Abstract
A surface-labeled lyophilized lymphocyte (sLL) preparation has been developed using human peripheral blood mononuclear cells prelabeled with a fluorescein isothiocyanate conjugated anti-CD4 monoclonal antibody. The sLL preparation is intended to be used as a reference material for CD4+ cell counting including the development of higher order reference measurement procedures and has been evaluated in the pilot study CCQM-P102. This study was conducted across 16 laboratories from eight countries to assess the ability of participants to quantify the CD4+ cell count of this reference material and to document cross-laboratory variability plus associated measurement uncertainties. Twelve different flow cytometer platforms were evaluated using a standard protocol that included calibration beads used to obtain quantitative measurements of CD4+ T cell counts. There was good overall cross-platform and counting method agreement with a grand mean of the laboratory calculated means of (301.7 ± 4.9) μL−1 CD4+ cells. Excluding outliers, greater than 90% of participant data agreed within ±15%. A major contribution to variation of sLL CD4+ cell counts was tube to tube variation of the calibration beads, amounting to an uncertainty of 3.6%. Variation due to preparative steps equated to an uncertainty of 2.6%. There was no reduction in variability when data files were centrally reanalyzed. Remaining variation was attributed to instrument specific differences. CD4+ cell counts obtained in CCQM-P102 are in excellent agreement and show the robustness of both the measurements and the data analysis and hence the suitability of sLL as a reference material for interlaboratory comparisons and external quality assessment. © 2015 The Authors. Published by Wiley Periodicals, Inc.
Keywords: CD4+ cell counting, relative concentration measurement, lyophilized cells, flow cytometry, standard measurement procedure, measurement of uncertainty, human immunodeficiency virus-1, acquired immunodeficiency syndrome, reference material
Infection with HIV leads to the development of acquired immune deficiency syndrome (AIDS) characterized by a loss of CD4+ cells required to mount an effective immune response against infections (1,2). In 2005, the World Health Organization (WHO) issued an open letter to manufacturers of CD4+ cell enumeration technologies emphasizing the need for laboratory monitoring of immunological parameters to support the clinical monitoring of human immunodeficiency virus-1 (HIV-1) infected patients (Supporting Information Document S1). In particular, this letter states that “All CD4+ cell enumeration technologies need to be compatible with a form of an external quality assessment program.” Accurate CD4+ cell count measurements ensure that patients receive appropriate antiretroviral therapy (ART) against HIV-1 and chemoprophylaxis for opportunistic infections (3,4). In developed countries, CD4+ cell counts that fall below 350 and 200 cells μL−1 of blood are the triggers for ART and chemoprophylaxis, respectively (3,4). Whereas in resource-poor countries, it is recommend that ART is initiated only when CD4+ cell counts fall below 200 cells μL−1 of blood (5). A CD4+ cell count of less than 200 cells μL−1 of blood is diagnosed as AIDS (6,7). Current WHO guidelines recommend expanded eligibility for ART with treatment initiation below 500 CD4+ cells μL−1 of blood, but giving priority to those with less than 350 cells μL−1 of blood (8,9). It is believed that these guidelines could avert an additional 3 million deaths between 2012 and 2025 in low and middle income countries (10). Unfortunately, HIV-1 is able to mutate in the presence of ART and develop drug resistance which is associated with declining CD4+ cell counts (11). Similarly, failure to respond to first line therapy is associated with ART resistant HIV-1 infection. Decisions regarding the switching of patients to different ART regimens are based on CD4+ cell counts and clinical findings. Hence, reliable CD4+ cell counts are at the forefront of care for people living with HIV/AIDS (3,12).
Flow cytometry is regarded as the “gold standard” for measuring CD4+ cell counts, due to its accuracy and precision (12,13). Currently, there is no internationally recognized or validated reference standard and no commonly accepted method of quality control for cellular phenotyping by flow cytometry (14). Furthermore, the concept of metrological traceability (15), which is essential to ensure comparability and accuracy of measurement results, is not yet established in cell counting because of a lack of reference material and reference measurement procedures. We organized pilot study CCQM-P102 to evaluate surface-labeled lyophilized lymphocytes (sLL) as a potential international reference material that could be used for internal and external quality assurance (QA) for the harmonization of diagnostic CD4+ cell counting that would satisfy the requirements set out in the WHO open letter published in 2005. To allow reliable interpretation of the measurement results obtained in CCQM-P102, an analysis of the associated measurement uncertainties was included. In particular, a reference material would be beneficial for the development of higher order or reference measurement procedures to provide traceability. Reference measurement procedures are indispensable for the replacement of consensus values by reference measurement values. Consensus values can be influenced by a specific protocol or technique and may not reflect the “true value” or the “conventional quantity value” (16).
Current technology used to assure cross-laboratory flow cytometry quality control relies on either bead-based methods, use of cryopreserved PBMC, or stabilized blood controls (17–20). Although very stable, beads do not have the same characteristics as cells, in terms of size, internal complexity, and fluorescent properties. Thus, flow cytometric detection settings (e.g., photomultiplier tube voltages or thresholds) required to resolve a population of beads may not be appropriate for cells and application of incorrect settings can result in difficulties in color compensation as well as in the resolution between positive and autofluorescent cells. Cryopreserved PBMCs have the same characteristics as patient samples and are stable while frozen, but need to be constantly monitored to ensure that no degradation occurs due to inadvertent freeze-thawing. Consequently, cryopreserved PBMC incur high shipping and storage costs unsuitable for resource poor settings. Stabilized blood controls have the same characteristics as patient samples and only require refrigeration. However, commercially available stabilized blood controls only have a short shelf-life of up to 3 months. None of these materials are suitable for use as a traceable reference material necessary to ensure the robust standardization of CD4+ cell counting. A suitable traceable biological reference material should have the same characteristics as the biological sample of the patient in a clinically significant range, be well characterized, have long-term stability, yet be easy to store and distribute by a custodian laboratory (21).
Materials and Methods
Production of sLL
The sLL reference material was prepared from a pool of three buffy coats from normal human donors preselected for similar levels of CD4 expression. PBMC were separated from each buffy coat by density gradient (Lymphoprep™, Axis Shield, Norway), labeled with anti-CD4 fluorescein isothiocyanate (FITC) monoclonal antibody (clone OKT4, Biolegend, UK), washed in phosphate buffered saline (PBS), and resuspended in a fixative solution comprised of 10% Transfix (Cytomark, England) in PBS. After overnight fixation, PBMC were washed, pooled in a PBS lyophilization buffer; the CD4+ cell count adjusted to a clinically relevant range (300 CD4+ cells μL−1) and freeze-dried in screw top vials using a modified 3 day cycle. Each vial of sLL contained 0.5 g of cell suspension in buffer. The sample weight prior to lyophilization had a coefficient of variation (CV) of 0.48%. After lyophilization, the cakes had a mean weight of 0.0143 g with a CV of 1.2%. A total of six vials of sLL were supplied to each participant, along with the same number of TruCount™ tubes (Becton-Dickinson, CA) from a single batch (Lot Number: 610431). Each tube contained a pellet of lyophilized calibration beads. Typically, about 50,000 beads, homogenously stained with fluorescent dye are contained in such tubes. The particle diameters are approximately 3.8 μm.
Pilot Study
A pilot study was carried out under the auspices of the Bio Analysis Working Group (BAWG) of the Comité Consultatif pour la Quantité de Matière (CCQM) and was piloted jointly by the National Institute for Biological Standards and Control (NIBSC, UK), Physikalisch-Technische Bundesanstalt (PTB, Germany), and National Institute of Standards and Technology (NIST, USA). The potential reference material was assessed across a range of sites and flow cytometric-based counting technologies. Participants were requested to follow the standard protocol distributed with the sLL (Supporting Information Document S2). Briefly, each vial of sLL was reconstituted on the day of use by adding
of sLL was reconstituted on the day of use by adding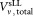 = 1 mL of sterile distilled water and gently mixing, preferably on a roller, for 10–30 min before use. All three vials were reconstituted on the same day and both dilutions assayed (tubes 1, 2, and 3) within 2 h. Two dilutions of the sLL in PBS, a 1 in 5 and a 1 in 20 dilution
= 1 mL of sterile distilled water and gently mixing, preferably on a roller, for 10–30 min before use. All three vials were reconstituted on the same day and both dilutions assayed (tubes 1, 2, and 3) within 2 h. Two dilutions of the sLL in PBS, a 1 in 5 and a 1 in 20 dilution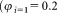 ,
,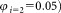 made up to
made up to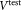 = 1 mL of test sample in each TruCount tube was requested for each set of three sLL vials sent to participants. A total of six test samples in two dilutions were used to perform CD4+ cell count measurements. Preparation of these two dilutions was requested to uncover possible impacts of influence quantities like counting loss in relative concentration measurements or cell adhesion. Flow cytometers used by participants are detailed in Supporting Information Table S1. Prior to acquisition, participants were required to perform routine instrument QA of their choice and to ensure that their flow cytometers were properly compensated for FITC and Phycoerythrin (PE) channels using their own choice of compensation method. The study protocol specified that forward scatter channel (FSC) and side scatter channel (SSC) needed to be set correctly for sample data acquisition to ensure a high signal to noise ratio for cells and beads. Each participant was requested to provide the registered counts
= 1 mL of test sample in each TruCount tube was requested for each set of three sLL vials sent to participants. A total of six test samples in two dilutions were used to perform CD4+ cell count measurements. Preparation of these two dilutions was requested to uncover possible impacts of influence quantities like counting loss in relative concentration measurements or cell adhesion. Flow cytometers used by participants are detailed in Supporting Information Table S1. Prior to acquisition, participants were required to perform routine instrument QA of their choice and to ensure that their flow cytometers were properly compensated for FITC and Phycoerythrin (PE) channels using their own choice of compensation method. The study protocol specified that forward scatter channel (FSC) and side scatter channel (SSC) needed to be set correctly for sample data acquisition to ensure a high signal to noise ratio for cells and beads. Each participant was requested to provide the registered counts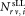 for the sLL and the corresponding number
for the sLL and the corresponding number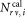 of calibration beads.
of calibration beads.
Calibration Bead Analysis
To estimate systematic deviations, the number of calibration beads was determined for 34 tubes from the TruCount LOT # 610431. For measurements of the concentration of calibration beads, a modified impedance counter with an integrated balance for mass determination (22) and an optical flow cytometer, equipped with a motor driven, gravimetrically calibrated 1-mL syringe were used. In both instruments, the sample was fed directly into the respective flow cell for impedance or optical particle counting through a platinum-iridium tube. This approach was chosen to minimize adhesion, as the tubing generally used in commercial instruments may cause significant loss of cells and calibration beads. The tube to tube variation of the number of calibration beads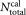 was determined to
was determined to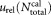 = 3.6% (Supporting Information Table S2 and Fig. S1). This value is in good agreement with the CV stated by the manufacturer (CV = 3.7%, Nicolas Quoix, BD Biosciences Scientific Support Europe, private communication).
= 3.6% (Supporting Information Table S2 and Fig. S1). This value is in good agreement with the CV stated by the manufacturer (CV = 3.7%, Nicolas Quoix, BD Biosciences Scientific Support Europe, private communication).
Data Analysis and Uncertainty Determination
In this paragraph, we give the relevant equations for data analysis and determination of uncertainty, which is essential to reliably compare the intralaboratory and interlaboratory results. A detailed mathematical derivation is summarized in the Supporting Information Document S3. A primary CD4 gating strategy, based on SSC versus CD4+ cells on the FITC channel with calibration beads in FITC channel versus PE channel, was provided to participants (Supporting Information Document S2). Participants were requested to collect 10,000 bead events from each sample before stopping acquisition of all events. The measurement volume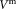 may be determined directly or indirectly using calibration beads, either of known concentration in suspension or as lyophilized pellet of known total number
may be determined directly or indirectly using calibration beads, either of known concentration in suspension or as lyophilized pellet of known total number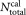 per test tube. TruCount calibration beads were used to indirectly determine the measurement volume
per test tube. TruCount calibration beads were used to indirectly determine the measurement volume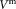 . The concentration of CD4+ cells
. The concentration of CD4+ cells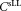 is calculated using the equation:
is calculated using the equation:
| (1) |
Equation (1) includes all quantities directly determined in each measurement (Supporting Information Document S3). This includes the number of events in the defined regions containing CD4+ cells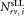 and calibration beads
and calibration beads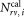 and the volume
and the volume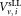 of CD4+ cells pipetted into a TruCount tube containing
of CD4+ cells pipetted into a TruCount tube containing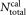 calibration beads. The indices r for the recorded events, v for the vial, i for the dilution, and j for the repeat measurement are required to unambiguously identify each data point. For the preparation of suspensions with two different volume fractions
calibration beads. The indices r for the recorded events, v for the vial, i for the dilution, and j for the repeat measurement are required to unambiguously identify each data point. For the preparation of suspensions with two different volume fractions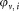 , the volumes
, the volumes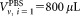 or
or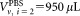 were added to provide a total volume of
were added to provide a total volume of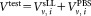 = 1 mL of the suspension used for measurement. Equation (1) reflects the advantage that for relative concentration measurements, the determination of the volume
= 1 mL of the suspension used for measurement. Equation (1) reflects the advantage that for relative concentration measurements, the determination of the volume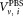 and hence the dilution factor
and hence the dilution factor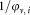 is not required, as both, the cell concentration and the concentration of calibration beads is changed accordingly.
is not required, as both, the cell concentration and the concentration of calibration beads is changed accordingly.
For reliable assessment of the results of this comparative study, it was essential to perform an uncertainty analysis. Various contributions to the uncertainty of relative concentration measurements must be considered when calculating CD4+ cell counts using Eq. (1). These include statistical uncertainties of the number of CD4+ cell events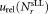 , tube to tube variation of the calibration beads
, tube to tube variation of the calibration beads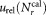 , and uncertainty of volume measurement
, and uncertainty of volume measurement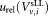 which all contribute to uncertainties
which all contribute to uncertainties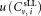 in the concentration measurements. When central analysis was carried out on measurements obtained using the standard protocol, the uncertainties were calculated using the standard relative uncertainty
in the concentration measurements. When central analysis was carried out on measurements obtained using the standard protocol, the uncertainties were calculated using the standard relative uncertainty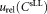 of the cell concentration
of the cell concentration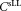 by applying the following quadratic propagation:
by applying the following quadratic propagation:
| (2) |
Typical values for the pipetting volume uncertainties returned by participants amounted to 1% (23) and the relative statistical uncertainties for the counted events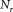 correspond to
correspond to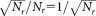 . For the total number of calibration beads, the nominal value supplied by the manufacturer was used,
. For the total number of calibration beads, the nominal value supplied by the manufacturer was used,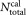 = 51,511. The relative uncertainty
= 51,511. The relative uncertainty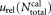 = 3.6% results from measurement data reported in Supporting Information Figure S1 and Table S2. For direct measurement of the concentration, the uncertainty of the volume fraction is needed, given in Supporting Information Document S3. Hence, the dilutions and corresponding uncertainties are
= 3.6% results from measurement data reported in Supporting Information Figure S1 and Table S2. For direct measurement of the concentration, the uncertainty of the volume fraction is needed, given in Supporting Information Document S3. Hence, the dilutions and corresponding uncertainties are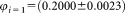 and
and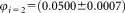 . From the three vials analyzed by each participant, the arithmetic average of
. From the three vials analyzed by each participant, the arithmetic average of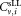 defined as
defined as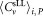 and the standard deviation
and the standard deviation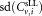 the uncertainty was derived by quadratic propagation according to:
the uncertainty was derived by quadratic propagation according to:
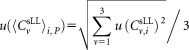 |
(3) |
The combined uncertainties, that is the standard deviation between the concentrations determined for three vials and the individual uncertainty of each measurement value is accounted for in Eq. (4):
| (4) |
The advantage of this approach is that individual uncertainties and the variation between different sLL vials are accounted for.
Central Analysis
To determine whether central analysis by a single operator would reduce variability, all raw data files were analyzed using FlowJo software (Flowjo LLC, OR) for CD4+ cell count determination. This third party software was used to analyze flow cytometry standard (FCS) files from a variety of sources as it is not restricted to specific instrument manufacturer data files. When performing central analysis, Grubbs' test for outliers was applied to exclude data influenced by technical or preparative issues. To justify the application of this approach, we plotted the frequency distribution off all concentration values (136 measurements). A Gaussian fit describes the data consistently, fulfilling the requirement of a normal distribution (Supporting Information Fig. S2).
Stability
Lot SS-194 sLL used in CCQM-P102 was filled in December 2010, distributed to participants in June 2011, and all data returned by October 2011. Stock sLL were kept in long-term storage at −20°C, shipped at ambient temperature and stored short term at 4°C by participants. Stock sLL were most recently assayed in June 2013, September 2013, and October 2014 to determine CD4+ cell count stability. The stability of lot 10-256, SS-319, and SS-320 sLL stored at −20, +4, +20, and +37°C for 6–12 months was compared.
Results
Analysis of Participant CD4+ Cell Concentrations and Sources of Uncertainty
The mean concentrations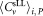 of CD4+ cells μL−1 for dilutions
of CD4+ cells μL−1 for dilutions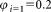 ,
,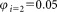 and the combined uncertainties were derived and summarized in Table 1. Example data obtained from participants A and B are shown in Supporting Information Table S3. The measurements results of other participants are shown in Supporting Information Table S4. Relative uncertainties associated with volume measurements lay within the range 1–1.5%. The major contribution to single vial analysis was from the tube to tube variation of the calibration beads, the corresponding fluctuation amounting to 3.6%.
and the combined uncertainties were derived and summarized in Table 1. Example data obtained from participants A and B are shown in Supporting Information Table S3. The measurements results of other participants are shown in Supporting Information Table S4. Relative uncertainties associated with volume measurements lay within the range 1–1.5%. The major contribution to single vial analysis was from the tube to tube variation of the calibration beads, the corresponding fluctuation amounting to 3.6%.
Table 1.
Mean CD4+ cell concentrations and combined uncertainties
| Dilution 1 |
Dilution 2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Labcode | Mean concentrations 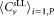
|
Combined uncertainties 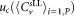
|
Mean concentrations 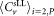
|
Combined uncertainties 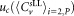
|
||||
| P | Standard protocol | Comment | Outliers excluded | μL−1 | μL−1 | μL−1 | μL−1 | |
| A | Yes | No | 266.8 | 8.3 | 253.1 | 14.6 | ||
| B | Yes | No | 274.8 | 7.0 | 276.4 | 7.4 | ||
| C1 | Yes | Yes | 270.4 | 7.6 | 285.8 | 14.6 | ||
| C2 | No | Volumetric count | Yes | 246.0 | 7.2 | 261.6 | 5.8 | |
| D | Yes | n.a. | 266.4 | 10.9 | 325.6 | 9.6 | ||
| E1 | Yes | No | 271.2 | 16.2 | 266.9 | 21.3 | ||
| E2 | No | Different “TrueCount” calibration particles used | No | 272.3 | 17.8 | 258.6 | 20.7 | |
| F | Yes | Yes | 390.8 | 22.7 | 356.5 | 11.9 | ||
| G1 | Yes | n.a. | ||||||
| G4 | No | Particle suspension “FlowCount” used for volume determination | No | 338.1 | 9.7 | 310.7 | 10.2 | |
| G5 | No | Yes | 314.0 | 18.3 | 328.6 | 18.3 | ||
| G6 | No | No | 330.7 | 13.9 | 306.3 | 10.3 | ||
| H1 | Yes | No | 313.9 | 10.2 | 306.5 | 10.0 | ||
| H2 | Yes | No | 321.6 | 18.3 | 314.4 | 10.6 | ||
| I1 | Yes | Yes | 291.9 | 62.5 | 323.5 | 9.7 | ||
| I2 | Yes | Measured sixteen days later due to postal delay | Yes | 285.3 | 8.2 | 300.9 | 13.3 | |
| J | No | Increased number of repeat measurements | No | 321.3 | 5.7 | 309.7 | 5.6 | |
| K | Yes | Yes | 310.6 | 8.9 | 311.7 | 9.4 | ||
| L | Yes | Yes | 339.1 | 9.8 | 324.0 | 9.8 | ||
| M | Yes | Yes | 433.5 | 31.4 | 360.7 | 11.0 | ||
| N | Yes | No | 335.1 | 8.2 | 304.3 | 13.7 | ||
| O1 | Yes | No | 318.0 | 10.5 | 289.0 | 6.7 | ||
| O2 | No | Gravimetrical dilution and bead based volume determination | No | 321.3 | 11.4 | 307.6 | 10.7 | |
| O3 | No | Gravimetrical dilution and direct volume measurement | Yes | 300.4 | 7.5 | 284.9 | 7.5 | |
The mean concentration of CD4+ SLL was derived from the arithmetic average of three vials of reconstituted sLLs, excluding outliers. The column “Standard protocol” indicates whether participants applied the standard or a modified protocol. Participants C2 and O3 measured the volume directly and hence volumes are given instead of the number of calibration bead counts. Participants E2, G4, G5 and G6 used either a different lot of lyophilized calibration beads or a different manufacturer's calibration kit. Participant J made up to three repeat measurements for both dilutions and all vials. To be consistent with the data of all participants, all counts from repeat measurements were summed. Raw data from participant G1 were reanalyzed and included in the centralized analysis. Measurements with instruments G2 and G3 were not suitable for either participants' or centralized analysis as the number of calibration tubes provided did not allow separate measurement on each instrument and using the same tube on different instruments does not provide accurate results.
The relative uncertainties derived by Eq. (4) typically cover the range from about 2.5(participant B, dilution 1) to 8% (participant E1). Generally, accuracy is increased when determining mean values. However, compared to uncertainties of about 4% observed for single vial analysis, smaller as well as higher fluctuations were found for the average concentrations. As only three vials were analyzed by each participant, the influence of the tube to tube variation may accidentally be smaller or larger resulting in corresponding smaller or larger uncertainties. Furthermore, the contribution of the uncertainty when reconstituting the sLLs by adding 1-mL water, not accounted for so far, enters in the analysis through the calculation of the standard deviation using Eq. (3) and increases the combined uncertainty. Statistical uncertainties, that is,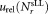 ,
,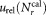 , uncertainty of volume measurement
, uncertainty of volume measurement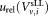 , and the uncertainty
, and the uncertainty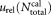 due to the tube to tube variation of the calibration beads all contribute to the uncertainties
due to the tube to tube variation of the calibration beads all contribute to the uncertainties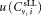 in concentration measurements.
in concentration measurements.
The ranked mean values of the data of all participants, for dilutions 1 and 2, are shown in Figure 1. For dilution 1, two outliers were identified (open circles), whereas for dilution 2 all measurements are included for further analysis. The weighted mean values amounts to a concentration of 300.4 CD4+ cells μL−1 for dilution 1 and 302.9 for dilution 2 (Table 2). Both values are in good agreement and indicated in Figure 1 as horizontal black lines. The combined uncertainties of the average values are shown as horizontal dashed lines. To prove consistency of the datasets, we applied the chi-squared test which relates the deviation of the participants' values to the respective estimated uncertainties (Table 2). For 21 degrees of freedom, the observed value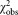 is expected to lie in between
is expected to lie in between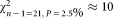 and
and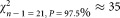 . However, the observed chi-squared (
. However, the observed chi-squared (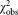 )-values are much greater than the upper limit, which means that the chi-squared consistency test failed. It follows that the uncertainty of the values for individual laboratories, averaged over the three vials, were underestimated by about a factor of 2.5. This indicates that other contributions, for example, caused by different adhesion behavior of calibration beads and cells are not accounted for. Dilution related additional influences can be excluded, as average concentration values for CD4+ cells μL−1 of (300.4 ± 7.3) for dilution 1 and (302.9 ± 6.4) for dilution 2 are in good agreement. Finally, by averaging these two values, we assign a CD4+ concentration value of (301.7 ± 4.9) μL−1 to the sLL (lot SS-194) distributed by NIBSC to the participants of CCQM-P102.
)-values are much greater than the upper limit, which means that the chi-squared consistency test failed. It follows that the uncertainty of the values for individual laboratories, averaged over the three vials, were underestimated by about a factor of 2.5. This indicates that other contributions, for example, caused by different adhesion behavior of calibration beads and cells are not accounted for. Dilution related additional influences can be excluded, as average concentration values for CD4+ cells μL−1 of (300.4 ± 7.3) for dilution 1 and (302.9 ± 6.4) for dilution 2 are in good agreement. Finally, by averaging these two values, we assign a CD4+ concentration value of (301.7 ± 4.9) μL−1 to the sLL (lot SS-194) distributed by NIBSC to the participants of CCQM-P102.
Figure 1.
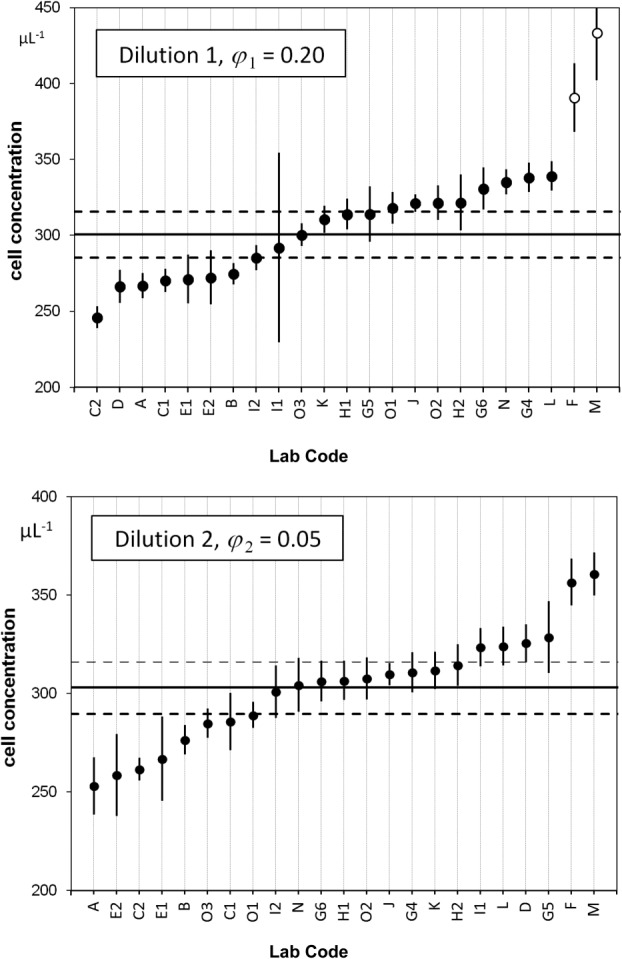
Ranked concentration of CD4+ cells for dilutions 1 and 2. Concentrations of CD4+ sLL measured by the participants for dilution 1 (1:5, upper graph) and dilution 2 (1:20, lower graph). The straight lines represent the mean values and the dashed lines indicate the expanded uncertainties of the mean values corresponding to a level of confidence of 95%. For dilution 1, two outliers (open circles) were identified and not used when determining the mean value. Participant lab codes shown on the x-axis are ranked from lowest to highest mean CD4+ cell concentration for dilutions 1 and 2.
Table 2.
Summary of mean CD4+ cell counts, uncertainties and the observed χ2 values
Weighted mean value 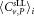 μL−1 μL−1
|
Uncertainty of mean value 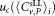 μL−1 μL−1
|
Standard deviation 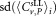 μL−1 μL−1
|
Number of measurement values n | Expansion factor k | χ2 | Consistency test | |
|---|---|---|---|---|---|---|---|
| Dilution 1 | 300.4 | 7.3 | 28.2 | 21 | 2.080 | 199.7 | Negative |
| Dilution 2 | 302.9 | 6.4 | 28.3 | 23 | 2.069 | 163.5 | Negative |
| Dilution 1 | 302.5 | 7.4 | 30.5 | 24 | 2.064 | 121.6 | Negative |
| Dilution 2 | 286.2 | 5.5 | 22.5 | 23 | 2.069 | 91.3 | Negative |
The first two lines represent the values calculated from data reported by participants and the last two lines show values from the centralized analysis. Results for the weighted mean values, combined uncertainties, standard deviation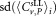 for a single participants' result, the number of measurement results, the corresponding expansion factor for a 95% level of confidence and chi-squared (χ2) test for consistency are given.
for a single participants' result, the number of measurement results, the corresponding expansion factor for a 95% level of confidence and chi-squared (χ2) test for consistency are given.
Analysis of Laboratory to Laboratory Variation
Although all vials were filled with the same number of sLL, participants prepared two different dilutions to examine the linearity of the measurement procedures applied. The results for each participant were plotted in the Youden-diagram depicted in Figure 2, where each point represents a single laboratory. No instrumental clusters were identified. The rectangle delineated by dashed lines, represents deviations from the average values of ±15%. Apart from the two outliers two additional values lie outside this rectangular, that is, 19 laboratories out of 23 corresponding to 83% are within a range of ±15%. When increasing the limit to ±20%, 21 values from 23 (91%) are within this range, a quotas typical for external quality assessment schemes. If the outliers are disregarded, then >90% of the participants' results are within a limit of ±15%. As most of the participants CD4+ cell counts agree within ±15% and type A (Statistical), expanded uncertainties are about 10%, which were derived from the standard deviation of repeated measurements, then type B (Systematic) uncertainties are expected to have approximately the same value based on the authors scientific judgment of the available information.
Figure 2.
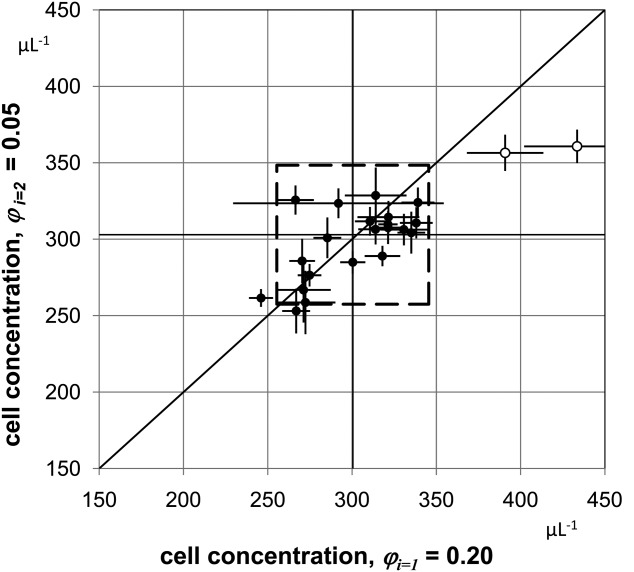
Youden-diagram of CD4+ cell concentrations for dilutions 1 and 2. Youden-diagram of the results obtained for both dilutions. The two outliers are indicated by open circles. The mean values of participants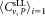 = 300.4 μL−1 and
= 300.4 μL−1 and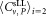 = 302.9 μL−1 are plotted as vertical and horizontal lines, respectively. The diagonal with unity slope was inserted to illustrate that the results obtained from both dilutions are consistent with a linear relation within the uncertainties reached. The middle dashed square indicates boundaries corresponding to ±15% deviation from the respective mean values. If the outliers are disregarded, 2 out of 21 values are outside of this range, that is, 90% of the participants' results are within a limit of ±15%.
= 302.9 μL−1 are plotted as vertical and horizontal lines, respectively. The diagonal with unity slope was inserted to illustrate that the results obtained from both dilutions are consistent with a linear relation within the uncertainties reached. The middle dashed square indicates boundaries corresponding to ±15% deviation from the respective mean values. If the outliers are disregarded, 2 out of 21 values are outside of this range, that is, 90% of the participants' results are within a limit of ±15%.
Analysis of Vial to Vial Variation
Vial to vial variations of all data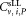 including outliers for each of the six dilution/vial combinations are shown in Supporting Information Figure S3. Except for dilution 1, vial 1 all median values and mean values are in good agreement and show a small scatter around a value of
including outliers for each of the six dilution/vial combinations are shown in Supporting Information Figure S3. Except for dilution 1, vial 1 all median values and mean values are in good agreement and show a small scatter around a value of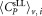 ≈ 300 CD4+ cells μL−1 (Table 3). A slight reduction in the mean CD4+ cell count across the vials was noted by some participants. To investigate this, we selected the data shown in Supporting Information Figure S3 and replotted it on order of analysis (Supporting Information Fig. S4). Analysis of variance of the CD4+ concentrations based on the order in which the vials were analyzed and the time elapsed between readings found no significant differences for either dilution 1 or 2 (Supporting Information Fig. S4).
≈ 300 CD4+ cells μL−1 (Table 3). A slight reduction in the mean CD4+ cell count across the vials was noted by some participants. To investigate this, we selected the data shown in Supporting Information Figure S3 and replotted it on order of analysis (Supporting Information Fig. S4). Analysis of variance of the CD4+ concentrations based on the order in which the vials were analyzed and the time elapsed between readings found no significant differences for either dilution 1 or 2 (Supporting Information Fig. S4).
Table 3.
Mean CD4+ cell counts and uncertainties derived for different vials and dilutions
Non weighted mean value 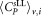 μL−1 μL−1
|
Uncertainty of mean value 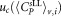 μL−1 μL−1
|
Standard deviation 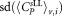 μL−1 μL−1
|
Number of measurement values n | Expansion factor k | 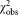 |
Consistency test | |
|---|---|---|---|---|---|---|---|
| Dilution 1, vial 1 | 327.9 | 9.1 | 39.6 | 21 | 2.080 | 243.1 | Negative |
| Dilution 1, vial 2 | 297.4 | 7.4 | 31.0 | 20 | 2.086 | 445.7 | Negative |
| Dilution 1, vial 3 | 288.3 | 10.0 | 43.5 | 20 | 2.086 | 495.4 | Negative |
| Dilution 2, vial 1 | 307.7 | 6.3 | 26.8 | 22 | 2.074 | 158.6 | Negative |
| Dilution 2, vial 2 | 305.3 | 6.0 | 25.0 | 22 | 2.074 | 105.1 | Negative |
| Dilution 2, vial 3 | 284.4 | 9.9 | 44.8 | 22 | 2.074 | 374.3 | Negative |
Values for each of six vial / dilution combinations were averaged over all participants.
Central Analysis of CD4+ Cell Concentrations
The mean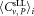 of centrally analyzed average values
of centrally analyzed average values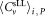 for CD4+ cells μL−1 was 302.5 for dilution 1 and 286.2 for dilution 2 (Table 2). When centrally analyzed data were plotted in a Youden-diagram, apart from one outlier four additional of the 24 values fell outside of the ±15% limits of the means, meaning that 80% of participants' results were within (Supporting Information Fig. S5). Additional data points, excluded by participants, but recovered from data files were included in the central analysis. It follows that no significant differences with respect to the mean values, uncertainties, and standard deviations between both central and participant analyses were observed. This result confirms that both, individual analysis with instrument manufacturer specific/restricted software and centralized analysis applying FlowJo software are well established and exchange of data in FCS data format is straightforward. The agreement between both approaches corresponds to a mutual validation of the data analysis protocols and software used.
for CD4+ cells μL−1 was 302.5 for dilution 1 and 286.2 for dilution 2 (Table 2). When centrally analyzed data were plotted in a Youden-diagram, apart from one outlier four additional of the 24 values fell outside of the ±15% limits of the means, meaning that 80% of participants' results were within (Supporting Information Fig. S5). Additional data points, excluded by participants, but recovered from data files were included in the central analysis. It follows that no significant differences with respect to the mean values, uncertainties, and standard deviations between both central and participant analyses were observed. This result confirms that both, individual analysis with instrument manufacturer specific/restricted software and centralized analysis applying FlowJo software are well established and exchange of data in FCS data format is straightforward. The agreement between both approaches corresponds to a mutual validation of the data analysis protocols and software used.
Stability of sLL CD4+ Cell Concentrations
The stability of sLL lot ss-194 used in CCQM-P102 and lots SS-319, SS-320, and 10-256 have been compared. The CD4+ cell counts and fluorescence intensity of ss-194 stored at −20°C has remained stable to date since 2010 (Supporting Information Fig. S6). Similarly, the CD4+ cell count of sLL lot 10-256, stored at −20, 4, and 20°C remained stable for 12 months, but were not stable when stored for 3 months at +37°C (Supporting Information Table S5). However, an increase in the fluorescence of sLL 10-256 CD4− cells was observed after 12 months storage at +4°C and after 6 months storage at +20 and +37°C (Supporting Information Table S5 and Fig. S7). After 12 months storage at +37°C, it was no longer possible to distinguish CD4− and CD4+ populations of sLL 10-256 (Supporting Information Fig. S7). Likewise, analysis of sLL lots SS-319 and SS-320 following 6 month storage at −20, +4, and +20°C did not reveal any significant change in CD4+ cell counts. Although no change in FSC or SSC was observed in SS-319 and SS-320 stored at −20°C, a slight decrease in FSC and increase in SSC was observed after 6 months storage at +4°C, a difference that was more marked after 6 months storage at +20°C (Supporting Information Fig. S8).
Discussion
International biological reference standards are the highest order standards for the calibration of measurement procedures and play a critical role in the standardization, harmonization, and quality control of diagnostic procedures (21). They are fundamental for the regulation of in vitro diagnostic tests and to standardize treatment procedures but are primarily intended for the calibration of secondary standards. Secondary standards are defined as either regional or national reference preparations or working reference materials used routinely with different laboratories and different platforms that are defined in units traceable to the higher order international standard. Controls are defined as manufacturer, platform, or laboratory specific reference preparations that are not defined in units traceable to a higher order international standard. A prerequisite for the calibration of a potential international reference candidate is commutability; a reference material should be recognized by a wide range of tests or test platforms with nearly the same efficiency. If a material shows significant intermethod variability in concentration then it is not suitable as a reference material. A reference material should be well characterized and include an estimate of uncertainty; that is, the range of values within which the true value is asserted to lie. A potential reference material should also be representative of the performance with patient samples validated for the assays used.
The aim of pilot study CCQM-P102 was to determine the potential of sLL to be used as international reference materials and as secondary standards for flow cytometry instrument control, preparation control, and data evaluation. Good overall cross-laboratory, cross-platform, and counting method agreement were achieved in CCQM-P102, with mean CD4+ cell counts in good agreement. Furthermore, the concentrations of CD4+ cells, determined to be (301.7 ± 4.9) μL−1, closely matched the target value of 300 μL−1 set during filling. As >90% of participants' results agreed within ±15% (excluding outliers), this value could be used as a starting limit for future studies with similar reference materials. This level of variability is similar to that previously reported by 10 year reviews of external quality assessment of CD4+ lymphocyte subsetting (24,25). Considering that the participants of CCQM-P102 consisted of mostly metrology laboratories, not experienced in routinely running CD4+ cell counting assays, a target of at least ±10% may be more appropriate in a clinical setting. The sLL reference material described here could be suitable for use both for external QA and as a performance monitoring tool, requirements set out in the WHO open letter to manufactures of CD4+ cell enumeration technologies. An international reference material for CD4+ cell enumeration based on lyophilized lymphocytes could lead to further improvements in intermethod and interlaboratory variability. It is important that laboratories minimize variation in CD4+ cell counts to ensure that HIV-1 patients receive appropriate ART to minimize the risk of mortality (26,27).
It is reported that interlaboratory variation in clinical flow cytometry can be reduced through use of standardized gating templates and centralized analysis (16,18,28). However, when central analysis with a single gating template was performed on raw data from all CCQM-P102 participants then no reduction in variability was obtained. In flow cytometry, the exact positions of the gates as suggested in the protocol are open to interpretation by the operator. As cell populations are rarely discrete, two approaches may be taken, a large gate may be drawn to capture all probable events, or a smaller gate used to capture only those events that are without doubt from the population of interest. In this study, central analysis was carried out using the second approach and a tight gate used. Conversely, as the localization of cell clusters in flow cytometric scatter diagrams depends on the instrument, its alignment and amplifier settings, experienced operators might be able to select the optimal gate to identify the lymphocyte subpopulation. However, the average CD4+ cell counts obtained by central analysis were in good agreement with results from participants' analysis for dilution 1, but about 5% lower for dilution 2 suggesting some under estimation had occurred.
Stabilized whole blood such as BD Multi-Check™, CD-Chex Plus™ and Immuno-Trol™ are successfully used as laboratory controls but are not suitable for use as an international reference standard as they typically have a closed vial stability of 3 months. In contrast, we have shown that sLL can remain stable for at least 4 years when stored at −20°C, so they are suitable for use as an international reference standard. Due to the short shelf life of stabilized blood controls, each batch is produced roughly every 2 months so can only be compared to the previous batch which can then lead to stochastic drift over time. Validation of new batches of stabilized blood controls against an international reference standard would eliminate any potential for drift. The robustness of sLL, compared to stabilized blood, favors their use as secondary controls in a third world context where cold chain or special transportation may be a challenge or for instrument validation in the field. There is no red blood cell lysis step with sLL, they are removed prior to lyophilization as they do not survive the freezing step, so if a lysis step is required then a stabilized blood control is preferential. However, like stabilized blood, unlabeled lyophilized lymphocytes can be used as a staining control, an approach currently being pursued for a CD34+ cell counting study (Supporting Information Fig. S9). Although frozen fixed cells (18) are likely to have the same stability as lyophilized lymphocytes while stored at −20°C, the advantage of the latter being the ability to ship at ambient temperature (+20°C) or on cool packs for hotter climates and the reduced storage requirements for end-users. The reproducibility of this approach to manufacture reference material with a clinically relevant CD4+ cell count is demonstrated by the results obtained with four different lots of sLL, SS-194, 10-256, SS-319, and SS-320.
Primary CD4 gating is a single color strategy that employs a CD4 versus SSC gating strategy to generate absolute CD4+ cell counts while minimizing the number of expensive reagents used, an important consideration in resource-poor countries (29,30). More widely advocated for resource-poor settings is the two color pan-leucocyte gating strategy, immunophenotyping for CD4 and CD45 (18,28,31). Whereas in developed countries, the approved method for assessing CD4+ cell counts relies upon multiparameter analysis of CD3, CD4, CD8, and CD45 (32–34). The format of an international reference standard for CD4+ cell counting would have to reconcile these different protocols, but once agreed might promote method harmonization. Another option is post reconstitution staining of lyophilized lymphocytes to support multiple immunophenotyping formats. However, post reconstitution staining would introduce an additional source of variation that would need to be assessed. Alternatively, producing different sLL prelabeled for all required markers is another approach, but one that would greatly increase costs.
The lyophilized lymphocytes evaluated in CCQM-P102 worked with a range of different manufacturers flow cytometers including volumetric instruments demonstrating commutability, with the exception of a single automated instrument. This was likely caused by the automated instruments' use of a viability dye which would have excluded sLL from analysis as these are dead cells due to fixation. With a manual flow cytometer it would only be necessary to open the viability gate in order for sLL to work, but typically settings are fixed on automated instruments. Similarly, a dual platform approach in which a hematology analyzer is used to obtain the total viable white cell count and a flow cytometer to calculate the percentage of CD4+ cells would not be suitable for this reference material for the same reason. However, it is generally not recommended to calculate the absolute CD4+ cell count using a dual platform system as differences in variation between laboratories is compounded (35,36).
A trend toward a reduction in CD4+ cell counts across vials for both dilutions was suggested by certain participants' data, but when all the results were tested by analysis of variance no significant vial to vial difference was found. Nevertheless, a post fill vial weight CV of 0.48% does contribute to vial to vial variability and is another source of uncertainty. Sedimentation of cells with time in sequentially read samples is another possibility that may explain reduced CD4+ cell counts across vials. However, analysis of variance of the time elapsed between tubes and CD4+ cell counts did not reveal any significant difference. When all uncertainties were taken into account the results were in good agreement for both dilutions and all tubes. Differences in the adhesion of sLL and calibration beads, dependent upon the type of containers used and the length of tubing within different instruments was not assessed in this pilot study, but it is suggested that this a major source of unaccounted for variation although no instrument clusters were identified. Neither was variation associated with different counting technologies or beads assessed, although it would be useful to know if these differ significantly. A well-characterized reference material for CD4+ cell counting could highlight issues with different approaches and instruments combinations potentially under or overestimating CD4+ cell counts. Harmonization of certain instrument specific differences between manufacturers may be another approach to reduce variation, for example, the length of tubing from the sample to the flow cell.
An internationally recognized reference standard for CD4+ cell counting with a defined clinically relevant range for HIV/AIDS does not exist. The sLL reference material described here could potentially meet that need. A proposal to the WHO Expert Committee on Biological Standardization to produce a lyophilized lymphocyte reference material for CD4+ cell counting has been accepted and is currently underway. Lyophilized lymphocytes compared to external quality assessment schemes with stabilized blood controls achieve similar outcomes; the advantage of lyophilized lymphocytes is their greater stability which reduces the challenges associated with shipping reference materials internationally.
Acknowledgments
The authors are indebted to Helen Parkes, Chair of CCQM/BAWG for her strong support of the study that lasted 2 years. The authors would like to thank Dr Sandrine Vessillier and Dr Luisa De Jesus Saraiva for their contribution to long-term stability studies of sLL. We acknowledge the contribution of Dr Carla Divieto, INRIM, Italy.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Supporting Information
Supporting Information Figures.
Supporting Information Tables.
Literature Cited
- 1.Fauci AS, Macher AM, Longo DL, Lane HC, Rook AH, Masur H, Gelmann EP. NIH conference. Acquired immunodeficiency syndrome: Epidemiologic, clinical, immunologic, and therapeutic considerations. Ann Intern Med. 1984;100:92–106. doi: 10.7326/0003-4819-100-1-92. [DOI] [PubMed] [Google Scholar]
- 2.Fahey JL, Taylor JM, Detels R, Hofmann B, Melmed R, Nishanian P, Giorgi JV. The prognostic value of cellular and serologic markers in infection with human immunodeficiency virus type 1. N Engl J Med. 1990;322:166–172. doi: 10.1056/NEJM199001183220305. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for a Public Health Approach. Geneva: World Health Organization; 2010. Available at: http://whqlibdoc.who.int/publications/2010/9789241599764_eng.pdf (last accessed on January 5, 2015) [PubMed] [Google Scholar]
- 4.Kaplan JE, Benson C, Holmes KK, Brooks JT, Pau A, Masur H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: Recommendations from CDC, the National Institutes of Health (NIH), the Centers for Disease Control and Prevention (CDC), and the HIV Medicine Association of the Infectious Disease Society of America (HIVMA/IDSA) Morbidity Mortality Weekly Rep. 2009;15:RR4. [PubMed] [Google Scholar]
- 5.Gilks CF, Crowley S, Ekpini R, Gove S, Perriens J, Souteyrand Y, Sutherland D, Vitoria M, Guerma T, De Cock K. The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet. 2006;368:505–510. doi: 10.1016/S0140-6736(06)69158-7. [DOI] [PubMed] [Google Scholar]
- 6.Schneider E, Whitmore S, Glynn MK, Dominguez K, Mitsch A, McKenna MT. Revised surveillance case definitions for HIV infection among adults, adolescents, and children aged <18 months and for HIV infection and AIDS among children aged 18 months to <13 years—United States, 2008. MMWR Recomm Rep. 2008;57:RR10. [PubMed] [Google Scholar]
- 7.World Health Organization. WHO Case Definitions of HIV for Surveillance and Revised Clinical Staging and Immunological Classification of HIV-Related Disease in Adults and Children. Geneva: World Health Organization; 2007. Available at: http://www.who.int/hiv/pub/guidelines/HIVstaging150307.pdf (last accessed on January 5, 2015) [Google Scholar]
- 8.Hirnschall G, Harries AD, Easterbrook PJ, Doherty MC, Ball A. The next generation of the World Health Organization's global antiretroviral guidance. J Int AIDS Soc. 2013;16:18757. doi: 10.7448/IAS.16.1.18757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. Geneva: World Health Organization; 2013. Available at: http://www.who.int/hiv/pub/guidelines/arv2013/en/index.html (last accessed on January 5, 2015) [PubMed] [Google Scholar]
- 10.Doherty M, Ford N, Vitoria M, Weiler G, Hirnschall G. The 2013 WHO guidelines for antiretroviral therapy: Evidence-based recommendations to face new epidemic realities. Curr Opin HIV AIDS. 2013;8:528–534. doi: 10.1097/COH.0000000000000008. [DOI] [PubMed] [Google Scholar]
- 11.Ogino MT, Dankner WM, Spector SA. Development and significance of zidovudine resistance in children infected with human immunodeficiency virus. J Pediatr. 1993;123:1–8. doi: 10.1016/s0022-3476(05)81529-x. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Laboratory Guidelines for enumerating CD4 T Lymphocytes in the context of HIV/AIDS. Geneva: World Health Organization; 2007. Available at: http://www.who.int/hiv/amds/LaboratoryGuideEnumeratingCD4TLymphocytes.pdf (last accessed on January 5, 2015) [Google Scholar]
- 13.Sherman GG, Galpin JS, Patel JM, Mendelow BV, Glencross DK. CD4+ T cell enumeration in HIV infection with limited resources. J Immunol Methods. 1999;222:209–221. doi: 10.1016/s0022-1759(98)00172-0. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Abbasi F, Ornatsky O, Cole KD, Misakian M, Gaigalas AK, He HJ, Marti GE, Tanner S, Stebbings R. Human CD4+ lymphocytes for antigen quantification: Characterization using conventional flow cytometry and mass cytometry. Cytometry Part A. 2012;81A:567–75. doi: 10.1002/cyto.a.22060. [DOI] [PubMed] [Google Scholar]
- 15. Technical Committee CEN/TC 140. In vitro diagnostic medical devices: Measurement of quantities in biological samples – Metrological traceability of values assigned to calibrators and control materials. EN ISO 17511:2003. Available at: http://www.iso.org/iso/catalogue_detail.htm?csnumber=30716 (last accessed on January 5, 2015)
- 16.Bureau International des Point et Mesures. Guidance document of the Joint Committee for Guides in Metrology JCGM 200:2012. 3rd. International vocabulary of metrology – Basic and general concepts and associated terms (VIM) Available at: http://www.bipm.org/utils/common/documents/jcgm/JCGM_200_2012.pdf (last accessed on January 5, 2015) [Google Scholar]
- 17.Lacroix R, Robert S, Poncelet P, Kasthuri RS, Key NS, Dignat-George F ISTH SSC Workshop. Standardization of platelet-derived microparticle enumeration by flow cytometry with calibrated beads: Results of the International Society on Thrombosis and Haemostasis SSC Collaborative workshop. J Thromb Haemost. 2010;8:2571–2574. doi: 10.1111/j.1538-7836.2010.04047.x. [DOI] [PubMed] [Google Scholar]
- 18.Maecker HT, Rinfret A, D'Souza P, Darden J, Roig E, Landry C, Hayes P, Birungi J, Anzala O, Garcia M, et al. Standardization of cytokine flow cytometry assays. BMC Immunol. 2005;6:13. doi: 10.1186/1471-2172-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicholson JK, Hubbard M, Dawson CD. Evaluation of stabilized whole blood control materials for lymphocyte immunophenotyping. Cytometry. 1999;38:268–273. doi: 10.1002/(sici)1097-0320(19991215)38:6<268::aid-cyto2>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 20.Glencross D, Scott LE, Jani IV, Barnett D, Janossy G. CD45-assisted PanLeucogating for accurate, cost-effective dual-platform CD4+ T-cell enumeration. Cytometry. 2002;50:69–77. doi: 10.1002/cyto.10068. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. 2006. Recommendations for the preparation, characterization and establishment of international and other biological reference standards (revised 2004). WHO Technical Report Series, No. 932. Available at: http://www.who.int/bloodproducts/publications/TRS932Annex2_Inter_biolefstandardsrev2004.pdf?ua=1 (last accessed on January 5, 2015)
- 22.Kammel M, Kummrow A, Neukammer J. Reference measurement procedure for the accurate determination of cell concentrations: Present status and future developments. J Lab Med. 2012;36:25–35. [Google Scholar]
- 23.Michel F, Sommer K, Spieweck F. Untersuchungen zur Ermittlung der Messunsicherheit von Kolbenhubpipetten mit Volumina von 1 μ L bis 50 μ L. PTB Mitt. 1995;105:437–444. [Google Scholar]
- 24.Levering WH, van Wieringen WN, Kraan J, van Beers WA, Sintnicolaas K, van Rhenen DJ, Gratama JW. Flow cytometric lymphocyte subset enumeration: 10 years of external quality assessment in the Benelux countries. Cytometry Part B Clin Cytom. 2008;74B:79–90. doi: 10.1002/cyto.b.20370. [DOI] [PubMed] [Google Scholar]
- 25.Bainbridge J, Wilkening CL, Rountree W, Louzao R, Wong J, Perza N, Garcia A, Denny TN. The immunology quality assessment proficiency testing program for CD3+4+ and CD3+8+ lymphocyte subsets: A ten year review via longitudinal mixed effects modeling. J Immunol Methods. 2014;409:82–90. doi: 10.1016/j.jim.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Detels R, Muñoz A, McFarlane G, Kingsley LA, Margolick JB, Giorgi J, Schrager LK, Phair JP. Effectiveness of potent antiretroviral therapy on time to AIDS and death in men with known HIV infection duration. JAMA. 1998;280:1497–1503. doi: 10.1001/jama.280.17.1497. [DOI] [PubMed] [Google Scholar]
- 27.Rodger AJ, Lodwick R, Schechter M, Deeks S, Amin J, Gilson R, Paredes R, Bakowska E, Engsig FN, Phillips A, et al. Mortality in well controlled HIV in the continuous antiretroviral therapy arms of the SMART and ESPRIT trials compared with the general population. AIDS. 2013;27:973–979. doi: 10.1097/QAD.0b013e32835cae9c. [DOI] [PubMed] [Google Scholar]
- 28.Pattanapanyasat K, Shain H, Noulsri E, Lerdwana S, Thepthai C, Prasertsilpa V, Likanonsakul S, Yothipitak P, Nookhai S, Eksaengsri A. A multicenter evaluation of the PanLeucogating method and the use of generic monoclonal antibody reagents for CD4 enumeration in HIV-infected patients in Thailand. Cytometry Part B Clin Cytom. 2005;65B:29–36. doi: 10.1002/cyto.b.20052. [DOI] [PubMed] [Google Scholar]
- 29.Janossy G, Jani IV, Bradley NJ, Bikoue A, Pitfield T, Glencross DK. Affordable CD4(+)-T-cell counting by flow cytometry: CD45 gating for volumetric analysis. Clin Diagn Lab Immunol. 2002;9:1085–1094. doi: 10.1128/CDLI.9.5.1085-1094.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lynen L, Teav S, Vereecken C, De Munter P, An S, Jacques G, Kestens L. Validation of primary CD4 gating as an affordable strategy for absolute CD4 counting in Cambodia. J Acquir Immune Defic Syndr. 2006;43:179–85. doi: 10.1097/01.qai.0000242447.82403.c2. [DOI] [PubMed] [Google Scholar]
- 31.Sippy-Chatrani N, Marshall S, Branch S, Carmichael-Simmons K, Landis RC, Abayomi A. Performance of the panleucogating protocol for CD4+ T cell enumeration in an HIV dedicated laboratory facility in Barbados. Cytometry Part B Clin Cytom. 2008;74B:S65–S68. doi: 10.1002/cyto.b.20406. [DOI] [PubMed] [Google Scholar]
- 32.Gelman R, Wilkening C. Analyses of quality assessment studies using CD45 for gating lymphocytes for CD3+4+% Cytometry. 2000;42:1–4. doi: 10.1002/(sici)1097-0320(20000215)42:1<1::aid-cyto1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 33.Mandy FF, Nicholson JK, McDougal JS CDC. Guidelines for performing single-platform absolute CD4+ T-cell determinations with CD45 gating for persons infected with human immunodeficiency virus. Centers for Disease Control and Prevention. MMWR Recomm Rep. 2003;52:1–13. [PubMed] [Google Scholar]
- 34.Barnett D, Bird G, Hodges E, Linch DC, Matutes E, Newland AC, Reilly JT. Guidelines for the determination of CD4+ T lymphocytes in immunosuppressed individuals. Clin Lab Haematol. 1997;19:231–241. doi: 10.1046/j.1365-2257.1997.00091.x. [DOI] [PubMed] [Google Scholar]
- 35.Robinson G, Morgan L, Evans M, McDermott S, Pereira S, Wansbrough-Jones M, Griffin G. Effect of type of haematology analyser on CD4 count. Lancet. 1992;340:485. doi: 10.1016/0140-6736(92)91807-k. [DOI] [PubMed] [Google Scholar]
- 36.Barnett D, Granger V, Whitby L, Storie I, Reilly JT. Absolute CD4+ T-lymphocyte and CD34+ stem cell counts by single platform flow cytometry: The way forward. Br J Haematol. 1999;106:1059–1062. doi: 10.1046/j.1365-2141.1999.01632.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information Figures.
Supporting Information Tables.


