Abstract
Some pathogens can use host suppressor of cytokine signaling 1 (SOCS-1), an important negative-feedback molecule, as the main mode of immune evasion. Here we found that group A Streptococcus (GAS) is capable of inducing SOCS-1 expression in RAW264.7 and BMDM macrophages. IFN-β plays a role in GAS-induced SOCS-1 expression in macrophages following the induction of cytokine expression by GAS, representing the classical pathway of SOCS-1 expression. However, GAS also induced STAT1 activation and SOCS-1 expression when GAS-infected cells were incubated with anti-IFN-β monoclonal antibody in this study. Moreover, upon comparing TLR4−/− BMDM macrophages with wild-type (WT) cells, we found that TLR4 also plays an essential role in the induction of SOCS-1. MyD88, which is an adaptor protein for TLR4, contributes to STAT1 activation and phosphorylation by forming a complex with Janus kinase 1 (JAK1) and signal transducer and activator of transcription 1 (STAT1) in macrophages. GAS-stimulated expression of STAT1 was severely impaired in MyD88−/− macrophages, whereas expression of JAK1 was unaffected, suggesting that MyD88 was involved in STAT1 expression and phosphorylation. Together, these data demonstrated that in addition to IFN-β signaling and MyD88 complex formation, JAK1 and STAT1 act in a novel pathway to directly induce SOCS-1 expression in GAS-infected macrophages, which may be more conducive to rapid bacterial infection.
Keywords: Group A Streptococcus, macrophages, SOCS-1, TLR4/MyD88 signaling
Introduction
Group A Streptococcus (GAS), a Gram-positive pathogen, is the causative agent of ‘pharyngitis', impetigo and many other human respiratory tract or soft tissue infections. On infection, some components of GAS are recognized by Toll-like receptors (TLRs) that are expressed by macrophages. The process results in marked secretion of inflammatory cytokines, including IFN-β,1,2,3 which is important in inducing adaptive immunity. However, studies have previously shown that there are almost no first-line immune defense cells, such as macrophages and neutrophils, in serious GAS infections.4,5 These studies suggested that survival and multiplication of GAS might utilize a novel strategy to combat host innate and adaptive immunity.
Suppressor of cytokine signaling (SOCS) is one such type of intracellular negative-feedback molecule, which was initially identified based on its inhibitory effects on Janus kinase (JAK) and signal transducer and activator of transcription (STAT). The SOCS family of proteins consists of eight members: SOCS1-7 and CIS. All members share the same common structure with a central Src homology-2 domain, a C-terminal SOCS box, and an N-terminus that varies in length.6 SOCS-1 binds to JAKs by means of its Src homology-2 domain and a proximal kinase inhibitor region and directly inhibits kinase activity.6 In addition to inhibiting JAK/STAT signaling, SOCS-1 limits NF-κB signaling by destabilizing p65/RelA.7,8 Recent reports have suggested that pathogens could induce endogenous host SOCS-1 and exploit it for their mode of immune evasion. For example, Toxoplasma gondii induces expression of SOCS-1, which contributes to the proliferation of the bacteria within the hostile environment of macrophages.9,10 Mycobacteria and Burkholderia pseudomallei increase the expression of SOCS-1 in macrophages—a process that has been linked to immune escape.11,12
IFN-β is clearly one of the major activators of SOCS-1.13,14,15,16,17 Upon ligand binding, receptor-associated tyrosine kinases (JAK-1 and Tyk2) are activated, which is followed by phosphorylation of STAT1. Activated STAT1 then translocates to the nucleus to activate the transcription of target genes and induce protein expression of SOCS-1. However, LPS induces SOCS-1 expression without cytokine secretion, and based on experiments conducted in our laboratory, we determined that induction of SOCS-1 is a direct consequence of TLR stimulation.18,19
Here, we found that GAS enhances SOCS-1 levels in macrophages; however, SOCS-1 expression at the early stage of infection does not completely depend on activation of IFN-β. Thus, we speculate that there are alternative mechanisms to induce SOCS-1 expression in GAS-infected macrophages. By comparing wild-type (WT) BMDMs with TLR4−/− and MyD88−/− BMDMs, we determined that GAS itself induces SOCS-1 expression early during the process of infection through the TLR/MyD88 pathway, specifically forming a complex of MyD88, JAK1 and STAT1 in macrophages, as opposed to the classic pathway of IFN-β activation. Furthermore, we found that SOCS-1 expression was affected by activation of NF-κB.
Materials and methods
Reagents and antibodies
An antibody (Ab) to neutralize mouse IFN-β was obtained from R&D Systems, (Emeryville, CA, USA). Antibodies targeted to p-p65, STAT1, SOCS-1, JAK1, p-JAK1 and phosphotyrosine-specific STAT1 (Tyr701) Ab were obtained from Cell Signaling Technology (Boston, Massachusetts, USA). Anti-MyD88 Ab was obtained from Santa Cruz biotechnology, Inc. (Santa Cruz, CA, USA). Cycloheximide (CHX) was obtained from Sigma (St. Louis, MO, USA). The NF-κB activation inhibitor JSH-23 was obtained from Sigma (St. Louis, MO, USA), and control IgG antibody was obtained from Cell Signaling Technology.
Cell culture
The mouse macrophage RAW 264.7 cell-line was cultured in Dulbecco's modified Eagle's medium (Carlsbad, CA, USA) supplemented with 10% heat-inactivated fetal bovine serum (Logan, UT, USA) at 37 °C under a 5% CO2 atmosphere. Primary BMDMs were obtained from the femur bone marrow of 6- to 10-week-old C57BL/6 mice. Cells were cultured in RPMI-1640 medium supplemented with 10% fetal calf serum in the presence of L-cell derived CSF-1. TLR4−/−, MyD88−/− and control WT mice, all of which were on the C57BL/6 genetic background, were housed under specific pathogen-free conditions.
Streptococcus pyogenes (GAS, strain M190-226) were grown in Todd-Hewitt broth containing 1% yeast extract at 37 °C. Solid media used for culturing GAS were either Todd-Hewitt or sheep blood agar. GAS cells were washed twice with PBS before use. Non-viable GAS were prepared by heating bacteria to 70 °C for 60 min.4,5
Macrophages infected with GAS
An overnight culture of RAW 264.7 cells (at a density of 2×106 cells/well) in a six-well plate was cocultured with bacteria at a multiplicity of infection (MOI) of 100∶1 for 1 h. To remove extracellular bacteria, the cells were washed five times in PBS and were then incubated in DMEM containing 1000 U/ml penicillin and 1000 U/ml streptomycin for 1 h. The remaining infected cells were allowed to grow in DMEM. BMDMs were infected by GAS as described above.
RNA preparation and quantitative real-time RT-PCR
Total RNA was isolated using an RNeasy kit (Waltham, MA, USA) in accordance with the manufacturer's protocol. Real-time PCRs were performed in 20 µl reaction volumes containing 2.0 µl cDNA, 0.4 µl of each primer, 6.0 µl dH2O, 0.4 µl ROX reference dye, and 10 µl fluorescent SYBGREEN (TaKaRa Bio Inc. Tokyo, Japan.). Amplification was carried out in 96-well optical plates on a 7300 real-time PCR system (Carlsbad, CA, USA) with a 30-s incubation at 95 °C followed by 45 cycles of 95 °C for 5 s and 60 °C for 60 s. The sequences of the primers used were as follows:
β-actin-F: TACCCAGGCATTGCTGACAGG
β-actin-R: ACTTGCGGTGCACGATGGA
SOCS-1-F: CTCCGTGACTACCTGAGTTCCT
SOCS-1-R: ATCTCACCCTCCACAACCACT
TLR-2-F: TTCAACAAGATCACCTACATTGGC
TLR-2-R: CAAGACTGCCCAGAGAATAAAAGG
TLR-4-F: CTGATGACATTCCTTCTTCAACC
TLR-4-R: TTTCCTGTCAGTATCAAGTTTGAG
IFN-β-F: AGAGTTACACTGCCTTTGCCATCC
IFN-β-R: CCACGTCAATCTTTCCTCTTGCTT
Each sample was analyzed in triplicate. The 2−ΔΔCt method of relative quantification was used to calculate changes in the expression of target genes.
Western blotting
Following infection with GAS, cells (at a density of 2×106 cells/well) were lysed for 50 min on ice in 100 µl of lysis buffer (containing PMSF and a phosphatase inhibitor). Lysates were centrifuged at 12 000g for 15 min at 4 °C. Equal amounts of cell lysates were separated by 10% SDS-PAGE and electrotransferred to polyvinylidene difluoride membranes. Proteins were detected using an ECL system (Logan, UT, USA). The intensity of each blot was measured with the densitometry program Quantity One (Bio-Rad Laboratories, Hercules, CA, USA). Each experiment was repeated three times, and similar results were obtained.
Immunofluorescence microscopy
Cells were grown on glass coverslips. After overnight incubation, the cells were fixed in 4% paraformaldehyde and blocked in 1% BSA (St. Louis, MO, USA) for 1 h at 37 °C. After four rinses, cells were incubated with monoclonal anti-MyD88 (1∶100), polyclonal anti-JAK1 or anti-STAT1 (1∶100) antibodies overnight at 4 °C. After four rinses, FITC-conjugated and TRITC-conjugated secondary antibodies were used to visualize the proteins by fluorescence microscopy (Tokyo, Japan). The nuclei were counter-stained with DAPI.
Immunoprecipitation (IP)
The lysed cell extracts (100 µg total protein) were combined with 50 µl of protein G sepharose beads and 2 µg of primary antibody and incubated under continuous rotation at 4 °C overnight. Beads were washed four times with IP buffer for 5 min at 2500g and at 4 °C. Next, 1×SDS buffer was added to the samples, which were then heated at 100 °C for 10 min. Immunoprecipitated samples were analyzed by western blotting using anti-MyD88, anti-Jak1, anti-Stat1, anti-pJAK1 or anti-pSTAT1 Abs.
Statistical analysis
Statistical analysis was performed using the SPSS 15.0 software package. Results were considered statistically significant if a probability of less than 0.05 was obtained.
Results
GAS induces significant SOCS-1 up-regulation in macrophages at the early stage
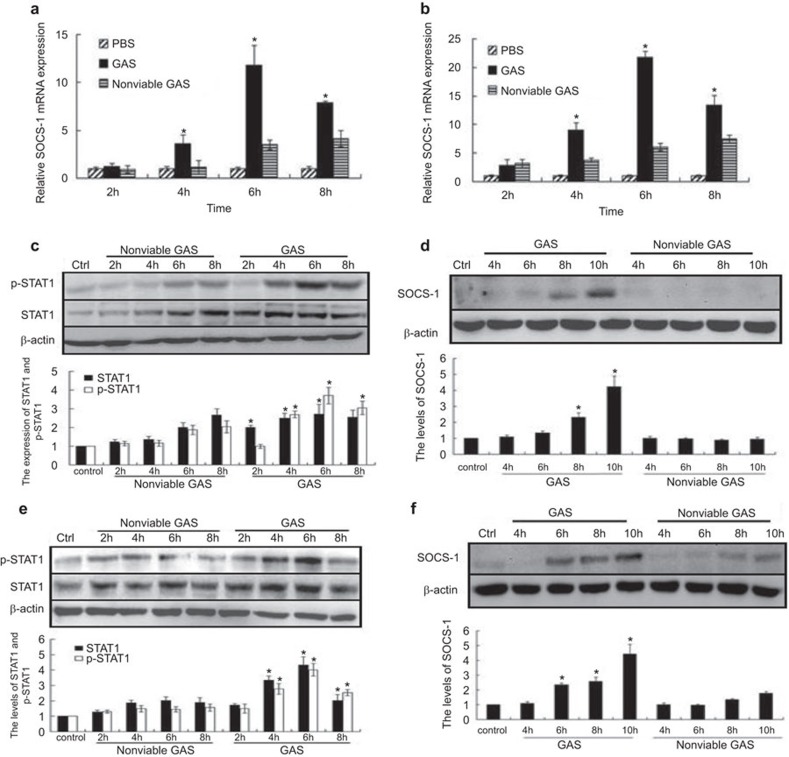
We showed that GAS reduced the levels of inflammatory factors (IL-6, IL-1β and TNF-α) 18–24 h post-infection in macrophages compared with the Staphylococcus aureus group (G+ bacterial control) (Supplementary Figure 1), although the mechanism responsible remains unclear. It has been previously confirmed that SOCS-1 proteins regulate cytokine communication in macrophages, and microbes exploit the host SOCS system to evade immunity.9,10,11,12,20 To investigate whether SOCS-1 proteins were produced in GAS-induced macrophages, we looked for SOCS-1 expression in GAS-infected RAW 264.7 cells, which is a well-described mouse macrophage model. The results demonstrated that the SOCS-1 mRNA level was elevated at 4 h after GAS infection and peaked at 6 h (reaching an approximate 12-fold induction). After 8 h, SOCS-1 expression began to decline. Peak levels of SOCS-1 mRNA in non-viable GAS-treated cells was reached at a later time point (elevated at 6–8 h) and was lower (approximately fourfold at the peak) compared to GAS-induced infection (Figure 1a). We observed a consistent trend of SOCS-1 mRNA expression in BMDMs, where the elevation in the SOCS-1 mRNA level was more pronounced and peaked with an approximate 21-fold induction (Figure 1b).
Figure 1.
Expression of SOCS-1 and STAT-1 phosphorylation in GAS-induced macrophages. RAW 264.7 macrophages and BMDMs from wild-type mice were infected with GAS or non-viable GAS at an MOI of 100∶1 for 1 h. At different post-infection times, the levels of SOCS-1 mRNAs were determined by RT-PCR in RAW 264.7 cells (a) or BMDMs (b), and SOCS-1 protein expression were detected by western blot respectively in RAW 264.7 cells (d) or BMDMs (f). Under the same condition, the levels of STAT-1 phosphorylation were determined by western blot analysis in RAW264.7 cells (c) or BMDMs (e). *P<0.05 vs. nonviable GAS group. Each experiment was repeated three times and similar results were obtained. GAS, group A Streptococcus; SOCS, suppressor of cytokine signaling; STAT, signal transducer and activator of transcription.
Previous studies have shown that SOCS-1 expression is induced in a STAT1-dependent manner.15,16,21,22 We therefore looked at levels of phosphorylation of STAT1 in GAS-infected macrophages. As shown in Figure 1c, an increase in STAT1 phosphorylation after GAS stimulation was seen at 4 h, with further elevation seen at 6 h. Phosphorylation of STAT-1 was decreased at 8 h post-stimulation. By contrast, STAT1 phosphorylation was delayed and lower in BMDMs stimulated with control non-viable GAS (Figure 1c and e). This observation showed that STAT1 was promptly phosphorylated in GAS-infected macrophages.
Next, we measured SOCS-1 protein expression by western blotting and found that SOCS-1 protein expression was detectable at 8 h post-infection and peaked at 10 h (Figure 1d). It should be noted that unlike macrophages infected with GAS, macrophages infected with non-viable GAS did not shown any apparent increase in SOCS-1 protein expression even after 10 h of stimulation (Figure 1d). These experiments were repeated in BMDMs and similar results were obtained. (Figure 1f), suggesting that GAS induces SOCS-1 upregulation significantly in macrophages in early stage infection.
IFN-β is not a prerequisite for early macrophage GAS-induced SOCS-1 expression
Previous reports have shown that RAW 264.7 macrophages infected with GAS induce IFN-β secretion1,2,3 and that the major pathway of intracellular signaling used by IFN-β and their receptors involves the tyrosine kinases JAK1 and TYK2, leading to activation of STAT-1, which is associated with induction of SOCS-1 gene expression in macrophages.16
However, others studies have found that IFNAR−/− mice had no deficiency in induction of SOCS-1,23 thereby suggesting that IFN-β was not necessary for SOCS-1 induction. To investigate whether the expression of SOCS-1 depends on the autocrine or paracrine involvement of IFN-β in the initial phases of GAS infection, we performed the experiments described below.
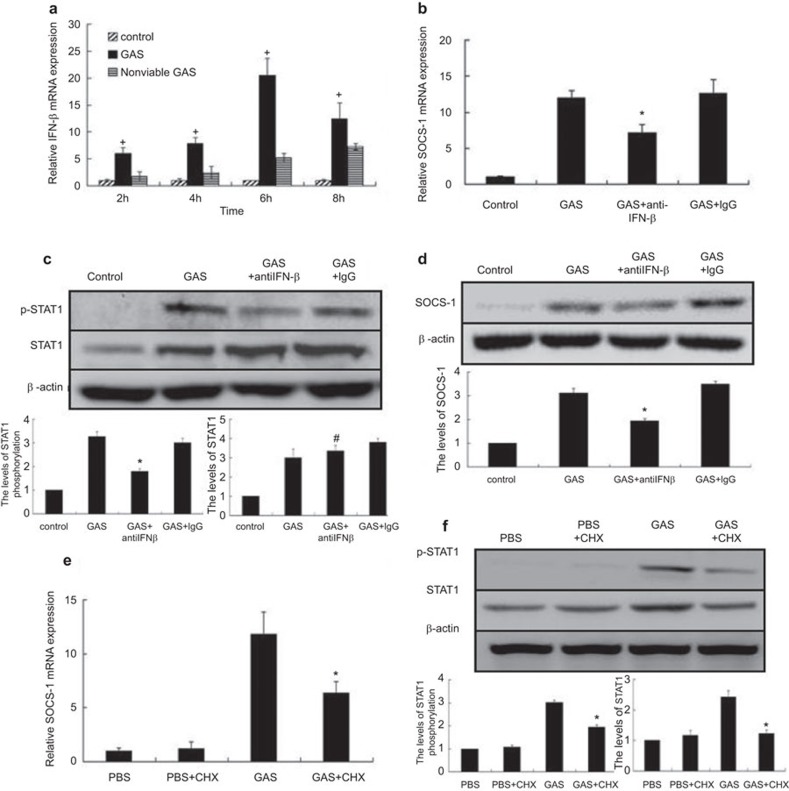
RAW 264.7 macrophages were stimulated with GAS at an MOI of 100∶1 for 1 h and continuously incubated, and IFN-β mRNA collected at various time points was analyzed. As shown in Figure 2a, IFN-β mRNA was detectable at 2 h, peaked at 6 h and then decreased at 8 h. The mRNA levels of IFN-β in the non-viable GAS-treated control group cells were strongly diminished. This finding is consistent with the expression of SOCS-1. However, whether SOCS-1 expression was completely IFN-β-dependent was tested by infection of macrophages with GAS in the presence of a neutralizing antibody targeted against IFN-β (10 µg/ml). As shown in Figure 2b–d, in GAS-infected RAW 264.7 macrophages, the activation of STAT1 and expression of SOCS-1 decreased in the presence of anti-IFN-β however, it did not diminish completely. Thus, we hypothesized that in addition to IFN-β, another pathway might exist for the induction of SOCS-1 expression in response to GAS.
Figure 2.
GAS induced IFN-β, SOCS-1 and p-STAT1 expression in RAW 264.7 macrophages in the presence of neutralizing anti-IFN-β or cycloheximide. RAW 264.7 macrophages were infected with GAS or non-viable GAS at an MOI of 100∶1 for 1 h. At different time points post-infection, IFN-β mRNA expression was determined by real-time PCR (a). After RAW 264.7 macrophages were infected with GAS in the presence of neutralizing anti-IFN-β (10 µg/ml) for 6 h of incubation, SOCS-1 mRNA expression was detected by real-time PCR (b) and p-STAT1 expression was analyzed by western blot (c). Following 10 h of incubation as described above, SOCS-1 expressions were analyzed by western blot (d). RAW 264.7 macrophages were incubated with cycloheximide (10 µM) for 30 min prior to GAS infection and then the expressions of SOCS-1 were determined by RT-PCR (e) and the expressions of p-STAT1 were detected by western blot following 6 h of infection (f). *P<0.05 vs. GAS group. +P<0.05 vs. nonviable GAS group. #P>0.05 vs. GAS group. Each experiment was repeated three times and similar results were obtained. GAS, group A Streptococcus; MOI, multiplicity of infection; SOCS, suppressor of cytokine signaling; STAT, signal transducer and activator of transcription.
According to previous studies, a number of cytokines are involved in SOCS-1 expression, including IL-10,24 IL-225 and colony-stimulating factors.26 In our next experiments, we used the protein synthesis inhibitor CHX to determine whether GAS could induce SOCS-1 expression without IFN-β- and/or other cytokines. RAW 264.7 macrophages were co-incubated with PBS, GAS, PBS plus CHX (10 µM) or GAS plus CHX (10 µM) for 6 h, after which RNA was extracted and analyzed by RT-PCR. The inclusion of CHX reduced GAS-induced SOCS-1 mRNA expression, but SOCS-1 expression was not completely abolished (Figure 2e), suggesting that there was another non-cytokine-dependent pathway involved in the induction of SOCS-1 expression, in addition to IFN-β activation. When STAT1 activation was analyzed, we found that in spite of the presence of CHX, STAT1 continued to be activated (Figure 2f). These data suggested that in addition to cytokine activation, GAS itself induces SOCS-1 expression through a novel pathway in which activation of STAT1 might play a critical role.
TLR4 plays an important role in GAS-induced SOCS-1 early expression
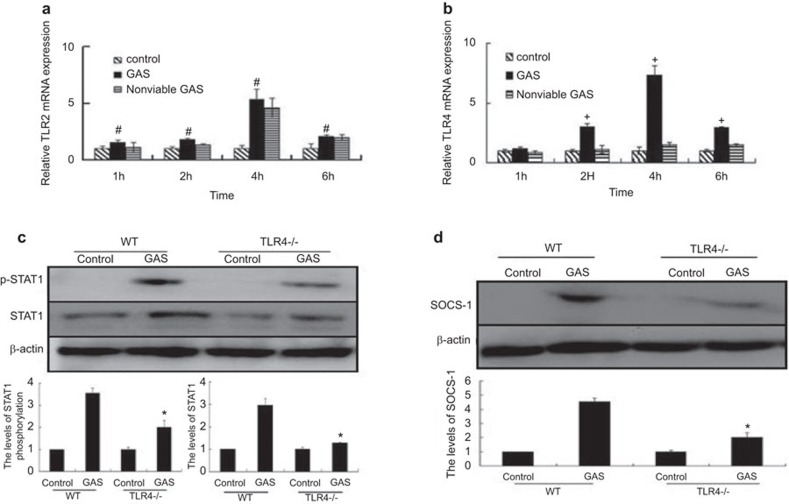
A number of studies have shown that LPS induces SOCS-1 expression through a direct TLR4-mediated pathway.18,19 For example, it was previously found that TLR2 and TLR4 activate STAT1 phosphorylation by distinct mechanisms in macrophages. In contrast to TLR2, only TLR4 engagement induced STAT1 phosphorylation at tyrosine 701 in murine macrophages.23,27,28 This led us to hypothesize that GAS induces early SOCS-1 expression through direct activation of TLR4. We have previously shown that both TLR2 and TLR4 were activated in GAS infected RAW 264.7 macrophages (data not shown). To study the possible regulation of TLR2- or TLR4-induced SOCS-1 gene expression, TLR2 and TLR4 mRNAs from GAS infected RAW 264.7 macrophages was collected at several time points and their expression was analyzed. After GAS infection at 1, 2, 4 and 6 h, the relative mRNA levels of TLR2 and TLR4 in the infected cells were determined by RT-PCR. Expression of TLR4 was induced and peaked at 4 h post-infection with GAS (equal to about a sevenfold induction) and began to decline at 6 h (Figure 3b). By contrast, RAW 264.7 macrophages infected with non-viable GAS did not show induction of TLR4 mRNA expression within 6 h post-infection (Figure 3b). However, TLR2 was found to be induced after both GAS and non-viable GAS infection (Figure 3a). Thus, we proposed that GAS might activate SOCS-1 quickly through the TLR4 pathway. In further experiments, BMDMs from TLR4−/− mice were stimulated with GAS, and the expression of p-STAT1, STAT1 and SOCS-1 was analyzed by western blotting. It was found that activation of STAT1 and expression of SOCS-1 was reduced in TLR4−/− BMDMs (Figure 3c and d). These data indicate that the TLR4 signaling pathway is required for the induction of SOCS-1 expression.
Figure 3.
Activation of TLR4 and TLR2 expression by RAW 264.7 macrophages and SOCS-1 expression in BMDMs from TLR4−/− knockout mice infected by GAS. RAW 264.7 cells were infected with GAS or non-viable GAS at an MOI of 100∶1. At different time points, the mRNA levels of TLR2 (a) and TLR4 (b) were assayed by real-time PCR. BMDMs from TLR4−/− knockout mice were stimulated with GAS at the same MOI for 1 h, and then washed GAS with PBS. Six hours later, treated cells were lysed, and equal amounts of cell lysate were blotted with anti-pSTAT-1, anti-STAT-1 and β-actin (c), and 10 h later, the treated cells were collected and determined by western blot using antibody targeted to SOCS-1 (d). *P<0.05 vs. WT group. +P<0.05 vs. nonviable GAS group. #P>0.05 vs. nonviable GAS group. Each experiment was repeated three times and similar results were obtained. GAS, group A Streptococcus; MOI, multiplicity of infection; SOCS, suppressor of cytokine signaling; STAT, signal transducer and activator of transcription; TLR, Toll-like receptor; WT, wild-type.
GAS-induced SOCS-1 early expression depend on MyD88
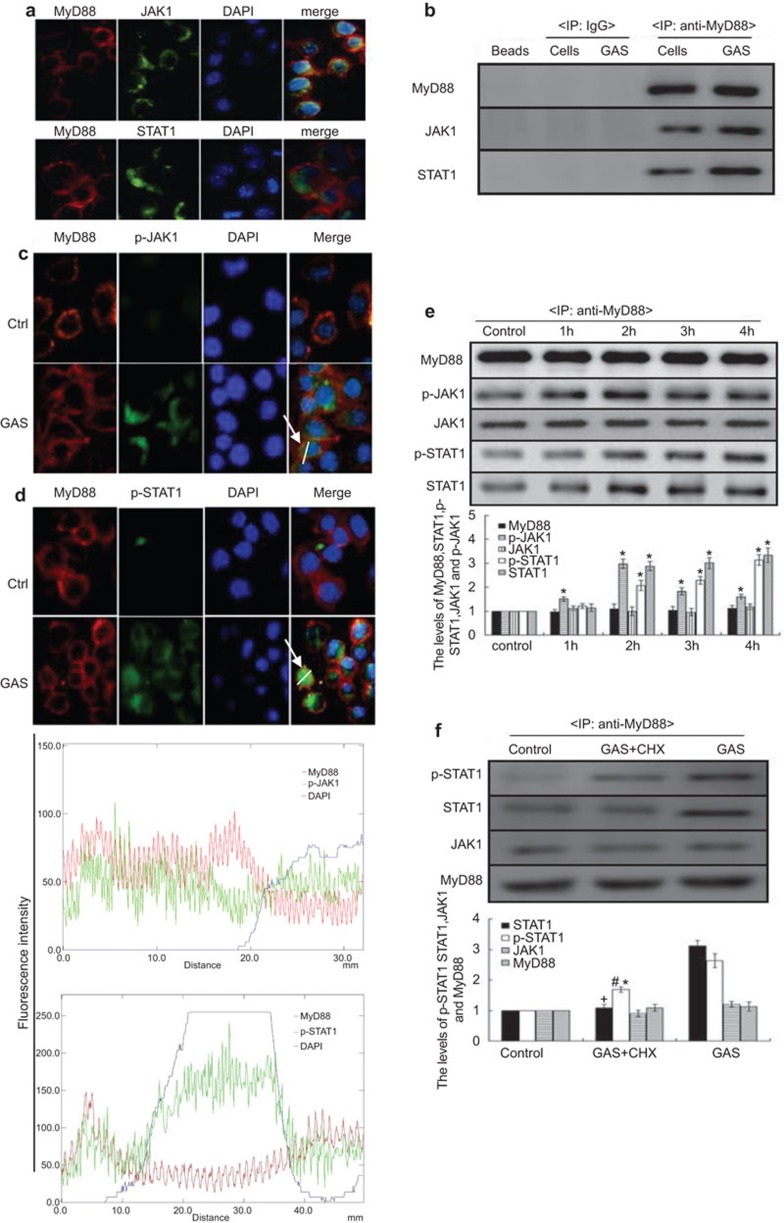
MyD88 is an adaptor protein for TLR4, and an association between JAK2 and the TLR4/MyD88 complex was found previously.29 Thus, we presumed that MyD88 might play an important role in STAT1 activation or SOCS-1 expression. To test whether JAK1/STAT1 associates with TLR4/MyD88, uninfected RAW 264.7 macrophages were first analyzed by immunofluorescence microscopy. The result revealed that JAK1 might associate with STAT1 and MyD88 (Figure 4a). Thus, we next confirmed by immunoprecipitation analysis that JAK1, STAT1 and MyD88 exist as a complex in RAW 264.7 macrophages (Figure 4b).
Figure 4.
JAK1 and STAT1 interaction with MyD88 in RAW 264.7 macrophages with/without cycloheximide treatment following GAS infection. The interaction of MyD88 (red) with JAK1 (green) or STAT1 (green) was detected by immunofluorescence assay (magnification, ×100) (a) and IP (b) in RAW 264.7 cells. And cells were infected with GAS at an MOI 100∶1. Two hours post-infection or 4 h post-infection, Expressions of MyD88, p-JAK1(green) (c) or p-STAT1 (green) (d) were detected by immunofluorescence assay (magnification, ×100) and following 1–4 h GAS post- infection, MyD88, JAK1, STAT1, p-JAK1 and p-STAT1 levels were determined by IP (e). Representative cells from the same field for each group are shown. Blue represents DAPI staining. (f) The cycloheximide-treated RAW 264.7 cells were infected by GAS, and 4 h post-infection, the expression levels of MyD88, JAK1, STAT1 and p-STAT1 in these cells was detected by IP. *P<0.05 vs. control group, +P<0.05 vs. GAS group. Each experiment was repeated for three times and similar results were obtained. GAS, group A Streptococcus; IP, immunoprecipitation; MOI, multiplicity of infection; SOCS, suppressor of cytokine signaling; STAT, signal transducer and activator of transcription.
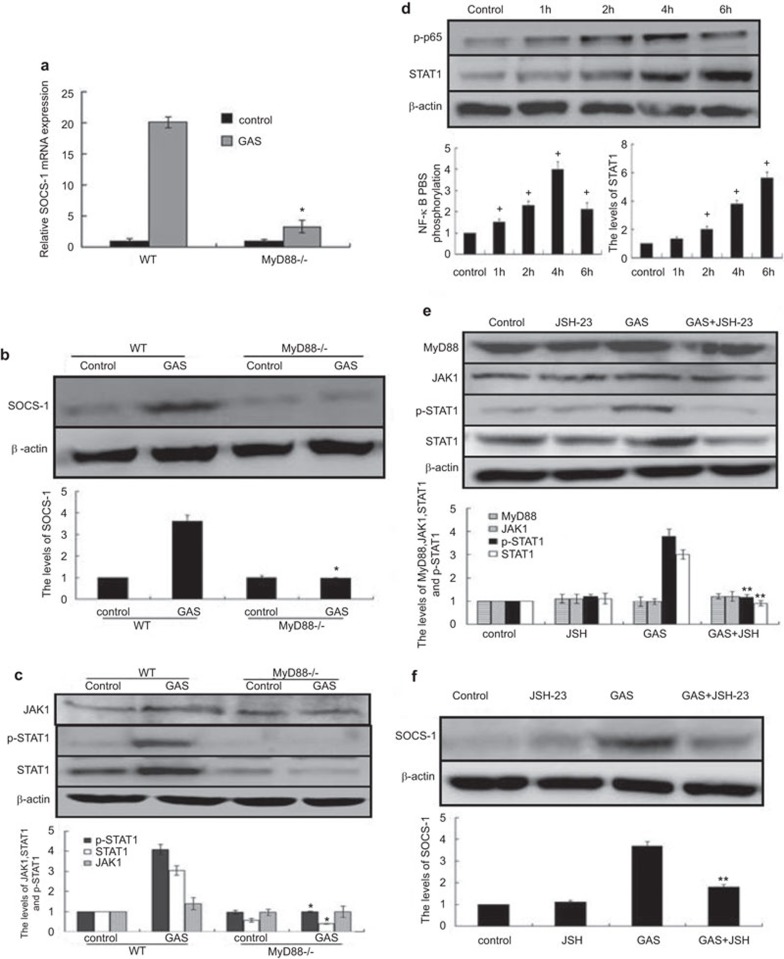
Because this complex existed in RAW 264.7 cells, it remained uncertain how only STAT1 of the complex was activated in GAS-infected cells at an early stage. Thus, we devised experiments in which RAW 264.7 cells were infected with GAS and the p-JAK1 or p-STAT1 levels combined with MyD88 were examined by immunofluorescence (at 2 h post-infection) and by immunoprecipitation analysis at 1 h, 2 h, 3 h and 4 h. It was found that when JAK1 or STAT1 associated with MyD88, phosphorylation rapidly occurred in macrophages at 2 h post-infection with GAS (Figure 4c–d). To further test whether STAT1 was phosphorylated through the MyD88–JAK1–STAT1 complex formation triggered by GAS rather than through cytokine activity, we examined p-STAT1 levels combined with MyD88 in the presence of CHX by immunoprecipitation assays. The cells were infected with GAS or with GAS plus CHX, and the p-STAT/MyD88 complex was then detected by immunoprecipitation analysis. As shown in Figure 4f, the p-STAT1 levels combined with MyD88 were partly affected in the presence of CHX. These findings suggest that in GAS-infected macrophages, GAS itself promptly activated STAT1 through TLR4/MyD88–JAK1/STAT1 complex formation. We next obtained BMDM macrophages from WT (C57BL/6) and MyD88−/− mice, and the cells were stimulated with GAS as indicated (Figure 5a–c). We found that Stat1 levels were diminished in MyD88−/− BMDMs compared with WT controls, and STAT1 phosphorylation was abolished completely. However, JAK1 was unaffected in MyD88−/− BMDMs, which showed that MyD88 was a key player in SOCS-1 expression and STAT1 phosphorylation.
Figure 5.
GAS-induced SOCS-1 and p-STAT1 expressions in macrophages knocked out MyD88 or treated with JSH23. BMDMs from WT or MyD88−/− mice were stimulated with GAS, 6 h or 10 h later, the mRNA or protein expressions of SOCS-1 were detected by RT-PCR (a) or western blot (b), and 6 h later, the expressions of p-STAT1, STAT1 or JAK1 were detected by western blot (c). BMDMs from WT mice with/without NF-κB inhibitor JSH-23 (20 µM) were infected with GAS. At post-infection times of 1 h, 2 h, 4 h and 6 h, the treated cells were lysed, and equal amounts were blotted with NF-kB (p-p65), STAT-1 and β-actin Ab (d), at 6 h of post infection, p-STAT-1, STAT-1 and β-actin were detected by western blot (e), and at 10 h of post-infection, SOCS-1 were detected by western blot (f). *P<0.05 vs. WT group; +P<0.05 vs. control group, **P<0.05 vs. GAS group. Each experiment was repeated for three times and similar results were obtained. Ab, antibody; GAS, group A Streptococcus; SOCS, suppressor of cytokine signaling; STAT, signal transducer and activator of transcription; WT, wild-type.
NF-κB activation affected early expression of SOCS-1 through reduction of STAT1 expression levels
In GAS-infected macrophages, we found that the p65 subunit of NF-κB was phosphorylated, which resulted in nuclear translocation and effects on gene and protein expression. From the kinetic changes in NF-κB activation in BMDMs, we found that GAS induced rapid and potent activation of NF-κB (Figure 5d). An increase in NF-κB activation after GAS stimulation was seen at 2 h, with further elevation at 4 h, which was then found to be decreased at 6 h. The expression of STAT1/JAK1 increased following NF-κB activation and peaked at 6 h (Figure 5d). Having established that GAS infection induces NF-κB activation and leads to increased STAT1/JAK1 expression; we questioned whether there was a link between NF-κB activation and STAT1/JAK1 expression. JSH-23 (an inhibitor of NF-κB activation) inhibits nuclear translocation of the p65 subunit of NF-κB but does not affect IκB degradation or recovery. Thus, we designed an experiment in which we pretreated BMDMs with JSH-23 (20 µM) for 30 min prior to infection and incubated the infected cells with JSH-23 for the indicated times. As shown in Figure 5e, the levels of STAT1 were strongly diminished, and the phosphorylation of STAT-1 was almost completely abolished in BMDMs treated with JSH-23. Next, we further examined the expression of SOCS-1 in BMDMs treated with JSH-23. GAS-induced expression of SOCS-1 was completely abolished in cells treated with JSH-23 (Figure 5f). However, JSH-23 had no effect on the expression of either MyD88 or JAK1 (Figure 5e). The experiment revealed that NF-κB activation promotes the expression of SOCS-1 by increasing STAT1 expression and phosphorylation.
Discussion
GAS is a Gram-positive bacterium and the causative agent of several systemic diseases. It is equipped with various surface-displayed and secreted virulence factors, such as M proteins,31,32 Fba proteins,33,34 C5a and SpeB,35,36 and these proteins play a vital role in streptococcal colonization, invasion, dissemination and host damage.37 GAS can survive and multiply in host cells, which requires suppression of host innate immunity.38,39,40,41
In our previous studies, we confirmed that secretion of pro-inflammatory cytokines (e.g., IL-1β and TNF-α) associated with GAS was lower than that associated with Staphylococcus aureus; thus, we supposed that there might exist a high expression of inhibited proteins in the early stages of GAS infection. SOCS-1 proteins have been identified as negative-feedback inhibitors for cytokine communication and are crucially important in limiting inflammatory responses in macrophages.42 SOCS-1 could also act as a direct inhibitor of TLR signaling, thus revealing a further role in the down-regulation of inflammatory cytokine secretion.47,48
In this study, we demonstrated that GAS had the capacity to induce murine macrophage protein expression of SOCS-1 within 6–8 h post-infection, which might be important in helping GAS evade immune responses during the early stages of infection (Figure 1d and f). Furthermore, studies have shown that other pathogens induce SOCS-1 protein expression, which would be particularly beneficial to pathogens infecting and replicating in macrophages.9,10,11,12 We also found that viable GAS-induced SOCS-1 expression was much higher and occurred earlier in murine macrophages than in non-viable GAS infection. This observation revealed that some heat-sensitive molecules of GAS were very important in inducing SOCS-1 protein expression.
Cytokine activation is a prerequisite for the expression of SOCS-1 in most cases, especially in the case of IFN-β. Moreover, induction of high levels of IFN-β expression following GAS infection was observed in our experiments. However, based on the results of experiments involving co-incubation with anti-IFN-β, we surmise that there is likely to be another pathway responsible for inducing SOCS-1 expression in addition to IFN-β binding its receptor. Raw264.7 cells were treated with CHX, which inhibited protein translation and blocked other cytokine activation pathways; we found that SOCS-1 mRNA was also expressed in this context. These results support the existence of another pathway through which GAS might induce a rapid and direct expression of SOCS-1 in early infection.
SOCS-1 is a cytokine-inducible inhibitor of the JAK-STAT signaling pathway,43,44 but recently, some studies have reported that SOCS-1 is directly induced by TLR signaling, although the mechanisms responsible remain unclear. We have shown that in GAS-infected macrophages, both TLR2 and TLR4 were activated simultaneously. However, TLR4 mRNA levels remained almost unaltered in non-viable GAS-infected macrophages, and the expression of SOCS-1 induced by viable GAS was higher than that induced by non-viable GAS, suggesting that some heat-sensitive molecules of GAS play an important role in activating TLR4 and inducing SOCS-1. Others have reported that both TLR2 and TLR4 activate STAT1 phosphorylation and do so by distinct mechanisms in macrophages.27 We thus considered the possibility that a direct interaction between TLR4 and SOCS-1 might exist. In view of the possibility of TLR4-mediated activation of STAT1, we examined TLR4−/− BMDMs with the aim of studying the relationship between SOCS-1 expression and TLR4 receptor signaling. In TLR4−/− BMDMs, SOCS-1 expression was reduced (Figure 4d), and these observations confirmed that SOCS-1 expression was closely linked to TLR4 activation in macrophages.
It has also been found that MyD88 promotes the association of JAK2 with TLR4 to form a complex in macrophages.28 Thus, we explored the possibility that MyD88 might link to adaptor proteins as a complex in macrophages. As expected, through immunofluorescence and IP analyses, we confirmed that JAK1 and STAT1 were linked in a complex with MyD88 in RAW 264.7 cells. In the early stages of GAS-induced infection (1–4 h), once the TLR4 signaling pathway was activated via MyD88, the STAT1/MyD88 complex was promptly activated. Subsequently, the phosphorylated STAT1 rapidly translocated to the nucleus and induced early expression of SOCS-1.
In the presence of CHX, the levels of p-STAT1 combined with MyD88 were only partly affected, suggesting that a direct TLR4/MyD88/STAT1 pathway existed and played a vital role in the induction of SOCS-1 in GAS-infected macrophages. Chhatwal and McMilan5 have previously reported that IFN-β production stimulated by GAS also depends on MyD88 signaling. Therefore, MyD88 acts as a central molecule in SOCS-1 induction through either the IFN-β-receptor pathway or the TLR4/MyD88 pathway. Subsequently, macrophages from MyD88−/− knockout mice were used in our experiments, and the lack of SOCS-1 expression in these cells following GAS infection (Figure 5a–c) supported the critical role of MyD88 in SOCS-1 expression.
The importance of NF-κB in regulating the expression of inflammatory genes has been shown by many studies.45,46 In the present study, we confirmed that the p65 subunit of NF-κB was phosphorylated at 1 h and peaked at 4 h in response to GAS infection, leading to nuclear translocation and STAT1 transcription. In the presence of JSH-23, the nuclear translocation of p65 was blocked, and the levels of STAT1 were subsequently reduced. This in turn led to reduced expression of SOCS-1 (Figure 5e and f).
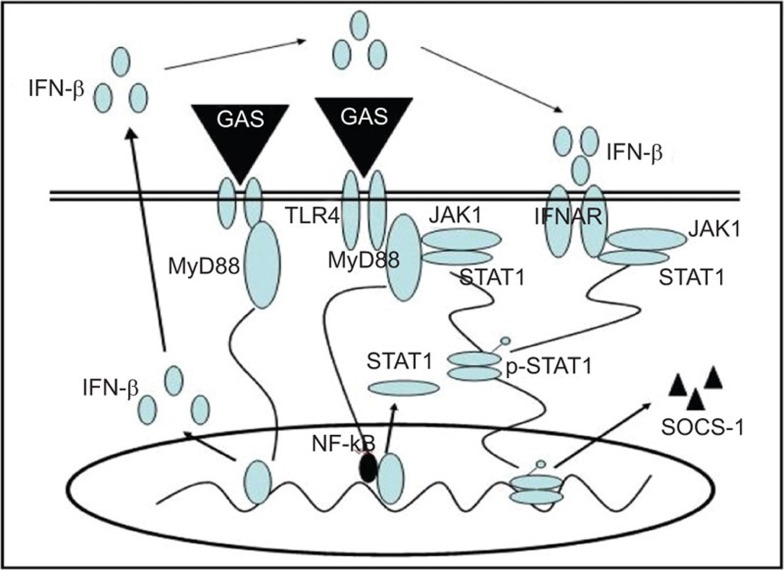
In summary, we have demonstrated that in addition to IFN-β signaling, the TLR4/MyD88 signaling pathway is involved in augmenting the expression of SOCS-1 in GAS infection. We have found that a complex of MyD88, JAK1 and STAT1 are formed in this pathway (Figure 6), which might be a novel and direct negative-feedback mechanism to block cytokine expression in GAS infection and would be more conducive to bacterial infection and evasion of host immunity.
Funding
This study was supported by the National Natural Science Foundation of China (Grant Nos. 31370914, 30901350 and 81172810).
Figure 6.
Schematic model of SOCS-1 induction in a TLR4/MyD88-dependent and IFN-β-dependent signaling pathway. SOCS, suppressor of cytokine signaling; TLR, Toll-like receptor.
All authors declare that they have no conflict of interests.
Footnotes
Supplementary Information accompanies the paper on Cellular & Molecular Immunology's website. (http://www.nature.com/cmi).
Supplementary Information
References
- 1Miettinen M, Lehtonen A, Julkunen I, Matikainen S. Lactobacilli and Streptococci activate NF-kappa B and STAT signaling pathways in human macrophages. J Immunol 2000; 164: 3733–3740. [DOI] [PubMed] [Google Scholar]
- 2Gratz N, Hartweger H, Matt U, Kratochvill F, Janos M, Sigel S et al. Type I interferon production induced by Streptococcus pyogenes-derived nucleic acids is required for host protection. PLoS Pathog 2011; 7: e1001345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Gratz N, Siller M, Schaljo B, Pirzada ZA, Gattermeier I, Vojtek I et al. Group A streptococcus activates type I interferon production and MyD88-dependent signaling without involvement of TLR2, TLR4, and TLR9. J Biol Chem 2008; 283: 19879–19887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Bisno AL, Brito MO, Collins CM. Molecular basis of group A streptococcal virulence. Lancet Infect Dis 2003; 3: 191–200. [DOI] [PubMed] [Google Scholar]
- 5Chhatwal GS, McMillan DJ. Uncovering the mysteries of invasive streptococcal diseases. Trends Mol Med 2005; 11: 152–155. [DOI] [PubMed] [Google Scholar]
- 6Ryo A, Suizu F, Yoshida Y, Perrem K, Liou YC, Wulf G et al. Regulation of NF-kappaB signaling by Pin1-dependent prolyl isomerization and ubiquitin-mediated proteolysis of p65/RelA. Mol Cell 2003; 12: 1413–1426. [DOI] [PubMed] [Google Scholar]
- 7Maine GN, Mao X, Komarck CM, Burstein E. COMMD1 promotes the ubiquitination of NF-kappaB subunits through a Cullin-containing ubiquitin ligase. EMBO J 2007; 26: 436–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Kubo M, Hanada T, Yoshimura A. Suppressors of cytokine signaling and immunity. Nat Immunol 2003; 4: 1169–1176. [DOI] [PubMed] [Google Scholar]
- 9Zimmermann S, Murray PJ, Heeg K, Dalpke AH. Induction of suppressor of cytokine signaling-1 by Toxoplasma gondii contributes to immune evasion in macrophages by blocking IFN-gamma signaling. J Immunol 2006; 176: 1840–1847. [DOI] [PubMed] [Google Scholar]
- 10Mun HS, Aosai F, Norose K, Piao LX, Fang H, Akira S et al. Toll-like receptor 4 mediates tolerance in macrophages stimulated with Toxoplasma gondii-derived heat shock protein 70. Infect Immun 2005; 73: 4634–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Manca C, Tsenova L, Freeman S, Barczak AK, Tovey M, Murray PJ et al. Hypervirulent M. tuberculosis W/Beijing strains upregulate type I IFNs and increase expression of negative regulators of the Jak–Stat pathway. J Interferon Cytokine Res 2005; 25: 694–701. [DOI] [PubMed] [Google Scholar]
- 12Ekchariyawat P, Pudla S, Limposuwan K, Arjcharoen S, Sirisinha S, Utaisincharoen P. Burkholderia pseudomallei-induced expression of suppressor of cytokine signaling 3 and cytokine-inducible src homology 2-containing protein in mouse macrophages: a possible mechanism for suppression of the response to gamma interferon stimulation. Infect Immun 2005; 73: 7332–7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13Frey KG, Ahmed CM, Dabelic R, Jager LD, Noon-Song EN, Haider SM et al. HSV-1-induced SOCS-1 expression in keratinocytes: use of a SOCS-1 antagonist to block a novel mechanism of viral immune evasion. J Immunol 2009; 183: 1253–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Waiboci LW, Ahmed CM, Mujtaba MG, Flowers LO, Martin JP, Haider MI et al. Both the suppressor of cytokine signaling 1 (SOCS-1) kinase inhibitory region and SOCS-1 mimetic bind to JAK2 autophosphorylation site: implications for the development of a SOCS-1 antagonist. J Immunol 2007; 178: 5058–5068. [DOI] [PubMed] [Google Scholar]
- 15Naka T, Fujimoto M, Tsutsui H, Yoshimura A. Negative regulation of cytokine and TLR signalings by SOCS and others. Adv Immunol 2005; 87: 61–122. [DOI] [PubMed] [Google Scholar]
- 16Qin H, Wilson CA, Lee SJ, Benveniste EN.IFN-beta-induced SOCS-1 negatively regulates CD40 gene expression in macrophages and microglia. FASEB J 2006; 20: 985–987. [DOI] [PubMed] [Google Scholar]
- 17Lesinski GB, Zimmerer JM, Kreiner M, Trefry J, Bill MA, Young GS et al. Modulation of SOCS protein expression influences the interferon responsiveness of human melanoma cells. BMC Cancer 2010; 10: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Nakagawa R, Naka T, Tsutsui H, Fujimoto M, Kimura A, Abe T et al. SOCS-1 participates in negative regulation of LPS responses. Immunity 2002; 17: 677–687. [DOI] [PubMed] [Google Scholar]
- 19Kinjyo I, Hanada T, Inagaki-Ohara K, Mori H, Aki D, Ohishi M et al. SOCS1/JAB is a negative regulator of LPS-induced macrophage activation. Immunity 2002; 17: 583–591. [DOI] [PubMed] [Google Scholar]
- 20Imai K, Kurita-Ochiai T, Ochiai K. Mycobacterium bovis bacillus Calmette-Guerin infection promotes SOCS induction and inhibits IFN-gamma-stimulated JAK/STAT signaling in J774 macrophages. FEMS Immunol Med Microbiol 2003; 39: 173–180. [DOI] [PubMed] [Google Scholar]
- 21Naka T, Narazaki M, Hirata M, Matsumoto T, Minamoto S, Aono A et al. Structure and function of a new STAT-induced STAT inhibitor. Nature 1997; 387: 924–929. [DOI] [PubMed] [Google Scholar]
- 22Cunningham MW. Pathogenesis of group A streptococcal infections and their sequelae. Adv Exp Med Biol 2008; 609: 29–42. [DOI] [PubMed] [Google Scholar]
- 23Baetz A, Frey M, Heeg K, Dalpke AH. Suppressor of cytokine signaling (SOCS) proteins indirectly regulate Toll-like receptor signaling in innate immune cells. J Biol Chem 2004; 279: 54708–54715. [DOI] [PubMed] [Google Scholar]
- 24Ding Y, Chen D, Tarcsafalvi A, Su R, Qin L, Bromberg JS. Suppressor of cytokine signaling 1 inhibits IL-10-mediated immune responses. J Immunol 2003; 170: 1383–1391. [DOI] [PubMed] [Google Scholar]
- 25Sporri B, Kovanen PE, Sasaki A, Yoshimura A, Leonard WJ. JAB/SOCS1/SSI-1 is an interleukin-2-induced inhibitor of IL-2 signaling. Blood 2001; 97: 221–226. [DOI] [PubMed] [Google Scholar]
- 26Stevenson NJ, Haan S, McClurg AE, McGrattan MJ, Armstrong MA, Heinrich PC et al. The chemoattractants, IL-8 and formyl-methionyl-leucyl-phenylalanine, regulate granulocyte colony-stimulating factor signaling by inducing suppressor of cytokine signaling-1 expression. J Immunol 2004; 173: 3243–3249. [DOI] [PubMed] [Google Scholar]
- 27Bhattacharyya S, Zhao Y, Kay TW, Muglia LJ. Glucocorticoids target suppressor of cytokine signaling 1 (SOCS1) and type 1 interferons to regulate Toll-like receptor-induced STAT1 activation. Proc Natl Acad Sci USA 2011; 108: 9554–9559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28Rhee SH, Jones BW, Toshchakov V, Vogel SN, Fenton MJ. Toll-like receptors 2 and 4 activate STAT1 serine phosphorylation by distinct mechanisms in macrophages. J Biol Chem 2003; 278: 22506–22512. [DOI] [PubMed] [Google Scholar]
- 29Kimura A, Naka T, Muta T, Takeuchi O, Akira S, Kawase I et al. Suppressor of cytokine signaling-1 selectively inhibits LPS-induced IL-6 production by regulating JAK–STAT. Proc Natl Acad Sci USA 2005; 102: 17089–17094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Thulin P, Johansson L, Low DE, Gan BS, Kotb M, McGeer A et al. Viable group A streptococci in macrophages during acute soft tissue infection. PLoS Med 2006; 3: e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Cunningham MW. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev 2000; 13: 470–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32Ringdahl U, Sjobring U. Analysis of plasminogen-binding M proteins of Streptococcus pyogenes. Methods 2000; 21: 143–150. [DOI] [PubMed] [Google Scholar]
- 33Pandiripally V, Gregory E, Cue D. Acquisition of regulators of complement activation by Streptococcus pyogenes serotype M1. Infect Immun 2002; 70: 6206–6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34Terao Y, Kawabata S, Kunitomo E, Murakami J, Nakagawa I, Hamada S. Fba, a novel fibronectin-binding protein from Streptococcus pyogenes, promotes bacterial entry into epithelial cells, and the fba gene is positively transcribed under the Mga regulator. Mol Microbiol 2001; 42: 75–86. [DOI] [PubMed] [Google Scholar]
- 35Lamont RJ, Gil S, Demuth DR, Malamud D, Rosan B. Molecules of Streptococcus gordonii that bind to Porphyromonas gingivalis. Microbiology 1994; 140(Pt 4): 867–872. [DOI] [PubMed] [Google Scholar]
- 36Lyon WR, Caparon MG. Role for serine protease HtrA (DegP) of Streptococcus pyogenes in the biogenesis of virulence factors SpeB and the hemolysin streptolysin S. Infect Immun 2004; 72: 1618–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37Nobbs AH, Lamont RJ, Jenkinson HF. Streptococcus adherence and colonization. Microbiol Mol Biol Rev 2009; 73: 407–450, Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38Staali L, Morgelin M, Bjorck L, Tapper H. Streptococcus pyogenes expressing M and M-like surface proteins are phagocytosed but survive inside human neutrophils. Cell Microbiol 2003; 5: 253–265. [DOI] [PubMed] [Google Scholar]
- 39Nakagawa I, Amano A, Mizushima N, Yamamoto A, Yamaguchi H, Kamimoto T et al. Autophagy defends cells against invading group A Streptococcus. Science 2004; 306: 1037–1040. [DOI] [PubMed] [Google Scholar]
- 40Hakansson A, Bentley CC, Shakhnovic EA, Wessels MR. Cytolysin-dependent evasion of lysosomal killing. Proc Natl Acad Sci USA 2005; 102: 5192–5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41Saitoh T, Fujita N, Jang MH, Uematsu S, Yang BG, Satoh T et al. Loss of the autophagy protein Atg16 L1 enhances endotoxin-induced IL-1beta production. Nature 2008; 456: 264–268. [DOI] [PubMed] [Google Scholar]
- 42Elliott J, Johnston JA. SOCS: role in inflammation, allergy and homeostasis. Trends Immunol 2004; 25: 434–440. [DOI] [PubMed] [Google Scholar]
- 43Alexander WS, Hilton DJ. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annu Rev Immunol 2004; 22: 503–529. [DOI] [PubMed] [Google Scholar]
- 44Ilangumaran S, Ramanathan S, Rottapel R. Regulation of the immune system by SOCS family adaptor proteins. Semin Immunol 2004; 16: 351–365. [DOI] [PubMed] [Google Scholar]
- 45Beutler B. 2004. Inferences, questions and possibilities in Toll-like receptor signalling. Nature 2004; 430: 257–263. [DOI] [PubMed] [Google Scholar]
- 46Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med 2007; 13: 460–469. [DOI] [PubMed] [Google Scholar]
- 47Kinjyo I, Hanada T, Inagaki-Ohara K, Mori H, Aki D, Ohishi M et al. SOCS1/JAB is a negative regulator of LPS-induced macrophage activation. Immunity 2002; 17: 583–591. [DOI] [PubMed] [Google Scholar]
- 48Nakagawa R, Naka T, Tsutsui H, Fujimoto M, Kimura A, Abe T et al. SOCS-1 participates in negative regulation of LPS responses. Immunity 2002; 17: 677–687. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.