Abstract
Type 1 interferon (IFN-I) promotes antigen-presenting cell maturation and was recently shown to induce hepatic IL-7 production during infection. Herein, we further explored the underlying mechanisms used by IFN-I to orchestrate antiviral immune responses in the liver. Acute viral hepatitis was induced by i.v. injection of adenovirus (Ad) in IFN-α receptor knockout (IFNAR−/−) and control mice. To disrupt signaling, monoclonal antibodies (mAbs) against IL-7 receptor alpha (IL-7Rα) or PD-L1 were i.p. injected. We found that CD8+ T cells in IFNAR−/− mice were less effective than those in control mice. The reduced T-cell function was accompanied by increased levels of PD-1 expression, apoptosis and decreased IFN-γ production. The lack of IFN-I signaling also impaired the expression of accessory molecules in both intrahepatic dendritic cell (DCs) and hepatocytes. PD-L1 was comparably and highly expressed on hepatocytes in both IFNAR−/− and control mice. Injection of PD-L1-specific mAb in IFNAR−/− mice reversed the compromised immune responses in the liver. Further investigation showed that hepatic IL-7 elevation was less pronounced in IFNAR−/− mice compared to the controls. A treatment with recombinant IL-7 suppressed PD-1 expression on CD8+ T cells in vitro. Accordingly, blocking IL-7R signaling in vivo resulted in increased PD-1 expression on CD8+ T cells in Ad-infected mice. Collectively, the results suggest that IFN-I-induced hepatic IL-7 production maintains antiviral CD8+ T-cell responses and homeostasis by suppressing PD-1 expression in acute viral hepatitis.
Keywords: CD8+ T cell, interleukin-7, PD-1, type 1 interferon, viral hepatitis
Introduction
Following acute viral infection, CD8+ T cells rapidly proliferate and produce cytokines and cytotoxic molecules to clear virus-infected cells and control viral replication.1,2 However, in several chronic virus infections such as hepatitis C virus infection in humans, virus-specific CD8+ T cells become functionally exhausted. The T cells have a reduced capacity to produce cytokines and effector molecules and exhibit impaired proliferation and survival. High expression of, program death 1 (PD-1), an inhibitory receptor in the CD28 superfamily, is the hallmark of the exhausted CD8+ T cells and contributes to the exhaustion of CD8+ T cells during chronic viral infection.3,4,5,6,7 In contrast to a relatively narrow range of PD-1 expression on activated T cells, its ligand, program death ligand 1 (PD-L1), was broadly expressed on a wide variety of cell types including hepatocytes.8,9,10,11,12 Ligation of PD-1 and PD-L1 reduces the host immune reaction against pathogens and maintains immune tolerance.4,13,14 Blocking the PD-1/PD-L1 pathway in mice chronically infected with lymphocytic choriomeningitis virus (LCMV) restores exhausted CD8+ T-cell proliferation, cytokine production, and cytotoxic activity. These changes lead to a reduced viral load.13 Although PD-1 expression is a hallmark of exhausted CD8+ T cells in chronic viral infection, the mechanisms that contribute to PD-1 expression in acute viral hepatitis are poorly understood, especially in the liver microenvironment.
Type 1 interferon (IFN-I) controls both acute and chronic viral infections. Currently, long-acting pegylated IFN is an important part of combination therapy with ribavirin and direct-acting hepatitis C virus protease inhibitors.15 In addition to directly suppressing viral replication and enhancing dendritic cell (DC) maturation,16 IFN-I is also able to stimulate hepatic IL-7 production.17 We have recently demonstrated that IFN-β induces hepatic IL-7 production shortly after adenoviral infection in the liver.18 However, it is unclear whether hepatic IL-7 expression is linked to local CD8+ T-cell functions or homeostasis. Emerging evidence suggests that in autoimmune type 1 diabetes, IL-7 suppressed PD-1 expression and maintained the survival of autoreactive T cells. Additionally, the inhibition of IL-7 alleviated diabetic diseases.19,20 Coincidently, clinical investigations reported that exhausted CD8+ T cells downregulated their cell surface expression of the α chain of the IL-7 receptor (IL-7R; CD127) in chronic hepatitis B virus and hepatitis C virus patients.14,21,22 Consistent with these observations, Pellegrini and colleagues23 demonstrated that IL-7 treatment decreased PD-1 expression and reversed T-cell exhaustion in chronic LCMV infection. These reports imply that IL-7/IL-7R signaling in CD8+ T cells may also affect their functions and fate in acute viral hepatitis.
Based on these reports,17,18,24 we hypothesized that IFN-I-induced IL-7 secretion in the liver suppresses PD-1 expression in CD8+ T cells and maintains their function and survival following acute viral infection. We tested this hypothesis using both wild type and IFN-α receptor knockout (IFNAR−/−) mice that were infected with hepatotropic adenovirus (Ad). In this model, liver injury reaches its peak in C57BL/6 mice on days 5–6 and then resolves spontaneously approximately 3–4 weeks later. We found that in the absence of IFN-I signaling, intrahepatic CD8+ T cells exhibited higher PD-1 expression and lower viability compared to wild type controls. The blockade of PD-1/PD-L1 significantly exacerbated Ad-induced liver injury in IFNAR−/− mice. Further studies revealed that hepatic IL-7 regulated PD-1 expression on CD8+ T cells. Collectively, the results of this study suggest that hepatic IFN-I-induced IL-7 maintains CD8+ T-cell responses and homeostasis by downregulating PD-1 expression in viral hepatitis.
Materials and methods
Animals
Breeding pairs of IFN-α receptor (IFNAR)−/− mice with a C57BL/6 background were kindly provided by Dr. Gregg N. Milligan (Department of Microbiology and Immunology, University of Texas Medical Branch, Galveston, TX, USA). Wild-type C57BL/6 mice were purchased from Taconic (Germantown, NY, USA). All mice were maintained and bred under specific pathogen-free conditions at the UTMB animal care facility and used at 7–10 weeks of age according to NIH Guidelines and with the approval of the IACUC. Ad carrying the lacZ gene (AdLacZ) was i.v. injected at 2×109 pfu/mouse to induce hepatitis as described previously.18
In vivo antibody blocking
To block the PD-1/PD-L1 pathway at the effector phase of Ad infection, Ad-infected mice were i.p. injected with 50 µg, monoclonal antibody (mAb) against PD-L1 (Clone: 10F.9G2, Biolegend, San Diego, CA, USA) on days 4 and 5 post-infection. To block the IL-7/IL-7R pathway, Ad-infected mice were i.p. injected with 100 µg anti-mIL-7Rα mAb (clone: SB/14, BD Bioscience, Franklin Lakes, NJ, USA) at days 0, 1, 3, and 5, post-infection. Mice were euthanized at day 6 post-infection. Normal rat IgG (Sigma, St. Louis, MO, USA) was administered as an isotype control.
Isolation of intrahepatic lymphocytes
Intrahepatic lymphocytes were isolated according to our previous method with slight modifications.18,25 Briefly, liver tissues were digested by collagenase IV (0.05%, Roche), followed by centrifugation (400g) at room temperature for 30 min over a 30%/70% discontinuous Percoll gradient (Sigma). The cells were collected from the interphase and then thoroughly washed. The cells were resuspended in complete RPMI-1640 medium containing 10% Fetal Bovine Serum (HyClone, Logan, UT, USA).
In vitro CD8+ T-cell stimulation
Splenocytes were incubated with biotin-labeled anti-CD4 mAb only or with biotin-labeled anti-CD4, anti-B220, anti-CD11b, anti-CD49b, anti-F4/80 and anti-CD11c mAbs together. The cells were then incubated with avidin-conjugated magnetic beads. The resulting fractions were labeled with Carboxyfluorescein succinimidyl ester (CFSE, 5 µM) according to a previously described method.25 Purified CD8+ T cells were stimulated with plate-coated anti-CD3 (2C11, 5 µg/ml) with or without anti-CD28 (37.51, plate-coated, 2 µg/ml). CD4+ T cell-depleted splenocytes were stimulated with plate-coated anti-CD3 for 72 h in the presence or absence of IL-7 (20 ng/ml; Peprotech, Rocky Hill, NJ, USA) or IFN-β (3 ng/ml; Peprotech).
Intracellular cytokine detection
The methods of intracellular staining were consistent with those of previous reports.18 Briefly, cells were incubated for 4 h with PMA (50 ng/ml) and ionomycin (750 ng/ml) in the presence of GolgiStop (BD Bioscience). After incubation, the cells were collected for further surface and intracellular staining before analysis by flow cytometry.
Flow cytometry
Murine lymphocytes were blocked with anti-mCD16/CD32 (eBioscience, San Diego, CA, USA) and stained with fluorochrome-labeled antibodies or biotinylated mAbs. The cells were then incubated with fluorochrome-conjugated streptavidin before analysis using a LSRII FACSFortessa cell analyzer (Becton Dickinson, San Jose, CA, USA). The data were analyzed with FlowJo software (TreeStar, Ashland, OR, USA). All fluorochrome-labeled mAbs and their corresponding isotype controls were purchased from BD Pharmingen (San Diego, CA, USA) and eBioscience.
Real-time PCR
Total RNA was extracted from the hepatocytes with an RNAqueous kit and digested with DNase I (Ambion, Austin, TX, USA).26 Quantitative RT-PCR assays were performed with iQ SYBR Green Supermix and a CFX96 Touch Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). The sequences of the forward and reverse gene-specific primers used were the following: GAPDH: forward 5′-TGGAAAGCTGTGGCGTGAT-3′, reverse 5′-TGCTTCACCACCTTCTTGAT-3′ IL-7: forward 5′-TTCCTCCACTGATCCTTGTTCT-3′, reverse 5′-AGCAGCTTCCTTTGTATCATCAC-3′ IL-15: forward 5′-CATCCATCTCGTGCTACTTGTG-3′, reverse 5′-GCCTCTGTTTTAGGGAGACCT-3′.
Statistical analysis
A two-tailed Student's t-test was used for statistical analyses. Analysis of variance was performed using ANOVA. All P values <0.05 were considered significant (*), and P<0.01 was considered highly significant (**).
Results
Lack of IFN-I signaling increases PD-1 level in hepatic CTLs
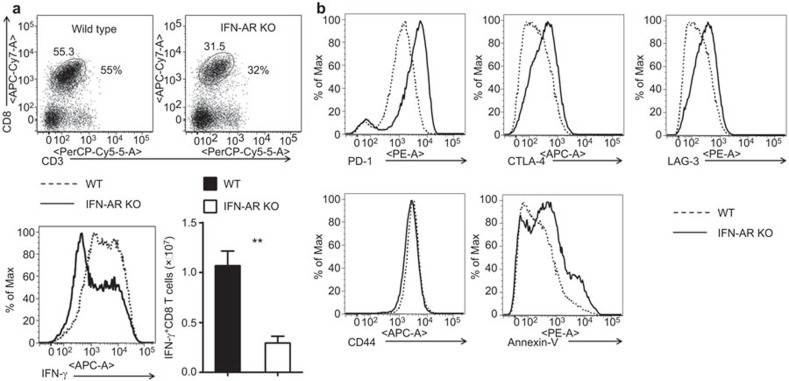
Ad infection in the control mice resulted in inflammatory infiltration, hepatocytes with megaloblastic changes and single-cell necrosis at day 6. However, the lack of IFN-I signaling in IFNAR−/− mice significantly decreased intrahepatic lymphocyte (IHL) infiltration and led to abated histopathological changes in the liver.18,27 IFNAR−/− mice showed a lower percentage of cytotoxic T lymphocyte (CTL) recruited into the liver (32% vs. 55%), and the liver-infiltrating cells had impaired IFN-γ production at day 6 post-infection (Figure 1a). Compared with wild-type controls, infiltrated intrahepatic CD8+ T cells in IFNAR−/− animals expressed significantly higher levels of inhibitory molecules such as PD-1, CTLA-4 and LAG-3 on their surface (Figure 1b). In contrast, CD44 expression on intrahepatic CD8+ T cells was comparable in WT and IFNAR−/− mice. Moreover, we found higher levels of Annexin-V staining among intrahepatic CD8+ T cell in IFNAR−/− animals, which indicated their pro-apoptotic status (Figure 1b).
Figure 1.
Intrahepatic CD8+ T cells expressed high level of PD-1 in IFNAR−/− mice infected by Ad. Wild-type and IFNAR−/− mice were i.v. infected with Ad and euthanized at day 6. (a) Top: flow cytometric examination of CD8+ T in IHLs; bottom-left: intracellular IFN-γ in CD3+CD8+ gate; bottom-right: numbers of IFN-γ+CD8+ T cells. (b) Surface staining of PD-1, CTLA-4, LAG-3, CD44 and Annexin V on CD8+ T in IHLs. N=6–8 mice per group. **P<0.01. Ad, adenovirus; IFN, interferon; IHL, intrahepatic lymphocyte; PD-1, program death 1.
Hepatocytes express PD-L1 in the liver
PD-L1 is expressed on APCs, T and B cells along with other hematopoietic cells. Upon activation, PD-L1 is also be expressed on endothelial, epithelial cells and hepatocytes.8,11,28 In this study, we isolated intrahepatic DCs from IFNAR−/− and control mice and analyzed their PD-L1 and other accessory molecular expression by flow cytometry at day 6 following Ad infection. The results indicate that DCs from IFNAR−/− mice expressed decreased levels of MHC-II and costimulatory molecules CD80, CD86 and CD40 compared with their wild-type counterparts (Figure 2a). PD-L1 expression levels on the intrahepatic DCs were also decreased due to the lack of IFN-I signaling.
Figure 2.
Differential PD-L1 expression on hepatic DCs and hepatocytes. Both wild-type mice and IFNAR−/− mice were injected i.v. with 2×109 pfu of AdLacZ and euthanized at day 6 post-infection. (a) Overlay analysis of MHC-II, CD80, CD86, CD40 and PD-L1 on hepatic DCs. (b) Overlay analysis of MHC-II, CD80, CD86, CD40 and PD-L1 on hepatocytes. Scaling was determined by isotype control staining in individual samples. Representative flow cytometric results are shown. N=6–8 mice per group. The experiments were repeated at least twice and resulted in the same pattern. Ad, adenovirus; DC, dendritic cell; IFN, interferon; PD-L1, program death ligand 1.
Hepatocytes are the most abundant cells in the liver. Upon inflammatory stimulation, hepatocytes act as antigen-presenting cells through the expression of accessory molecules. We purified primary hepatocytes from Ad-infected mice and found that hepatocytes in wild type mice expressed MHC-II, co-stimulatory molecules CD80, CD86 and PD-L1 (Fig. 2B, upper panel) at day 6 post-infection. However, in IFNAR−/− mice there were lower levels of hepatic CD80, CD86, CD40 and MHC-II expression but higher levels of PD-L1 expression (Figure 2b, lower panel).
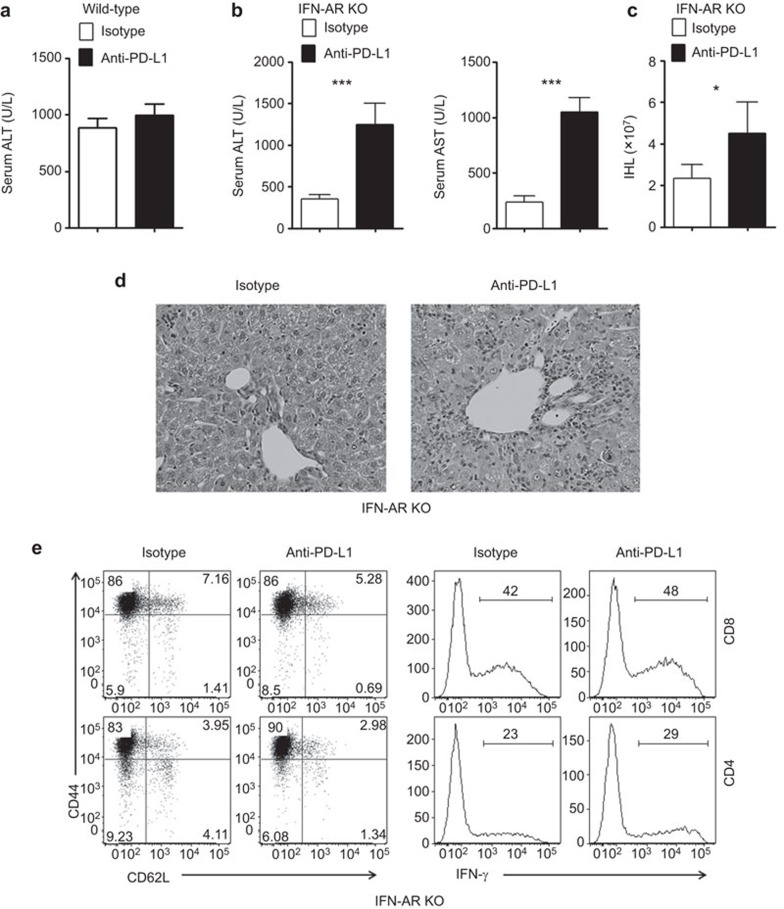
Blockade of PD-1/PD-L1 interaction reverses CD8+ T-cell compromise in IFNAR−/− mice
PD-1 was highly expressed on intrahepatic CD8+ T cells in IFNAR−/− mice after Ad infection. To confirm the relative contributions of PD-1/PD-L1 interaction in vivo, we infected the wild-type and IFNAR−/− mice and then treated the animals with two i.p. injections of anti-PD-L1 mAb on days 4 and 5. All animals were euthanized on day 6. We found that anti-PD-L1 treatment had no significant impact on hepatitis in wild-type animals (Figure 3a). However, anti-PD-L1 treatment significantly increased the levels of serum ALT, AST and IHL numbers (Figure 3b and c). PD-1/PD-L1 signaling blockade partially reversed a compromised T cell-mediated inflammatory response in the liver of IFNAR−/− mice (Figure 3d). The blockade of PD-L1/PD-1 signaling only slightly expanded CD44hiCD62LloCD4+ T cells and IFN-γ+ CD4+ and CD8+ T-cell populations (Figure 3e). Together, these results suggest that the PD-L1/PD-1 interaction modulates CTL recruitment and survival but not their effector functions in the inflammatory liver lesions.
Figure 3.
PD-L1 antibody strengthened the intrahepatic antiviral immune reaction in IFN-AR KO mice. Wild-type (a) and IFNAR−/− mice (b–e) were injected i.v. with 2×109 pfu of AdLacZ and i.p. with anti-PD-L1 at days 4 and 5 post-infection. Mice were euthanized at day 6 post-infection. (a) Serum ALT of wild type mice. (b) Serum ALT and AST. (c) Intrahepatic lymphocyte numbers. The mean±s.d. is shown. (d) Representative H&E staining. (e) Flow cytometric dot plot showing the CD44 and CD62L expression (left) or the intracellular IFN-γ expression (right) of intrahepatic CD8+ and CD4+ cells. N=6–8 mice per group. *P<0.01, ***P<0.001. Ad, adenovirus; DC, dendritic cell; IFN, interferon; KO, knockout; PD-L1, program death ligand 1.
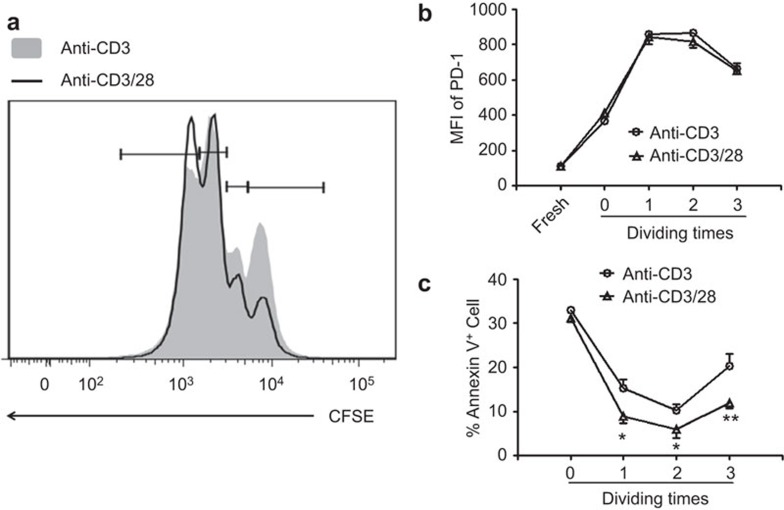
Lack of costimulatory signaling impair the survival but not the PD-1 expression of activated CD8+ T cells
Type I IFN signaling is important for the maturation and activation of antigen-presenting cells in viral hepatitis.18,27,29 We then hypothesized that insufficient co-stimulatory signaling might be responsible for this high expression of PD-1 on CD8+ T cells. We found that without accessory signaling, CD8+ T cells had robust proliferation (Figure 4a). In addition to T cell activation, PD-1 expression was increased on CD8+ T cells. There were no differences of PD-1 expression found between anti-CD3 alone and anti-CD3 together with anti-CD28 stimulations (Figure 4b). However, Annexin V staining revealed that in the absence of accessory signaling, the survival of CD8+ T cells was significantly decreased compared to fully activated cells (Figure 4c).
Figure 4.
Accessory signaling did not influence PD-1 expression on activated CD8+ T cells. Purified naive CD8 T cells were stimulated with anti-CD3 in the presence or absence of anti-CD28. (a) CFSE dilution of CD8 T+ cells. The experiments were repeated at least three times with a similar pattern. Representative overlay pictures are shown. (b) MFI of PD-1 expression on CD8+ T cells. (c) Living cell percentage determined by Annexin-V staining. For b and c, the cumulative statistical results pooled from three experiments are shown. *P<0.01, ***P<0.001. MFI, mean fluorescence intensity; PD-1, program death 1.
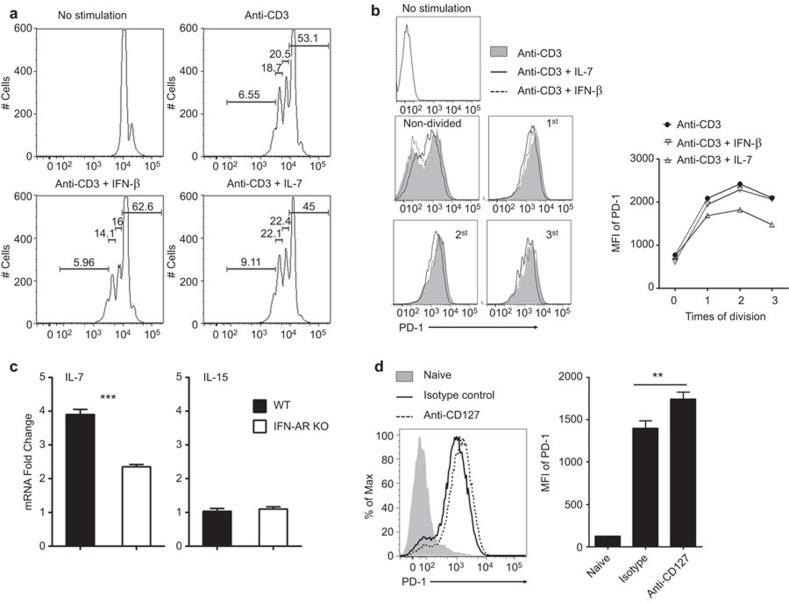
IL-7 signaling represses PD-1 expression during CD8+ T-cell activation in Ad-induced hepatitis
We and others have recently found that hepatocytes produce large amounts of IL-7, a pleiotropic cytokine regulating T-cell homeostasis,30,31 in response to Toll-like receptor 4 (TLR4) and Type I interferon (IFN-I) signaling.17,18 To further explore its modulatory effects on T-cell function in viral hepatitis, we examined whether IL-7 inhibits PD-1 expression on T cells. We partially purified splenic CD8+ T cells from C57BL/6 mice and in vitro stimulated them with anti-CD3 mAb in the presence or absence of either IFN-β or IL-7. We found that anti-CD3 stimulation resulted in 47% CD8+ T-cell proliferation in control groups (Figure 5a). IFN-β slightly suppressed the proliferation of CD8+ T cells with 37% proliferating (Figure 5a). IFN-β exerted no influence on PD-1 expression in activated CD8+ T cells (Figure 5b). In contrast to IFN-β, IL-7 stimulation enhanced the proliferation of CD8+ T cells (55% proliferated) and simultaneously suppressed PD-1 expression (Figure 5a and b).
Figure 5.
IL-7 suppressed PD-1 expression on CD8+ T cells. For a and b, CD4-depleted splenocytes from naive C57BL/6 mice were stimulated with anti-CD3 in the presence of IFN-β or IL-7. (a) CFSE dilution of CD8 T cells. (b) The flow cytometric histogram and MFI of PD-1 expression on CD8+ T cells. The experiments were repeated at least 3 times with a similar pattern. For c and d, wild-type and IFNAR−/− mice were i.v. infected with Ad and euthanized at day 6. (c) IL-7 and IL-15 mRNA expression in the liver, compared with naïve control, respectively. (d) PD-1 expression (left: flow cytometric histogram; right: MFI) on intrahepatic CD8+ T cells from anti-IL-7Rα mAb-treated C57BL/6 mice infected with Ad. The cumulative statistical results are shown. N=5 to 8 mice per group. **P<0.01. Ad, adenovirus; IFN, interferon; mAb, monoclonal antibody; MFI, mean fluorescence intensity; PD-1, program death 1.
To examine the potential cell types that produced IL-7 in the liver, we infected wild-type C57BL/6 mice with 2×109 pfu adenovirus. At 12 h post-infection, we purified hepatocytes, total intrahepatic lymphocytes, splenic CD4+ cells, CD8+ cells and natural killer (NK) cells. We found that hepatocytes expressed the highest levels of IL-7 mRNA (data not shown). Based on the data indicating that hepatocytes could produce IL-7 in response to IFN-I signaling 17,18 and that IL-7 suppressed PD-1 expression on CD8+ T cells in vitro (Figure 5a and b), we then asked whether IL-7 affects PD-1 expression on CD8+ T cells in vivo during acute viral infection. We found that on day 6 of infection, hepatic IL-7 expression levels increased significantly in the control mice. However, the lack of IFN-I signaling significantly dampened IL-7 expression in the liver (Figure 5c). This IFN-I-related cytokine dysregulation was not ubiquitous in the liver because IL-15, a cytokine known to maintain the peripheral T-cell homeostasis, was not affected in the liver of IFNAR−/− mice (Figure 5c). We then generated Ad-induced hepatitis in wild-type mice and treated these animals with anti-CD127 mAb. As shown in Figure 5c, in vivo blockade of IL-7/IL-7R interaction significantly increased the expression of PD-1 on the surface of intrahepatic CD8+ T cells on day 6 post-infection.
Discussion
CD8+ T cells are primed by APCs in the draining lymph nodes following hepatotropic Ad infection. After activation, these T cells infiltrate the liver and clear both infected cells and bystanders.32,33,34 During the contraction phase, CD8+ T cells undergo apoptosis and are deleted in the liver. If this process is selectively impeded, then accelerated hepatocyte damage may occur.25,35 Many cell types and pathways are involved in regulating T-cell functions and homeostasis in the liver. However, the local molecular interactions that determine hepatic T-cell homeostasis are poorly understood. For instance, it is not clear whether local IFN-I maintains effector functions and viability of activated T cells in the liver beyond its direct antiviral and DC-licensing activities.16
IL-7 is a key cytokine used to maintain the homeostasis and survival of effector T cells in the periphery.36,37,38 It is secreted by stromal cells in the bone marrow and thymus.30 Recently, we and others reported that hepatocytes could produce IL-7 that functions both locally and systemically.17,18,24 In murine autoimmune diabetes models, the blockade of IL-7R upregulated PD-1 expression in autoreactive T cells, and the administration of recombinant IL-7 suppressed PD-1 levels.19,20 In chronic LCMV infection, recombinant IL-7 treatment decreased PD-1 expression and reversed the exhaustion of antiviral CD8+ T cells.23 Although animals showed clinical signs with the systemic administration of IL-7, there is no direct evidence that endogenous IL-7 regulates antiviral immune responses in the liver. Therefore, additional research is required to further elucidate the role of hepatocyte-derived IL-7 in viral hepatitis. In this study, we found that in the absence of IFN-I signaling, intrahepatic CD8+ T cells expressed high levels of PD-1 and were functionally impaired and apoptotic (Figure 1). We also analyzed the PD-1 expression on splenic CD8+ T cells in both groups of mice. Interestingly, wild-type mice had a higher percentage of PD-1hiCD8+ T cells than IFNAR−/− animals (data not shown). These results clearly suggest that in response to viral infection, CD8+ T cells display unique phenotypes and functions in different organs. This conclusion is consistent with the data indicating that the liver is the primary target organ of i.v. infused adenovirus, and the spleen is an important peripheral lymphoid organ where antigen-presentation and T-cell priming take place. Thus, it is possible that high percentages of antigen-specific CD8+ effector cells are recruited to the infected mouse liver and upregulate PD-1. In the spleen, CD8+ T cells from wild-type animals proliferate, and clonal expansion occurs following encounters with professional APCs. These cells display low levels of PD-1 in the presence of IL-2 and serve as an important source of antiviral T cells for the peripheral organs such as the liver. Based on these results and previous studies,3,4,5,6,7 we speculated that the observed CD8+ T-cell impairment in IFNAR−/− mice was partially attributable to insufficient hepatic IL-7. We found that hepatic IL-7 expression was significantly reduced in these mice following viral infection, and PD-1 levels on intrahepatic CD8+ T cells were increased (Figure 5c and b). Coculture experiments revealed that IL-7 could directly suppress PD-1 expression and maintain the survival of activated CD8+ T cells (Figure 5, and data not shown). In vivo IL-7R signaling inhibition significantly increased the PD-1 levels on CD8+ T cells during Ad infection in the liver (Figure 5).
PD-1 can bind to two ligands, PD-L1 and PD-L2. PD-L1 is expressed on a variety of cell types including many hematopoietic cells and hepatocytes. PD-L2 is a secondary ligand for PD-1, and its expression is restricted to DCs and a few tumor cell lines.8,9,10,11,12 Emerging evidence suggests that PD-L1 expression on parenchymal cells protects against autoimmune diseases and mediates tissue tolerance.3,8 The same strategy was used by tumor cells to suppress adaptive immunity and avoid immune surveillance. The liver is a quiescent and immune-tolerant organ. Dong and colleagues39 reported that in PD-L1-deficient mice, antigen-specific CD8+ T cells proliferated normally. However, during the immune contraction phase, their apoptosis was selectively impaired in the liver. Furthermore, Mühlbauer and colleagues11 provided the in vitro evidence that a hepatocyte-like cell line expressed PD-L1 in response to viral infection and induced T cell apoptosis. In this study, we found that IFNAR−/− mice upregulate PD-L1 expression on hepatocytes, and the level was comparable to control animals. However, their MHC-II and costimulatory molecular expression was more dependent on the IFN-I signaling pathway (Figure 2). The immune impairment in IFNAR−/− mice can be reversed by blocking PD-L1 and PD-1 interaction (Figure 3). Interestingly, this disruption of PD-1 engagement sustained and augmented the numbers of highly activated CD8+ and CD4+ T cells and other cells expressing IFN-γ. Based on these results, we conclude that in the absence of IFN-I signaling, hepatocytes do not fully support highly activated CD8+ T cells in the liver by decreasing IL-7 secretion and increasing PD-L1 expression. Hepatic PD-L1 expression and its engagement with elevated PD-1 on CD8+ T cells are partially responsible for the immune contraction and T-cell exhaustion following viral clearance.
We compared the viral clearance at 6 day post-infection and found no statistically significant difference in viral copy numbers between IFNAR−/− and control mice (data not shown). These findings are consistent with previous reports that most adenovirus is eliminated rapidly by the innate immune system 24 h post-infection. Although day 6 was the peak of adaptive immune response and clinical manifestations, nearly all of the adenovirus had been cleared at this stage. The remaining virus was slowly cleared by T cells in the following weeks. Due to the lack of differences in viral load between IFNAR−/− and control mice, the elevated levels of PD-1 on T cells in IFNAR−/− mice were not attributable to the viral load.
Collectively, this study showed that in addition to enhancing DCs maturation, IFN-I directly affects hepatocytes and the adaptive anti-viral immune response through a highly coordinated expression of surface molecules and secretion of survival cytokines such as IL-7. Additional investigations are needed to further explore the impact of the hepatic IFN-I/IL-7 cascade on the function and fate of intrahepatic CD8+ T cells in other chronic viral hepatitis models.
Acknowledgments
We thank Ms Mardelle Susman for her editorial assistance. None of the authors has a conflict of interest. LH, ZJ, YL and DM performed the experiments; LH, ZJ, YL, LS and JS analyzed the data: LH, YL, DM, LS and JS wrote the paper. This work was supported by the National Institutes of Health grant AI109100 (JS). ZJ was in part supported by the James W. McLaughlin Fellowship and the UTMB Graduate School of Biomedical Sciences.
The authors have declared no competing interests.
References
- 1Yang Y, Ertl HC, Wilson JM. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity 1994; 1: 433–442. [DOI] [PubMed] [Google Scholar]
- 2Guidotti LG, Borrow P, Hobbs MV, Matzke B, Gresser I, Oldstone MB et al. Viral cross talk: intracellular inactivation of the hepatitis B virus during an unrelated viral infection of the liver. Proc Natl Acad Scie USA 1996; 93: 4589–4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Iwai Y, Terawaki S, Ikegawa M, Okazaki T, Honjo T. PD-1 inhibits antiviral immunity at the effector phase in the liver. J Exp Med 2003; 198: 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J Virol 2007; 81: 9249–9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Hofmeyer KA, Jeon H, Zang X. The PD-1/PD-L1 (B7-H1) pathway in chronic infection-induced cytotoxic T lymphocyte exhaustion. J Biomed Biotechnol 2011; 2011: 451694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol 2003; 77: 4911–4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Day CP. From fat to inflammation. Gastroenterology 2006; 130: 207–210. [DOI] [PubMed] [Google Scholar]
- 8Mueller SN, Vanguri VK, Ha SJ, West EE, Keir ME, Glickman JN et al. PD-L1 has distinct functions in hematopoietic and nonhematopoietic cells in regulating T cell responses during chronic infection in mice. J Clin Invest 2010; 120: 2508–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Blackburn SD, Crawford A, Shin H, Polley A, Freeman GJ, Wherry EJ. Tissue-specific differences in PD-1 and PD-L1 expression during chronic viral infection: implications for CD8 T-cell exhaustion. J Virol 2010; 84: 2078–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med 2006; 203: 883–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Mühlbauer M, Fleck M, Schütz C, Weiss T, Froh M, Blank C et al. PD-L1 is induced in hepatocytes by viral infection and by interferon-α and -β and mediates T cell apoptosis. J Hepatol 2006; 45: 520–528. [DOI] [PubMed] [Google Scholar]
- 12Pinchuk IV, Saada JI, Beswick EJ, Boya G, Qiu SM, Mifflin RC et al. PD-1 ligand expression by human colonic myofibroblasts/fibroblasts regulates CD4+ T-cell activity. Gastroenterology 2008; 135: 1228–1237.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006; 439: 682–687. [DOI] [PubMed] [Google Scholar]
- 14Radziewicz H, Ibegbu CC, Fernandez ML, Workowski KA, Obideen K, Wehbi M et al. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J Virol 2007; 81: 2545–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15Pawlotsky JM, de Erik C. Advances in Pharmacology. Vol. 67. Chapter 5: Hepatitis C Virus: Standard-of-Care Treatment.New York: Academic Press, 2013: 169–215. [DOI] [PubMed] [Google Scholar]
- 16Le Bon A, Etchart N, Rossmann C, Ashton M, Hou S, Gewert D et al. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat Immunol 2003; 4: 1009–1015. [DOI] [PubMed] [Google Scholar]
- 17Sawa Y, Arima Y, Ogura H, Kitabayashi C, Jiang JJ, Fukushima T et al. Hepatic interleukin-7 expression regulates T cell responses. Immunity 2009; 30: 447–457. [DOI] [PubMed] [Google Scholar]
- 18Hou L, Jie Z, Desai M, Liang Y, Soong L, Wang T et al. Early IL-17 production by intrahepatic T cells is important for adaptive immune responses in viral hepatitis. J Immunol 2013; 190: 621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Lee LF, Logronio K, Tu GH, Zhai W, Ni I, Mei L et al. Anti-IL-7 receptor-alpha reverses established type 1 diabetes in nonobese diabetic mice by modulating effector T-cell function. Proc Natl Acad Sci USA 2012; 109: 12674–12679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20Penaranda C, Kuswanto W, Hofmann J, Kenefeck R, Narendran P, Walker LS et al. IL-7 receptor blockade reverses autoimmune diabetes by promoting inhibition of effector/memory T cells. Proc Natl Acad Sci USA 2012; 109: 12668–12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21Golden-Mason L, Burton JR Jr, Castelblanco N, Klarquist J, Benlloch S, Wang C et al. Loss of IL-7 receptor alpha-chain (CD127) expression in acute HCV infection associated with viral persistence. Hepatology 2006; 44: 1098–109. [DOI] [PubMed] [Google Scholar]
- 22Lv G, Ying L, Ma WJ, Jin X, Zheng L, Li L et al. Dynamic analysis of CD127 expression on memory CD8 T cells from patients with chronic hepatitis B during telbivudine treatment. Virol J 2010; 7: 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23Pellegrini M, Calzascia T, Toe JG, Preston SP, Lin AE, Elford AR et al. IL-7 engages multiple mechanisms to overcome chronic viral infection and limit organ pathology. Cell 2011; 144: 601–613. [DOI] [PubMed] [Google Scholar]
- 24Tang XZ, Jo J, Tan AT, Sandalova E, Chia A, Tan KC et al. IL-7 licenses activation of human liver intrasinusoidal mucosal-associated invariant T cells. J Immunol 2013; 190: 3142–3152. [DOI] [PubMed] [Google Scholar]
- 25Yan J, Jie Z, Hou L, Wanderley JL, Soong L, Gupta S et al. Parenchymal expression of CD40 exacerbates adenovirus-induced hepatitis in mice. Hepatology 2011; 53: 1455–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26Liang Y, Jie Z, Hou L, Aguilar-Valenzuela R, Vu D, Soong L et al. IL-33 Induces nuocytes and modulates liver injury in viral hepatitis. J Immunol 2013; 190: 5666–5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27Zhu J, Huang X, Yang Y. Type I IFN signaling on both B and CD4 T cells is required for protective antibody response to adenovirus. J Immunol 2007; 178: 3505–3510. [DOI] [PubMed] [Google Scholar]
- 28Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol 2002; 169: 5538–5545. [DOI] [PubMed] [Google Scholar]
- 29Zhu J, Huang X, Yang Y. A critical role for type I IFN-dependent NK cell activation in innate immune elimination of adenoviral vectors in vivo. Mol Ther 2008; 16: 1300–1307. [DOI] [PubMed] [Google Scholar]
- 30Jiang Q, Li WQ, Aiello FB, Mazzucchelli R, Asefa B, Khaled AR et al. Cell biology of IL-7, a key lymphotrophin. Cytokine Growth Factor Rev 2005; 16: 513–533. [DOI] [PubMed] [Google Scholar]
- 31Mazzucchelli R, Durum SK. Interleukin-7 receptor expression: intelligent design. Nat Rev Immunol 2007; 7: 144–154. [DOI] [PubMed] [Google Scholar]
- 32Barbier L, Tay SS, McGuffog C, Triccas JA, McCaughan GW, Bowen DG et al. Two lymph nodes draining the mouse liver are the preferential site of DC migration and T cell activation. J Hepatol 2012; 57: 352–358. [DOI] [PubMed] [Google Scholar]
- 33Jooss K, Ertl HC, Wilson JM. Cytotoxic T-lymphocyte target proteins and their major histocompatibility complex class I restriction in response to adenovirus vectors delivered to mouse liver. J Virol 1998; 72: 2945–2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34Sun J, Tumurbaatar B, Jia J, Diao H, Bodola F, Lemon SM et al. Parenchymal expression of CD86/B7.2 contributes to hepatitis C virus-related liver injury. J Virol 2005; 79: 10730–10739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Huang L, Soldevila G, Leeker M, Flavell R, Crispe IN. The liver eliminates T cells undergoing antigen-triggered apoptosis in vivo. Immunity 1994; 1: 741–749. [DOI] [PubMed] [Google Scholar]
- 36Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol 2000; 1: 426–432. [DOI] [PubMed] [Google Scholar]
- 37Rubinstein MP, Lind NA, Purton JF, Filippou P, Best JA, McGhee PA et al. IL-7 and IL-15 differentially regulate CD8+ T-cell subsets during contraction of the immune response. Blood 2008; 112: 3704–3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38Schluns KS, Williams K, Ma A, Zheng XX, Lefrancois L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J Immunol 2002; 168: 4827–4831. [DOI] [PubMed] [Google Scholar]
- 39Dong H, Zhu G, Tamada K, Flies DB, van Deursen JM, Chen L. B7-H1 determines accumulation and deletion of intrahepatic CD8+ T lymphocytes. Immunity 2004; 20: 327–336. [DOI] [PubMed] [Google Scholar]