Abstract
Hepatocellular carcinoma (HCC) is currently the third leading cause of cancer mortality and a common poor-prognosis malignancy due to postoperative recurrence and metastasis. There is a significant correlation between chronic hepatitis B virus (HBV) infection and hepatocarcinogenesis. As the first line of host defense against viral infections and tumors, natural killer (NK) cells express a large number of immune recognition receptors (NK receptors (NKRs)) to recognize ligands on hepatocytes, liver sinusoidal endothelial cells, stellate cells and Kupffer cells, which maintain the balance between immune response and immune tolerance of NK cells. Unfortunately, the percentage and absolute number of liver NK cells decrease significantly during the development and progression of HCC. The abnormal expression of NK cell receptors and dysfunction of liver NK cells contribute to the progression of chronic HBV infection and HCC and are significantly associated with poor prognosis for liver cancer. In this review, we focus on the role of NK cell receptors in anti-tumor immune responses in HCC, particularly HBV-related HCC. We discuss specifically how tumor cells evade attack from NK cells and how emerging understanding of NKRs may aid the development of novel treatments for HCC. Novel mono- and combination therapeutic strategies that target the NK cell receptor–ligand system may potentially lead to successful and effective immunotherapy in HCC.
Keywords: activating receptor, hepatocellular carcinoma, inhibitory receptor, natural killer cell, natural killer receptor
Introduction
Hepatocellular carcinoma (HCC) is currently the third leading cause of cancer mortality and a common poor-prognosis malignancy due to postoperative recurrence and metastasis.1 Common clinical risk factors for HCC include hepatitis B virus (HBV)/hepatitis C virus (HCV) infection, heavy alcohol intake, steatohepatitis and diabetes.2 Approximately 50% of HCC cases worldwide can be attributed to chronic HBV infection (CHB) and almost 75% of HCC cases occur in developing countries where HBV is endemic.3,4 According to statistics, older male patients who are infected with HBV genotype C or co-infected with HCV, have high levels of viral load and have been exposed to the aflatoxin, or older male patients who have a family history of HCC tend to have a high risk of developing HCC.5,6,7 Accumulating clinical and epidemiological evidence shows a significant correlation between chronic HBV infection and hepatocarcinogenesis. However, the underlying mechanisms are still not completely understood. It has been demonstrated that tumor-infiltrating immune cells play an important role in host immune defense against tumor progression in various cancers.8,9,10,11 These observations seem to provide a reasonable treatment direction and impel an increasing number of researchers to focus on immune responses in the tumor microenvironment.
The first line of host defense against viral infections and tumors is innate immunity. Interestingly, innate immune lymphocytes (including natural killer (NK), NK T and γδT) account for more than half of the total number of liver lymphocytes, of which NK cells account for a large proportion.12 More importantly, the percentage of NK cells in the liver is known to be nearly five times greater than the percentages in blood or spleen.13,14 These liver-residing NK cells exert unique phenotypic and functional characteristics, such as immunological memory, suggesting the critical role of NK cells in the liver.15,16 However, the percentage and absolute number of liver NK cells decrease significantly during the development and progression of HCC. Lower NK cell numbers are significantly associated with poor prognosis for liver cancer.8,17 Furthermore, NK cell-related genes are closely associated with NK cell function, and data from microarray experiments suggest that a series of NK cell-associated genes accurately predicts the clinical outcome of HCC patients, especially in early stages.8 NK cells express a large number of immune recognition receptors (NKRs) to recognize ligands on hepatocytes, liver sinusoidal endothelial cells, stellate cells and Kupffer cells, which maintain the balance between immune response and immune tolerance of NK cells. One particular strategy that tumors have to escape the immune system is to target receptor–ligand systems to impair NK cytotoxicity. Consistent with the view that NKRs are important components of anti-tumor immune responses, several mechanisms that tumors use to evade NKR-induced NK cell-mediated killing of tumor cells have been identified.18,19,20,21,22,23 Despite these associations, the mechanisms linking NKRs and HCC are not completely understood. In this review, we focus on the role of these NK cell receptors in anti-tumor immune responses. We discuss specifically how tumor cells evade NK cell attack and how the emerging understanding of NKRs may aid the development of novel treatments of HCC.
NK receptors in tumors
Historically, the term ‘natural killer cells' came from their ability to recognize and kill invading pathogens and cancer cells. This capability depends on the integrated balance of activating and inhibitory receptor signaling. NK cells express a wide range of activating and inhibitory receptors to recognize specific ligands on target cells. The main triggering activating receptors expressed by NK cells include CD16, NKG2D, NKG2C, CD226 (DNAM-1), CD244 (2B4) and the natural cytotoxicity receptors.18,24 The ligands for the activating receptor NKG2D are cellular stress inducible molecules: MICA, MICB, and ULBPs.25 The main inhibitory receptors are killer cell immunoglobulin-like receptors (KIRs), CD94/NKG2A and leukocyte immunoglobulin-like receptor 1 (CD85), most of which recognize MHC class I molecules. The activation of NK cells is dominated by inhibitory receptors that bind to MHC-I molecules on target cells. Normal ‘self' cells can abundantly express MHC-I molecules, leading to de-activation of NK cells. The missing self-recognition is one common process by which NK cells recognize target tumor cells deficient in MHC-I expression.
Although NK cells have powerful cytotoxic activity against tumor cells, tumor cells and the tumor microenvironment have evolved several mechanisms to negatively influence the activity and function of NK cells, thus leading to impairment of NK cell-mediated anti-tumor function. More and more experimental evidence has shown that cancer cells evade NK cell anti-tumor immunity by modulating expression of NK cell receptors and their ligands (Table 1). Decreased expression of NKG2D has been observed in most cancer patients, which include breast cancer (BC), lung cancer (LC), colorectal cancer (CRC), cervical cancer (CC), pancreatic cancer (PC), gastric cancer (GC) and HCC.26,27,28,29,30,31 With disease progression, decreased expression of NKG2D can be correlated with decreased NK cell function, most notably reduced cytotoxicity. The significantly downregulated expression of NKp30 represents an escape mechanism associated with low NK cell activity and with tumor progression. Downregulation of NKp30 has been observed in both solid tumors and hematological malignancies.20,26,27,28,32,33 Studies on patients with PC, GC, CRC and acute myeloid leukemia (AML) indicate that the impaired function of NK cells in patients with these cancers arises from a decreased percentage of NKp46+ NK cells.27,28 Similar downregulation of other activating receptors, such as CD16, NKG2C, CD226, CD244 and NKp44, have been observed in both solid tumors and hematological malignancies.22,28,34,35,36
Table 1. NK cell receptors and function in tumors.
| Receptors | Ligand | Tumor | Expression | Outcome | Refs |
|---|---|---|---|---|---|
| Activating | |||||
| NKp30 | B7-H6, BAG6 | BC, HCC, PC, GC, CRC, LCC, CLL, AML, CC-2 | Decreased | Decreased NK cell function, most notably cytotoxicity | 20,26–28,32,33 |
| NKp44 | PCNA | Hela Cells | Decreased NK cell functions | 36 | |
| NKp46 | Vimentin | PC, GC, CRC, AML, CC-2 | Decreased | Indicators of disease progression | 27,28,33 |
| NKG2D | MICs, ULBPs | BC, LC, CRC, CC-1, PC, GC, CC-2 | Decreased | Decreased NK cell function, most notably cytotoxicity; indicators of disease progression | 26–31,41 |
| NKG2C | HLA- E | AML | Decreased | Inadequate tumor immune surveillance by NK cells | 33 |
| CD226 | CD155, CD112 | BC, CC-1, AML | Decreased | Decreased NK cell function, most notably cytotoxicity; suppress the activation of NK cells | 26,33,41 |
| CD16 | IgG | BC, MM | Decreased | Decreased NK cell function, most notably cytotoxicity. | 26,34 |
| CD244 | CD48 | AML | Decreased | Inadequate tumour immunosurveillance by NK cells | 33 |
| Inhibitory | |||||
| NKG2A | HLA- E | BC, CRC | Increased | Poor prognosis | 26,37 |
| KIR3DL1 | HLA- A, -B, -C | PC, GC, CRC | Increased | Not associated with disease progression | 27 |
| KIR2DL2/L3 | HLA-C | Melanoma | Increased | 35 | |
| CD200R | CD200 | AML, CLL, MM | Suppression of natural killer cell function; CD200 blocking antibody recovers NK cell activity | 38–40 | |
Abbreviations: AML, acute myeloid leukemia; BC, breast cancer; CC-1, colon carcinoma; CC-2, cervical cancer; CLL, chronic lymphocytic leukemia; CRC, colorectal carcinoma; GC, gastric cancer; LC, lung cancer; MM, multiple myeloma; NK, natural killer; PC, pancreatic cancer; PCNA, proliferating cell nuclear antigen.
Moreover, these alterations are not limited to activating receptors on NK cells. There are several studies that show that NK cell dysfunction is correlated with increased expression of the inhibitory receptor NKG2A and HLA-E.26,37 Another study provided evidence of a significantly increased level of KIR3DL1-positive NK cells in patients with PC, GC and CRC. However, this increase was not associated with tumor progression.27 It was shown that an increased level of CD158b was observed in involved lymph nodes compared to uninvolved lymph nodes of melanoma patients.35 In addition, CD200, the ligand of the inhibitory receptor CD200R, has been found to be over-expressed in multiple hematological malignancies, such as B-cell chronic lymphocytic leukemia, multiple myeloma and acute myeloid leukemia.38,39,40 CD200 has a direct suppressive effect on NK cell anti-tumor activity in AML patients and contributes to the increased relapse rate in CD200+ patients.38 These data therefore demonstrate that cancer cells may alter their expression of surface ligands for NK receptors to escape from NK cell-mediated anti-tumor immunity.
Furthermore, release of soluble activating NKR ligands has been frequently observed in most tumors.20,29,32,37,41,42,43 NKG2D ligands are often upregulated on a wide range of tumor cells in response to cellular stress during malignant transformation. However, as tumors progress, soluble MICA/B or ULBPs are often shed from the membranes of tumor cells. The shedding of NKG2D ligands has been frequently observed in most tumors, and leads to downregulation of NKG2D on NK cells and reduced NK cell cytotoxicity.32,44,45 It has also been found that downregulation of B7-H6 (a ligand for the activating receptor NKp30) on various tumor cell lines or release of BAG6/BTA3 (a soluble ligand for NKp30) from chronic lymphocytic leukemia cells reduces the NKp30-dependent effector functions of NK cells.20 Suppressive cytokines (such as transforming growth factor (TGF)-β and IL-10) and regulatory T (Treg) cells within tumor microenvironments have been shown to suppress NKG2D and NKp30 expression on NK or CD8+ T cells and also reduce MICA and ULBP expression on malignant cells.46,47,48 These cytokines and Treg cells strongly contribute to the tumor's escape from NK cell-mediated anti-tumor immune response.
Abnormal NK cells in HCC patients
Depressed NK cytolytic activity has been found in various mouse models of cancer and is associated with poor clinical outcome in patients.18,49,50,51,52,53 NK cell dysfunction in patients with HCC and liver cirrhosis has been observed since the 1980s.54 The cytolytic activity of NK cells from HCC patients was significantly lower than that of NK cells from LC patients or healthy controls.55 Importantly, NK cell dysfunction plays key roles in controlling liver carcinogenesis, as decreased NK activity appears to be one of the promoting factors for HCC development and also acts as a predictor for HCC.56,57
Circulating NK cells in HCC
The percentage and absolute number of circulating NK cells have been found to be reduced in HCC patients. Though an earlier investigation from 28 HCC patients in TNM stage I showed that the proportion of NK cells in the peripheral blood mononuclear cells (20.51%±10.24%) did not differ from that of healthy controls (20.71%±7.96%),57 most of the subsequent studies have found a reduction in the proportion of NK cells in peripheral blood of HCC patients. A reduction of NK cells has been observed in 51 HCC patients (18.77%±10.67%) with stage III compared with healthy subjects (20.71%±7.96).58 Another study found a dramatic reduction in the NK proportion of total peripheral blood (14.29%±0.96%) in 110 HCC patients at various stages compared with healthy control subjects (23.41%±1.59%). Moreover, the ratio of CD56bright and CD56dim NK cells was dramatically increased in HCC patients because CD56brightCD16− NK cells were expanded and CD56dimCD16+ NK cells were reduced.59 The absolute counts of CD56bright and CD56dim NK cells were found reduced in peripheral blood from 20 Egyptian patients with HCC compared to 152 healthy control subjects.60
Liver NK cells in HCC
Due to the limited supply of fresh liver tissue samples from patients, the majority of NK cells that have been studied were derived from circulating NK cells in HCC. However, more studies on intrahepatic NK cells are needed. Some papers have reported elevated infiltration by NK cells of tumor tissue compared to that of non-tumor tissue in HCC patients at various stages (II/III/IV).61 However, other groups disputed these findings. Cai et al.,59 in a study of 110 HCC patients at various stages (I/II/III: 20/35/55), found that NK cells accumulated largely in liver tissues of HCC patients whose total peripheral NK cell numbers were significantly decreased. In addition, non-tumor infiltrating lymphocytes contained more NK cells (approximately 19.7%) than tumor infiltrating lymphocytes (TILs) (approximately 5.34%) in HCC. Furthermore, the frequency of NK cells in TILs was found to be significantly decreased compared with the frequency in non-tumor infiltrating lymphocytes in six stage III HCC patients. This decrease was mainly due to the reduction of CD56dimCD16+ NK cells.59 Similar evidence was also provided by another study with 50 HCC patients at various stages (I/II/III/IV:15/23/8/4).62 Results from immunohistochemical staining showed that NK cells were predominant in normal liver, chronic hepatitis liver and non-tumor liver but not in tumor tissue. Such reductions in NK cell numbers are particularly characteristic of advanced stages of HCC.63 These results suggest that the proportion of NK cells in TILs tends to significantly decrease in advanced TNM stage patients. Taken together, these observations suggest that the reduced frequency of intrahepatic NK cells in HCC patients might be one of the mechanisms by which tumors escape from NK cell-mediated anti-tumor immune responses, which contributes to the progression of HCC.
Functional impairment of NK cells from CHB and HCC patients
Chronic HBV infection is the key driving force for the development of hepatic cirrhosis and HCC. In developing countries, there is a 70% risk factor for developing HBV-related HCC. The frequencies, activation and cytokine production of circulating NK cells were significantly reduced in CHB patients compared with healthy controls. Higher secretion of the immunosuppressive cytokine IL-10 in CHB patients suppresses NK cell function, which contributes to immune tolerance and facilitates viral persistence. Blocking IL-10 or administering anti-viral therapy restored NK cell activation and interferon gamma (IFN-γ) production.64,65 Impaired NK cell function induced by persistent HBV infection and chronic inflammation contributes to the progression of HCC.
Accumulating evidence from peripheral blood mononuclear cells and TILs indicates that NK cells in HCC patients have functional defects in cytotoxicity and cytokine secretion.17,54,55,56,57,59,66,67 Cytotoxicity of NK cells from both peripheral blood and TILs of HCC patients was markedly reduced compared to healthy controls.17 The production of cytoplasmic granules (which involve granzyme A, granzyme B and perforin) by circulating NK cells was markedly decreased in advanced patients (stages II and III) compared with healthy donors. A similar decrease was also observed when comparing NK cells from TILs with those from non-tumor infiltrating lymphocytes or comparing NK cells from stage II patients with stage I patients.59 It has been found that the patients with lower NK cell activity tend to have venous invasion or have both lobes involved, which indicates poor clinical outcome.67 A recent detailed study of 294 untreated HCC patients showed that functional NK cell accumulation in HCC tissues can predict improved survival of patients.67 These data strongly suggest that the functional impairment of NK cells might contribute to the pathogenesis of HCC.
NK receptors and their ligands in HCC
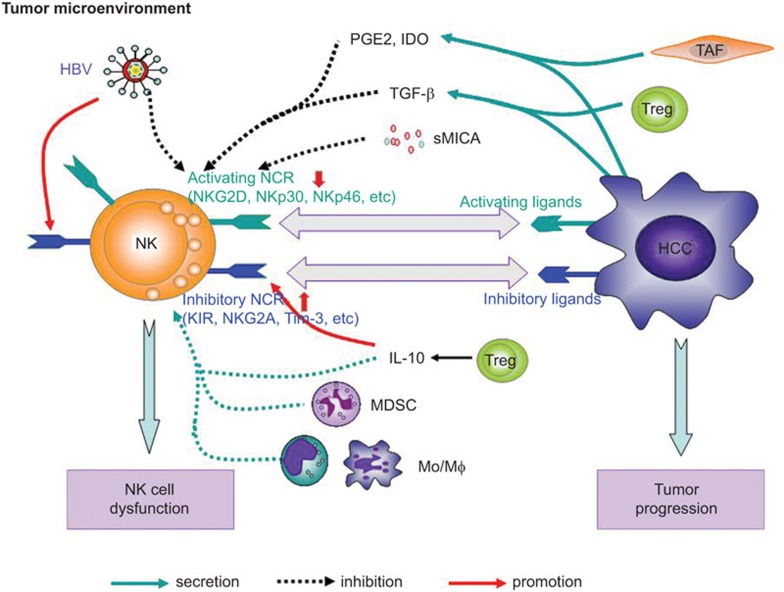
The development and function of immune cells is largely influenced by microenvironmental factors. The inflammatory microenvironment in the livers of HCC patients can also impair the function of intrahepatic immune cells. As a large proportion of lymphocytes in the liver, hepatic NK cells exhibit unique phenotypic and functional characteristics due to the surrounding liver microenvironment and are also influenced by the inflammatory tumor microenvironment, which modulates expression of activating and inhibitory NK cell receptors (Table 2), thereby contributing to impaired NK cell function and to HCC progression (Figure 1).
Table 2. NK cell receptors and function in HCC.
| Source | Age (years) | Sex (M/F) | No. of cases | TNM (I/II/III/IV) | Receptors | Ligand | Cytolysis | References |
|---|---|---|---|---|---|---|---|---|
| Activating receptors | ||||||||
| Blood | 68±9 | 177∶55 | 232 | 59/68/64/39 | NKG2D↓ | sMICA/B↑ | 98 | |
| 52∶8 | 60 | NKG2D↓ | sMICA↑ | 96 | ||||
| Blood | 26 | NKG2D↓ | sMICA↑ | Cytolysis↓ | 66 | |||
| Blood | 70.2±7.99 | 33∶28 | 61 | KIR2DS5↑ (with TTR↑, OS↑)KIR3DS1↑ | HLA-Bw4T80↑ | 67 | ||
| Tissue | 65.4±10.8 | 44∶10 | 54 | NKG2D↓ | ULBP1↑ | CD107a↓ | 99 | |
| 68±9 | 177∶55 | 232 | 59/68/64/39 | NKG2D↓ | sMICA/B↑ | 98 | ||
| Inhibitory receptors | ||||||||
| Blood | 57.1 (42–74) | 17∶1 | 18 | KIR, KIR2DL, and CD94: NS | 80 | |||
| 70.2±7.99 | 33∶28 | 61 | KIR2DL2 (with OS↓, TTR↑) | HLA-C1 | 67 | |||
| Tissue | 57.1 (49–68) | 5∶2 | 7 | KIR↓ (8.9% vs. 37.85%), CD94↓ (21% vs. 45.95%) compared to HC | 80 | |||
| Tissue | 22 | KIR↓, CD94↓ | 79 | |||||
| Tissue | 46 | KIR↓ | Cytolysis↓ | 81 | ||||
Abbreviations: HC, healthy control; HCC, hepatocellular carcinoma; NK, natural killer; NS, not significant; OS, overall survival; TTR, time to recurrence.
Figure 1.
The imbalance of NK cell receptors and dysfunction of NK cells in the HCC microenvironment. The phenotype and function of NK cells are influenced by the surrounding liver and tumor microenvironment. The imbalance of NK cell receptors is characterized by downregulated activating receptors and increased expression of inhibitory receptors, which contribute to the dysfunction of NK cells and progression of HCC. For example, TGF-β secreted by Treg or HCC cells downregulates the surface expression of NKG2D or other activating NK cell receptors. PGE2 and IDO derived from tumor cells or activated TAFs can also downregulate NKG2D expression. sMICA or sULBP shed from tumor cells is associated with downregulated NKG2D expression and impairs the activation of NK cells. The suppressive cells—MDSCs, monocytes/macrophages from intratumoral tissues and M2-polarized TAMs—also suppress the expression of NK cell-activating receptors and impair NK cell function by secreting TGF-β and IL-10 or IDO. In addition, persistent HBV infection, the main risk factor for HCC, increases the expression of inhibitory receptors (e.g., NKG2A and Tim-3) and reduces the expression of activating receptors on NK cells. HBV, hepatitis B virus; HCC, hepatocellular carcinoma; MDSC, myeloid-derived suppressor cell; NK, natural killer; TAF, tumor-associated fibroblast; TAM, tumor-associated macrophage; TGF, transforming growth factor; Treg, regulatory T.
Downregulated activating receptors
Accumulating evidence indicates that the expression of NK cell activating receptors is often decreased during the development and progression of cancers such as HCC.22,23,24,25,58 The immunosuppressive cytokine TGF-β and the cells that are its main source, Treg cells, have been reported to downregulate surface expression of NKG2D and other activating NK cell receptors in the tumor microenvironment, thereby impairing NK cell function and further promoting tumor progression.46,68 PGE2 and IDO derived from tumor cells can also downregulate NKG2D expression.69,70 Elevated levels of sMICA are associated with downregulated NKG2D expression and impaired activation of NK cells in advanced HCC patients.66 Another suppressive cell, the myeloid-derived suppressor cell, in patients with HCC induced NK cell dysfunction characterized by impaired cytotoxicity and cytokine secretion. Furthermore, the suppression of NK cells is dependent on cell contact mediated by the NKp30 receptor on NK cells.17 Macrophage infiltration of peritumoral stroma in HCC patients was recently reported to positively correlate with NK cell defects in intratumoral areas and to lead to impaired production of tumor necrosis factor alpha (TNF-α) and IFN-γ.71 Moreover, NK cell dysfunction induced by monocytes/macrophages is mediated by CD48/2B4 interactions but not by NKG2D and NKp30. Monocytes isolated from intratumoral tissues express significantly higher levels of the CD48, the ligand for 2B4. Expression of Ki67, CD69, TRAIL and granzyme B in NK cells was significantly reduced after NK cells were co-cultured with monocytes from tumor areas for 8 days.63 Tumor progression is now recognized as an outcome of evolving crosstalk between different cell types within tumors and in the tumor-surrounding stroma. Fibroblasts are recognized as the dominant tumor-surrounding stromal cell type important for tumorigenesis. Several studies have indicated that fibroblasts derived from HCC tissues suppress the function of NK cells. It has been shown that PGE2 and IDO derived from activated fibroblasts impair cytotoxicity and cytokine production by NK cells. Exposing HCC-associated fibroblasts to anti-PGE2 and anti-IDO antibodies significantly restored NK cell function.72,73 These results indicate that fibroblasts in HCC patients play an important role in triggering NK cell dysfunction in HCC. In addition to killing tumor cells, NK cells also downregulate fibrosis by inducing apoptosis of activated stellate cells without affecting quiescent stellate cells.74,75,76
Changes of inhibitory receptors
Binding of killer inhibitory receptors (e.g., KIR, KIR2DL and CD94 family) to their respective ligands on target cells can inhibit the cytolytic responses of NK cells. It is generally accepted that cancer cells induce downregulation of NK-activating receptors as well as upregulation of inhibitory receptors to evade NK cell-mediated anti-tumor immune responses.16,20,22,28 Importantly, anti-KIR antibodies that block KIR-mediated inhibition of NK cells has shown therapeutic anti-tumor effects especially for patients with hematopoietic malignancy.23,77,78 However, there is nearly no direct data showing increased expression of inhibitory NK cell receptors on hepatic NK cells in HCC patients. On the contrary, NK cells in TILs from primary HCC patients have shown significantly decreased expression of KIR2DL1 (p58.1) and CD94 compared to hepatic lymphocytes from control subjects. Similarly, NK T cells in TILs have also shown remarkably lower expression of KIR2DL1 and KIR2DL2 (p58.2) compared to control subjects.79,80 However, no differences in the expression of KIR2DL1, KIR2DL2 and CD94 were found on NK cells in peripheral blood mononuclear cells from HCC patients compared to control subjects.80 In another study, human liver NK cells from 46 metastatic and primary HCC patients had reduced lytic potential compared with NK cells from blood due to their limited expression of inhibitory KIRs, which suggested a decreased number of licensed NK cells that express inhibitory receptors for self MHC-I in liver.81
Notably, persistent HBV or HCV infection, which is the main risk factor for HCC, has been shown to influence NK cell phenotype, especially by increasing expression of inhibitory receptors and reducing expression of activating receptors (Table 3).82,83,84,85,86,87,88,89 In one study, NK cells from chronic HCV patients had a significantly reduced expression of NKp46 and NKp30 and an increased expression of NKG2A compared with NK cells from healthy and HBV infected subjects.88 Our group recently found a higher percentage of NKG2A+ NK cells in peripheral blood from patients with active CHB patients than from patients with inactive CHB or from control patients. The percentage of NKG2A+ NK cells was decreased in patients who had received antiviral therapy and had reduced HBV loads. The increased NKG2A expression was upregulated by IL-10 produced by hepatic Treg cells. Importantly, blocking the interaction between NKG2A and HLA-E or Qa-1 restored NK cell cytotoxicity and promoted viral clearance in an NK cell-dependent manner.90 The expression of the co-inhibitory receptor Tim-3 was significantly increased on circulating NK cells and liver-infiltrating lymphocytes from 40 CHB patients compared to those of 18 healthy controls and 9 patients with fatty liver disease. Blocking Tim-3 signaling with anti-Tim-3 antibodies or with Tim-3-Fc fusion proteins resulted in increased cytolysis and elevated IFN-γ production by NK cells.83 The imbalance of NK cell receptor expression and dysfunction of NK cells during persistent HBV/HCV infection in the premalignant stage of HCC might promote the development and progression of HCC.
Table 3. NK cell receptors and function in HBV or HCV infection.
| Source | Age (years) | Sex (M/F) | % or no. of NK cells | Receptors | Cytokine | Cytolysis | No. of cases | References |
|---|---|---|---|---|---|---|---|---|
| HBV | ||||||||
| Blood | % Reduced | Higher NKp30, NKp46 and NKG2C than HCV, lower NKG2A than HCV | IFN-γ↓TNF-α↓ | Increased | 22 | 82 | ||
| Blood | 34.5±13.6 | 55∶21 | TIM3↑ | 76 | 83 | |||
| Blood | Lower % NK cells in active CHB patients | NKG2A↑ | IL-10↑ | Decreased | 73 | 90 | ||
| Tissue | 34.5±13.6 | 55∶21 | TIM3↑ | 76 | 83 | |||
| HCV | ||||||||
| Blood | 56 (25–88) | 40∶30 | ↑NKp44, NKp46, CXCR4, NKG2A↓NKp30, KIR3DL1 | 70 | 84 | |||
| Blood | 50 (26–64) | 17∶11 | Reduced (8.6% vs. 13.3%) | 28 | 85 | |||
| Blood | ↑NKp30, NKp46 | 15 | 86 | |||||
| Blood | ↓NKp30, NKp46↑NKG2A | 30 | 87 | |||||
| Blood | Reduced % | KIR3DL1↓ | Increased | 35 | 82 | |||
| Blood | Reduced % | ↓Cytolysis | 36 | 88 | ||||
| Blood | Reduced % | Not differ | 28 | 89 | ||||
| Tissue | 50 (26–64) | 17∶11 | ↓CD158a,h+ and ↓CD158b,j+ | 28 | 85 | |||
| Tissue | 56 (25–88) | 40∶30 | ↑NKp44, NKp46, CXCR4, NKG2A↓NKp30, KIR3DL1 | ↓TRAIL↓CD107a | 70 | 84 | ||
Abbreviations: AN, absolute number; CHB, chronic hepatitis B; HBV, hepatitis B virus; HC, healthy controls; HCV, hepatitis C virus; IFN, interferon; LC, liver cirrhosis; NK, natural killer; TNF, tumor necrosis factor.
Soluble ligands of NKG2D
In most instances, the mechanisms by which tumor cells escape from NK cell-mediated surveillance include quantitative and qualitative deficiencies of NK cells that are caused by increased inhibition or decreased activation of signaling pathways via engagement of inhibitory and activating NK receptors. NKG2D, an activating receptor whose ligands include MICA, MICB and the ULBPs, has a crucial role in NK cell activation. Although NKG2D ligands are usually upregulated on a wide range of tumor cells due to cellular stress during malignant transformation, accumulating evidence indicates that soluble MICA/B or ULBPs can be shed from the membranes of tumor cells. This shedding reduces the expression of NKG2D ligands on NK cells and, in turn, severely impairs the responsiveness of NK cells.53,91,92,93,94 These findings have been widely confirmed in different tumor models including HCC.66,95,96 Serum sMICA was studied in 26 patients with HCC and significant amounts of sMICA were detected in 11 of the patients, while no sMICA was detected in chronic HBV/HCV patients or healthy controls except for five cases with marginal positivity. More importantly, the percentage of sMICA-positive patients is higher among advanced HCC patients (stage III or IV) than in stage I or II patients (71% versus 8.4%). Not only reduced NKG2D expression on NK cells but also impaired NKG2D-mediated cytolytic activity of NK cells was observed in these sMICA-positive patients.66 The expression level of NKG2D on NK cells was negatively correlated with the sMICA level. Survival analysis also showed that higher sMICA levels were related with poor prognosis in HCC patients, and sMICA is regarded as a predictive biomarker for HBV-induced HCC.96,97 Significantly higher levels of sMICA/B were also observed in patients with chronic liver disease compared with healthy volunteers.98 Reduced expression of another NKG2D ligand, ULBP1, has also been observed in HCC, and soluble ULBP1 prevented effective NKG2D-mediated killing and led to early recurrence of HCC after hepatectomy.99
NKR expression by activating cytokines
The expression of NK cell receptor and their ligands is influenced by factors in the tumor microenvironment such as activating and inhibitory cytokines. It has been shown that IL-12, IL-15 and IFN-α are capable of inducing the expression of NKG2D on NK cells.100,101,102 IFN-α stimulates the expression of NKG2D but inhibits the expression of the inhibitory receptor NKG2A. IFN-α therefore alters the balance of stimulatory and inhibitory receptors in favor of activation, leading to NK cell-mediated cytotoxicity, whereas IFN-γ exerts the opposite effect.103 We also found similar opposing effects of these two types of interferons on the expression of MICA, the ligand for NKG2D.104 Cytokine-induced changes in the tumor microenvironment in HCC patients modulate expression of NK cell receptors and their ligands, thus influencing NK cell anti-tumor responses and HCC progression. It has been found that higher levels of IL-2 and IL-15, which can enhance NK cell proliferation and cytotoxicity, in peritumoral hepatic tissues compared to tumor tissues, were associated with a low incidence of recurrence and a prolonged overall survival of HBV-related HCC patients.105 Univariate analysis in tumor and non-tumor liver tissues identified the expression of TNF, IL-6 and CCL2 within tumors as positively associated with the survival of HCC patients. TNF was determined to be an independent predictor of survival in a multivariate analysis.106 The pro-inflammatory cytokines IL-1, IFN-γ and TNF were downregulated in livers with metastatic HCC, while the anti-inflammatory cytokines IL-4, IL-5, IL-8 and IL-10 were upregulated.107
NKR expression by inhibitory cytokines
Immunosuppressive cytokines (such as TGF-β and IL-10) that are upregulated in tumors are involved in inhibiting NK cell-activating receptors and enhancing the expression of inhibitory receptors. High levels of TGF-β1 have been found in HCC patients with severely impaired NK cell function. Blocking TGF-β by using small molecule inhibitors of TGF-β or anti-TGF antibodies appears to inhibit HCC cell migration and to attenuate tumor cell-mediated NK-cytotoxicity inhibition.108,109,110 Interestingly, in human lung cancer or colorectal cancer patients, elevated TGF-β1 secretion is inversely correlated with the expression of NKG2D and cytotoxicity of NK cells. TGF-β1 neutralization by anti-TGF-β1 monoclonal antibodies in vitro can restore NKG2D expression.111 Likewise, TGF-β1 downregulates the expression of NKG2D and 2B4 on NK cells and subsequently impairs NK cell function in persistent HBV infection.112 Previous studies have shown that the impairment of both peripheral and tumor-infiltrating NK cells is associated with increased CD4+CD25+ Treg cells in HCC patients.59 Accumulating evidence suggests that Tregs suppress NK cell function by expressing high levels of membrane-bound TGF-β or secreting high levels of soluble TGF-β, which downregulates the expression of NKG2D and other activating receptors on NK cells. These observations indicate that inhibiting Tregs and TGF-β can restore NK-mediated anti-tumor immune responses in HCC patients.59,113,114
Other suppressive cells that are abundant in tumors including HCC, such as myeloid-derived suppressor cells and M2-polarized tumor-associated macrophages, also suppress the expression of NK cell-activating receptors and impair NK cell function by secreting TGF-β and IL-10 or IDO.17,48,115 In addition, tumor-associated fibroblasts prevent IL-2-induced upregulation of the activating receptors NKp44, DNAM-1 and NKp30, which depend on cell-to-cell contact and PGE2 release; thus, tumor-associated fibroblasts strongly inhibit NK-cell function.116 Similar studies have also reported that HCC-associated fibroblasts trigger NK cell dysfunction via PGE2 and IDO.73 Studies on the roles of fibroblast growth factor-2 in HCC development have indicated that fibroblast growth factor-2 levels in patients with liver cirrhosis or HCC were significantly higher than in healthy controls. Fibroblast growth factor-2 stimulation increased the expression of the MICA and decreased the expression of HLA class I molecules on HCC cells, resulting in enhanced NK sensitivity against HCC cells.117
NK function and chemokines
Chemokines also stimulate NK cell cytotoxicity and attract NK cells to the tumor microenvironment.118 High NK cell infiltration in tumor sites correlates with a better prognosis and improved survival.11 NK cells express chemokine receptors, such as CCR2, CCR5, CXCR3 and CX3CR1, and can migrate to inflamed sites or to tumors in response to their respective chemokines. CXCR3 and CX3CR1 are key chemokine receptors responsible for NK cell migration to tumors. The tumor type and chemokine profile in the tumor microenvironment also influence the recruitment of NK cells.48 CD56dim NK cells with high cytolytic potential are preferentially recruited to inflamed peripheral tissues, e.g., inflamed liver and lungs, most likely aided by the CXCR1/CXCL8 axis.118 The recently identified chemokines CCL5, CCL2 and CXCL10 drive NK-cell infiltration into tumors, leading to cancer cell death in HCC. TNF-α, IFN-γ and TLR3 ligands stimulate intratumoral production of CXCL10 and CCL5, which induces infiltration of tumors by CD8+ T and NK cells. Furthermore, enhancement of IFN-γ expression by NK cells further augments CXCL10 production, resulting in a positive feedback loop.8 CCL2 and TNF-α or IL-6 in the inflammatory environment of HCC were shown to be associated with the infiltration and proliferation of NK and T cells, which are predictors of increased survival in HCC patients.77 Similarly, in a mouse model of liver carcinoma, CCL2 secreted by senescent tumor cells was shown to play a predominant role in recruiting NK cells to tumors, leading to NKG2D-dependent elimination of senescent tumors by NK cells.119 Therefore, new strategies to increase the recruitment of NK or T cells will improve tumor elimination.
Conclusion and perspective
The balance between activating and inhibitory receptor signaling is a complex process requiring several NK cell surface receptors acting synergistically. Tumor cells can become ‘invisible' to immune surveillance by inducing the upregulation of inhibitory receptors and downregulation of activating receptors on NK cells. Conversely, NK cell dysfunction may promote the escape of tumor cells and indicates a poor prognosis of patients with cancer.
In the last few years, several developments have been made on the characterization of receptor–ligand interactions involved in NK cell activation. Several therapeutic strategies have been explored to enhance NK cell immunity by interfering with the expression of activating and inhibitory receptors. One challenge these strategies face is avoiding systemic toxicity, which requires optimal dosage and timing of treatment. Further research is required to determine the molecular mechanisms by which these activating and inhibitory receptors are regulated in patients to design new therapeutic strategies to enhance NK cell function in patients with cancer. Although studies to date have shown promising progress in understanding the activation of NK cells in the context of anti-tumor immune responses, there are still many important questions to explore. Some recent studies indicated that NK cells have a suppressive role in anti-viral immunity (e.g., during LCMV and HBV infection).120,121 NK cells were shown to negatively regulate antiviral immunity in chronic HBV infection by eliminating HBV-specific CD8+ T cells in a contact-dependent manner by inducing apoptosis.121 Apoptosis induction by NK cells might be one of the reasons HCC-specific CD8+ T cells are reduced in HBV-related HCC.122 Further studies are needed to delve into the opposing roles of NK cells in CHB to substantively exploit the potential of NK cells to treat chronic HBV infection and HCC. In addition, how NK cells quantitatively evaluate the strength of activating and inhibitory signals is unknown, as is the mechanism by which NK cells set their own activation threshold or become self-tolerant during tumor evasion. A better understanding of these issues will certainly contribute to developing successful therapies for chronic HBV infection and HCC.
In summary, NK cell surface receptors play a pivotal role in NK cell-mediated immunity against HCC. Emerging knowledge of the molecular mechanisms of activating and inhibitory receptors functions will allow novel mono- and combination therapeutic strategies to be envisioned, which can potentially lead to successful and effective immunotherapies for HCC.
Acknowledgments
This work was supported by grants from the Chinese Government Department of Science & Technology of China (2012ZX10002006; 2012ZX10002014; 2013ZX10002002; 2012AA020901; 2010CB911901; 2013CB944901) and the Natural Science Foundation of China (81273220; 81472646).
The authors declare that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
References
- 1El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011; 365: 1118–1127. [DOI] [PubMed] [Google Scholar]
- 2El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007; 132: 2557–2576. [DOI] [PubMed] [Google Scholar]
- 3Ferenci P, Fried M, Labrecque D, Bruix J, Sherman M, Omata M et al. Hepatocellular carcinoma (HCC): a global perspective. J Clin Gastroenterol 2010; 44: 239–245. [DOI] [PubMed] [Google Scholar]
- 4El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012; 142: 1264–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Ferenci P, Fried M, Labrecque D, Bruix J, Sherman M, Omata M et al. World Gastroenterology Organisation Guideline. Hepatocellular carcinoma (HCC): a global perspective. J Gastrointestin Liver Dis 2010; 19: 311–317. [PubMed] [Google Scholar]
- 6Welzel TM, Graubard BI, Quraishi S, Zeuzem S, Davila JA, El-Serag HB et al. Population-attributable fractions of risk factors for hepatocellular carcinoma in the United States. Am J Gastroenterol 2013; 108: 1314–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Harkisoen S, Arends JE, van Erpecum KJ, van den Hoek A, Hoepelman AI. Hepatitis B viral load and risk of HBV-related liver disease: from East to West? Ann Hepatol 2012; 11: 164–171. [PubMed] [Google Scholar]
- 8Chew V, Chen J, Lee D, Loh E, Lee J, Lim KH et al. Chemokine-driven lymphocyte infiltration: an early intratumoural event determining long-term survival in resectable hepatocellular carcinoma. Gut 2012; 61: 427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 2013; 14: 1014–1022.24048123 [Google Scholar]
- 10Fu YX. New immune therapy targets tumor-associated environment: from bone marrow to tumor site. Cell Mol Immunol 2012; 9: 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Wang H, Chen L. Tumor microenviroment and hepatocellular carcinoma metastasis. J Gastroenterol Hepatol 2013; 28(Suppl 1): 43–48. [DOI] [PubMed] [Google Scholar]
- 12Gao B, Jeong WI, Tian Z. Liver: an organ with predominant innate immunity. Hepatology 2008; 47: 729–736. [DOI] [PubMed] [Google Scholar]
- 13Sun H, Sun C, Tian Z, Xiao W. NK cells in immunotolerant organs. Cell Mol Immunol 2013; 10: 202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Li F, Tian Z. The liver works as a school to educate regulatory immune cells. Cell Mol Immunol 2013; 10: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15Peng H, Jiang X, Chen Y, Sojka DK, Wei H, Gao X et al. Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. J Clin Invest 2013; 123: 1444–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16Jiang X, Chen Y, Peng H, Tian Z. Memory NK cells: why do they reside in the liver? Cell Mol Immunol 2013; 10: 196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H et al. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology 2009; 50: 799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Koch J, Steinle A, Watzl C, Mandelboim O. Activating natural cytotoxicity receptors of natural killer cells in cancer and infection. Trends Immunol 2013; 34: 182–191. [DOI] [PubMed] [Google Scholar]
- 19Sabry M, Lowdell MW. Tumor-primed NK cells: waiting for the green light. Front Immunol 2013; 4: 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20Fiegler N, Textor S, Arnold A, Rolle A, Oehme I, Breuhahn K et al. Downregulation of the activating NKp30 ligand B7-H6 by HDAC inhibitors impairs tumor cell recognition by NK cells. Blood 2013; 122: 684–693. [DOI] [PubMed] [Google Scholar]
- 21Tallerico R, Todaro M, Di Franco S, Maccalli C, Garofalo C, Sottile R et al. Human NK cells selective targeting of colon cancer-initiating cells: a role for natural cytotoxicity receptors and MHC class I molecules. J Immunol 2013; 190: 2381–2390. [DOI] [PubMed] [Google Scholar]
- 22Chinnery F, King CA, Elliott T, Bateman AR, James E. Viral antigen mediated NKp46 activation of NK cells results in tumor rejection via NK–DC crosstalk. Oncoimmunology 2012; 1: 874–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23Thielens A, Vivier E, Romagne F. NK cell MHC class I specific receptors (KIR): from biology to clinical intervention. Curr Opin Immunol 2012; 24: 239–245. [DOI] [PubMed] [Google Scholar]
- 24Malhotra A, Shanker A. NK cells: immune cross-talk and therapeutic implications. Immunotherapy 2011; 3: 1143–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25Gonzalez S, Groh V, Spies T. Immunobiology of human NKG2D and its ligands. Curr Top Microbiol Immunol 2006; 298: 121–138. [DOI] [PubMed] [Google Scholar]
- 26Mamessier E, Sylvain A, Thibult ML, Houvenaeghel G, Jacquemier J, Castellano R et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest 2011; 121: 3609–3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27Peng YP, Zhu Y, Zhang JJ, Xu ZK, Qian ZY, Dai CC et al. Comprehensive analysis of the percentage of surface receptors and cytotoxic granules positive natural killer cells in patients with pancreatic cancer, gastric cancer, and colorectal cancer. J Transl Med 2013; 11: 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28Garcia-Iglesias T, del Toro-Arreola A, Albarran-Somoza B, del Toro-Arreola S, Sanchez-Hernandez PE, Ramirez-Duenas MG et al. Low NKp30, NKp46 and NKG2D expression and reduced cytotoxic activity on NK cells in cervical cancer and precursor lesions. BMC Cancer 2009; 9: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29de Kruijf EM, Sajet A, van Nes JG, Putter H, Smit VT, Eagle RA et al. NKG2D ligand tumor expression and association with clinical outcome in early breast cancer patients: an observational study. BMC Cancer 2012; 12: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Shen Y, Lu C, Tian W, Wang L, Cui B, Jiao Y et al. Possible association of decreased NKG2D expression levels and suppression of the activity of natural killer cells in patients with colorectal cancer. Int J Oncol 2012; 40: 1285–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31He S, Yin T, Li D, Gao X, Wan Y, Ma X et al. Enhanced interaction between natural killer cells and lung cancer cells: involvement in gefitinib-mediated immunoregulation. J Transl Med 2013; 11: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32Reiners KS, Topolar D, Henke A, Simhadri VR, Kessler J, Sauer M et al. Soluble ligands for NK cell receptors promote evasion of chronic lymphocytic leukemia cells from NK cell anti-tumor activity. Blood 2013; 121: 3658–3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33Sanchez-Correa B, Morgado S, Gayoso I, Bergua JM, Casado JG, Arcos MJ et al. Human NK cells in acute myeloid leukaemia patients: analysis of NK cell-activating receptors and their ligands. Cancer Immunol Immunother 2011; 60: 1195–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34Treon SP, Hansen M, Branagan AR, Verselis S, Emmanouilides C, Kimby E et al. Polymorphisms in FcgammaRIIIA (CD16) receptor expression are associated with clinical response to rituximab in Waldenstrom's macroglobulinemia. J Clin Oncol 2005; 23: 474–481. [DOI] [PubMed] [Google Scholar]
- 35Vuletic A, Jurisic V, Jovanic I, Milovanovic Z, Nikolic S, Konjevic G. Distribution of several activating and inhibitory receptors on CD3−CD56+ NK cells in regional lymph nodes of melanoma patients. J Surg Res 2013; 183: 860–868. [DOI] [PubMed] [Google Scholar]
- 36Rosental B, Hadad U, Brusilovsky M, Campbell KS, Porgador A. A novel mechanism for cancer cells to evade immune attack by NK cells: the interaction between NKp44 and proliferating cell nuclear antigen. Oncoimmunology 2012; 1: 572–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37Bossard C, Bezieau S, Matysiak-Budnik T, Volteau C, Laboisse CL, Jotereau F et al. HLA-E/beta2 microglobulin overexpression in colorectal cancer is associated with recruitment of inhibitory immune cells and tumor progression. Int J Cancer 2012; 131: 855–863. [DOI] [PubMed] [Google Scholar]
- 38Coles SJ, Wang EC, Man S, Hills RK, Burnett AK, Tonks A et al. CD200 expression suppresses natural killer cell function and directly inhibits patient anti-tumor response in acute myeloid leukemia. Leukemia 2011; 25: 792–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39Kretz-Rommel A, Qin F, Dakappagari N, Cofiell R, Faas SJ, Bowdish KS. Blockade of CD200 in the presence or absence of antibody effector function: implications for anti-CD200 therapy. J Immunol 2008; 180: 699–705. [DOI] [PubMed] [Google Scholar]
- 40Kretz-Rommel A, Qin F, Dakappagari N, Ravey EP, McWhirter J, Oltean D et al. CD200 expression on tumor cells suppresses antitumor immunity: new approaches to cancer immunotherapy. J Immunol 2007; 178: 5595–5605. [DOI] [PubMed] [Google Scholar]
- 41Zhang Z, Su T, He L, Wang H, Ji G, Liu X et al. Identification and functional analysis of ligands for natural killer cell activating receptors in colon carcinoma. Tohoku J Exp Med 2012; 226: 59–68. [DOI] [PubMed] [Google Scholar]
- 42Bedel R, Thiery-Vuillemin A, Grandclement C, Balland J, Remy-Martin JP, Kantelip B et al. Novel role for STAT3 in transcriptional regulation of NK immune cell targeting receptor MICA on cancer cells. Cancer Res 2011; 71: 1615–1626. [DOI] [PubMed] [Google Scholar]
- 43Kloess S, Huenecke S, Piechulek D, Esser R, Koch J, Brehm C et al. IL-2-activated haploidentical NK cells restore NKG2D-mediated NK-cell cytotoxicity in neuroblastoma patients by scavenging of plasma MICA. Eur J Immunol 2010; 40: 3255–3267. [DOI] [PubMed] [Google Scholar]
- 44Wu JD, Atteridge CL, Wang X, Seya T, Plymate SR. Obstructing shedding of the immunostimulatory MHC class I chain-related gene B prevents tumor formation. Clin Cancer Res 2009; 15: 632–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45Waldhauer I, Goehlsdorf D, Gieseke F, Weinschenk T, Wittenbrink M, Ludwig A et al. Tumor-associated MICA is shed by ADAM proteases. Cancer Res 2008; 68: 6368–6376. [DOI] [PubMed] [Google Scholar]
- 46Castriconi R, Cantoni C, Della Chiesa M, Vitale M, Marcenaro E, Conte R et al. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: consequences for the NK-mediated killing of dendritic cells. Proc Natl Acad Sci USA 2003; 100: 4120–4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47Eisele G, Wischhusen J, Mittelbronn M, Meyermann R, Waldhauer I, Steinle A et al. TGF-beta and metalloproteinases differentially suppress NKG2D ligand surface expression on malignant glioma cells. Brain 2006; 129(Pt 9): 2416–2425. [DOI] [PubMed] [Google Scholar]
- 48Vitale M, Cantoni C, Pietra G, Mingari MC, Moretta L. Effect of tumor cells and tumor microenvironment on NK-cell function. Eur J Immunol 2014; 44: 1582–1592. [DOI] [PubMed] [Google Scholar]
- 49Farnault L, Sanchez C, Baier C, Le Treut T, Costello RT. Hematological malignancies escape from NK cell innate immune surveillance: mechanisms and therapeutic implications. Clin Dev Immunol 2012; 2012: 421702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N et al. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity 2008; 28: 571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51Sanchez CJ, Le Treut T, Boehrer A, Knoblauch B, Imbert J, Olive D et al. Natural killer cells and malignant haemopathies: a model for the interaction of cancer with innate immunity. Cancer Immunol Immunother 2011; 60: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52Cerwenka A, Lanier LL. Natural killer cells, viruses and cancer. Nat Rev Immunol 2001; 1: 41–49. [DOI] [PubMed] [Google Scholar]
- 53Mincheva-Nilsson L, Baranov V. Cancer exosomes and NKG2D receptor-ligand interactions: impairing NKG2D-mediated cytotoxicity and anti-tumour immune surveillance. Semin Cancer Biol 2014; in press. [DOI] [PubMed]
- 54Morizane T, Watanabe T, Tsuchimoto K, Tsuchiya M. Impaired T cell function and decreased natural killer activity in patients with liver cirrhosis and their significance in the development of hepatocellular carcinoma. Gastroenterol Jpn 1980; 15: 226–232. [DOI] [PubMed] [Google Scholar]
- 55Dunk AA, Novick D, Thomas HC. Natural killer cell activity in hepatocellular carcinoma. In vitro and in vivo responses to interferon. Scand J Gastroenterol 1987; 22: 1245–1250. [DOI] [PubMed] [Google Scholar]
- 56Hirofuji H, Kakumu S, Fuji A, Ohtani Y, Murase K, Tahara H. Natural killer and activated killer activities in chronic liver disease and hepatocellular carcinoma: evidence for a decreased lymphokine-induced activity of effector cells. Clin Exp Immunol 1987; 68: 348–356. [PMC free article] [PubMed] [Google Scholar]
- 57Nakajima T, Mizushima N, Kanai K. Relationship between natural killer activity and development of hepatocellular carcinoma in patients with cirrhosis of the liver. Jpn J Clin Oncol 1987; 17: 327–332. [PubMed] [Google Scholar]
- 58Liao Y, Wang B, Huang ZL, Shi M, Yu XJ, Zheng L et al. Increased circulating Th17 cells after transarterial chemoembolization correlate with improved survival in stage III hepatocellular carcinoma: a prospective study. PloS One 2013; 8: e60444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59Cai L, Zhang Z, Zhou L, Wang H, Fu J, Zhang S et al. Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients. Clin Immunol 2008; 129: 428–437. [DOI] [PubMed] [Google Scholar]
- 60Fathy A, Eldin MM, Metwally L, Eida M, Abdel-Rehim M. Diminished absolute counts of CD56dim and CD56bright natural killer cells in peripheral blood from Egyptian patients with hepatocellular carcinoma. Egypt J Immunol 2009; 16: 17–25. [PubMed] [Google Scholar]
- 61Liu Y, Poon RT, Hughes J, Feng X, Yu WC, Fan ST. Chemokine receptors support infiltration of lymphocyte subpopulations in human hepatocellular carcinoma. Clin Immunol 2005; 114: 174–182. [DOI] [PubMed] [Google Scholar]
- 62Guo CL, Yang HC, Yang XH, Cheng W, Dong TX, Zhu WJ et al. Associations between infiltrating lymphocyte subsets and hepatocellular carcinoma. Asian Pac J Cancer Prev 2012; 13: 5909–5913. [DOI] [PubMed] [Google Scholar]
- 63Wu Y, Kuang DM, Pan WD, Wan YL, Lao XM, Wang D et al. Monocyte/macrophage-elicited natural killer cell dysfunction in hepatocellular carcinoma is mediated by CD48/2B4 interactions. Hepatology 2013; 57: 1107–1116. [DOI] [PubMed] [Google Scholar]
- 64Peppa D, Micco L, Javaid A, Kennedy PT, Schurich A, Dunn C et al. Blockade of immunosuppressive cytokines restores NK cell antiviral function in chronic hepatitis B virus infection. PLoS Pathog 2010; 6: e1001227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65Tjwa ET, van Oord GW, Hegmans JP, Janssen HL, Woltman AM. Viral load reduction improves activation and function of natural killer cells in patients with chronic hepatitis B. J Hepatol 2011; 54: 209–218. [DOI] [PubMed] [Google Scholar]
- 66Jinushi M, Takehara T, Tatsumi T, Hiramatsu N, Sakamori R, Yamaguchi S et al. Impairment of natural killer cell and dendritic cell functions by the soluble form of MHC class I-related chain A in advanced human hepatocellular carcinomas. J Hepatol 2005; 43: 1013–1020. [DOI] [PubMed] [Google Scholar]
- 67Chuang WL, Liu HW, Chang WY. Natural killer cell activity in patients with hepatocellular carcinoma relative to early development and tumor invasion. Cancer 1990; 65: 926–930. [DOI] [PubMed] [Google Scholar]
- 68Ghiringhelli F, Menard C, Terme M, Flament C, Taieb J, Chaput N et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med 2005; 202: 1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69Pietra G, Manzini C, Rivara S, Vitale M, Cantoni C, Petretto A et al. Melanoma cells inhibit natural killer cell function by modulating the expression of activating receptors and cytolytic activity. Cancer Res 2012; 72: 1407–1415. [DOI] [PubMed] [Google Scholar]
- 70Hayakawa Y. Targeting NKG2D in tumor surveillance. Expert Opin Ther Targets 2012; 16: 587–599. [DOI] [PubMed] [Google Scholar]
- 71Wu Y, Kuang DM, Pan WD, Wan YL, Lao XM, Wang D et al. Monocyte/macrophage-elicited natural killer cell dysfunction in hepatocellular carcinoma is mediated by CD48/2B4 interactions. Hepatology 2013; 57: 1107–1116. [DOI] [PubMed] [Google Scholar]
- 72Jia CC, Wang TT, Liu W, Fu BS, Hua X, Wang GY et al. Cancer-associated fibroblasts from hepatocellular carcinoma promote malignant cell proliferation by HGF secretion. PLoS One 2013; 8: e63243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73Li T, Yang Y, Hua X, Wang G, Liu W, Jia C et al. Hepatocellular carcinoma-associated fibroblasts trigger NK cell dysfunction via PGE2 and IDO. Cancer Lett 2012; 318: 154–161. [DOI] [PubMed] [Google Scholar]
- 74Radaeva S, Sun R, Jaruga B, Nguyen VT, Tian Z, Gao B. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology 2006; 130: 435–452. [DOI] [PubMed] [Google Scholar]
- 75Mehal W, Imaeda A. Cell death and fibrogenesis. Semin Liver Dis 2010; 30: 226–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76Melhem A, Muhanna N, Bishara A, Alvarez CE, Ilan Y, Bishara T et al. Anti-fibrotic activity of NK cells in experimental liver injury through killing of activated HSC. J Hepatol 2006; 45: 60–71. [DOI] [PubMed] [Google Scholar]
- 77Vey N, Bourhis JH, Boissel N, Bordessoule D, Prebet T, Charbonnier A et al. A phase 1 trial of the anti-inhibitory KIR mAb IPH2101 for AML in complete remission. Blood 2012; 120: 4317–4323. [DOI] [PubMed] [Google Scholar]
- 78Benson DMJr, Hofmeister CC, Padmanabhan S, Suvannasankha A, Jagannath S, Abonour R et al. A phase 1 trial of the anti-KIR antibody IPH2101 in patients with relapsed/refractory multiple myeloma. Blood 2012; 120: 4324–4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79Norris S, Doherty DG, Curry M, McEntee G, Traynor O, Hegarty JE et al. Selective reduction of natural killer cells and T cells expressing inhibitory receptors for MHC class I in the livers of patients with hepatic malignancy. Cancer Immunol Immunother 2003; 52: 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80Yuen MF, Norris S. Expression of inhibitory receptors in natural killer (CD3−CD56+) cells and CD3+CD56+ cells in the peripheral blood lymphocytes and tumor infiltrating lymphocytes in patients with primary hepatocellular carcinoma. Clin Immunol 2001; 101: 264–269. [DOI] [PubMed] [Google Scholar]
- 81Burt BM, Plitas G, Zhao Z, Bamboat ZM, Nguyen HM, Dupont B et al. The lytic potential of human liver NK cells is restricted by their limited expression of inhibitory killer Ig-like receptors. J Immunol 2009; 183: 1789–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82Oliviero B, Varchetta S, Paudice E, Michelone G, Zaramella M, Mavilio D et al. Natural killer cell functional dichotomy in chronic hepatitis B and chronic hepatitis C virus infections. Gastroenterology 2009; 137: 1151–1160. [DOI] [PubMed] [Google Scholar]
- 83Ju Y, Hou N, Meng J, Wang X, Zhang X, Zhao D et al. T cell immunoglobulin- and mucin-domain-containing molecule-3 (Tim-3) mediates natural killer cell suppression in chronic hepatitis B. J Hepatol 2010; 52: 322–329. [DOI] [PubMed] [Google Scholar]
- 84Varchetta S, Mele D, Mantovani S, Oliviero B, Cremonesi E, Ludovisi S et al. Impaired intrahepatic natural killer cell cytotoxic function in chronic hepatitis C virus infection. Hepatology 2012; 56: 841–849. [DOI] [PubMed] [Google Scholar]
- 85Bonorino P, Ramzan M, Camous X, Dufeu-Duchesne T, Thelu MA, Sturm N et al. Fine characterization of intrahepatic NK cells expressing natural killer receptors in chronic hepatitis B and C. J Hepatol 2009; 51: 458–467. [DOI] [PubMed] [Google Scholar]
- 86De Maria A, Fogli M, Mazza S, Basso M, Picciotto A, Costa P et al. Increased natural cytotoxicity receptor expression and relevant IL-10 production in NK cells from chronically infected viremic HCV patients. Eur J Immunol 2007; 37: 445–455. [DOI] [PubMed] [Google Scholar]
- 87Nattermann J, Feldmann G, Ahlenstiel G, Langhans B, Sauerbruch T, Spengler U. Surface expression and cytolytic function of natural killer cell receptors is altered in chronic hepatitis C. Gut 2006; 55: 869–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88Meier UC, Owen RE, Taylor E, Worth A, Naoumov N, Willberg C et al. Shared alterations in NK cell frequency, phenotype, and function in chronic human immunodeficiency virus and hepatitis C virus infections. J Virol 2005; 79: 12365–12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89Morishima C, Paschal DM, Wang CC, Yoshihara CS, Wood BL, Yeo AE et al. Decreased NK cell frequency in chronic hepatitis C does not affect ex vivo cytolytic killing. Hepatology 2006; 43: 573–580. [DOI] [PubMed] [Google Scholar]
- 90Li F, Wei H, Gao Y, Xu L, Yin W, Sun R et al. Blocking the natural killer cell inhibitory receptor NKG2A increases activity of human natural killer cells and clears hepatitis B virus infection in mice. Gastroenterology 2013; 144: 392–401. [DOI] [PubMed] [Google Scholar]
- 91Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature 2002; 419: 734–738. [DOI] [PubMed] [Google Scholar]
- 92Salih HR, Rammensee HG, Steinle A. Cutting edge: down-regulation of MICA on human tumors by proteolytic shedding. J Immunol 2002; 169: 4098–4102. [DOI] [PubMed] [Google Scholar]
- 93Waldhauer I, Steinle A. Proteolytic release of soluble UL16-binding protein 2 from tumor cells. Cancer Res 2006; 66: 2520–2526. [DOI] [PubMed] [Google Scholar]
- 94Song H, Kim J, Cosman D, Choi I. Soluble ULBP suppresses natural killer cell activity via down-regulating NKG2D expression. Cell Immunol 2006; 239: 22–30. [DOI] [PubMed] [Google Scholar]
- 95Jinushi M, Takehara T, Tatsumi T, Kanto T, Groh V, Spies T et al. Expression and role of MICA and MICB in human hepatocellular carcinomas and their regulation by retinoic acid. Int J Cancer 2003; 104: 354–361. [DOI] [PubMed] [Google Scholar]
- 96Lin H, Yan J, Wang Z, Hua F, Yu J, Sun W et al. Loss of immunity-supported senescence enhances susceptibility to hepatocellular carcinogenesis and progression in Toll-like receptor 2-deficient mice. Hepatology 2013; 57: 171–182. [DOI] [PubMed] [Google Scholar]
- 97Kumar V, Yi Lo PH, Sawai H, Kato N, Takahashi A, Deng Z et al. Soluble MICA and a MICA variation as possible prognostic biomarkers for HBV-induced hepatocellular carcinoma. PLoS One 2012; 7: e44743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98Kohga K, Takehara T, Tatsumi T, Ohkawa K, Miyagi T, Hiramatsu N et al. Serum levels of soluble major histocompatibility complex (MHC) class I-related chain A in patients with chronic liver diseases and changes during transcatheter arterial embolization for hepatocellular carcinoma. Cancer Sci 2008; 99: 1643–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99Kamimura H, Yamagiwa S, Tsuchiya A, Takamura M, Matsuda Y, Ohkoshi S et al. Reduced NKG2D ligand expression in hepatocellular carcinoma correlates with early recurrence. J Hepatol 2012; 56: 381–388. [DOI] [PubMed] [Google Scholar]
- 100Zhang C, Zhang J, Niu J, Tian Z. Interleukin-15 improves cytotoxicity of natural killer cells via up-regulating NKG2D and cytotoxic effector molecule expression as well as STAT1 and ERK1/2 phosphorylation. Cytokine 2008; 42: 128–136. [DOI] [PubMed] [Google Scholar]
- 101Chretien AS, Le Roy A, Vey N, Prebet T, Blaise D, Fauriat C et al. Cancer-induced alterations of NK-mediated target recognition: current and investigational pharmacological strategies aiming at restoring NK-mediated anti-tumor activity. Front Immunol 2014; 5: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102Raulet DH, Gasser S, Gowen BG, Deng W, Jung H. Regulation of ligands for the NKG2D activating receptor. Annu Rev Immunol 2013; 31: 413–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103Zhang C, Zhang J, Sun R, Feng J, Wei H, Tian Z. Opposing effect of IFNgamma and IFNalpha on expression of NKG2 receptors: negative regulation of IFNgamma on NK cells. Int Immunopharmacol 2005; 5: 1057–1067. [DOI] [PubMed] [Google Scholar]
- 104Zhang C, Niu J, Zhang J, Wang Y, Zhou Z, Tian Z. Opposing effects of interferon-alpha and interferon-gamma on the expression of major histocompatibility complex class I chain-related A in tumors. Cancer Sci 2008; 99: 1279–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105Zhou H, Huang H, Shi J, Zhao Y, Dong Q, Jia H et al. Prognostic value of interleukin 2 and interleukin 15 in peritumoral hepatic tissues for patients with hepatitis B-related hepatocellular carcinoma after curative resection. Gut 2010; 59: 1699–1708. [DOI] [PubMed] [Google Scholar]
- 106Chew V, Tow C, Teo M, Wong HL, Chan J, Gehring A et al. Inflammatory tumour microenvironment is associated with superior survival in hepatocellular carcinoma patients. J Hepatol 2010; 52: 370–379. [DOI] [PubMed] [Google Scholar]
- 107Budhu A, Forgues M, Ye QH, Jia HL, He P, Zanetti KA et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell 2006; 10: 99–111. [DOI] [PubMed] [Google Scholar]
- 108Fransvea E, Mazzocca A, Santamato A, Azzariti A, Antonaci S, Giannelli G. Kinase activation profile associated with TGF-beta-dependent migration of HCC cells: a preclinical study. Cancer Chemother Pharmacol 2011; 68: 79–86. [DOI] [PubMed] [Google Scholar]
- 109Dituri F, Mazzocca A, Peidro FJ, Papappicco P, Fabregat I, de Santis F et al. Differential inhibition of the TGF-beta signaling pathway in HCC cells using the small molecule inhibitor LY2157299 and the D10 monoclonal antibody against TGF-beta receptor type II. PLoS One 2013; 8: e67109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110Wilson EB, El-Jawhari JJ, Neilson AL, Hall GD, Melcher AA, Meade JL et al. Human tumour immune evasion via TGF-beta blocks NK cell activation but not survival allowing therapeutic restoration of anti-tumour activity. PLoS One 2011; 6: e22842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111Lee JC, Lee KM, Kim DW, Heo DS. Elevated TGF-beta1 secretion and down-modulation of NKG2D underlies impaired NK cytotoxicity in cancer patients. J Immunol 2004; 172: 7335–7340. [DOI] [PubMed] [Google Scholar]
- 112Sun C, Fu B, Gao Y, Liao X, Sun R, Tian Z et al. TGF-beta1 down-regulation of NKG2D/DAP10 and 2B4/SAP expression on human NK cells contributes to HBV persistence. PLoS Pathog 2012; 8: e1002594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113Ghiringhelli F, Menard C, Martin F, Zitvogel L. The role of regulatory T cells in the control of natural killer cells: relevance during tumor progression. Immunol Rev 2006; 214: 229–238. [DOI] [PubMed] [Google Scholar]
- 114Bergmann C, Wild CA, Narwan M, Lotfi R, Lang S, Brandau S. Human tumor-induced and naturally occurring Treg cells differentially affect NK cells activated by either IL-2 or target cells. Eur J Immunol 2011; 41: 3564–3573. [DOI] [PubMed] [Google Scholar]
- 115Sprinzl MF, Reisinger F, Puschnik A, Ringelhan M, Ackermann K, Hartmann D et al. Sorafenib perpetuates cellular anticancer effector functions by modulating the crosstalk between macrophages and natural killer cells. Hepatology 2013; 57: 2358–2368. [DOI] [PubMed] [Google Scholar]
- 116Balsamo M, Scordamaglia F, Pietra G, Manzini C, Cantoni C, Boitano M et al. Melanoma-associated fibroblasts modulate NK cell phenotype and antitumor cytotoxicity. Proc Natl Acad Sci USA 2009; 106: 20847–20852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117Tsunematsu H, Tatsumi T, Kohga K, Yamamoto M, Aketa H, Miyagi T et al. Fibroblast growth factor-2 enhances NK sensitivity of hepatocellular carcinoma cells. Int J Cancer 2012; 130: 356–364. [DOI] [PubMed] [Google Scholar]
- 118Maghazachi AA. Role of chemokines in the biology of natural killer cells. Curr Top Microbiol Immunol 2010; 341: 37–58. [DOI] [PubMed] [Google Scholar]
- 119Iannello A, Thompson TW, Ardolino M, Lowe SW, Raulet DH. p53-dependent chemokine production by senescent tumor cells supports NKG2D-dependent tumor elimination by natural killer cells. J Exp Med 2013; 210: 2057–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120Waggoner SN, Daniels KA, Welsh RM. Therapeutic depletion of natural killer cells controls persistent infection. J Virol 2014; 88: 1953–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121Peppa D, Gill US, Reynolds G, Easom NJ, Pallett LJ, Schurich A et al. Up-regulation of a death receptor renders antiviral T cells susceptible to NK cell-mediated deletion. J Exp Med 2013; 210: 99–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122Gehring AJ, Ho ZZ, Tan AT, Aung MO, Lee KH, Tan KC et al. Profile of tumor antigen-specific CD8 T cells in patients with hepatitis B virus-related hepatocellular carcinoma. Gastroenterology 2009; 137: 682–690. [DOI] [PubMed] [Google Scholar]