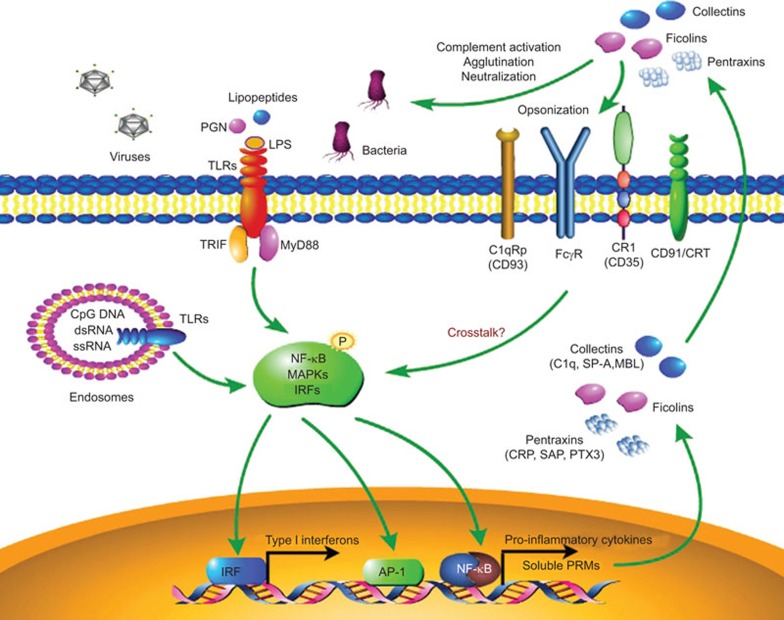
The initiation of innate immune response relies on the recognition of pathogen-associated molecular patterns by pattern recognition molecules (PRMs), including the cellular pattern recognition receptors and extracellular soluble PRMs. Pattern recognition receptors such as Toll-like receptors (TLRs), NOD-like receptors and RIG-I like receptors are well documented cell-associated PRMs that sense microbial components on the cell surface, in the cytoplasm or in the nucleus of immune or non-immune cells to initiate the innate response to eliminate the invading pathogens.1,2 However, there is limited evidence of an extracellular PRM-mediated innate response to disease. In response to pathogen invasion, humoral PRMs are rapidly produced by a variety of cells and tissues, most notably by innate immune cells such as neutrophils, dendritic cells and macrophages. Extracellular humoral PRMs can be divided into different molecular families, primarily including pentraxins,3 collectins and ficolins.4 Accumulating evidence indicates that extracellular PRMs are important components of the humoral arm of innate immunity. They are able to recognize a variety of pathogenic agents and eliminate them through shared common mechanisms including complement activation, opsonization, agglutination, neutralization and regulation of inflammation (Figure 1). Moreover, extracellular PRMs also interact with cellular TLRs and regulate their function, jointly contributing to the recognition of microbial patterns and modulation of the innate immune responses.5,6,7,8
Figure 1.
Innate immune responses initiated by cellular TLRs and extracellular PRMs. Cellular TLRs and extracellular soluble PRMs are critical for sensing evading pathogens and triggering the innate immune responses. Pathogen-associated molecular patterns can be recognized by cellular TLRs in different cellular compartments. After ligation, TLRs can initiate downstream activation of NF-κB, MAPKs and IRFs through different signaling pathways that lead to the production of pro-inflammatory cytokines and type I interferon. Soluble PRMs are rapidly produced and secreted into the extracellular fluid in response to various danger signals and TLR engagement. Soluble PRMs generally belong to different molecular classes, including pentraxins (CRP, SAP, PTX3), collectins (C1q, SP-A, SP-D, MBL) and ficolins. These humoral PRMs primarily act through complement activation, agglutination and neutralization. They also have cellular receptors such as CR1 (CD35), CD91/CRT, C1qRp (CD93) and FcγR. After binding to these receptors, soluble PRMs can facilitate the recognition and phagocytosis of a microorganism by immune cells (opsonization) and can regulate the functions of cellular TLRs via mechanisms that remain unclear. CRP, C-reactive protein; IRF, IFN regulatory factor; MBL, mannan-binding lectin; MyD88, myeloid differentiation factor 88; PRM, pattern recognition molecule; SAP, serum amyloid P component; SP-A, surfactant protein A; TLR, Toll-like receptor; TRIF, Toll-IL-1 receptor domain-containing adaptor-inducing interferon-β.
Pentraxins are a superfamily of evolutionarily conserved PRMs that can be divided into short and long pentraxins on a structural basis. Pentraxin 3 (PTX3) is the first identified member of the long pentraxin subfamily. It can be induced in somatic and immune cells following the stimulation of pro-inflammatory signals and the engagement of TLRs.9 It has been suggested that PTX3 is a multifunctional extracellular PRM involved in innate immunity, inflammation, matrix deposition and female fertility.10,11 Urinary tract infections (UTIs) remain a common and quite costly medical problem and are primarily caused by uropathogenic strains of Escherichia coli (UPEC).12 The role of the innate immune system in UTIs remains unclear. In a recent issue of Immunity, Jaillon et al.13 explored the involvement of the soluble PRM PTX3 in UTIs. They found that in UPEC-infected mice, the expression of PTX3 was significantly elevated in the blood and in the urinary organs such as the bladder and kidneys. PTX3-deficient mice were revealed to be much more susceptible to UTIs and displayed an increased bacterial burden in bladders and kidneys, as well as significant body weight loss. Thus, PTX3 is crucial for the host defense against UPEC infection.
Next, the authors investigated how PTX3 protected the host from UPEC infection. They found that PTX3 bound to the surface of E. coli and served as opsonins to promote phagocytosis and phagosome maturation in human and mouse neutrophils. Opsonization of UPEC by PTX3 dramatically increased phagocytosis by neutrophils in vitro. Then, the authors sought to explore the cellular source and signaling pathway responsible for PTX3 production during UTIs. PTX3 was found to be primarily produced by infiltrating leukocytes and epithelial cells in the urinary tract through the TLR4–MyD88 axis.
The authors then analyzed the inflammatory response in PTX3-deficient mice. Compared with wild-type mice, increased neutrophil recruitment in the urine was observed in PTX3-deficient mice with much higher levels of chemokines. In consideration of higher bacterial burden in PTX3-deficient mice, the above findings indicated that the loss of PTX3 resulted in the defective clearance of pathogens and exacerbated inflammation during UTIs. Intriguingly, the authors further analyzed the function of PTX3 in human UTIs. By immunohistochemistry, PTX3 was found to have a diffuse expression in the bladder sections from UTI patients, while almost no expression was observed in healthy donors. PTX3 production was also significantly elevated in serum and urine samples from patients suffering from UTIs compared with healthy donors. Strikingly, increased PTX3 production could be reduced by antibiotic treatment. The authors also found that PTX3 gene polymorphisms might correlate with susceptibility to acute pyelonephritis and cystitis in cohorts of UTI-prone patients. Therefore, PTX3 may be a good prognostic and therapeutic target for UTI patients.
This article provides compelling evidence demonstrating the critical role of PTX3 in UTIs and sheds new light on the previously ignored role of humoral PRMs in innate immunity. However, several questions remain. First, although PTX3-deficient mice exhibited much more neutrophil recruitment in the urine compared with wild-type mice during UTIs, the levels of bacteria in the bladders and kidneys were still significantly higher. This indicated that the clearance of the bacteria by neutrophils was defective in PTX3-deficient mice. The authors believed that PTX3 molecules could serve as opsonins and that the loss of PTX3 caused impaired phagocytosis of neutrophils. In addition to phagocytosis, neutrophils possess other mechanisms of eliminating invading pathogens, including the elaboration of reactive oxygen species and antimicrobial cytokines and the release of neutrophil extracellular DNA traps.14 It would be interesting to further explore whether and how PTX3 might influence other functions of neutrophils during UTIs. Second, innate immune cells such as macrophages and dendritic cells are important effectors in infection, and the effect of PTX3 on the functions of these cells remains unclear. Moreover, it was implicated that PTX3 had cellular receptors.5,15 During infection, it is possible that PTX3 directly binds to its receptor, triggers downstream signaling and exerts a regulatory role in inflammation that needs to be further characterized. Last but not least, TLRs are also activated to initiate a robust innate immune response during UTIs. Because PTX3 was produced through TLR4 signaling, it would be interesting to investigate the crosstalk between PTX3 and the TLR-mediated inflammatory response during UTIs.
As important components of the humoral innate immune system, PRMs are virgin fields to be further explored. In addition to the well-characterized PRMs, the functions of many PRMs are still unknown. Unidentified members of the PRM family may also exist, and it would be interesting to investigate their functions. Furthermore, emerging evidence suggests that extracellular PRMs may play a regulatory role during the cellular TLR-mediated innate response. The lung collectin, surfactant protein A, was able to upregulate TLR2 expression and dampen TLR2 and TLR4 signaling in human macrophages.6 In addition to initiating complement activation, mannan-binding lectin facilitated TLR2/6 signaling from the phagosome during Staphylococcus aureus infection.7 A recent report found that mannan-binding lectin could interact with poly(I∶C) and TLR3, thus suppressing the activation of the TLR3 pathway and subsequent cytokine production.8 The internal signaling pathway involved after PRMs bind to their membrane receptors and the detailed mechanisms of how PRMs interact with TLR signaling remain unknown. It is also possible that PRMs interfere with other pattern recognition receptors such as RLRs and NLRs, and this possibility needs to be studied further. Moreover, as an important modulator of inflammation, PRMs have been suggested to participate in the pathogenesis of autoimmune and inflammatory diseases.5,11 For example, a lack of PTX3 was shown to aggravate autoimmune lung disease in a murine model of systemic lupus erythematosus. PTX3 fostered the rapid clearance of apoptotic T cells by macrophages, thereby keeping lupus autoantigens away from dendritic cells and avoiding the activation of autoreactive T cells.16 PTX3 also showed cardiovascular protective effects during atherosclerosis and cardiovascular diseases.17 However, the effecter mechanisms of PRMs in these diseases remain unclear. Further exploration of the functions of PRMs in different experimental models is necessary to identify their roles in the development of different diseases; these studies may have clinical applications.
In summary, this study reveals that soluble PTX3 is critical for the innate immune response to UTIs. Future studies of PTX3 and other extracellular PRMs may help us understand how the cellular and humoral arms of innate immunity work together to eliminate invading pathogens and will help clarify the roles of extracellular PRMs in health and disease.
References
- 1O'Neill LA, Golenbock D, Bowie AG. The history of Toll-like receptors-redefining innate immunity. Nat Rev Immunol 2013; 13: 453–460. [DOI] [PubMed] [Google Scholar]
- 2Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 2010; 140: 805–820. [DOI] [PubMed] [Google Scholar]
- 3Mantovani A, Garlanda C, Doni A, Bottazzi B. Pentraxins in innate immunity: from C-reactive protein to the long pentraxin PTX3. J Clin Immunol 2008; 28: 1–13. [DOI] [PubMed] [Google Scholar]
- 4Holmskov U, Thiel S, Jensenius JC. Collections and ficolins: humoral lectins of the innate immune defense. Annu Rev Immunol 2003; 21: 547–578. [DOI] [PubMed] [Google Scholar]
- 5Bottazzi B, Doni A, Garlanda C, Mantovani A. An integrated view of humoral innate immunity: pentraxins as a paradigm. Annu Rev Immunol 2010; 28: 157–183. [DOI] [PubMed] [Google Scholar]
- 6Henning LN, Azad AK, Parsa KV, Crowther JE, Tridandapani S, Schlesinger LS. Pulmonary surfactant protein A regulates TLR expression and activity in human macrophages. J Immunol 2008; 180: 7847–7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Ip WK, Takahashi K, Moore KJ, Stuart LM, Ezekowitz RA. Mannose-binding lectin enhances Toll-like receptors 2 and 6 signaling from the phagosome. J Exp Med 2008; 205: 169–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Liu H, Zhou J, Ma D, Lu X, Ming S, Shan G et al. Mannan binding lectin attenuates double-stranded RNA-mediated TLR3 activation and innate immunity. FEBS Lett 2014; 588: 866–872. [DOI] [PubMed] [Google Scholar]
- 9Mantovani A, Valentino S, Gentile S, Inforzato A, Bottazzi B, Garlanda C. The long pentraxin PTX3: a paradigm for humoral pattern recognition molecules. Ann NY Acad Sci 2013; 1285: 1–14. [DOI] [PubMed] [Google Scholar]
- 10Garlanda C, Bottazzi B, Bastone A, Mantovani A. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu Rev Immunol 2005; 23: 337–366. [DOI] [PubMed] [Google Scholar]
- 11Cieślik P, Hrycek A. Long pentraxin 3 (PTX3) in the light of its structure, mechanism of action and clinical implications. Autoimmunity 2012; 45: 119–128. [DOI] [PubMed] [Google Scholar]
- 12Sivick KE, Mobley HL. Waging war against uropathogenic Escherichia coli: winning back the urinary tract. Infect Immun 2010; 78: 568–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13Jaillon S, Moalli F, Ragnarsdottir B, Bonavita E, Puthia M, Riva F et al. The humoral pattern recognition molecule PTX3 is a key component of innate immunity against urinary tract infection. Immunity 2014; 40: 621–632. [DOI] [PubMed] [Google Scholar]
- 14Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol 2011; 11: 519–531. [DOI] [PubMed] [Google Scholar]
- 15Lu J, Marnell LL, Marjon KD, Mold C, Du Clos TW, Sun PD. Structural recognition and functional activation of FcgammaR by innate pentraxins. Nature 2008; 456: 989–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16Lech M, Römmele C, Kulkarni OP, Susanti HE, Migliorini A, Garlanda C et al. Lack of the long pentraxin PTX3 promotes autoimmune lung disease but not glomerulonephritis in murine systemic lupus erythematosus. PLoS One 2011; 6: e20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Norata GD, Garlanda C, Catapano AL. The long pentraxin PTX3: a modulator of the immunoinflammatory response in atherosclerosis and cardiovascular diseases. Trends Cardiovasc Med 2010; 20: 35–40. [DOI] [PubMed] [Google Scholar]