± 32.5 | |
All data are represented as mean ± s.d. HTR, holding time ratio; s.d., standard deviation.
The 24-h HTR increased dose dependently and similarly in both studies.
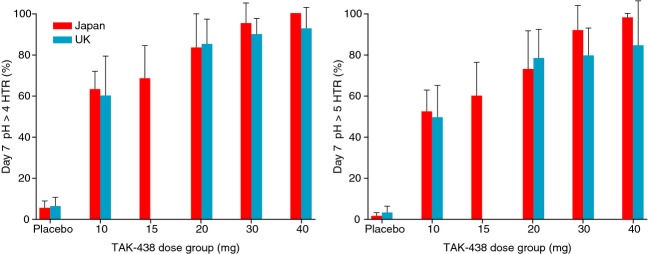
Mean pH >4 and pH >5 HTR after the 40 mg dose on Day 1 were 85.3% and 78.3% respectively (Japan) and 85.6% and 73.1% respectively (UK). Corresponding results for mean pH >4 and pH >5 HTR on Day 4 were 94.0% and 88.6% respectively (UK only) and on Day 7 were 100% and 98.6% respectively (Japan) and 93.2% and 85.0% respectively (UK) (Figure5; Table 2).
Figure 5.
Dose–response for pH >4 HTR (left) and pH>5 HTR (right) on Day 7 in the Japanese and UK studies in healthy male subjects receiving TAK-438 10–40 mg once daily at a fixed dose level for 7 consecutive days (Pharmacodynamic analysis set: Japan N = 60; UK N = 48).
Night-time acid suppression was greatest during the 40 mg dose regimen: mean pH >4 and pH >5 HTR from 12 to 24 h post-dose on Day 1/7 were 86.5%/100.0% and 73.6%/97.2% respectively, in the Japanese study and from 20:00 to 08:00 hours on Day 1/4/7 were 87.7%/91.0%/90.4% and 65.6%/84.1%/77.5% respectively, in the UK study (Figure4).
Safety
Nine of 60 subjects in the Japanese study experienced one or more treatment-emergent adverse events [one event each of increased serum uric acid (placebo), increased neutrophil count (placebo), increased serum triglycerides (TAK-438 15 mg), decreased white blood cell count (TAK-438 15 mg), a fall (TAK-438 15 mg)], influenza (TAK-438 20 mg), nasopharyngitis (TAK-438 30 mg), increased eosinophil count (TAK-438 30 mg) and pharyngitis (TAK-438 40 mg). Ten of 48 subjects in the UK study experienced one or more treatment-emergent adverse events [four events of headache (one with placebo, one with TAK-438 10 mg and two with TAK-438 20 mg), three of abdominal pain (one with placebo and two with TAK-438 10 mg), two of oropharyngeal pain (one with placebo and one with TAK-438 30 mg) and one each of cough (placebo), nasal congestion (placebo), contact dermatitis (placebo), nasopharyngitis (TAK-438 20 mg), toothache (TAK-438 30 mg), oral herpes (TAK-438 30 mg) and neck pain (TAK-438 30 mg)], with no relationship between dose and the incidence of adverse events. There were no serious adverse events in either study.
There were no abnormal changes in urinalysis, vital signs, medical examination or ECG. In the Japanese study, there was one occurrence each of increased serum uric acid and one of increased neutrophil count (placebo), one each of increased serum triglycerides and decreased white blood cell count (TAK-438 15 mg) and one of increased eosinophil count (TAK-438 30 mg). These were recorded as adverse events (see above). In the UK study, there were no clinically relevant changes from baseline in any laboratory value from Days 2 to 9 at any dose level of TAK-438. There were no clinically important increases in ALT, AST or total bilirubin in either study. ALT, AST and bilirubin baseline data and changes from baseline on Day 8 are given in Table S2.
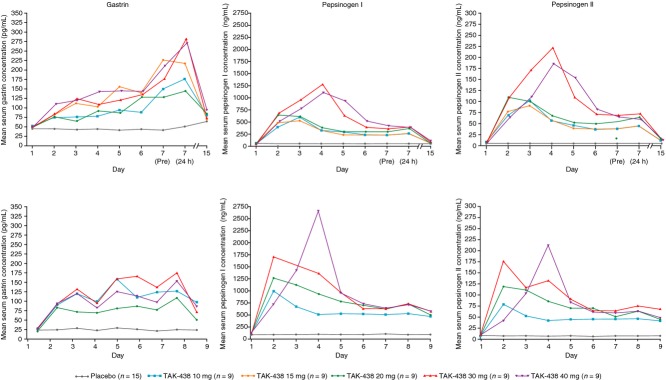
Serum concentrations of gastrin and pepsinogen I and II increased in both studies during treatment with TAK-438 at all dose levels studied (Figure6); however, there was no clear dose–response relationship for this treatment-related effect. Mean concentrations of gastrin, but not of pepsinogen I or II, were approximately twofold higher in the Japanese subjects than in the UK subjects, both at baseline and after repeated dosing with TAK-438. In the UK study, gastrin and pepsinogen I and II concentrations remained elevated at 24 and 48 h after the final dose. In the Japanese study, all three analytes had returned to baseline levels by the follow-up visit on Day 15 or 16.
Figure 6.
Time course of mean serum gastrin (left), pepsinogen I (middle) and pepsinogen II (right) concentrations in healthy male subjects receiving TAK-438 10–40 mg once daily at a fixed dose level for 7 consecutive days in the Japanese (top three panels) and UK (bottom three panels) studies (Pharmacodynamic analysis set: Japan N = 60; UK N = 48).
Discussion
We evaluated the repeated-dose safety, tolerability, pharmacokinetics and pharmacodynamics of TAK-438, a novel P-CAB, in healthy adult male subjects in two double-blind, placebo-controlled studies in Japan and the UK. As the results of both studies were very similar, they are reviewed together in this section. TAK-438 was safe and well tolerated in multiple rising dose levels of 10–40 mg once daily for 7 days.
Median Tmax of TAK-438 was ≤2 h and estimated median T½ was ≤9 h. Plasma concentrations of TAK-438 increased slightly more than dose proportionally. The mean AUC of TAK-438 increased between Days 1 and 7, but AI was <1.3 for all doses and so the accumulation was minor and unlikely to be clinically relevant. As noted in a previous single-dose study, the pharmacokinetics of TAK-438 were not sensitive to CYP2C19 polymorphism.19
The intragastric pH–time profiles after the first dose of TAK-438 showed a rapid onset of acid suppression that persisted for the entire 24-h monitoring interval at all dose levels studied, and there was a clear dose–response relationship for mean pH ≥4 and pH ≥5 HTR. Profound acid suppression was achieved by the 40 mg dose level: pH >4 HTR was >85% on Day 1, 94% on Day 4 (UK only) and >93% on Day 7. pH>5 HTR was >73% on Day 1, 89% on Day 4 (UK only) and ≥85% on Day 7. Of particular note, the pH >4 HTR during night-time on Days 1, 4 and 7 at the 40 mg dose was ≥87%, 91% (UK only) and >90% respectively.
Intragastric pH >4 HTR is a widely accepted predictor of efficacy of treatments for GERD.21,22 In models based on clinical trial data from patients with erosive oesophagitis using a range of acid-suppressing drugs with different mechanisms of action, pH >4 HTR has been shown to be a good predictor of healing at 4 or 8 weeks.23 Based on those models, a 100% rate of healing would be predicted after 4 weeks with a pH >4 HTR of ≥90% or after 8 weeks with a pH >4 HTR of ≥75%.23
In both studies, the normal tendency for intragastric pH to fall at times when nocturnal acid breakthrough would be expected in healthy people and in those with GERD (22:00–06:00 hours)24 was attenuated by TAK-438 in a strongly dose-dependent fashion (Figures2 and 3).
As regards the time dependence of the effect on intragastric pH, the suppression of gastric acidity tended to increase from Day 1 to Day 7 at the 20, 30 and 40 mg dose levels, and was fully maintained on Day 7 at the 10 and 15 mg dose levels. Thus, there was no evidence of the development of tolerance to TAK-438.
Serum gastrin concentrations in these studies were similar to those seen during PPI therapy.25,26 The higher concentrations in the Japanese subjects are unexplained, but might simply reflect random variation; the effect of treatment on gastrin concentration was not a prior hypothesis. Pepsinogen I and II concentrations did not indicate corpus atrophy in any subject at any dose level. Importantly, TAK-438 did not increase serum ALT levels, probably because its chemical structure differs significantly from other P-CABs, such as AZD0865, which increased ALT in some patients with non-erosive reflux disease.12
The steady-state 24-h pH HTRs in our studies (mean pH >4 HTR for the 40 mg dose on Day 7: Japan, ∼100%; UK, ∼93%) suggest that TAK-438 at the 40 mg dose level may cause more profound gastric acid suppression than that reported after PPIs.26 Thus, the results reported here suggest that TAK-438 could surpass the best currently available gastric acid-reducing treatments in terms of early healing rates and fewer severe cases of erosive oesophagitis.
Although the two studies reported here were conducted in healthy male subjects, the rapid and sustained inhibition of gastric acid secretion by TAK-438 justifies the further investigation of its efficacy in the treatment of patients with acid-related disorders. The results were used to guide the choice of doses for evaluation in a Phase II trial of the efficacy and safety of TAK-438 5–40 mg compared with lansoprazole 30 mg in patients with erosive oesophagitis.27 The current profile suggests that TAK-438 might have advantages over existing acid-suppressing drugs, such as maximum efficacy after the first dose, prevention of nocturnal acid breakthrough, reduction in night-time gastric acidity and the ability to dose at any time regardless of meals.
In conclusion, in these two Phase I studies in healthy male subjects, TAK-438 at multiple rising dose levels of 10–40 mg once daily for 7 days produced rapid, profound and sustained suppression of gastric acid secretion and was well tolerated. The intragastric pH HTRs on Day 7 showed good pH control throughout the 24-h dosing interval (pH >4 HTR >83%), including during the night-time period (pH >4 HTR ≥73%) at the clinically recommended dose of 20 mg, with even greater acid suppression observed with daily doses >20 mg. The results justify further investigation of TAK-438 in patients with acid-related disorders.
Authorship
Guarantor of the article: Helen Jenkins.
Author contributions: Akira Nishimura, Richard Jenkins, Mark Hibberd, Kiyoshi Ashida, Yuuichi Sakurai and Helen Jenkins were involved in the study concept and design. Tomoki Yoneyama was involved with the bioanalytical methodology. Yoichiro Ogama and Steve Warrington conducted the studies. Yuuichi Sakurai and Hiryoyuki Okamoto were involved in the statistical analysis. Yuuichi Sakurai, Richard Jenkins, Helen Jenkins and Steve Warrington were involved in the drafting and critical revision of the manuscript. All authors approved the final version of this manuscript.
Acknowledgments
Writing support was provided by Hiroaki Itoh of Interface, Kanagawa, Japan and funded by Takeda Pharmaceutical Company Ltd and by Susan Crawford of Absolute Healthcare Communications, London, UK and funded by Takeda Pharmaceuticals International, Inc.
Declaration of personal interests: Kiyoshi Ashida is a paid consultant to Takeda Pharmaceutical Company Ltd. Akira Nishimura, Yuuichi Sakurai and Hiryoyuki Okamoto are employees of Takeda Pharmaceutical Company Ltd. Mark Hibberd, Richard Jenkins and Helen Jenkins are employees of Takeda Development Centre Europe Ltd, London, UK. Yoichiro Ogama is an employee of Medical Co. LTA Honjo Clinic (current Sumida Hospital), Tokyo, Japan, which received funding for the Japanese study. Steve Warrington is an employee of Hammersmith Medicines Research, London, UK, which received funding for the UK study.
Declaration of funding interests: These studies were funded in full by Takeda Pharmaceutical Company Ltd, Tokyo, Japan, and Takeda Development Centre Europe Ltd, London, UK.
SUPPORTING INFORMATION
Table S1. Demographic and baseline characteristics of healthy male subjects receiving TAK-438 10–40 mg once daily at a fixed dose level for 7 consecutive days in Japanese and UK studies (Safety analysis set: N = 60; UK N = 48).
Table S2. Bilirubin, AST and ALT at baseline, and change from baseline on Day 7/8 for placebo and TAK-438 in healthy male subjects receiving TAK-438 10–40 mg once daily at a fixed dose level for 7 consecutive days in Japanese and UK studies (Safety analysis set: Japan N = 60; UK N = 48).
Figure S1. Dose proportionality of AUC0–tau (left) and Cmax (right) on Day 7 for TAK-438 in Japanese and UK repeated-dose studies in healthy male subjects receiving TAK-438 10–40 mg once daily at a fixed dose level for 7 consecutive days (Pharmacokinetic analysis set: Japan N = 60; UK N = 48, each point represents a single subject).
Figure S2. Relationship between CYP2C19 genotypes and dose-normalised AUC0–tau of TAK-438 in healthy male subjects receiving TAK-438 10–40 mg once daily at a fixed dose level for 7 consecutive days in the Japanese study (Pharmacokinetic analysis set: Japan N = 60; UK N = 48).
References
- 1.Mejia A, Kraft WK. Acid peptic diseases: pharmacological approach to treatment. Expert Rev Clin Pharmacol. 2009;2:295–314. doi: 10.1586/ecp.09.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Modlin IM, Sachs G. Acid Related Diseases: Biology and Treatment. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2004. [Google Scholar]
- 3.Shin JM, Vagin O, Munson K, et al. Molecular mechanisms in therapy of acid-related diseases. Cell Mol Life Sci. 2008;65:264–81. doi: 10.1007/s00018-007-7249-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cicala M, Emerenziani S, Guarino MPL, Ribolsi M. Proton pump inhibitor resistance, the real challenge in gastro-esophageal reflux disease. World J Gastroenterol. 19:6529–35. doi: 10.3748/wjg.v19.i39.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sachs G, Shin JM, Vagin O, et al. The gastric H, K ATPase as a drug target: past, present, and future. J Clin Gastroenterol. 2007;41(Suppl. 2):S226–42. doi: 10.1097/MCG.0b013e31803233b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vakil NB, Shaker R, Johnson DA, et al. The new proton pump inhibitor esomeprazole is effective as a maintenance therapy in GERD patients with healed erosive oesophagitis: a 6-month, randomized, double-blind, placebo-controlled study of efficacy and safety. Aliment Pharmacol Ther. 2001;15:927–35. doi: 10.1046/j.1365-2036.2001.01024.x. [DOI] [PubMed] [Google Scholar]
- 7.Fennerty MB, Johanson JF, Hwang C, et al. Efficacy of esomeprazole 40 mg vs. lansoprazole 30 mg for healing moderate to severe erosive oesophagitus. Aliment Pharmacol Ther. 2005;21:455–63. doi: 10.1111/j.1365-2036.2005.02339.x. [DOI] [PubMed] [Google Scholar]
- 8.Scarpignato C, Pelosini I. Review article: the opportunities and benefits of extended acid suppression. Aliment Pharmacol Ther. 2006;23(Suppl. 2):23–34. doi: 10.1111/j.1365-2036.2006.02945.x. [DOI] [PubMed] [Google Scholar]
- 9.Katz PO, Castell DO, Chen Y, et al. Intragastric acid suppression and pharmacokinetics of twice-daily esomeprazole: a randomized, three-way crossover study. Aliment Pharmacol Ther. 2004;20:399–406. doi: 10.1111/j.1365-2036.2004.02079.x. [DOI] [PubMed] [Google Scholar]
- 10.Chey WD, Mody R, Chen L, et al. Nighttime symptoms and sleep impairment among patients with gastro-esophageal reflux disease (GERD) receiving prescription (Rx) proton pump inhibitors (PPIs) Gastroenterology. 2008;134(4 Suppl. 1):A323–4. [Google Scholar]
- 11.Shin JM, Sachs G. Long lasting inhibitors of the gastric H,K-ATPase. Expert Rev Clin Pharmacol. 2009;2:461–8. doi: 10.1586/ecp.09.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dent J, Kahrilas PJ, Hatlebakk J, et al. A randomized, comparative trial of a potassium-competitive acid blocker (AZD0865) and esomeprazole for the treatment of patients with nonerosive reflux disease. Am J Gastroenterol. 2008;103:20–6. doi: 10.1111/j.1572-0241.2007.01544.x. [DOI] [PubMed] [Google Scholar]
- 13.Arikawa Y, Nishida H, Kurasawa O, et al. Discovery of a novel pyrrole derivative 1-[5-(2-fluorophenyl)-1(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine fumarate (TAK-438) as a potassium-competitive acid blocker (P-CAB) J Med Chem. 2012;55:4446–56. doi: 10.1021/jm300318t. [DOI] [PubMed] [Google Scholar]
- 14.Nishida H, Hasuoka A, Arikawa Y, et al. Discovery, synthesis, and biological evaluation of novel pyrrole derivatives as highly selective potassium-competitive acid blockers. Bioorg Med Chem. 2012;20:3925–38. doi: 10.1016/j.bmc.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Shin JM, Inatomi N, Muson K, et al. Characterization of a novel potassium-competitive acid blocker of the gastric H, K-ATPase, 1-[5-(2-fluorophenyl)-1(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine monofumarate (TAK-438) J Pharmacol Exp Ther. 2011;339:412–20. doi: 10.1124/jpet.111.185314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsukawa J, Hori Y, Nishida H, et al. A comparative study on the modes of action of TAK-438, a novel potassium-competitive acid blocker, and lansoprazole in primary cultured rabbit gastric glands. Biochem Pharmacol. 2011;81:1145–51. doi: 10.1016/j.bcp.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Hori Y, Matsukawa J, Takeuchi T, et al. A study comparing the antisecretory effect of TAK-438, a novel potassium-competitive acid blocker, with lansoprazole in animals. J Pharmacol Exp Ther. 2011;337:797–804. doi: 10.1124/jpet.111.179556. [DOI] [PubMed] [Google Scholar]
- 18.Hori Y, Imanishi A, Matsukawa J, et al. 1-[5-(2-Fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine monofumarate (TAK-438), a novel and potent potassium-competitive acid blocker for the treatment of acid-related diseases. J Pharmacol Exp Ther. 2010;335:231–8. doi: 10.1124/jpet.110.170274. [DOI] [PubMed] [Google Scholar]
- 19.Sakurai Y, Nishimura A, Kennedy G, et al. Gastric acid suppression effect of TAK-438, a potassium-competitive acid blocker following ascending single doses in healthy subjects. Abstract presentation at UEGW 2012.
- 20.Gough K, Hutchison M, Keene O, et al. Assessment of dose proportionality: report from pharmaceutical industry. Drug Inf J. 1995;29:1039–48. [Google Scholar]
- 21.Bell NJV, Burget D, Howden CW, et al. Appropriate acid suppression for the management of gastro-oesophageal reflux disease. Digestion. 1992;51(Suppl. 1):59–67. doi: 10.1159/000200917. [DOI] [PubMed] [Google Scholar]
- 22.Kalaitzakis E, Björnsson E. A review of esomeprazole in the treatment of gastroesophageal reflux disease. Ther Clin Risk Manag. 2007;3:653–63. [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan Y, Hunt RH. Intragastric pH holding time of pH <4 predicts low erosive esophagitis (EE) healing rate. Gastroenterology. 2010;138(5 Suppl. 1):S651. [Google Scholar]
- 24.Tutuian R, Castell DO. Nocturnal acid breakthrough — approach to management. MedGenMed. 2004;6:11. [Published online Oct 26, 2004]. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1480544/ [PMC free article] [PubMed] [Google Scholar]
- 25.Orlando LA, Lenard L, Orlando RC. Chronic hypergastrinemia: causes and consequences. Dig Dis Sci. 2007;52:2482–9. doi: 10.1007/s10620-006-9419-3. [DOI] [PubMed] [Google Scholar]
- 26.Biemond I, Klinkenberg-Knol E, Lamers CBHW, Meuwissen SGM. Serum pepsinogens after interruption of long-term maintenance therapy with omeprazole in patients with reflux esophagitis. Dig Dis Sci. 1993;38:932–6. doi: 10.1007/BF01295923. [DOI] [PubMed] [Google Scholar]
- 27.Chiba T, Sakurai Y, Nishimura A, et al. A phase 2, active comparative, randomized, double-blind, parallel-group, multicenter, dose-ranging study to evaluate the efficacy and safety of a novel potassium-competitive acid blocker (P-CAB) TAK-438 in patients with erosive esophagitis. Abstract presentation at DDW 2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Demographic and baseline characteristics of healthy male subjects receiving TAK-438 10–40 mg once daily at a fixed dose level for 7 consecutive days in Japanese and UK studies (Safety analysis set: N = 60; UK N = 48).
Table S2. Bilirubin, AST and ALT at baseline, and change from baseline on Day 7/8 for placebo and TAK-438 in healthy male subjects receiving TAK-438 10–40 mg once daily at a fixed dose level for 7 consecutive days in Japanese and UK studies (Safety analysis set: Japan N = 60; UK N = 48).
Figure S1. Dose proportionality of AUC0–tau (left) and Cmax (right) on Day 7 for TAK-438 in Japanese and UK repeated-dose studies in healthy male subjects receiving TAK-438 10–40 mg once daily at a fixed dose level for 7 consecutive days (Pharmacokinetic analysis set: Japan N = 60; UK N = 48, each point represents a single subject).
Figure S2. Relationship between CYP2C19 genotypes and dose-normalised AUC0–tau of TAK-438 in healthy male subjects receiving TAK-438 10–40 mg once daily at a fixed dose level for 7 consecutive days in the Japanese study (Pharmacokinetic analysis set: Japan N = 60; UK N = 48).