Abstract
Interleukin (IL)-21, a cytokine produced by activated CD4+ T cells, has broad pleiotropic actions that affect the functions of a variety of lymphoid cells. The roles of IL-21 in modulating immunity to infections are currently being defined. Notably, IL-21-mediated cellular and humoral immune responses play an important role in determining the outcome of viral infection. This article reviews the current knowledge on the critical role of IL-21 in hepatitis B virus (HBV) infection. As a competent intermediary, IL-21 derived from virus-specific CD4+ T cells plays key roles in sustaining CD8+ T cells and promoting B-cell responses that are essential for effective viral control. However, as a mediator of inflammation, IL-21 is also involved in the development of HBV-induced liver cirrhosis and exacerbating liver injury. Overall, the current data point to IL-21 as an immunomodulatory cytokine in HBV infection. Immunotherapeutic strategies aimed at optimizing the beneficial effects of IL-21 in HBV infection may prove to be a rigorous challenge in the future, as they should foster the strengths of IL-21 while circumventing potential drawbacks.
Keywords: hepatitis B virus, immunomodulatory, interleukin-21
Introduction
Hepatitis B virus (HBV) is a non-cytopathic virus that causes liver disease of varying severity, ranging from acute self-limiting infection to chronic liver injury, which may lead to liver fibrosis, cirrhosis and hepatocellular carcinoma. Emerging evidence has suggested that cytokine-mediated immune responses play a pivotal role in determining the clinical outcome of HBV infection.
Interleukin-21 (IL-21) is a member of a large family of cytokines (IL-2, IL-4, IL-7, IL-9 and IL-15) whose receptors share a common receptor γ chain (CD132). This cytokine is produced by activated CD4+ T cells, in particular follicular T helper (Tfh) cells, Th17 cells and activated natural killer T cells.1,2,3,4 The effects of IL-21 are pleiotropic because of the broad cellular distribution of IL-21 receptor (IL-21R).1,5 A deepened understanding of the broadly immunomodulatory activity of IL-21 has attracted great interest in this cytokine in different disease settings, including chronic virus infections. In mouse models of chronic lymphocytic choriomeningitis virus infection, IL-21 produced by virus-specific CD4+ T cells was essential for sustaining antiviral functions of CD8+ T cells and preventing them from immune exhaustion.6,7,8 Acutely-resolved hepatitis C virus was characterized by strong Th1/Th17 responses with specific expansion of IL-21-producing CD4+ T cells and increased IL-21 levels in the plasma.9 In patients with HIV-1 infection, reduced IL-21 production could be a contributing factor to the compromised cellular and humoral immune responses.10,11 Collectively, these studies indicate that IL-21may serve to limit viral persistence in chronic viral infection. Nevertheless, IL-21 could also trigger inflammatory pathways that promote tissue damage in various organs.12,13 Hence, it is possible that IL-21 exerts effects on inflammatory responses in the liver that promote the development of inflammatory disorders. Here, we review the current knowledge on the immunomodulatory effects of IL-21 in HBV infection.
Function of IL-21 in cellular immune responses
HBV-specific CD8+ T cells play a crucial role in controlling HBV progression. Polyclonal and multispecific cytotoxic T-lymphocyte activity against HBV could be detected in patients with acute self-limiting HBV infection.14,15 However, in chronically infected individuals, HBV-specific CD8+ T cells persist in a non-functional ‘exhausted' state, characterized by an inability to produce important antiviral cytokines (e.g., IFN-γ and TNF-α), low cytotoxic activities and low proliferation in response to cognate antigen.16 The exhaustion of specific cytotoxic CD8+ T-cell response mainly results from high levels of viral antigen and reduced CD4+ T helper cells, supporting a key role for CD4+ T helper cells in maintaining fully functional antigen-specific CD8+ T-cell responses.17,18,19 In a chimpanzee model of acute HBV infection, early activation of CD4+ T cells correlated with an influx of HBV-specific CD8+ T cells into the liver, and animals depleted of CD4+ T cells became persistently infected when inoculated with a dose of HBV.15,20 These results indicated that the induction of efficient antiviral cytotoxic T-lymphocyte responses depend on early CD4+ T-cell priming to HBV infection.
Recent studies demonstrated that CD4+ T-cell ‘help' for the CD8+ T-cell response involved IL-21 as a mediator. IL-21 secreted by activated CD4+ T cells was essential for the maintenance of specific CD8+ T-cell functions and control of viremia in lymphocytic choriomeningitis virus infection.6,7,8 CD4+ T cells from individuals with sustained immune control of HIV-1 infection produced larger amounts of IL-21 upon stimulation with phorbol myristate acetate/ionomycin or HIV-1 gag peptides. Of note, exogenous IL-21 significantly increased HIV-1-specific CD8+ T-cell effector functions.10 These studies implicate the importance of CD4+ T cells and IL-21 in promoting and sustaining immunity to chronic infections.
Depending on the age of an individual infected with human HBV, two different immune response patterns are observed. Infection during infancy often results in chronic persistence, while infection during adulthood usually leads to an acute response and viral clearance. The impact of age in the immune response to HBV infection resulting in viral clearance or persistence was explored in a transgenic mouse model of HBV infection.21 Adult mice showed elevated HBV-specific IL-21 production in the liver, while young mice exhibited a paucity of IL-21-producing Tfh cells and fewer CD8+ T cells in the liver.21 Therefore, ineffective hepatic Tfh cell priming during early HBV infection can lead to decreased IL-21 production and hence, a reduced capacity to generate specific CD8+ T-cell responses that are essential for viral clearance. Mechanistic studies have identified that IL-21 was required to mediate the clearance of HBV antigen in adult mice, indicating that IL-21 plays an important role in the age-dependent response to HBV clearance. Although HBV transgenic mice could not be considered as an ideal model of vertical infection due to the lack of a bona fide HBV infection, the system did reveal the potential involvement of increased IL-21 expression for the effective control of HBV replication; and this was confirmed in samples taken from patients with HBV infection. IL-21 expression was found to be elevated in acutely infected adults, but was low in patients with chronic HBV infection, even during active disease.21
Cross-sectional data from our group showed that the inactive carrier (IC) patients, characterized by low or undetectable levels of HBV DNA, had higher serum IL-21 concentrations than patients in the immune tolerant stage.22 Although IL-21 levels in chronic hepatitis B (CHB) and IC patients were comparable, there was a higher frequency of IL-21+CD4+ T or IL-21+CXCR5+CD4+ T cells in the IC than the CHB group after either nonspecific (phorbol myristate acetate/ionomycin) or specific (HBV core antigen or peptides) stimulation.22,23 However, similar observations were not obtained in a recent study by Li et al.24 This discrepancy may be attributed to patient selection, as viral load or viral antigens could influence the HBV-specific CD4+ T-cell response in CHB patients. Interestingly, Li et al. found a significantly elevated frequency of HBcAg-specific IL-21+CD4+ T cells in patients with acute hepatitis B (AHB).24 This cell subset was negatively correlated with HBV DNA levels, but was positively correlated with HBc18-27-specific IFN-γ-producing CD8+ T cells in CHB patients. Moreover, HBcAg-specific CD4+ T cells derived IL-21 from patients with AHB could enhance IFN-γ and perforin expression by CD8+T cells from CHB patients.24 These data indicated that HBcAg-specific IL-21-producing CD4+ T-cell responses could promote the antiviral activity of CD8+ T cells through IL-21 signaling. Notably, IL-21 in vitro could sustain the survival and function of HBc18-27-specific CD8+ T cells in this study; while Ren et al.25 reported that the addition of IL-21 in vitro could partially restore the function of HBV-specific CD8+ T-cell responses in HBV/HIV-1-coinfected patients, but not in HBV-monoinfected patients.25 Therefore, the precise effects of IL-21 on CD8+ T-cell responses and the relevant mechanisms warrant further investigation.
Function of IL-21 in effective humoral immunity
The appearance of antibodies against HBsAg and HBeAg indicate a favorable outcome of chronic HBV infection. The most critical site for the generation of T-dependent antibody responses is the germinal centers within lymphoid follicles in secondary lymphoid organs. A CD4+ T-cell subset, named Tfh cells, resides primarily in B-cell follicles, and specializes in helping B cells develop into antibody-producing cells in germinal centers by secreting high levels of IL-21.26,27 In fact, accumulating evidence has supported the concept of the liver as a secondary lymphoid organ, which can support the priming of classical CD4+ and CD8+ T-cell responses.28,29,30,31 Although hepatic immune priming is generally considered to favor inherent tolerogenicity of the liver,32,33,34 recent data from experimental animal models of human HBV have strongly demonstrated that age-dependent immune responses contributed to effective immune activation and antiviral immunity of the liver.21,35 Additionally, the hepatic lymphoid structures possessed the capacity to support the differentiation and maturation of B-cell responses, as well as the priming of Tfh cells.35 Because of the elevated IL-21 expression from liver Tfh cells and the increased numbers of IgG-expressing B cells, adult recipient mice could generate an effective humoral immune response that resulted in life-long clearance of HBsAg.21 However, in the absence of IL-21R on donor splenocytes, adult mice were unable to produce HBsAb, leading to HBV antigen persistence.21 Despite being phenotypically Tfh-like, IL-21R knockout CD4+ T cells were functionally devoid of B helper cell activity.36 Therefore, IL-21R knockout mice with lymphocytic choriomeningitis virus infection exhibited a profound defect in the generation of long-lived plasma cells and in the maintenance of antiviral antibody response over time, highlighting the importance of IL-21 in humoral immunity to viral infection.36
Using a longitudinal cohort under telbivudine treatment, our group found that HBeAg seroconversion during antiviral therapy for HBeAg positive patients with CHB was associated with high serum IL-21 concentrations at the week 12 time point: a strong predictor independent of other common parameters, such as HBV DNA and ALT levels.22 The data suggested that serum IL-21 levels may be identified as an immune biomarker for HBeAg seroconversion.37 However, in HIV/HBV-coinfected patients placed under HBV-active combination antiretroviral therapy, there was little evidence to support the association between IL-21 and HBeAg seroconversion.38
HBeAg loss was associated with enhanced CD4+ T cells responses to HBV in adefovir dipivoxil-treated HBeAg-positive patients with CHB.39 In a cross-sectional study, the IC patients characterized by the presence of anti-HBe showed an increased frequency of HBV-specific IL-21+CD4+ T cells.22 Thus, the source of IL-21 in patients who achieved HBeAg seroconversion was likely derived from HBV-specific CD4+ T cells, especially Tfh cells, which are the predominant producers of IL-21. Recently, the importance of Tfh cells at other sites has become evident.26 Several studies have demonstrated that CXCR5+CD4+ T cells in the peripheral circulation shared some properties with Tfh cells.40,41,42 Moreover, CXCR5+CD4+ T cells derived from both the circulation and germinal centers potently induced antibody production in in vitro cocultures with autologous B cells.42,43 Thus, circulating CXCR5+CD4+ T cells, which are easily accessible in the peripheral blood, may serve as surrogates for assessing the function of Tfh cells. This concept was further supported by our observation that circulating CXCR5+CD4+ T cells were closely related to Tfh cells of the lymphoid organs in patients with chronic HBV infection.23
Peripheral blood CXCR5+CD4+ T cells also participate in HBV-related immune responses. In a study by Feng et al.,44 immune active CHB patients had increased frequencies of peripheral blood CXCR5+CD4+ T cells. Consistent with this observation, we recently found that both the overall and HBV-specific circulating CXCR5+CD4+ T cells were expanded in chronic HBV infection, and a high frequency of this cell subset was associated with HBeAg seroconversion in both cross-sectional and longitudinal studies.23 More importantly, the CXCR5+CD4+ T cells from HBeAg seroconverters could promote anti-HBe production by autologous B cells in vitro in an IL-21-dependent manner.23 These data support the notion that IL-21 represents a major mediator for the function of CXCR5+CD4+ T cells in the induction and maintenance of HBeAg seroconversion.
Function of IL-21 in liver injury and disease progression
In addition to Tfh cells, IL-17-producing-CD4+ T cells (Th17) also secrete IL-21. Meanwhile, retinoic acid receptor-related orphan receptor gamma t, a transcription factor induced by IL-21, is essential for Th17 development;45 this autocrine property can also support high levels of persistent (and perhaps detrimental) Th17 cells. The highly increased frequency of Th17 cells in peripheral blood mononuclear cell has been observed in CHB patients with severe liver damage,46 strongly supporting the hypothesis that Th17-related cytokines, such as IL-21, maybe involved in the pathogenesis of hepatitis B and promote HBV infection-related liver injury.
In patients with hepatitis B-related acute-on-chronic liver failure, the serum levels of IL-21 and the frequencies of IL-21-producing-CD4+ T cells were significantly elevated, and both of these factors declined with clinical improvement in surviving subjects.47 Moreover, a high level of serum IL-21 was identified to be positively associated with increased model for end-stage liver disease score and mortality. In vitro, IL-21 could upregulate the expression of the pro-inflammatory cytokines IL-1β, IL-6 and TNF-α in peripheral blood mononuclear cell from both healthy subjects and CHB patients.47 These results suggest that IL-21may have a causal role in the development of severe liver inflammation. However, there was no correlation between serum IL-21 and ALT levels in this study, which is in agreement with our subsequent finding from immune activated CHB patients;22 therefore, IL-21 production may be related to the immune response to HBV, rather than to the degree of liver inflammation. In contrast, a recent study from Pan et al.48 found that IL-21 expression in serum and liver tissues were positively correlated with serum ALT and AST levels in CHB. Meanwhile, the intensity of IL-21 staining in liver tissues was associated with the grade of liver damage; this observation was consistent with a previous report showing that Th17 cells were largely accumulated in the liver of CHB patients and correlated positively with the histological activity index.46 These findings support the potentiality that serum IL-21 levels may serve as a biomarker for evaluating the severity of hepatic inflammation as well as liver injury in CHB. Taken together, collective evidence provided by current studies suggest that aberrant IL-21 responses may be associated with the liver injury during the pathogenesis of chronic HBV infection.
Chronic inflammation and liver injury initiated by HBV infection have been considered major factors leading to liver fibrosis. The host immune response is pivotal for fibrogenesis in liver cirrhosis. Increased peripheral and intrahepatic Th17 cells have been detected in patients with HBV-associated liver cirrhosis (HBV-LC) and have been found to contribute to the severity of disease progression through induction of hepatic stellate cell activation,49 raising the possibility that IL-21 may be involved in the process of liver fibrogenesis. Indeed, studies in animal models have demonstrated that IL-21 could act as a vital profibrogenic factor in vivo by promoting fibrosis through facilitating the development of CD4+ T helper 2 responses.50 In patients with HBV-LC, increased peripheral numbers of IL-21+CD4+ T cells and elevated plasma levels of IL-21 were observed. Meanwhile, consistent with the findings from CHB, liver IL-21+ cells were positively correlated with fibrotic staging scores, and clinical progression from CHB to LC.51 Mechanisms underlying this process were attributed to IL-21, which could promote activation, inhibit apoptosis and enhance collagen production, without affecting the proliferation of hepatic stellate cell.51 These observations indicated that IL-21 may participate in the fibrogenesis of liver cirrhosis. Therefore, therapeutic strategies targeting the neutralization of IL-21 might be a viable treatment for liver cirrhosis.
Conclusions and future perspectives
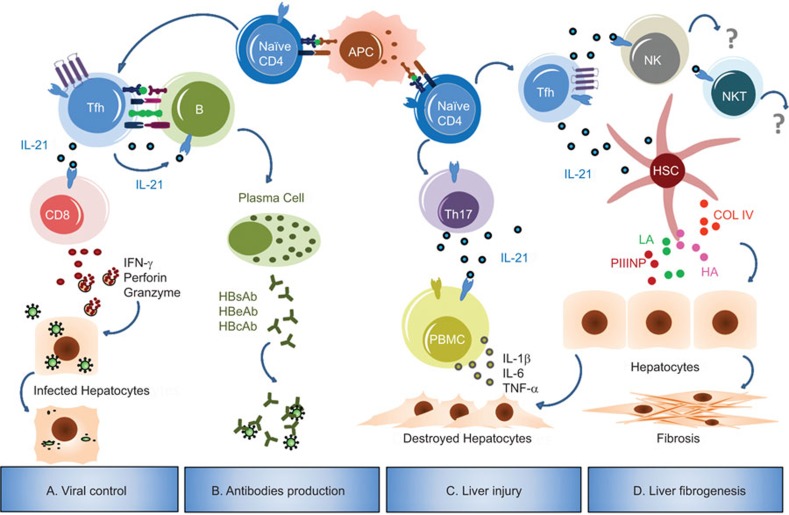
IL-21 is an important cytokine that regulates both cellular and humoral immune responses. In this review, we highlighted some properties of IL-21 in the context of HBV infection in murine models and humans. As a critical helper factor, IL-21 improves the functional quality of the antiviral CD8+ T cells response that is required for viral control. High levels of serum IL-21 after antiviral therapy correlate with the development of HBV-specific antibody responses. Therefore, IL-21 derived from CD4+ T cells acts as an effective intermediary for the optimal generation of specific CD8+ T and B-cell responses that are crucial for viral clearance and disease outcome, indicating a beneficial role of increasing IL-21 levels in HBV infection. However, elevated IL-21 expression may also aggravate the development of HBV-induced liver cirrhosis and the severity of liver injury. Taken together, IL-21 exerts a diverse range of effects at different stages of HBV infection (Figure 1).
Figure 1.
Pleiotropic effects of IL-21 in HBV infection. During the response to HBV, APCs present HBV-derived antigens to naive CD4+ T cells, thereby inducing the differentiation of these T cells into functional T helper cells. (a) Activated CD4+ T cells (Tfh cells in mouse models) secrete IL-21, which sustains antiviral functions of CD8+ T cells by enhancing IFN-γ, perforin and granzyme expression, eventually leading to viral control.21,24,25 (b) In combination with costimulatory signals, IL-21 secreted by Tfh cells regulates B-cell proliferation and differentiation into antibody-secreting plasma cells, thus facilitating the production of HBV-specific antibodies.21,23 (c) Aberrant IL-21 derived from Th17 cells (perhaps Tfh cells) can upregulate the expression of the pro-inflammatory cytokines IL-1β, IL-6 and TNF-α in PBMC from CHB patients, resulting in immune-mediated liver injury and inflammation.46,47,48 (d) IL-21 contributes to the severity of disease progression through HSC activation, and might be involved in the process of liver fibrogenesis.49,50,51 Currently, little information is known regarding the immune effects of IL-21 on NK or NKT cells in the context of HBV infection. APC, antigen-presenting cell; COL IV, collagen type IV; HA, hyaluronic acid; HSC, hepatic stellate cell; LN, laminin; PBMC, peripheral blood mononuclear cell; PIIINP, procollagen type III N-terminal peptide; Tfh, follicular T helper; TNF-α, tumor necrosis factor-alpha.
While current findings have improved our understanding regarding the immunomodulatory effects of IL-21 in HBV infection, many unresolved issues still remain. For example, it is necessary to dissect how IL-21 interacts with other components of the immune system, due to the pleiotropic effects of this cytokine. Additionally, it is unknown whether the predictive value of IL-21 is specific to telbivudine treatment in CHB patients. Since patients with IFN-α treatment have relatively higher rates of HBeAg seroconversion, it will be interesting to investigate whether the Tfh/IL-21/B cells axis plays an important role in determining this outcome, and to uncover the relevant immune and molecular mechanisms underlying this process. Furthermore, that IL-21 is over-expressed in HBV-LC raised a question meriting attention to the role of IL-21 in HBV-related hepatocellular carcinoma. Future work is needed to elucidate whether IL-21 can function as a suppressive or stimulatory factor in the development of HBV-related HCC, while this has yet to be explored, it will definitely provide insights to the development of new therapeutic interventions for this malignant cancer.
Although the precise role of IL-21 during HBV infection needs to be explored in depth, recent findings from animal models and clinical cohorts have provided a promising prospect for the application of an IL-21-based immunotherapy approach in the treatment of chronic HBV infection. However, it is conceivable that manipulating the effects of IL-21 may be a double-edged sword. In this context, the targeting of IL-21 in vivo to optimize therapeutic efficacy will be a future challenge in this field.
Acknowledgments
The authors express their sincere thanks to Drs Xiaoyong Zhang and Chris Kafai Li for their critical comments. This work was supported by the Major Science and Technology Special Project of China (2008ZX10002-004, 2012ZX10002-003 and 2011CB946100) and the National Natural Science Foundation of China (81270025).
References
- 1Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 2000; 408: 57–63. [DOI] [PubMed] [Google Scholar]
- 2Bryant VL, Ma CS, Avery DT, Li Y, Good KL, Corcoran LM et al. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol 2007; 179: 8180–8190. [DOI] [PubMed] [Google Scholar]
- 3Wei L, Laurence A, Elias KM, O'Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem 2007; 282: 34605–34610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Coquet JM, Kyparissoudis K, Pellicci DG, Besra G, Berzins SP, Smyth MJ et al. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J Immunol 2007; 178: 2827–2834. [DOI] [PubMed] [Google Scholar]
- 5Parrish-Novak J, Foster DC, Holly RD, Clegg CH. Interleukin-21 and the IL-21 receptor: novel effectors of NK and T cell responses. J Leukoc Biol 2002; 72: 856–863. [PubMed] [Google Scholar]
- 6Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science 2009; 324: 1569–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science 2009; 324: 1572–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Frohlich A, Kisielow J, Schmitz I, Freigang S, Shamshiev AT, Weber J et al. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science 2009; 324: 1576–1580. [DOI] [PubMed] [Google Scholar]
- 9Kared H, Fabre T, Bedard N, Bruneau J, Shoukry NH. Galectin-9 and IL-21 mediate cross-regulation between Th17 and Treg cells during acute hepatitis C. PLoS Pathog 2013; 9: e1003422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10Chevalier MF, Julg B, Pyo A, Flanders M, Ranasinghe S, Soghoian DZ et al. HIV-1-specific interleukin-21+ CD4+ T cell responses contribute to durable viral control through the modulation of HIV-specific CD8+ T cell function. J Virol 2011; 85: 733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Cubas RA, Mudd JC, Savoye AL, Perreau M, van Grevenynghe J, Metcalf T et al. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nat Med 2013; 19: 494–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12Kwok SK, Cho ML, Park MK, Oh HJ, Park JS, Her YM et al. Interleukin-21 promotes osteoclastogenesis in humans with rheumatoid arthritis and in mice with collagen-induced arthritis. Arthritis Rheum 2012; 64: 740–751. [DOI] [PubMed] [Google Scholar]
- 13Fantini MC, Monteleone G, MacDonald TT. IL-21 comes of age as a regulator of effector T cells in the gut. Mucosal Immunol 2008; 1: 110–115. [DOI] [PubMed] [Google Scholar]
- 14Maini MK, Boni C, Ogg GS, King AS, Reignat S, Lee CK et al. Direct ex vivo analysis of hepatitis B virus-specific CD8+ T cells associated with the control of infection. Gastroenterology 1999; 117: 1386–1396. [DOI] [PubMed] [Google Scholar]
- 15Thimme R, Wieland S, Steiger C, Ghrayeb J, Reimann KA, Purcell RH et al. CD8+ T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol 2003; 77: 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16Chisari FV, Isogawa M, Wieland SF. Pathogenesis of hepatitis B virus infection. Pathol Biol (Paris) 2010; 58: 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 2003; 300: 337–339. [DOI] [PubMed] [Google Scholar]
- 18Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 2003; 421: 852–856. [DOI] [PubMed] [Google Scholar]
- 19Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat Immunol 2004; 5: 927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20Asabe S, Wieland SF, Chattopadhyay PK, Roederer M, Engle RE, Purcell RH et al. The size of the viral inoculum contributes to the outcome of hepatitis B virus infection. J Virol 2009; 83: 9652–9662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21Publicover J, Goodsell A, Nishimura S, Vilarinho S, Wang ZE, Avanesyan L et al. IL-21 is pivotal in determining age-dependent effectiveness of immune responses in a mouse model of human hepatitis B. J Clin Invest 2011; 121: 1154–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22Ma SW, Huang X, Li YY, Tang LB, Sun XF, Jiang XT et al. High serum IL-21 levels after 12 weeks of antiviral therapy predict HBeAg seroconversion in chronic hepatitis B. J Hepatol 2012; 56: 775–781. [DOI] [PubMed] [Google Scholar]
- 23Li Y, Ma S, Tang L, Li Y, Wang W, Huang X et al. Circulating chemokine (C–X–C Motif) receptor 5+ CD4+ T cells benefit hepatitis B e antigen seroconversion through IL-21 in patients with chronic hepatitis B virus infection. Hepatology 2013; 58: 1277–1286. [DOI] [PubMed] [Google Scholar]
- 24Li L, Liu M, Cheng LW, Gao XY, Fu JJ, Kong G et al. HBcAg-specific IL-21-producing CD4+ T cells are associated with relative viral control in patients with chronic hepatitis B. Scand J Immunol 2013; 78: 439–446. [DOI] [PubMed] [Google Scholar]
- 25Ren G, Esser S, Jochum C, Schlaak JF, Gerken G, Schadendorf D et al. Interleukin 21 augments the hepatitis B virus-specific CD8+ T-cell response in vitro in patients coinfected with HIV-1. AIDS 2012; 26: 2145–2153. [DOI] [PubMed] [Google Scholar]
- 26Crotty S. Follicular helper CD4 T cells (TFH). Annu Rev Immunol 2011; 29: 621–663. [DOI] [PubMed] [Google Scholar]
- 27Ozaki K, Spolski R, Feng CG, Qi CF, Cheng J, Sher A et al. A critical role for IL-21 in regulating immunoglobulin production. Science 2002; 298: 1630–1634. [DOI] [PubMed] [Google Scholar]
- 28Klein I, Crispe IN. Complete differentiation of CD8+ T cells activated locally within the transplanted liver. J Exp Med 2006; 203: 437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29Wuensch SA, Pierce RH, Crispe IN. Local intrahepatic CD8+ T cell activation by a non-self-antigen results in full functional differentiation. J Immunol 2006; 177: 1689–1697. [DOI] [PubMed] [Google Scholar]
- 30Greter M, Hofmann J, Becher B. Neo-lymphoid aggregates in the adult liver can initiate potent cell-mediated immunity. PLoS Biol 2009; 7: e1000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol 2009; 27: 147–163. [DOI] [PubMed] [Google Scholar]
- 32Thomson AW, Knolle PA. Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol 2010; 10: 753–766. [DOI] [PubMed] [Google Scholar]
- 33Calne RY. Immunological tolerance—the liver effect. Immunol Rev 2000; 174: 280–282. [DOI] [PubMed] [Google Scholar]
- 34Knolle PA, Gerken G. Local control of the immune response in the liver. Immunol Rev 2000; 174: 21–34. [DOI] [PubMed] [Google Scholar]
- 35Publicover J, Gaggar A, Nishimura S, Van Horn CM, Goodsell A, Muench MO et al. Age-dependent hepatic lymphoid organization directs successful immunity to hepatitis B. J Clin Invest 2013; 123: 3728–3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36Rasheed MA, Latner DR, Aubert RD, Gourley T, Spolski R, Davis CW et al. Interleukin-21 is a critical cytokine for the generation of virus-specific long-lived plasma cells. J Virol 2013; 87: 7737–7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37Lau GK, Wang FS. Uncover the immune biomarkers underlying hepatitis B e antigen (HBeAg) seroconversion: a need for more translational study. J Hepatol 2012; 56: 753–755. [DOI] [PubMed] [Google Scholar]
- 38Giarda P, Avihingsanon A, Sasadeusz J, Audsley J, Marks P, Matthews G et al. CXCL-10, interleukin-12 and interleukin-21 are not immunological predictors of HBeAg seroconversion in HIV-1-HBV coinfection following HBV-active antiretroviral therapy. Antivir Ther 2014; 19: 429–433. [DOI] [PubMed] [Google Scholar]
- 39Cooksley H, Chokshi S, Maayan Y, Wedemeyer H, Andreone P, Gilson R et al. Hepatitis B virus e antigen loss during adefovir dipivoxil therapy is associated with enhanced virus-specific CD4+ T-cell reactivity. Antimicrob Agents Chemother 2008; 52: 312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40Simpson N, Gatenby PA, Wilson A, Malik S, Fulcher DA, Tangye SG et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum 2010; 62: 234–244. [DOI] [PubMed] [Google Scholar]
- 41Linterman MA, Vinuesa CG. Signals that influence T follicular helper cell differentiation and function. Semin Immunopathol 2010; 32: 183–196. [DOI] [PubMed] [Google Scholar]
- 42Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G et al. Human blood CXCR5+CD4+ T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 2011; 34: 108–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43Chevalier N, Jarrossay D, Ho E, Avery DT, Ma CS, Yu D et al. CXCR5 expressing human central memory CD4 T cells and their relevance for humoral immune responses. J Immunol 2011; 186: 5556–5568. [DOI] [PubMed] [Google Scholar]
- 44Feng J, Lu L, Hua C, Qin L, Zhao P, Wang J et al. High frequency of CD4+ CXCR5+ TFH cells in patients with immune-active chronic hepatitis B. PLoS One 2011; 6: e21698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45Dong C. Regulation and pro-inflammatory function of interleukin-17 family cytokines. Immunol Rev 2008; 226: 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46Zhang JY, Zhang Z, Lin F, Zou ZS, Xu RN, Jin L et al. Interleukin-17-producing CD4+ T cells increase with severity of liver damage in patients with chronic hepatitis B. Hepatology 2010; 51: 81–91. [DOI] [PubMed] [Google Scholar]
- 47Hu X, Ma S, Huang X, Jiang X, Zhu X, Gao H et al. Interleukin-21 is upregulated in hepatitis B-related acute-on-chronic liver failure and associated with severity of liver disease. J Viral Hepat 2011; 18: 458–467. [DOI] [PubMed] [Google Scholar]
- 48Pan Q, Yu Y, Tang Z, Xi M, Jiang H, Xun Y et al. Increased levels of IL-21 responses are associated with the severity of liver injury in patients with chronic active hepatitis B. J Viral Hepat 2014; 21: e78–e88. [DOI] [PubMed] [Google Scholar]
- 49Sun HQ, Zhang JY, Zhang H, Zou ZS, Wang FS, Jia JH. Increased Th17 cells contribute to disease progression in patients with HBV-associated liver cirrhosis. J Viral Hepat 2012; 19: 396–403. [DOI] [PubMed] [Google Scholar]
- 50Pesce J, Kaviratne M, Ramalingam TR, Thompson RW, Urban JJ, Cheever AW et al. The IL-21 receptor augments Th2 effector function and alternative macrophage activation. J Clin Invest 2006; 116: 2044–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51Feng G, Zhang JY, Zeng QL, Yu X, Zhang Z, Lv S et al. Interleukin-21 mediates hepatitis B virus-associated liver cirrhosis by activating hepatic stellate cells. Hepatol Res 2013; in press. [DOI] [PubMed]