Abstract
Hepatitis B virus (HBV) reactivation and recurrence are common in patients undergoing immunosuppression therapy. Tumor necrosis factor (TNF) blockage therapy is effective for the treatment of many autoimmune inflammatory diseases. However, the role of TNF-α blockage therapy in the innate and adaptive immune responses against HBV is still not clear. A detailed analysis of HBV infection under TNF-α blockage therapy is essential for the prophylaxis and therapy for HBV reactivation and recurrence. In this study, HBV clearance and T-cell responses were analyzed in a HBV-transfected mouse model under anti-TNF blockage therapy. Our results demonstrated that under TNF-α blockage therapy, HBV viral clearance was impaired with persistent elevated HBV viral load in a dose- and temporal-dependent manner. The impairment of HBV clearance under anti-TNF-α blockage therapy occurred at early time points after HBV infection. In addition, TNF-α blockade maintained a higher serum HBV viral load and increased the number of intrahepatic programmed cell death (PD)-1highCD127low exhausted T cells. Furthermore, TNF-α blockade abolished Toll-like receptor 9 (TLR9) ligand-induced facilitation of HBV viral clearance. Taken together, TNF-α blockade impairs HBV clearance and enhances viral load, and these effects depend on early administration after HBV infection. Our results here demonstrate that early TNF-α blockade reduces viral clearance and persistently maintains elevated HBV viral load in a mouse model, suggesting that HBV may reactivate during therapy with TNF-α-blocking agents.
Keywords: HBV viral load, hepatitis B virus, tumor necrosis factor
Introduction
Hepatitis B virus (HBV) infection causes acute and chronic necroinflammatory hepatitis in humans. Approximately 5%–10% of HBV infections in adults become chronic.1 An efficient clearance of HBV requires coordinated actions of both the innate and adaptive immune responses.2,3 Innate immunity induces an antiviral effect on infected cells by producing type I interferon and promotes the efficient maturation and local recruitment of adaptive immunity through the production of pro-inflammatory cytokines, especially tumor necrosis factor-alpha (TNF-α).4 However, in chronic HBV infection, impaired HBV-specific immune responses fail to eliminate infected hepatocytes, resulting in the persistence of HBV. Although the activation of the innate immune response has a key role in initiating the adaptive immune response, experimental results of HBV infection in both chimpanzees and woodchucks demonstrated non- or limited activation of innate immunity in acute HBV infection.5,6 A number of recent studies7,8,9,10 have investigated and suggested the involvement of innate cells, such as nature killer and nature killer T cells, in chronic HBV infection and suggested that these cells could play a role in liver damage during reactivation. Nevertheless, the role of innate immunity in viral clearance during HBV infection is still not clear.
TNF-α is known for its pro-inflammatory property in antitumor activity, immune modulation, and the integrated host defense system against infectious diseases. In HBV infection, TNF-α supports HBV-specific cytotoxic T lymphocytes to abolish HBV replication in hepatocytes11 and delivers non-cytopathic antiviral signals to hepatocytes to degrade the cytoplasmic transcript and the nucleocapsid particles of HBV.12 In adoptively transferred HBV-specific cytotoxic T lymphocytes in an HBV-transgenic mouse model, TNF-α can abolish HBV gene expression and replication in the liver without killing the hepatocytes.13 In an acutely HBV-infected chimpanzee model, HBV DNA was shown to largely disappear from the liver and the blood long before the peak of T-cell infiltration, showing a non-cytopathic role for TNF-α in HBV viral clearance.14 In addition, studies from TNF-α knockout mice have also demonstrated that lack of the effect of TNF-α would impair the CD8+ T-cell response to HBV, leading to ineffectiveness of viral clearance.15,16 These observations indicate that TNF-α is a crucial innate cytokine for clearing HBV and eliminating HBV viral load in the host immune response against HBV infection.
Persistent exposure to viral antigens leads to progressive functional impairment of T cells, so called T-cell exhaustion.17,18,19 Recent studies in animal models of viral infection indicated that the interaction between programmed cell death (PD)-1 on lymphocytes and its ligands plays a critical role in T-cell exhaustion by inducing T-cell inactivation. Persistent viral antigen stimulation correlates with the exhaustion of a previously stable primed T-cell population.17,18,19 HBV-specific PD-1-positive CD8-positive T cells of patients with chronic HBV infection also displayed lower levels of the interleukin (IL)-7 receptor, CD127, which was previously described in association with the exhausted phenotype.20 However, the effects of TNF-α blockage therapy on T-cell exhaustion and the adaptive immune response in HBV infection is still uncertain.
TNF-α blockage therapy is effective for the treatment of many autoimmune inflammatory diseases such as rheumatoid arthritis, ankylosing spondylitis, Crohn's disease or psoriasis.21,22,23 However, there are limited reports on its effect on HBV persistence and reactivation in chronic HBV infection during anti-TNF therapy.24,25 The role of anti-TNF therapy in chronic HBV infection is still not clear, and detailed analysis of HBV infection under TNF-α blockage therapy is essential for the prophylaxis and therapy for HBV reactivation and recurrence.
In this study, we investigated the dynamic change of HBV viral load and the clearance of HBV under the influence of TNF-α blockage therapy by using immunocompetent mice hydrodynamically transfected with an HBV DNA-expressing plasmid.26,27 The model was used to clarify the relationship between blockage of TNF-α and HBV viral load and to provide evidence that anti-TNF treatment leads to delayed clearance of HBV. We demonstrate here that early blockage of TNF-α reduces viral clearance and enhances T-cell exhaustion in this mouse model, suggesting that HBV may reactivate during therapy with TNF-α-blocking agents.
Materials and methods
Animals
BALB/c and C57BL/6 mice (male, 6–7 weeks) were obtained from the National Laboratory Animal Center, Taiwan. TNF-α knockout mice (C57BL/6 background; H-2b haplotype) were obtained from Dr Chun-Jen Chen (National Taiwan University, Taiwan). All the experimental mice were maintained in a specific pathogen-free environment. All animal experiments were approved by the Animal Ethical Committee of National Taiwan University.
Hydrodynamic injection
BALB/c or C57BL/6 mice were anesthetized with ketamine and xylazine. Ten micrograms of HBV DNA-expressing plasmid, pAAV/HBV1.2, in a volume equivalent to 8% of the mouse body weight was injected via tail veins within 5 s of anesthetization. The plasmid DNA was purified using the EndoFree Maxi plasmid kit (Qiagen, Hilden, Germany).
Reagents
Soluble TNF-α receptor, etanercept (Enbrel), was purchased from commercial sources (Pfizer, New York City, NY, USA). Toll-like receptor-9 (TLR9) ligand (CpG oligodeoxynucleotide 1668: 5′-S-TCCATGACGTTCCTGATGCT-3′) and control non-TLR9 ligand (non-CpG oligodeoxynucleotide 1720: 5′-S-TCCATGAGCTTCCTGATGCT-3′) were from Bioneer Inc. (Alameda, CA, USA).
Detection of serum HBV antigen, HBV DNA and ALT
Serum titers of HBV surface antigen (HBsAg) in mice were absolutely quantified using the ABBOTT PRISM HBsAg assay kit (Abbott Laboratories, North Chicago, IL, USA) and semi-quantified using the AXSYM system kit (Abbott Diagnostika, Wiesbaden Delkenheim, Germany). The obtained signal-to-noise (S/N) ratio for titer of HBsAg was compared with a standard curve with known concentrations of HBsAg. HBsAg-positivity was defined as an S/N ratio greater than 2. HBV DNA was measured by the extraction of total DNA of serum samples and then detected by real-time PCR as previously described.28 Serum alanine aminotransferase (ALT) was measured using ALT/GPT reagent (Denka Seiken, Tokyo, Japan) on the automated clinical chemistry analyzer TBA-200FR (Toshiba, Tokyo, Japan).
Immunohistochemistry
Liver tissues were collected from the euthanized mice at the indicated time points. Intrahepatic HBsAg or HBV core antigen was visualized by immunohistochemical staining of tissues embedded in paraffin by rabbit anti-HBc or anti-HBs antibodies (DAKO, Carpinteria, CA, USA; Biomeda, Foster City, CA, USA) and Envisio System, HRP (diaminobenzidine) (DAKO).
Isolation of intrahepatic leukocytes (IHLs)
Mice were anesthetized with tribromoethanol (Avertin) 0.2 ml/10 g. The chest and abdomen were opened. The inferior vena cava below the liver was cannulated with a scalp vein needle and then gripped by microclips. The inferior vena cava above the liver was clamped by microclips. The liver was perfused with 10 ml phosphate-buffered saline (PBS) at the rate of 5 ml/min. After the excision of whole liver, the liver was minced by a plunger passing through 70 µm nylon mesh to obtain a liver cell suspension. The liver cell suspension was collected and washed with HBSS to a final volume of 50 ml. Hepatocytes and large cell clumps were removed by 50g centrifugation at 4 °C twice, and then the pellet was discarded. The supernatant containing IHLs was pelleted by 300g centrifugation at 4 °C for 10 min. The cell pellet was resuspended in 40% HBSS and layered gently on top of a 70% Percoll solution (GE Healthcare, Piscataway, NJ, USA). Next, the cells were centrifuged at 1200g for 20 minutes at 25 °C. Viable IHLs were recoverable at the 40%/70% Percoll interphase. IHLs were collected, washed with 15 ml HBSS, and centrifuged at 300g for 10 min at 4 °C. The cell pellet was collected for downstream applications.
Flow cytometry
Multiparameter flow cytometry was performed according to a standard protocol. Surface staining was performed using the following anti-mouse monoclonal antibodies: allophycocyanin-conjugated anti-CD3, phycoerythrin-cy5.5-conjugated anti-CD8 (BD Biosciences, Palo Alto, CA, USA); fluorescein isothiocyanate-conjugated anti-CD127 (eBioscience, San Diego, CA, USA) and phycoerythrin-conjugated anti-PD-1 (eBioscience). Cells were labeled in fluorescence-activated cell sorter buffer (PBS/2% fetal calf serum/0.1% sodium azide). Flow cytometry was performed using a FACSCalibur machine, and data were analyzed with CellQuest software (Becton Dickinson, Mountain View, CA, USA). The gating strategy and isotype control for flow cytometry analysis are presented in Supplementary Figure 1.
Statistical analysis
Student's t-test was used to compare two experimental groups. A Spearman correlation test was applied to test the correlations between PD-1 expression of CD8+ T cells and HBV viral load. All the P values referred to two-tail tests, and a P value of less than 0.05 was defined as statistically significant. The analyses were conducted using SPSS (Statistical Package for the Social Sciences) software, version 16.0 (SPSS, Chicago, IL, USA).
Results
TNF-α blockade impaired HBV clearance and enhanced T-cell exhaustion in a dose-dependent manner
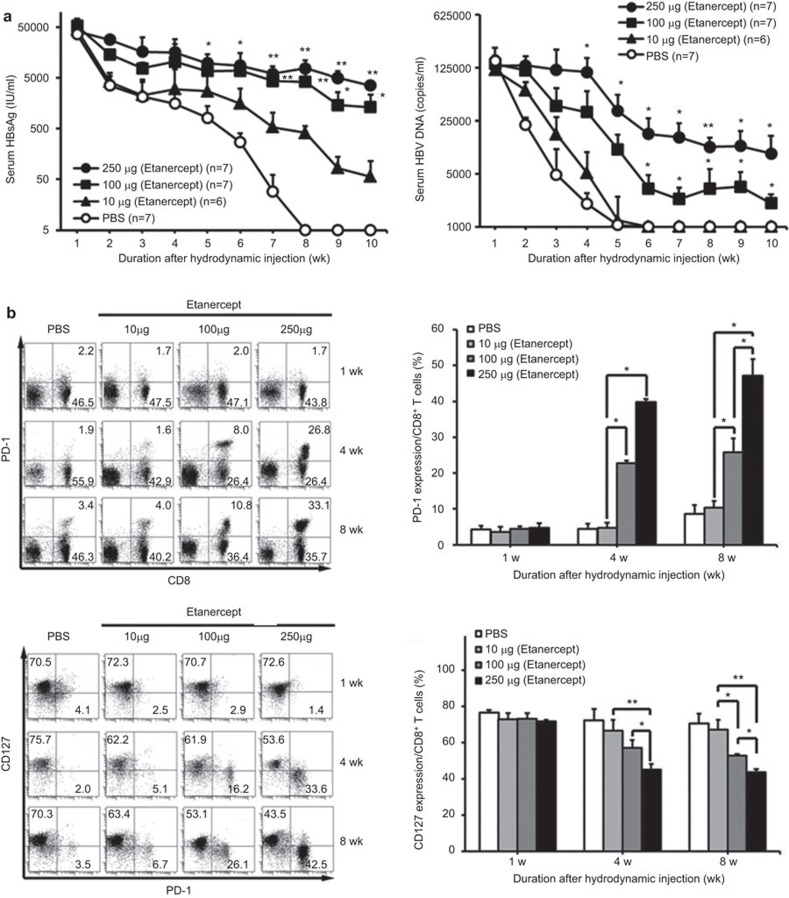
In our previous study, we have demonstrated that there was enhanced HBV persistence and delayed HBV clearance in TNF-α knockout mice, suggesting that TNF-α is crucial in the clearance of HBV in a mouse animal model.16 HBV clearance was determined by the mouse genetic background: this approach induced HBV persistence in C57BL/6 mice, but not in BALB/c mice.26,27 We further investigated the role of TNF-α blockade during HBV infection when TNF-α was neutralized with the soluble TNF receptor, etanercept, in this mouse model. The results demonstrated that TNF blockade significantly impaired HBV clearance in both mouse strains (Supplementary Figure 2). Furthermore, TNF-α blockade induced the elevation of serum HBV viral load and maintained HBV viral antigen expression in the liver (Supplementary Figure 3). All these results were consistent with those of our previous study.16 To further address the extent of TNF-α blockage therapy on HBV clearance and viral load, we investigated the dynamic change of the HBV viral load and T cell exhaustion phenotype under different dosages of etanercept in mice hydrodynamically injected with replication-competent HBV DNA. The results shown in Figure 1a demonstrated that TNF-α blockage therapy potently impairs HBV clearance and results in persistently maintained serum HBsAg and HBV DNA in a dose-dependent manner. In contrast, HBV was completely cleared in the eighth week in those mice without anti-TNF treatment.
Figure 1.
TNF-α blockade impairs HBV clearance in a dose-dependent manner. BALB/c mice were intraperitoneally administered recombinant soluble TNF-α receptor, etanercept, in different dosages on the day of hydrodynamic injection of pAAV/HBV1.2 plasmid. Etanercept was administered twice a week over the whole period. (a) Differences in titers of serum HBsAg (left) and serum HBV viral loads (right) in HBsAg-positive mice with different dosages of etanercept were quantified. *P<0.05; **P<0.01, Student's t-test, compared with the PBS group. (b) After injection, intrahepatic lymphocytes from mice receiving etanercept were isolated at the indicated time points, and the PD-1 and CD127 expression of liver-infiltrating CD8+ lymphocytes was analyzed by flow cytometry. *P<0.05; **P<0.01, Student's t-test. The data are representative of at least three independent experiments in each group. HBsAg, HBV surface antigen; HBV, hepatitis B virus; PBS, phosphate-buffered saline; PD-1, programmed cell death-1; TNF, tumor necrosis factor.
Recent studies on HBV infection indicate that the interaction between the PD-1 receptor on lymphocytes and its ligands plays a critical role in T-cell exhaustion by inducing T-cell inactivation.17,18,19 These exhausted T cells also display lower levels of IL-7 receptor, CD127.29,30 In mice receiving higher dosages of anti-TNF, there was a significant increase in PD-1 expression and a significant decrease in CD127 expression in liver-infiltrating CD8+ T cells (Figure 1b). The results shown in Supplementary Figure 4 further demonstrated that there was a significant negative correlation between HBV viral load and T-cell exhaustion in mice receiving TNF-α blockage treatment, indicating that TNF blockade enhances T-cell exhaustion in HBV infection (Supplementary Figure 4).To summarize, our results demonstrate that TNF blockade impairs HBV clearance, with increased liver-infiltrating PD-1highCD127low-exhausted CD8± T cells in a dose- dependent manner in vivo.
Blockage of TNF-α persistently maintained HBV viral load and enhanced T-cell exhaustion
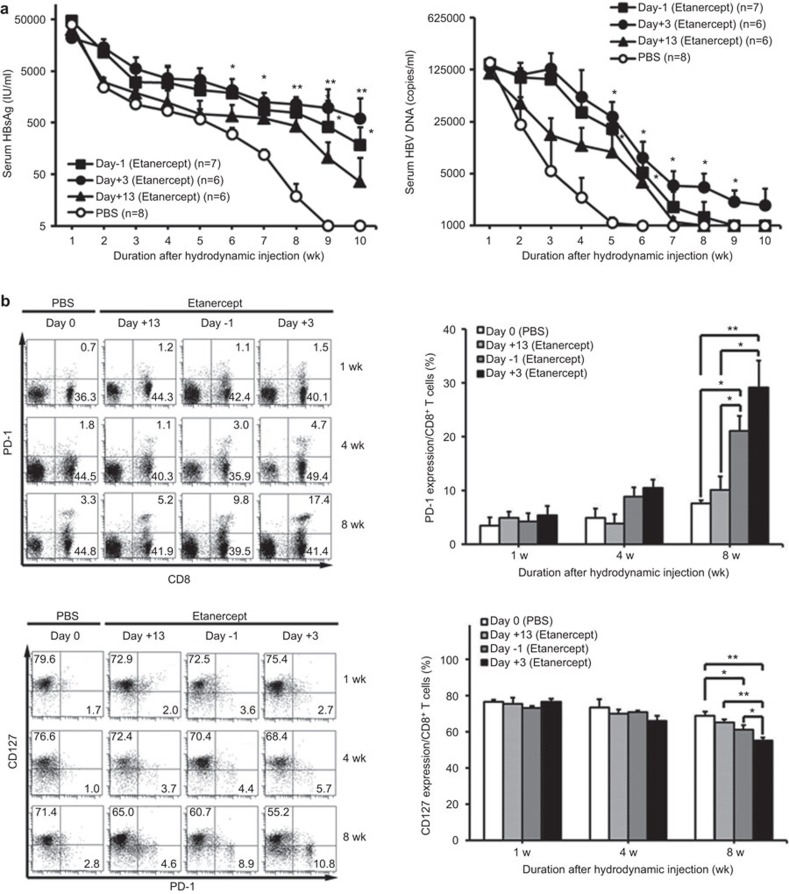
We further investigated the effects of different intervals of anti-TNF delivery on HBV viral clearance. The results shown in Figure 2 demonstrated that early sufficient blockage of TNF-α significantly impaired subsequent HBV clearance and maintained HBV viral load. Such effects were persistently maintained over a duration of 4 weeks or longer after a single injection, indicating that initial efficient blockage of TNF-α is crucial for HBV clearance. In addition, the effects of TNF blockade on the T-cell exhaustion phenotype is affected more by the intervals of anti-TNF delivery (Figure 2b). Overall, these results support the conclusion that early sufficient blockage of TNF-α maintains persistently elevated HBV viral load and enhanced T-cell exhaustion.
Figure 2.
Effects of anti-TNF interval dosing on HBV clearance, viral load and T-cell exhaustion phenotype. BALB/c mice were intraperitoneally treated with etanercept in different intervals on the day of pAAV/HBV1.2 hydrodynamic injection; 250 µg of etanercept was administered over the whole period. (a) Differences in titers of serum HBsAg (left) and serum HBV viral loads (right) of mice receiving etanercept were quantified. *P<0.05, **P<0.01, Student's t-test, compared with the PBS group. (b) After injection, intrahepatic lymphocytes from mice receiving etanercept at different time intervals were isolated at the indicated time point, and the PD-1 and CD127 expression of liver-infiltrating CD8+ lymphocytes was analyzed by flow cytometry *P<0.05, **P<0.01, Student's t-test. The data are representative of at least three independent experiments in each group. HBV, hepatitis B virus; PBS, phosphate-buffered saline; PD-1, programmed cell death-1; TNF, tumor necrosis factor.
The effect of anti-TNF on HBV clearance is determined at an early time point after HBV infection
Because an initial single dose of anti-TNF is sufficient to persistently impair HBV clearance, we then investigated the critical timing of anti-TNF delivery on HBV clearance. We administered a single dose of etanercept at different time points after hydrodynamically injecting BALB/c mice with HBV DNA. The results shown in Figure 3 revealed that only at an early time point after anti-TNF delivery did the anti-TNF lead to impaired HBV clearance with elevated viral load and enhanced T-cell exhaustion. In contrast, when the anti-TNF was delivered at a later time point (at day 13), the effects were significantly reduced. Taken together, these results indicate that anti-TNF affects the clearance of HBV at the early phase of induction of the immune response to HBV.
Figure 3.
Early time point of anti-TNF-α delivery is critical for HBV clearance. BALB/c mice were intraperitoneally administered a single injection of etanercept (250 µg) at different time points after pAAV/HBV1.2 hydrodynamic injection. (a) Differences in titers of serum HBsAg (left) and serum HBV viral loads (right) of HBsAg-positive mice with a single injection of etanercept at different time points were quantified. *P<0.05; **P<0.01, Student's t-test, compared with PBS group. (b) Intrahepatic lymphocytes from mice receiving a single etanercept injection at different time points were isolated, and the PD-1 and CD127 expression of liver-infiltrating CD8+ lymphocytes was analyzed by flow cytometry. *P<0.05; **P<0.01, Student's t-test. The data are representative of at least three independent experiments in each group. HBsAg, HBV surface antigen; HBV, hepatitis B virus; PBS, phosphate-buffered saline; PD-1, programmed cell death-1; TNF, tumor necrosis factor.
TNF blockade suppresses TLR9 ligand-induced facilitation of HBV viral clearance
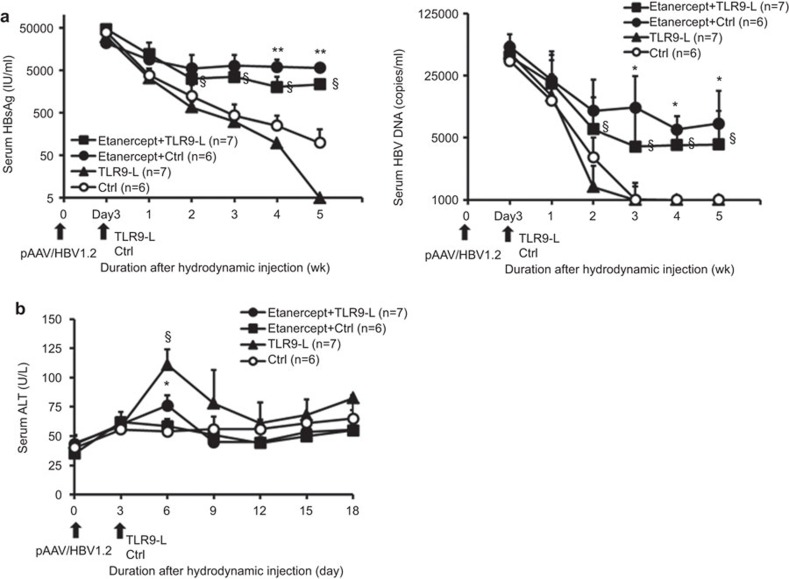
TNF-α is the key mediator that drives local intrahepatic proliferation of T cells (iMATEs: ‘intrahepatic myeloid-cell aggregates for T-cell population expansion'), and TLR9 ligands induce the formation of iMATEs to facilitate viral clearance via TNF.31 To further address how TNF blockade would impair TLR9 ligand-induced facilitation of HBV clearance, we administered TLR9 ligand after anti-TNF delivery in BALB/c mice receiving HBV DNA hydrodynamically. The results shown in Figure 4 demonstrated that TLR9 ligand enhances HBV clearance, but the administration of a TNF-α inhibitor, etanercept, significantly impaired the ability to clear virus, resulting in a higher HBV viral load and delayed clearance. These results indicate that TNF blockage abolishes TLR9 ligand-induced facilitation of HBV viral clearance. In addition to suppressing the effects of TLR9 ligand on viral clearance, TNF-α blockade also reduced the effects of TLR9 ligand-induced elevation of serum ALT from hepatocytes (Figure 4b). Altogether, these results indicate that TNF blockade abolishes TLR9 ligand-induced facilitation of HBV viral clearance.
Figure 4.
TNF blockage suppresses TLR9 ligand-induced facilitation of HBV viral clearance. BALB/c mice were either intraperitoneally administered etanercept or were untreated on the day before pAAV/HBV1.2 hydrodynamic injection. Etanercept treatment was administered twice a week over the whole period. At 3 days after hydrodynamic injection, the mice were treated with TLR9 ligand or non-TLR9-binding control oligonucleotide. (a) Differences in titers of serum HBsAg (left) and serum HBV viral loads (right) of mice that did or did not receive etanercept were quantified. §P<0.05, Student's t-test, compared with the TLR9 ligand group. *P<0.05; **P<0.01, Student's t-test, compared with the non-TLR9-binding control group. (b) Kinetics of serum ALT in mice challenged with TLR9 ligand or non-TLR9-binding control in the presence or absence of etanercept were quantified. §P<0.05, Student's t-test, compared with the TLR9 ligand group. *P<0.05, Student's t-test, compared with the non-TLR9-binding control group. HBsAg, HBV surface antigen; HBV, hepatitis B virus; TLR, Toll-like receptor.
Discussion
In this study, we used a mouse model of HBV infection to demonstrate that early blockage of TNF-α led to persistent impaired HBV clearance with enhanced T-cell exhaustion and maintained high HBV viral load. Previous studies have shown that the innate cytokine TNF-α plays a fundamental role in sustaining viral clearance 14,32 and inhibiting the transcriptional activity of the HBV core promoter in vitro.33 In the process of HBV viral clearance, TNF-α inhibits HBV replication by activating NF-κB signaling.34 TNF-α has been described as potentially having a potentially modulating effect on HBV gene expression35,36 and as being a key immune effector in HBV clearance in a study of a panel of immune effector-deficient mouse strains.16,37 As an innate cytokine, TNF-α causes iMATE formation, which facilitates the expansion of the cytotoxic T-lymphocyte population against HBV.31 Clinically, blockage of TNF-α with anti-TNF has been widely used in many immune-mediated diseases, including rheumatoid arthritis, ankylosing spondylitis, inflammatory bowel disease, psoriasis and psoriatic arthritis.21,38,39,40 Several studies reported that elevated HBV viral load subsequently leads to active hepatitis.24,25 A recent study analyzing patients with inflammatory arthritis also revealed an increased risk of HBV reactivation associated with anti-TNF therapy in patients with chronic HBV infection.41 However, due to limited well-controlled studies, the association between TNF-α blockage and HBV reactivation remains uncertain. In this study, we further addressed the dynamic change of HBV viral load in blockage of TNF-α. Our results support the notion that TNF plays a critical role in HBV clearance and that blockage of TNF-α reduces HBV clearance and maintains HBV viral load in this mouse model.
Previous studies have shown that the anti-inflammatory effect of TNF blockage is dose-dependent.42,43 Consistent with these previous results, our results showed that the HBV viral load was increased with higher doses of anti-TNF. Moreover, early sufficient blockage of TNF-α significantly impaired subsequent HBV clearance, resulting in an elevated HBV viral load, and such effects were prolonged in duration. In addition, a single dose of anti-TNF at an early time point instead of at a late time point led to delayed HBV clearance and persistent elevated viral load, indicating that TNF-α deficiency impairs an early phase of the immune response to HBV. These results also suggest that TNF-α may serve as a critical innate cytokine to activate the adaptive immune response to HBV and that TNF-α blockage affects the early phase of induction of the innate immune response to HBV. In addition to direct anti-viral effects on HBV,12,13 TNF-α is a key mediator that drives the formation of iMATEs to facilitate viral clearance.31 In this study, we further demonstrated that TNF-α blockade abolishes the effects of TLR9 ligand-induced facilitation of HBV viral clearance. Taken together, these results indicate that anti- TNF-α therapy impairs HBV clearance, maintains HBV viral load, and enhances T-cell dysfunction in this mouse model.
The induction of TNF-α can trigger both the innate and adaptive immune responses and regulate the balance of viral replication and clearance in the liver. The clearance of HBV particles in the liver is mainly mediated by HBV-specific CD8+ T cells triggered by TNF-α.14,44 Persistent HBV infection impairs HBV-specific CD8+ T-cell function and results in the exhausted-phenotype CD8+ cells that fail to clear virus.45,46 In our study, our results demonstrate that blockage of TNF-α leads to increasing liver-infiltrating PD-1highCD127low-exhausted phenotype CD8+ T cells in mice with HBV persistence. These results indicate that in HBV persistence, blockage of TNF-α will further impair the adaptive immune response and that TNF-α is crucial for maintaining an effective anti-HBV immune response.
In conclusion, blockage of TNF-α significantly reduces HBV clearance, maintains higher serum HBV viral load and impairs intrahepatic T-cell function in a mouse model, indicating that TNF-α is crucial in the immune response against HBV. Our results provide evidence that TNF-α blockage therapy reduces HBV viral clearance, enhances viral persistence and maintains HBV viral load, suggesting that HBV may reactivate during therapy with TNF-blocking agents.
Acknowledgments
We thank Dr Shie-Liang Hsieh for critically reviewing the manuscript and Ms W-L Wang and Y-L Chen for assistance. This work was supported by grants from the National Science Council, Taiwan (NSC98-3112-B-002-047, NSC101-2320-B-038-019-, NSC 101-2321-B-002-008- and 102-2320-B-038 -040-MY3).
Footnotes
Supplementary information accompanies the paper on Cellular & Molecular Immunology's website(http://www.nature.com/cmi/).
Supplementary Information
References
- 1Merican I, Guan R, Amarapuka D, Alexander MJ, Chutaputti A, Chien RN et al. Chronic hepatitis B virus infection in Asian countries. J Gastroenterol Hepatol 2000; 15: 1356–1361. [DOI] [PubMed] [Google Scholar]
- 2Bertoletti A, Ferrari C. Innate and adaptive immune responses in chronic hepatitis B virus infections: towards restoration of immune control of viral infection. Gut 2012; 61: 1754–1764. [DOI] [PubMed] [Google Scholar]
- 3Durantel D, Zoulim F. Innate response to hepatitis B virus infection: observations challenging the concept of a stealth virus. Hepatology 2009; 50: 1692–1695. [DOI] [PubMed] [Google Scholar]
- 4Bertoletti A, Ferrari C. Innate and adaptive immune responses in chronic hepatitis B virus infections: towards restoration of immune control of viral infection. Postgrad Med J 2013; 89: 294–304. [DOI] [PubMed] [Google Scholar]
- 5Dunn C, Peppa D, Khanna P, Nebbia G, Jones M, Brendish N et al. Temporal analysis of early immune responses in patients with acute hepatitis B virus infection. Gastroenterology 2009; 137: 1289–1300. [DOI] [PubMed] [Google Scholar]
- 6Guo H, Jiang D, Ma D, Chang J, Dougherty AM, Cuconati A et al. Activation of pattern recognition receptor-mediated innate immunity inhibits the replication of hepatitis B virus in human hepatocyte-derived cells. J Virol 2009; 83: 847–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Baron JL, Gardiner L, Nishimura S, Shinkai K, Locksley R, Ganem D. Activation of a nonclassical NKT cell subset in a transgenic mouse model of hepatitis B virus infection. Immunity 2002; 16: 583–594. [DOI] [PubMed] [Google Scholar]
- 8Bonorino P, Ramzan M, Camous X, Dufeu-Duchesne T, Thelu MA, Sturm N et al. Fine characterization of intrahepatic NK cells expressing natural killer receptors in chronic hepatitis B and C. J Hepatol 2009; 51: 458–467. [DOI] [PubMed] [Google Scholar]
- 9Gao B, Radaeva S, Park O. Liver natural killer and natural killer T cells: immunobiology and emerging roles in liver diseases. J Leukoc Biol 2009; 86: 513–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10Nattermann J, Feldmann G, Ahlenstiel G, Langhans B, Sauerbruch T, Spengler U. Surface expression and cytolytic function of natural killer cell receptors is altered in chronic hepatitis C. Gut 2006; 55: 869–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Chisari FV. Cytotoxic T cells and viral hepatitis. J Clin Invest 1997; 99: 1472–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12Guidotti LG, Chisari FV. Cytokine-induced viral purging—role in viral pathogenesis. Curr Opin Microbiol 1999; 2: 388–391. [DOI] [PubMed] [Google Scholar]
- 13Guidotti LG, Ishikawa T, Hobbs MV, Matzke B, Schreiber R, Chisari FV. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity 1996; 4: 25–36. [DOI] [PubMed] [Google Scholar]
- 14Guidotti LG, Rochford R, Chung J, Shapiro M, Purcell R, Chisari FV. Viral clearance without destruction of infected cells during acute HBV infection. Science 1999; 284: 825–829. [DOI] [PubMed] [Google Scholar]
- 15Kasahara S, Ando K, Saito K, Sekikawa K, Ito H, Ishikawa T et al. Lack of tumor necrosis factor alpha induces impaired proliferation of hepatitis B virus-specific cytotoxic T lymphocytes. J Virol 2003; 77: 2469–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16Tzeng HT, Tsai HF, Chyuan IT, Liao HJ, Chen CJ, Chen PJ et al. Tumor necrosis factor-alpha induced by hepatitis B virus core mediating the immune response for hepatitis B viral clearance in mice model. PLoS One 2014; 9: e103008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 2006; 443: 350–354. [DOI] [PubMed] [Google Scholar]
- 18Lang KS, Recher M, Navarini AA, Harris NL, Lohning M, Junt T et al. Inverse correlation between IL-7 receptor expression and CD8 T cell exhaustion during persistent antigen stimulation. Eur J Immunol 2005; 35: 738–745. [DOI] [PubMed] [Google Scholar]
- 19Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med 2006; 203: 2281–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20Ye P, Weng ZH, Zhang SL, Zhang JA, Zhao L, Dong JH et al. Programmed death-1 expression is associated with the disease status in hepatitis B virus infection. World J Gastroenterol 2008; 14: 4551–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21Mease PJ, Goffe BS, Metz J, VanderStoep A, Finck B, Burge DJ. Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. Lancet 2000; 356: 385–390. [DOI] [PubMed] [Google Scholar]
- 22Su CG, Judge TA, Lichtenstein GR. The role of biological therapy in inflammatory bowel disease. Drugs Today (Barc) 2001; 37: 121–133. [DOI] [PubMed] [Google Scholar]
- 23Taylor PC. Anti-TNF therapy for rheumatoid arthritis and other inflammatory diseases. Mol Biotechnol 2001; 19: 153–168. [DOI] [PubMed] [Google Scholar]
- 24Perez-Alvarez R, Diaz-Lagares C, Garcia-Hernandez F, Lopez-Roses L, Brito-Zeron P, Perez-de-Lis M et al. Hepatitis B virus (HBV) reactivation in patients receiving tumor necrosis factor (TNF)-targeted therapy: analysis of 257 cases. Medicine (Baltimore) 2011; 90: 359–371. [DOI] [PubMed] [Google Scholar]
- 25Tamori A, Koike T, Goto H, Wakitani S, Tada M, Morikawa H et al. Prospective study of reactivation of hepatitis B virus in patients with rheumatoid arthritis who received immunosuppressive therapy: evaluation of both HBsAg-positive and HBsAg-negative cohorts. J Gastroenterol 2011; 46: 556–564. [DOI] [PubMed] [Google Scholar]
- 26Huang LR, Wu HL, Chen PJ, Chen DS. An immunocompetent mouse model for the tolerance of human chronic hepatitis B virus infection. Proc Natl Acad Sci USA 2006; 103: 17862–17867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27Lin YJ, Huang LR, Yang HC, Tzeng HT, Hsu PN, Wu HL et al. Hepatitis B virus core antigen determines viral persistence in a C57BL/6 mouse model. Proc Natl Acad Sci USA 2010; 107: 9340–9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28Yeh SH, Tsai CY, Kao JH, Liu CJ, Kuo TJ, Lin MW et al. Quantification and genotyping of hepatitis B virus in a single reaction by real-time PCR and melting curve analysis. J Hepatol 2004; 41: 659–666. [DOI] [PubMed] [Google Scholar]
- 29Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol 2007; 81: 4215–4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Boettler T, Panther E, Bengsch B, Nazarova N, Spangenberg HC, Blum HE et al. Expression of the interleukin-7 receptor alpha chain (CD127) on virus-specific CD8+ T cells identifies functionally and phenotypically defined memory T cells during acute resolving hepatitis B virus infection. J Virol 2006; 80: 3532–3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Huang LR, Wohlleber D, Reisinger F, Jenne CN, Cheng RL, Abdullah Z et al. Intrahepatic myeloid-cell aggregates enable local proliferation of CD8+ T cells and successful immunotherapy against chronic viral liver infection. Nat Immunol 2013; 14: 574–583. [DOI] [PubMed] [Google Scholar]
- 32Guidotti LG, Chisari FV. Immunobiology and pathogenesis of viral hepatitis. Annu Rev Pathol 2006; 1: 23–61. [DOI] [PubMed] [Google Scholar]
- 33Romero R, Lavine JE. Cytokine inhibition of the hepatitis B virus core promoter. Hepatology 1996; 23: 17–23. [DOI] [PubMed] [Google Scholar]
- 34Biermer M, Puro R, Schneider RJ. Tumor necrosis factor alpha inhibition of hepatitis B virus replication involves disruption of capsid Integrity through activation of NF-kappaB. J Virol 2003; 77: 4033–4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Gilles PN, Fey G, Chisari FV. Tumor necrosis factor alpha negatively regulates hepatitis B virus gene expression in transgenic mice. J Virol 1992; 66: 3955–3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36Neta R, Oppenheim JJ, Douches SD. Interdependence of the radioprotective effects of human recombinant interleukin 1 alpha, tumor necrosis factor alpha, granulocyte colony-stimulating factor, and murine recombinant granulocyte-macrophage colony-stimulating factor. J Immunol 1988; 140: 108–111. [PubMed] [Google Scholar]
- 37Yang PL, Althage A, Chung J, Maier H, Wieland S, Isogawa M et al. Immune effectors required for hepatitis B virus clearance. Proc Natl Acad Sci USA 2010; 107: 798–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38Calin A, Dijkmans BA, Emery P, Hakala M, Kalden J, Leirisalo-Repo M et al. Outcomes of a multicentre randomised clinical trial of etanercept to treat ankylosing spondylitis. Ann Rheum Dis 2004; 63: 1594–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39Ledingham J, Deighton C. Update on the British Society for Rheumatology guidelines for prescribing TNFalpha blockers in adults with rheumatoid arthritis (update of previous guidelines of April 2001). Rheumatology (Oxford) 2005; 44: 157–163. [DOI] [PubMed] [Google Scholar]
- 40Siddiqui MA, Scott LJ. Spotlight on infliximab in Crohn disease and rheumatoid arthritis. BioDrugs 2006; 20: 67–70. [DOI] [PubMed] [Google Scholar]
- 41Ye H, Zhang XW, Mu R, Fang LK, Gu JR, Lin J et al. Anti-TNF therapy in patients with HBV infection—analysis of 87 patients with inflammatory arthritis. Clin Rheumatol 2014; 33: 119–123. [DOI] [PubMed] [Google Scholar]
- 42de la Torre I, Valor L, Nieto JC, Montoro M, Carreno L. Minimum effective dosages of anti-TNF in rheumatoid arthritis: a cross-sectional study. Reumatol Clin 2013; 10: 101–104. [DOI] [PubMed] [Google Scholar]
- 43Moots RJ, Haraoui B, Matucci-Cerinic M, van Riel PL, Kekow J, Schaeverbeke T et al. Differences in biologic dose-escalation, non-biologic and steroid intensification among three anti-TNF agents: evidence from clinical practice. Clin Exp Rheumatol 2011; 29: 26–34. [PubMed] [Google Scholar]
- 44Isogawa M, Furuichi Y, Chisari FV. Oscillating CD8+ T cell effector functions after antigen recognition in the liver. Immunity 2005; 23: 53–63. [DOI] [PubMed] [Google Scholar]
- 45Raziorrouh B, Schraut W, Gerlach T, Nowack D, Gruner NH, Ulsenheimer A et al. The immunoregulatory role of CD244 in chronic hepatitis B infection and its inhibitory potential on virus-specific CD8+ T-cell function. Hepatology 2010; 52:1934–1947. [DOI] [PubMed] [Google Scholar]
- 46Schurich A, Khanna P, Lopes AR, Han KJ, Peppa D, Micco L et al. Role of the coinhibitory receptor cytotoxic T lymphocyte antigen-4 on apoptosis-prone CD8 T cells in persistent hepatitis B virus infection. Hepatology 2011; 53: 1494–1503. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.