Abstract
Chronic hepatitis B virus (HBV) infection progresses through distinct disease phases that are strongly associated with patient age. The so-called immune tolerant (IT) phase represents the classical early phase of infection; it is associated with high levels of HBV replication and lack of clinical signs of liver Inflammation. Whether this phase of HBV infection is also associated with immunological features of “tolerance' has recently been challenged. Here, we review the data that dispute this concept of immune tolerance and then propose an alternative interpretation of the immunopathological events that take place during this early phase of CHB infection.
Keywords: Hepatitis B, antiviral immunity, T cells
Introduction
The development of chronic hepatitis B (CHB) is inextricably linked to the patient's age at the time of infection. Hepatitis B virus (HBV) is thought to exploit the immaturity of the neonatal immune system to establish a persistent infection, reflected in the 90% of neonates who develop chronicity following vertical or perinatal transmission, while children infected between the ages of 1 and 5 years are believed to have a 30% chance of developing chronic infection; conversely only 1%–5% of subjects infected in adulthood will develop CHB.1,2 Historically, fetal development of tolerance to the virus in utero, through the transplacental transfer of viral proteins, was cited as a possible explanation for the high rates of chronic infection in infants.3
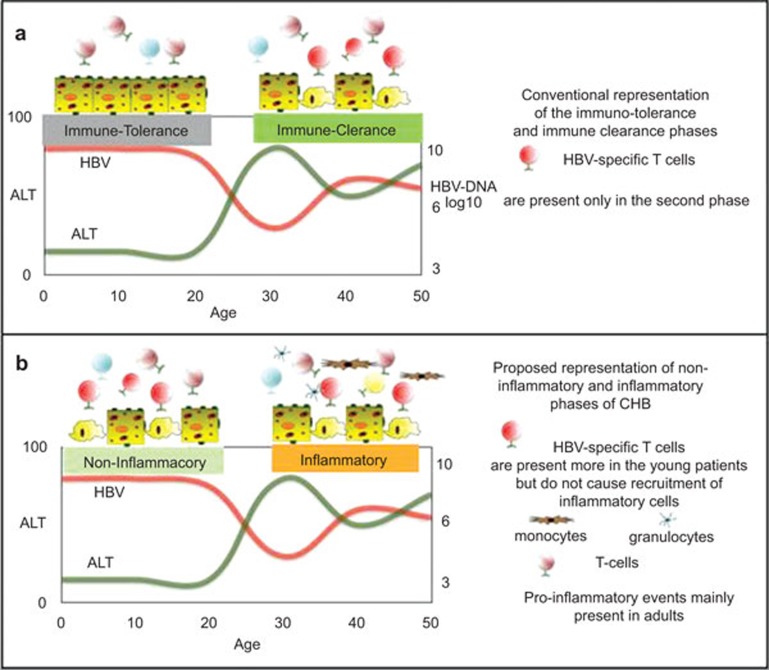
Patient age at the time of exposure does not only play a role in the outcome of infection, but also influences the clinical and virological features of the disease. Chronic HBV infection progresses through distinct disease phases that are strongly associated with patient age; the so called immune tolerant (IT) phase represents the classical early phase of infection, while the immune clearance and immune control phase are typically associated with adult patients (Figure 1a). These disease phases, which define the natural course of CHB, should mirror the different interaction between the host immune response and the virus.2 However, the concept of immunological tolerance, a basic premise on which the disease is managed and treatment decisions are made, is increasingly being challenged.
Figure 1.
Conventional (a) and proposed new representations (b) of the immunological events occurring during the natural history of HBV infection. HBV, hepatitis B virus.
A fundamental problem is that the IT phase, synonymous with HBV infection in young people, is a clinical categorisation used to define a disease state in an immune-mediated liver condition; broadly, it refers to hepatitis B e antigen positivity, high levels of HBV DNA and normal serum aminotransferases. The IT phase is usually asymptomatic and believed to be associated with minimal or no liver fibrosis on biopsy.1,2 However, whether this clinical and virological phenotype is really linked with genuine features of immune tolerance and a lack of liver damage is questionable. Several data can be used to challenge this concept. The efficacy of Peg IFN-alpha and Peg IFN-alpha+nucleos(t)ide analogue therapy has been reported to be superior in ‘immune tolerant' children than it is in adults.4,5 The concept of immune tolerance present in HBV-infected young people contrasts with the epidemiological observation in malaria-HBV co-infected young patients in whom reduced parasitemia6 and an increased incidence of cerebral malaria, a Th1-mediated malaria complication,7 have been reported. In addition, a recent immunological characterization of IT HBV-infected adolescents did not reveal any tolerogenic T-cell pattern.8 Finally, histologically active disease with evidence of minimal chronic hepatitis or nonspecific reactive hepatitis has been reported in CHB children under the age of 10–12 years, considered IT.9,10
Here, we will review the data that dispute this concept of immune tolerance and then propose an alternative interpretation of the immunopathological events that take place during this phase of CHB infection.
Does vertical or perinatal HBV infection induce immune tolerance?
HBV is thought to capitalize on the immaturity of the newborn immune system and establish a persistent infection through the induction of a state of low or absent virus-specific response.11 Acute hepatitis B infection in adults is almost invariably associated with control of HBV infection through the induction of an efficient HBV-specific T and B cell response.12 On the contrary, HBV infection in infants or young children rarely causes acute hepatitis and results in the asymptomatic disease phase characterized by high levels of HBV replication and a low incidence of liver inflammation defined clinically as immune tolerance.2 To explain this dichotomy, data from experimental animal models (i.e., HBV transgenic animals) have described the presence of immunological defects which impair HBV-specific T- and B-cell priming in neonatal animals;3,13,14 thus predisposing to HBV chronicity.
However, the concept that HBV exposure at birth is only associated with viral persistence and an inability to trigger an antigen-specific adaptive immunity is not unequivocal. First, the concept that the neonatal immune system is in some way ‘defective' appears more uncertain and there is growing evidence to suggest that neonatal immune responses defy such simple categorization. Both effector and regulatory immune responses are already in place during early fetal life.15,16 Newborns have been shown to mount a virus-specific T-cell response.17,18,19 The immune systems of newborns and infants are not ‘defective' per se, but appear less prone to trigger a pro-inflammatory reaction which is likely an evolutionary adaptation to avoid dangerous immune reactions in utero.20 Furthermore, a better analysis of data generated in natural HBV infection reveals that a proportion of neonates exposed to HBV at birth, mount a HBV-specific T-cell response. For example, two independent studies performed in HBsAg-negative children born to HBV-positive mothers21,22 have demonstrated the presence of core and polymerase-specific T cells. Neonates of HBV+ mothers have also been shown to have minimal or normal dendritic cell functions.23,24,25
Furthermore, the efficacy of HBV vaccination within the first year of life in HBV+ children26,27 raises considerable doubt that the state of HBV immune tolerance and defects in T–B cell interactions, detected in murine models,28,29 represent the inevitable consequence of HBV exposure in neonates and children.
Immunological and virological parameters in the immune tolerant phase of HBV infection
Most of the evidence supporting the existence of an IT disease phase of HBV infection is based on clinical and virological parameters. HBV is not directly cytopathic and since HBV-specific CD8 T cells control HBV replication by recognition and killing of the HBV infected hepatocytes,30 it is logical to interpret perturbations in alanine aminotransferase (ALT) level as a reflection of the presence or absence of HBV-specific T cells. Normal or minimal fluctuation in the liver enzymes has therefore been perceived as an indication of a lack of HBV-specific T-cell response. On the contrary, alterations in the liver enzymes and fluctuating levels of HBV DNA replication, which are observed more commonly in adulthood, are interpreted as an awakening of HBV-specific immunity.
In reality, both experimental data in animal models and HBV infection in humans have shown that ALT measurement cannot be used as a surrogate of a virus-specific T-cell response. Overt T-cell immunity against hepatocytes performed in adenovirus infected mice31 takes place without alteration in the serum ALT. Furthermore, adoptive transfer of HBV-specific T cells can cause substantial inhibition of HBV replication without increased levels of ALT, an observation that led to the understanding that efficient HBV clearance from infected hepatocytes is also mediated by a cytokine-mediated non-cythopathic effect.32,33
Besides, the quantification of circulating and intrahepatic HBV-specific T cells in CHB patients have shown that their quantity is not proportional to the level of biochemical activity reflected in the serum ALT.28,29 The robust inflammatory events in the liver that are associated with significant ALT elevations, are actually associated with an intrahepatic recruitment of granulocytes, monocytes and non-antigen-specific T cells.28,34,35,36,37
A direct demonstration that HBV immune response is not completely absent during the IT phase of CHB disease was shown in our recent work. We studied the immune response in CHB-infected adolescents with ostensible IT disease, based on their clinical and virological profile; analysis of the circulatory T cells demonstrated that these patients did not display any tolerogenic T-cell features. Moreover, they could mount a perfectly normal Th1 T-cell response and even harbour HBV-specific T cells. Notably, these HBV-specific T cells, though weak and functionally impaired as one would expect to see in patients with chronic hepatitis B, were in fact quantitatively and functionally superior to those found in CHB-infected adults in the ‘immune clearance' phase of disease.8 In addition to these immunological data, analysis of HBV quasispecies in children with an IT clinical profile showed significant HBV diversity.38 Despite this work focusing primarily on the analysis of HBV diversity in adult patients with CHB, the data collected in children with an IT clinical profile were compatible with the presence of a potential immune pressure against HBV during this initial phase of infection.
Does the immune tolerant phase of HBV infection stand up to closer scrutiny in the clinic?
We have discussed the limitations of serum ALT as a surrogate of HBV-specific T-cell activity and we summarized the data suggesting that the IT phase of disease is not associated with a complete absence of HBV-specific T-cell immunity. However, it is also important to understand whether normal liver enzymes and the potential presence of antiviral immunity can fully exclude ongoing disease activity within the liver.
Indeed, it has already been proposed by others that there is likely a low but persistent immune destruction of infected hepatocytes, by low-level cytotoxic T lymphocyte infiltration, leading over time to an adaptive response of the liver, with a possible clonal emergence of hepatocytes resistant to HBV infection; a scenario that could explain the progressive decrease of HBV replication levels over time.39
Studies in CHB-infected children under the age of 10–12 years, considered IT, were shown to have histologically active disease with evidence of minimal chronic hepatitis or nonspecific reactive hepatitis, despite ALT levels being described as within the normal range9,10 and clonal hepatocyte repopulation, an indirect measurement of targeted killing of HBV-infected hepatocytes, has been detected in patients in the IT disease phase (Kennedy and Mason, manuscript in preparation). Whether such low-level hepatocyte lysis can initiate the process for the development of liver cirrhosis and/or HCC later in adult life is at this juncture, difficult to predict.
Age-dependent changes in immunity
We have reviewed clinical and experimental evidence showing that HBV-specific T-cell immunity and some degree of liver damage are not completely absent in the initial phase of chronic HBV infection. However, how can we explain the differences in the clinical and virological profiles associated with age?
A possible explanation can be related to the fact that immune responses are not identical during the life of an individual. TLR-mediated immune function has been shown to change in different periods of life;40 the production of anti-inflammatory cytokines (e.g., IL-10) is high in preterm infants, then progressively declines over the first years of life, but is superior in children when compared to adults. Conversely, pro-inflammatory cytokines (e.g., IL-1beta, TNF-alpha) steadily increase during early life40 until they reach the state of chronic low-grade systemic inflammation called ‘inflammaging' that is characteristic of elderly subjects.41 Similarly, T-cell response shifts from a Th2/Treg type response in newborns to a more Th1 response in children/adults 20 with a progressive increase of effector memory T-cell pools, which are more sensitive to cytokine-mediated activation.42
Can the different virological and inflammatory patterns observed during the natural history of HBV infection be caused by the age-related modulation in immune function?
The concept that pathological processes might be modulated by age is established in other infections. For example, during the 1918 influenza pandemic, children, despite experiencing the same rate of clinical influenza, had a much lower mortality rate than young adults.43 This phenomenon has also been observed in other bacterial and viral infections44 and has been associated with the lower production of pro-inflammatory cytokines in children.
Interestingly, a detailed, albeit small, longitudinal study has shown that more dramatic changes in serum ALT levels occur in CHB patients around the 20- to 25-year-old age bracket,38 which is exactly the period where more pronounced pro-inflammatory events are observed in humans.
Immunological events in HBV infection: an alternative hypothesis
Based on the epidemiological, clinical and experimental data summarized above, we propose an alternative interpretation of the immune events occurring during the different phases of chronic HBV infection.
The state of high HBV replication and low ALT levels present in children and young CHB patients might be caused by the presence of an anti-viral ‘non-inflammatory' immune response (Figure 1b). As we have summarized above, data obtained from young patients with CHB (aged between 15 and 30 years) show that, at least in this period, the absence of serological markers of liver inflammation is not associated with a complete absence of HBV-specific T-cell response.8 Furthermore, signs of limited killing of hepatocytes9,10 and of virological immune escape38 can be detected in patients in the IT disease phase. Thus, in the initial phase of HBV infection, the few and functionally impaired HBV-specific T cells might, similar to adults, try to contain the HBV infection by cytokine-mediated control and by killing HBV-infected hepatocytes. However, as a consequence of the reduced pro-inflammatory cytokine milieu and of the limited pool of T cells responding to by-stander cytokine- mediated activation, this HBV-specific T-cell response does not trigger nonspecific inflammatory events. In contrast the so called ‘immune clearance phase' of chronic hepatitis B, characterized by abnormal serum ALT levels, fluctuations in the levels of HBV replication and clear histological signs of liver inflammation; may differ not by the number or function of HBV-specific T cells (that are more profoundly exhausted in CHB infected adult patients),8 but the higher propensity to trigger ‘inflammatory' events (Figure 1b). Liver inflammation can still be triggered by HBV-specific CD8 T cells, but it is unlikely a direct effect of a quantitative or functional recovery, which is in fact theoretical and has never actually been demonstrated in adult CHB patients. In these subjects, HBV-specific T-cell response has never been shown to be proportional to liver inflammation.28 Furthermore, episodes of heightened liver inflammation, like those observed during hepatic flares, occur without any detectable increase in circulating HBV-specific T cells,45 while they are associated with fluctuations in chemokines45 and natural killer cell activation.46 Alternatively, liver inflammatory events in adults may derive directly from pro-inflammatory reactions triggered, for example, by bacterial products as shown in a model of hepatic steatosis.47 It is notable that increased and persistent production of pro-inflammatory cytokines like IL-1beta and TNF-alpha can directly inhibit HBV replication48,49 and thus, explain the reduced HBV-DNA levels observed in adults patients.
Conclusions
CHB is a dynamic disease with an unpredictable course. The assumption that those infected at birth or in early childhood enter a phase of protracted immune tolerance with minimal or no disease progression over two to three decades appears increasingly implausible. Furthermore, as we discussed here, this clinical categorization lacks meaningful immunological data to support the concept of tolerance; while clinically the concept of a generic IT disease phase appears increasingly unsound.
Experimental and epidemiological data from HBV infection raise doubts that the natural progression of chronic HBV infection can be dictated by a simple quantitative difference in adaptive immunity against the virus. The oversimplistic view that vertical or perinatal transmission of HBV results in a phase of HBV-specific immune tolerance which is then lost in adulthood is not supported by our increased understanding of the effect of age on the immune response, nor is it supported by our better insight into the immune events triggered by HBV infection. The arguments presented here challenge the concept of a generic IT disease phase in young people and thus importantly, raise questions about the premise on which treatment decisions are made.
Additional data need to be gathered from HBV-exposed newborns and CHB-infected children, in whom limited studies have been performed to date. The potential role and function of many other components of the immune response, such as natural killer or natural killer T cells that are highly abundant in the liver, should also be analyzed in the context of age.50 Should more data support the proposed thinking, some practical consequences might arise. Firstly, if young CHB patients exhibit a more conserved HBV-specific immune response, than that observed in adult patients, but do not trigger a full blown pro-inflammatory reaction; therapy designed to boost HBV-specific immunity (vaccine therapy, check points inhibitors) is more likely to be successful and indeed less damaging in young patients. Secondly, we should start to consider and evaluate CHB in adults as an inflammatory rather than a viral induced disease. The efficacy of therapy based on suppressing platelet function in blocking the development of HCC in HBV transgenic mice represents the first step towards this analysis.51,52
References
- 1Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepatitis 2004; 11: 97–107. [DOI] [PubMed] [Google Scholar]
- 2Yim HJ, Lok AS. Natural history of chronic hepatitis B virus infection: what we knew in 1981 and what we know in 2005. Hepatology 2006; 43: S173–S181. [DOI] [PubMed] [Google Scholar]
- 3Milich DR, Jones JE, Hughes JL, Price J, Raney AK, McLachlan A et al. Is a function of the secreted hepatitis B e antigen to induce immunologic tolerance in utero? Proc Natl Acad Sci USA 1990; 87: 6599–6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4D'Antiga L, Aw M, Atkins M, Moorat A, Vergani D, Mieli-Vergani G. Combined lamivudine/interferon-α treatment in ‘immunotolerant' children perinatally infected with hepatitis B: a pilot study. J Pediatr 2006; 148: 228–233.e1. [DOI] [PubMed] [Google Scholar]
- 5Carey I, D'Antiga L, Bansal S, Longhi MS, Ma Y, Mesa IR et al. Immune and viral profile from tolerance to hepatitis B surface antigen clearance: a longitudinal study of vertically hepatitis B virus-infected children on combined therapy. J Virol 2011; 85: 2416–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Andrade BB, Santos CJ, Camargo LM, Souza-Neto SM, Reis-Filho A, Clarêncio J et al. Hepatitis B infection is associated with asymptomatic malaria in the Brazilian Amazon. PLoS ONE 2011; 6: e19841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Thursz MR, Kwiatkowski D, Torok ME, Allsopp CE, Greenwood BM, Whittle HC et al. Association of hepatitis B surface antigen carriage with severe malaria in Gambian children. Nat Med 1995; 1: 374–375. [DOI] [PubMed] [Google Scholar]
- 8Kennedy PT, Sandalova E, Jo J, Gill U, Ushiro-Lumb I, Tan AT et al. Preserved T-cell function in children and young adults with immune-tolerant chronic hepatitis B. Gastroenterology 2012; 143: 637–645. [DOI] [PubMed] [Google Scholar]
- 9Chang MH, Hwang LY, Hsu HC, Lee CY, Beasley RP. Prospective study of asymptomatic HBsAg carrier children infected in the perinatal period: clinical and liver histologic studies. Hepatology 1988; 8: 374–377. [DOI] [PubMed] [Google Scholar]
- 10Hsu HC, Lin YH, Chang MH, Su IJ, Chen DS. Pathology of chronic hepatitis B virus infection in children: with special reference to the intrahepatic expression of hepatitis B virus antigens. Hepatology 1988; 8: 378–382. [DOI] [PubMed] [Google Scholar]
- 11Prendergast AJ, Klenerman P, Goulder PJ. The impact of differential antiviral immunity in children and adults. Nat Rev Immunol 2012; 12: 636–648. [DOI] [PubMed] [Google Scholar]
- 12Bertoletti A, Ferrari C. Innate and adaptive immune responses in chronic hepatitis B virus infections: towards restoration of immune control of viral infection. Gut 2012; 61: 1754–1764. [DOI] [PubMed] [Google Scholar]
- 13Publicover J, Gaggar A, Nishimura S, van Horn CM, Goodsell A, Muench MO et al. Age-dependent hepatic lymphoid organization directs successful immunity to hepatitis B. J Clin Invest 2013; 123: 3728–3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Publicover J, Goodsell A, Nishimura S, Vilarinho S, Wang ZE, Avanesyan L et al. IL-21 is pivotal in determining age-dependent effectiveness of immune responses in a mouse model of human hepatitis B. J Clin Invest 2011; 121: 1154–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15Mold JE, Venkatasubrahmanyam S, Burt TD, Michaëlsson J, Rivera JM, Galkina SA et al. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science 2010; 330: 1695–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16Zhang X, Mozeleski B, Lemoine S, Dériaud E, Lim A, Zhivaki D et al. CD4 T cells with effector memory phenotype and function develop in the sterile environment of the fetus. Sci Transl Med 2014; 6: 238ra72–238ra72. [DOI] [PubMed] [Google Scholar]
- 17Marchant A, Appay V, van der Sande M, Dulphy N, Liesnard C, Kidd M et al. Mature CD8+ T lymphocyte response to viral infection during fetal life. J Clin Invest 2003; 111: 1747–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Vermijlen D, Brouwer M, Donner C, Liesnard C, Tackoen M, van Rysselberge M et al. Human cytomegalovirus elicits fetal T cell responses in utero. J Exp Med 2010; 207: 807–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Luzuriaga K, Holmes D, Hereema A, Wong J, Panicali DL, Sullivan JL. HIV-1-specific cytotoxic T lymphocyte responses in the first year of life. J Immunol 1995; 154: 433–443. [PubMed] [Google Scholar]
- 20Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol 2007; 7: 379–390. [DOI] [PubMed] [Google Scholar]
- 21Komatsu H, Inui A, Sogo T, Hiejima E, Tateno A, Klenerman P et al. Cellular immunity in children with successful immunoprophylactic treatment for mother-to-child transmission of hepatitis B virus. BMC Infect Dis 2010; 10: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22Koumbi L, Bertoletti A, Anastasiadou V, Machaira M, Goh W, Papadopoulos NG et al. Hepatitis B-specific T helper cell responses in uninfected infants born to HBsAg+/HBeAg− mothers. Cell Mol Immunol 2010; 7: 454–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23Koumbi LJ, Papadopoulos NG, Anastassiadou V, Machaira M, Kafetzis DA, Papaevangelou V. Dendritic cells in uninfected infants born to hepatitis B virus-positive mothers. Clin Vaccine Immunol 2010; 17: 1079–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24Guo J, Gao Y, Guo Z, Zhang LR, Wang B, Wang SP. Frequencies of dendritic cells and Toll-like receptor 3 in neonates born to HBsAg-positive mothers with different HBV serological profiles. Epidemiol Infect 2014; in press. [DOI] [PMC free article] [PubMed]
- 25Zheng Z, Chen D, Yao J, Zhang H, Jin L, Shi M et al. Increased infiltration of intrahepatic DC subsets closely correlate with viral control and liver injury in immune active pediatric patients with chronic hepatitis B. Clin Immunol 2007; 122: 173–180. [DOI] [PubMed] [Google Scholar]
- 26Beasley RP, Hwang LY, Lee GC, Lan CC, Roan CH, Huang FY et al. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet 1983; 2: 1099–1102. [DOI] [PubMed] [Google Scholar]
- 27Mackie CO, Buxton JA, Tadwalkar S, Patrick DM. Hepatitis B immunization strategies: timing is everything. Can Med Assoc J 2009; 180: 196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28Maini MK, Boni C, Lee CK, Larrubia JR, Reignat S, Ogg GS et al. The role of virus-specific CD8+ cells in liver damage and viral control during persistent hepatitis B virus infection. J Exp Med 2000; 191: 1269–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29Webster GJ, Reignat S, Brown D, Ogg GS, Jones L, Seneviratne SL et al. Longitudinal analysis of CD8+ T cells specific for structural and nonstructural hepatitis B virus proteins in patients with chronic hepatitis B: implications for immunotherapy. J Virol 2004; 78: 5707–5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Thimme R, Wieland S, Steiger C, Ghrayeb J, Reimann KA, Purcell RH et al. CD8+ T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol 2003; 77: 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Stabenow D, Frings M, Trück C, Gärtner K, Förster I, Kurts C et al. Bioluminescence imaging allows measuring CD8 T cell function in the liver. Hepatology 2010; 51: 1430–1437. [DOI] [PubMed] [Google Scholar]
- 32Guidotti LG, Ishikawa T, Hobbs MV, Matzke B, Schreiber R, Chisari FV. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity 1996; 4: 25–36. [DOI] [PubMed] [Google Scholar]
- 33Phillips S, Chokshi S, Riva A, Evans A, Williams R, Naoumov NV. CD8+ T cell control of hepatitis B virus replication: direct comparison between cytolytic and noncytolytic functions. J Immunol 2009; 184: 287–295. [DOI] [PubMed] [Google Scholar]
- 34Kakimi K, Lane TE, Wieland S, Asensio VC, Campbell IL, Chisari FV et al. Blocking chemokine responsive to gamma-2/interferon (IFN)-gamma inducible protein and monokine induced by IFN-gamma activity in vivo reduces the pathogenetic but not the antiviral potential of hepatitis B virus-specific cytotoxic T lymphocytes. J Exp Med 2011; 194: 1755–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Ando K, Moriyama T, Guidotti LG, Wirth S, Schreiber RD, Schlicht HJ et al. Mechanisms of class I restricted immunopathology. A transgenic mouse model of fulminant hepatitis. J Exp Med 1993; 178: 1541–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36Sitia G, Isogawa M, Iannacone M, Campbell IL, Chisari FV, Guidotti LG. MMPs are required for recruitment of antigen-nonspecific mononuclear cells into the liver by CTLs. J Clin Invest 2004; 113: 1158–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37Sitia G, Isogawa M, Kakimi K, Wieland SF, Chisari FV, Guidotti LG. Depletion of neutrophils blocks the recruitment of antigen-nonspecific cells into the liver without affecting the antiviral activity of hepatitis B virus-specific cytotoxic T lymphocytes. Proc Natl Acad Sci USA 2002; 99: 13717–13722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38Wang HY, Chien MH, Huang HP, Chang HC, Wu CC, Chen PJ et al. Distinct hepatitis B virus dynamics in the immunotolerant and early immunoclearance phases. J Virol 2010; 84: 3454–3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39Zoulim F, Mason WS. Reasons to consider earlier treatment of chronic HBV infections. Gut 2012; 61: 333–336. [DOI] [PubMed] [Google Scholar]
- 40Kollmann TR, Levy O, Montgomery RR, Goriely S. Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly. Immunity 2012; 37: 771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41Shaw AC, Joshi S, Greenwood H, Panda A, Lord JM. Aging of the innate immune system. Curr Opin Immunol 2010; 22: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4+ T cells. J Exp Med 2001; 194: 1711–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43Fedson DS. Was bacterial pneumonia the predominant cause of death in the 1918–1919 influenza pandemic? J Infect Dis 2009; 199: 1408–1509; author reply 1409–1410. [DOI] [PubMed] [Google Scholar]
- 44Fedson DS. Treating influenza with statins and other immunomodulatory agents. Antiviral Res 2013; 99: 417–435. [DOI] [PubMed] [Google Scholar]
- 45Tan AT, Koh S, Goh W, Zhe HY, Gehring AJ, Lim SG et al. A longitudinal analysis of innate and adaptive immune profile during hepatic flares in chronic hepatitis B. J Hepatol 2010; 52: 330–339. [DOI] [PubMed] [Google Scholar]
- 46Dunn C, Brunetto M, Reynolds G, Christophides T, Kennedy PT, Lampertico P et al. Cytokines induced during chronic hepatitis B virus infection promote a pathway for NK cell-mediated liver damage. J Exp Med 2007; 204: 667–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 2012; 482: 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48Watashi K, Liang G, Iwamoto M, Marusawa H, Uchida N, Daito T et al. Interleukin-1 and tumor necrosis factor-α trigger restriction of hepatitis B virus infection via a cytidine deaminase activation-induced cytidine deaminase (AID). J Biol Chem 2013; 288: 31715–31727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49Puro R, Schneider RJ. Tumor necrosis factor activates a conserved innate antiviral response to hepatitis B virus that destabilizes nucleocapsids and reduces nuclear viral DNA. J Virol 2007; 81: 7351–7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50Maini MK, Peppa D. NK cells: a double-edged sword in chronic hepatitis B virus infection. Front Immunol 2013; 4: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51Sitia G, Iannacone M, Muller S, Bianchi ME, Guidotti LG. Treatment with HMGB1 inhibitors diminishes CTL-induced liver disease in HBV transgenic mice. J Leuk Biol 2006; 81: 100–107. [DOI] [PubMed] [Google Scholar]
- 52Sitia G, Aiolfi R, Di Lucia P, Mainetti M, Fiocchi A, Mingozzi F et al. Antiplatelet therapy prevents hepatocellular carcinoma and improves survival in a mouse model of chronic hepatitis B. Proc Natl Acad Sci USA 2012; 109: E2165–E2172. [DOI] [PMC free article] [PubMed] [Google Scholar]