Abstract
B cells play an important role in the clearance of hepatitis B virus (HBV) and protection against reinfection. However, the functional characteristics of these cells that are associated with the outcome of chronic HBV infection remain unknown. We comprehensively investigated the frequency, phenotype, and function of peripheral B-cell subsets from CHB patients in different phases: immune tolerance (IT), immune activation (IA), immune clearance (IC), responders with HBsAg seroconversion (resolved patients, RP), and healthy controls (HC). IA patients displayed lower percentages of peripheral blood memory B cells compared with the other groups. Overall polyclonal activation of B cells, indicated by higher levels of activation markers and secretion of IgG and IgM, was observed in IA patients. This B-cell hyperactivation could be induced by increased IFN-α and soluble CD40 ligands in IA patients. Notably, the expression of the co-stimulator molecule CD80 and serum HBsAb and the frequency of HBsAg-specific B cells were significantly decreased in IT, IA, and IC patients compared with HC subjects. More importantly, the B-cell hyperactivation, co-stimulatory molecule downregulation and HBsAg-specific B-cell impairment were reversed in RP patients. The reversal of B-cell hyperactivation and functional impairment is associated with HBsAg seroconversion in chronic hepatitis B patients.
INTRODUCTION
The natural history of chronic hepatitis B virus (HBV) infection generally comprises four phases:1 immune tolerance (IT), immune activation (IA), immune clearance (IC), and non/low-replication (LR). Some patients might further clear hepatitis B surface antigen (HBsAg) either spontaneously or through antiviral therapy and then progress to the HBsAg seroconversion phase. During the course of HBV infection, host immunity against the virus is considered to play a key role in liver pathogenesis and disease prognosis.2 HBV-specific T cells are exhausted during chronic infection,2,3,4,5 whereas antibodies to HBsAg are key to HBV clearance. However, the nature of B cell-mediated humoral immunity remains obscure. Several clinical studies have demonstrated that in chronic HBV-infected patients receiving liver transplantation, the adoptive HBV humoral immunity from donors potentially clears the residual virus and protects the liver graft from HBV reinfection.6,7,8,9 In lymphoma patients with HBV infection, treatment with rituximab (a chimeric mouse human monoclonal antibody (mAb) against CD20 that induces profound and durable B-cell depletion) significantly increases HBV reactivation.10,11,12,13 Indeed, anti-HBsAg antibodies (HBsAb) can neutralize circulating HBsAg and clear infectious HBV particles in vivo, and the presence of HBsAb in serum is considered an indicator of the resolution of chronic HBV infection. These findings strongly highlight the importance of HBsAb in the clearance of HBV infection. However, few reports have characterized the HBsAb-producing B cells in chronic HBV infection.
Confined techniques in the analysis of B cells, particularly in the analysis of antigen-specific B cells in humans, have limited the study of B cells in HBV-infected patients. Several previous studies have indicated that HBsAg-specific B cells and HBsAb are lacking in CHB patients.14,15,16,17,18,19 A recent study indicated that B cells display enhanced differentiation and deficient proliferation in CHB patients.16 Although these studies characterized B cells in chronic HBV infection, the dynamics of B cells in the natural history of chronic HBV infection have not been well described. Therefore, it is important to correlate specific B-cell responses with the outcomes of chronic HBV infection, particularly with HBsAg seroconversion.
In the present study, B cells from patients in different phases of chronic HBV infection, therapy-induced responders (resolved patients, RP) with HBsAg seroconversion and healthy controls (HC) were comprehensively characterized. Increased total B-cell activation and decreased HBsAg-specific B-cell responses were associated with HBV persistence, whereas the restoration of B-cell hyperactivation and HBsAg-specific B-cell impairment was associated with HBsAg seroconversion in chronic HBV infection. These results highlight the active role of B cells in the immunopathogenesis of chronic HBV infection, and the reduction in B-cell hyperactivation and improvement in HBsAg-specific B-cell responses might facilitate HBsAg seroconversion.
PATIENTS AND METHODS
Patients
A total of 68 HBV-infected subjects and 22 vaccinated HC were enrolled in this study. These patients were either interferon (IFN)-α therapy-induced responders (RP) with HBsAg seroconversion for at least 1 year (n = 13) or chronic HBV patients categorized according to the disease phase: IT phase (n = 12), IA phase (n = 32), or IC phase (n = 11) patients. None of the patients had received antiviral treatment (IFN-α or nucleoside analogs) within the previous 6 months. The HC subjects had received three vaccinations with 10 μg recombinant HBsAg and were serum anti-HBsAg positive at enrollment. All individuals were negative for antibodies to hepatitis A virus, hepatitis C virus (HCV), hepatitis D virus, and human immunodeficiency virus (HIV). Further exclusion criteria were end-stage liver insufficiency, autoimmune disorders, immunosuppressive treatment and malignancies. The study protocol was approved by the local medical ethics committee. Written informed consent for the study was obtained from each subject. The clinical characteristics of these subjects are listed in Table 1.
Table 1. Clinical characteristics of enrolled subjects in the study.
| Group | HC | IT | IA | IC | RP |
|---|---|---|---|---|---|
| Case | 22 | 12 | 32 | 11 | 13 |
| Sex (male/female) | 11/11 | 11/1 | 30/2 | 9/2 | 12/1 |
| Age (years) | 28 (24–35) | 29 (16–60) | 34 (15–63) | 44(19–62) | 37 (22–53) |
| HBsAg/HBsAb | −/+ | +/− | +/− | +/− | −/+ |
| HBeAg/HBeAb | −/− | +/− | +/− | −/+ | −/− |
| ALT (U/L) | ND | 23 (13–37) | 292 (54–1885) | 28 (5–40) | 28 (15–45) |
| AST (U/L) | ND | 23 (16–32) | 128 (27–875) | 19 (6–38) | 19 (12–38) |
| HBV DNA (100 IU/ml) | ND | 8.2 (7.8–8.9) | 6.8 (3.3–8.8) | LDL | LDL |
Abbreviations: HC, healthy controls; IA, Immune activation; IC, immune clearance; IT, Immune tolerance; LDL, lower detection limitation; ND, not determined; RP, responders with HBsAg seroconversion.
Data are shown as median and range.
Flow cytometry
All mAbs were purchased from eBioscience (San Diego, CA, USA), except for anti-CD80, anti-CD86, and anti-CXCR3 mAbs (Becton Dickinson Pharmingen, San Jose, CA, USA). Peripheral blood mononuclear cells (PBMCs) were isolated and stained with the following mAbs conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE), allophycocyanin (APC), or peridinin chlorophyll protein (PerCP): anti-CD19-APC, anti-CD27-FITC, anti-CD38-PE, anti-CD69-PerCP, anti-CD71-PE, anti-CD80-PE, and anti-CXCR3-PE. The cells were subsequently analyzed using FACSCalibur (Becton Dickinson, San Diego, CA, USA) and Flowjo software (TreeStar, Ashland, OR, USA) as previously described.20 At least 200 000 events were acquired per run.
Cell culture
The cells were cultured in RPMI 1640 medium (GIBCO/BRL) supplemented with streptomycin, glutamine, and 10% fetal calf serum (FCS) and further stimulated in vitro using Phytolacca americana pokeweed mitogen (PWM) (Sigma, St. Louis, MO, USA) for 3 days. The cells were subsequently used for enzyme-linked immunospot (Elispot) detection, and the supernatants were collected for ELISA. Alternatively, PBMCs or B cells purified with CD19 MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany) were cultured with IL-6 (100 ng/ml), IL-17 (100 ng/ml), IFN-α (1000 IU/ml), CD40L (2 μg/ml, R&D Systems, Minneapolis, MN, USA), IL-21 (100 ng/ml), or IL-33 (100 ng/ml, Peprotech, Rocky Hill, NJ, USA) for 24 h. The cells were subsequently collected for the detection of B-cell activation.
Detection of anti-HBsAg IgG-secreting B cells using Elispot
The frequency of anti-HBsAg-secreting B cells was determined using Elispot assays. Briefly, PBMCs (5 × 105 cells/well) were cultured in 200 μl of medium containing PWM (5 μg/ml) for 3 days in a 96-well plate. During the culture period, Elispot plates (Millipore, Billerica, MA, USA) were coated with 10 μg/ml recombinant HBsAg overnight at 4 °C. After 3 days, the cells were transferred to Elispot plates and incubated with PWM for an additional 24 h. Wells coated with ovalbumin were used as negative controls. The plates were subsequently washed and incubated with biotin-labeled anti-IgG mAb (Sigma) and horseradish peroxidase (HRP)-conjugated streptavidin (Sigma). The plates were subsequently air-dried, and the spots were counted using an automated Elispot reader. The responses were expressed as spot-forming cells (SFCs) per million PBMCs. The assay was considered valid when the number of spots was at least twofold above the background level. All reactions were performed in triplicate.
ELISA
Plasma IFN-α (PBL Biomedical Laboratories, Piscataway, NJ, USA) and soluble CD40 ligand (sCD40L, eBioscience) were determined using a commercial ELISA kit. The total IgG, IgM, and anti-HBsAg-IgG levels in serum and culture supernatants were also quantified using ELISA. Briefly, recombinant HBsAg (US Biological, Swampscott, MA, USA), anti-human IgG or IgM antibodies (Bethyl Laboratories, Montgomery, TX, USA) were coated at a concentration of 3 μg/ml in 96-well flat-bottom ELISA plates (Nunc, Roskilde, Denmark) overnight at 4 °C. The plates were washed and incubated with the samples or human reference serum (Bethyl Laboratories) for 1 h. Subsequently, the plates were incubated with biotin-labeled anti-human IgG (Sigma) and HRP-conjugated anti-human IgG or IgM (Bethyl Laboratories) for 1 h at room temperature. The plates were sequentially washed and developed with TMB substrate.
Virological and immunological assessments
The levels of HBsAg, anti-HBsAg, HBeAg, anti-HBeAg, and anti-HBcAg were measured using commercial kits (Abbott Laboratories, North Chicago, IL, USA). HBV DNA levels were determined using quantitative real-time polymerase chain reaction (AMPLICOR, Roche) according to the manufacturer's instructions. The threshold of detection for HBV DNA was 100 IU/ml.
Statistical analysis
All data were analyzed using the SPSS software (SPSS Inc., Chicago, IL, USA). The Kruskal–Wallis H nonparametric test was performed for multiple comparisons among the five groups. Significant differences between two groups were determined using the Mann–Whitney nonparametric U test. Data from the same individuals were compared using the Wilcoxon matched-pairs t-test. Correlations between variables were evaluated using the Spearman's rank correlation test. For all tests, a P-value less than 0.05 was considered significant.
RESULTS
Naive B cells are increased but memory B cells are decreased in IA patients
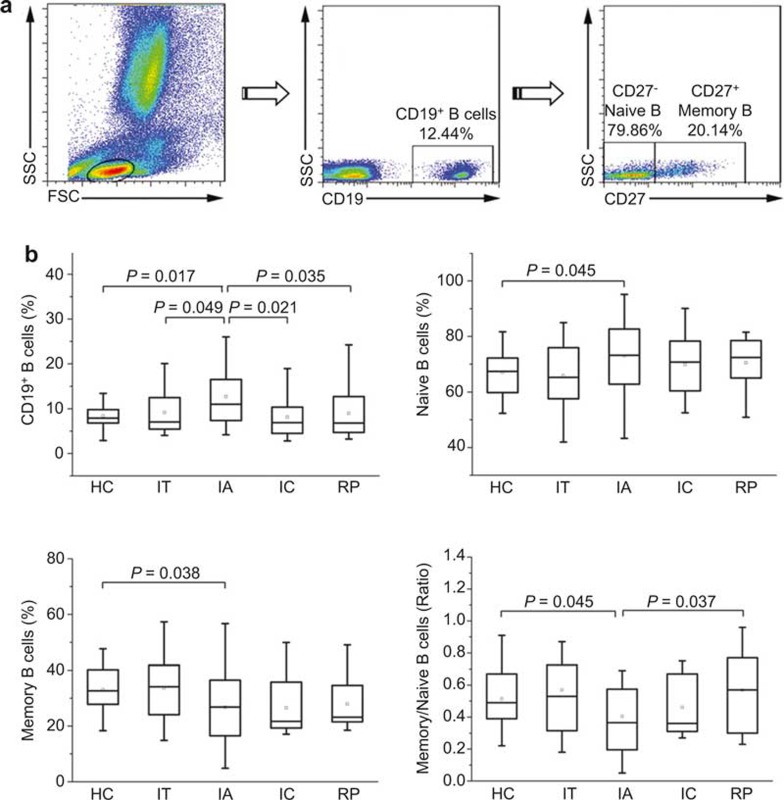
We first investigated the percentages of total B cells and naive and memory B-cell subsets based on CD27 expression using flow cytometric analysis in HC subjects and IT, IA, IC, and RP patients (Figure 1). The frequency of CD19+ total B cells in peripheral blood lymphocytes was significantly higher in IA patients than in HC subjects and IT patients (P = 0.017 and P = 0.049, respectively); however, this frequency was reduced in IC patients and RP subjects compared with IA patients (P = 0.021 and P = 0.035, respectively), showing levels similar to the level observed in HC patients (Figure 1B). Further analysis indicated that the percentages of CD27− naive and CD27+ memory B-cell subsets relative to the total B cells were altered in opposite directions: CD27− naive B cells were preferentially increased whereas CD27+ memory B cells were significantly reduced in IA patients compared with HC subjects and IT patients (Figure 1C and D). Accordingly, the ratio of memory to naive B cells was also decreased in IA patients (Figure 1E). More importantly, we observed that the increased ratio of memory to naive B cells in IA patients was significantly reversed in RP subjects. However, compared with IA patients, the frequency of naive, memory B cells and the ratio of memory to naive B cells in IC patients were not significantly changed (Figure 1C–E). Furthermore, no significant associations were observed between the proportions of these B-cell subsets and the plasma HBV DNA, serum alanine transaminase (ALT) and aspartate aminotransferase (AST) levels (data not shown). These data suggest that chronic HBV infection alters the distribution of naive and memory B cells and that these effects can be reversed in patients with HBsAg seroconversion but not in IC patients.
Figure 1.
Differential distribution of B-cell subsets in HBV-infected patients at various phases. (A) Representative dot plots showing the proportions of CD19+ total B cells, CD27− naive B cells and CD27+ memory B cells. The percentages of each B-cell subset in the dot plots indicate the relative proportion of the total B cells. (B–D) Pooled data indicating the total peripheral B cells as a proportion of total lymphocytes (B) or the naive (C) and memory B cells (D) as a proportion of total B cells and the ratio of memory to naive B cells (E) in healthy controls (HC), immune tolerant patients (IT), immune activated patients (IA), immune clearance (IC), and responders with HBsAg seroconversion (resolved patients, RP). The boxes represent the 5th, 25th, 75th, and 95th percentiles, and the solid line indicates the median value of each subset. P-values less than 0.05 are shown.
B cells are activated in IA patients but partially restored in RP subjects
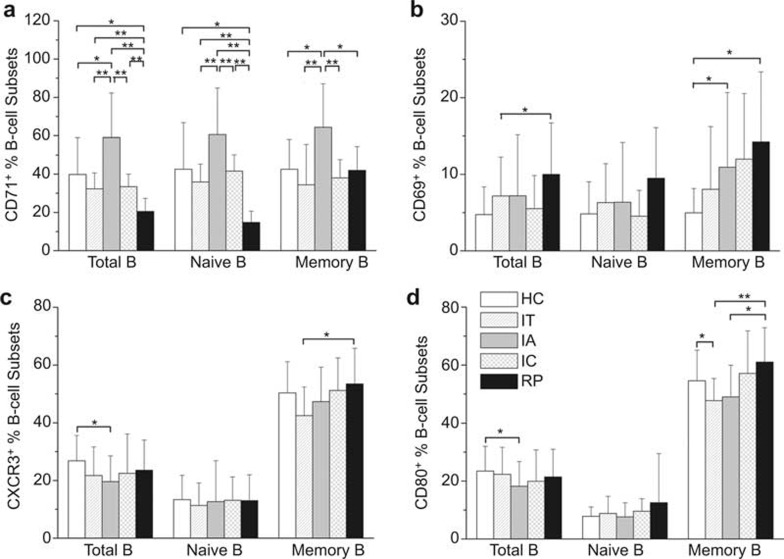
We next examined the expression of the early activation molecule CD69, the transferrin receptor CD71, the chemokine receptor CXCR3 (CD183), and the co-stimulatory molecule CD80 on the B-cell subsets in the five subject groups. As shown in Figure 2A, the CD71 expression levels on total B cells and naive and memory B-cell subsets were markedly elevated in IA patients compared with HC and IT subjects. This increase in CD71 expression, however, was significantly downregulated in IC and RP subjects to levels lower than that observed in HC subjects. Furthermore, the expression of CD71 in RP patients was even lower than in IC patients. CD69 expression on memory B cells was also significantly upregulated in IA patients compared with HC subjects. In contrast, CD69 expression on these B cells remained at a high level in RP patients (Figure 2B). Unexpectedly, CXCR3 expression on total B cells was decreased in IA patients, but upregulated on memory B cells in RP subjects compared with IT patients (Figure 2C). The expression of the co-stimulatory molecule CD80 was also markedly decreased on total B cells in IA patients and on memory B cells in IT patients compared with HC subjects. Importantly, this reduced CD80 expression on memory B cells was significantly recovered in RP subjects (Figure 2D). In addition, no significant correlations were observed between the markers expressed on B-cell subsets and HBV DNA, ALT, or AST levels in these patients (data not shown). These results indicated that although B cells are significantly activated in CHB patients, these cells lack the expression of the co-stimulatory molecule CD80, which indicates an intrinsic B-cell defect. Moreover, the hyperactivation and defective co-stimulatory molecule expression were reversed in RP subjects with HBsAg seroconversion.
Figure 2.
Expression of B-cell activation markers are upregulated in IA patients but recovered in RP subjects. The expression levels of activation markers CD71 (A), CD69 (B), CXCR3 (C), and the co-stimulator CD80 (D) on total B cells, naive and memory B cells were examined in healthy donors, IT, IA, and IC patients and RP subjects. The data are presented as the means ± standard deviation. *P < 0.05, **P < 0.01.
Functional B cells in CHB patients are significantly attenuated in RP subjects
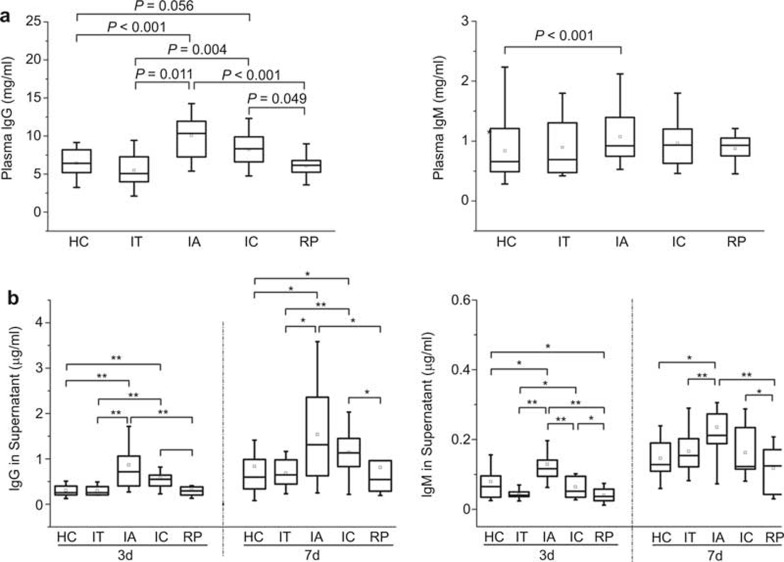
To investigate whether the activated B cells are functional, we detected total plasma IgG and IgM levels in these groups. As expected, the level of total plasma IgG was significantly higher in IA patients compared with IT patients and HC subjects. Compared with IA patients, the plasma IgG levels in IC patients decreased to some extent but remained higher than those in HC and IT subjects. In addition, the total plasma IgG levels were reduced to normal levels in RP subjects compared with HC subjects (Figure 3A). The total plasma IgM was increased only in IA patients compared with HC subjects.
Figure 3.
IgG and IgM production are increased in IA patients but reduced in RP subjects. (A) Plasma IgG and IgM levels in HC, IT, IA, IC, and RP subjects were determined using ELISA. P-values less than 0.05 are shown. (B) IgG and IgM production levels in B cells stimulated with PWM for 3 or 7 days in vitro were determined using ELISA. The boxes represent the 5th, 25th, 75th, and 95th percentiles, and the solid line indicates the median value of each subset. *P < 0.05, **P < 0.01.
To further investigate the intrinsic potential of B cells to secrete antibodies, we compared the IgG and IgM production from B cells in the various groups after stimulation with PWM for 3 and 7 days. The B cells from IA patients produced higher levels of IgG and IgM than those from HC subjects after both 3 and 7 days of stimulation. The levels of IgG and IgM in the supernatant of cells from IT patients, however, were similar to those of healthy individuals. The production of IgG, rather than IgM, in IC patients remained higher compared with HC subjects and IT patients. Compared with IA patients, only levels of IgM in the supernatant of cells from IC patients were significantly decreased after 3 days of stimulation. Notably, the ability of B cells to produce IgG and IgM was significantly restored in RP subjects (Figure 3B). These results indicated that the increased production of IgG and IgM in CHB patients also reflects the activation of B cells and that the hyper-Ig production by B cells was partially reduced in IC patients but reduced to normal levels in RP subjects.
IFN-α and soluble CD40L mediate B-cell activation in vitro
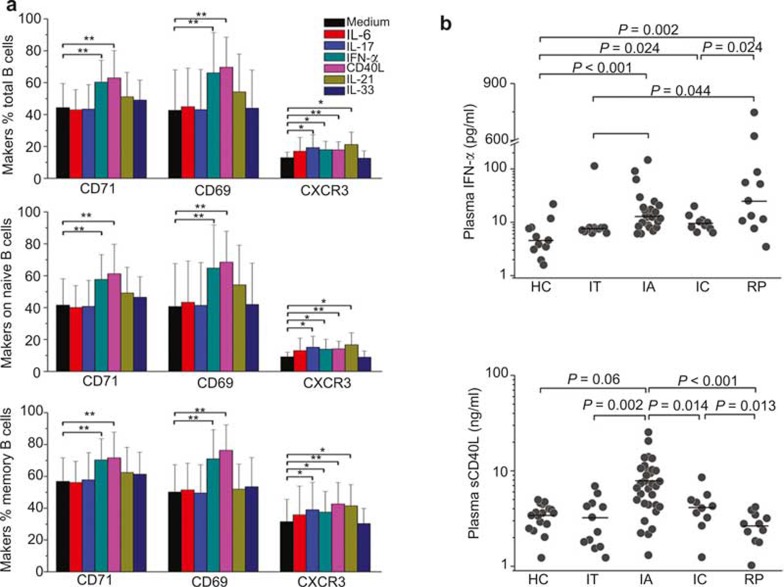
To determine which factors are potentially responsible for activating peripheral B cells, we detected the effects of IFN-α, sCD40L, IL-6, IL-17, IL-21, and IL-33 on purified B-cell activation in vitro. IFN-α and sCD40L significantly increased CD69 and CD71 expression on total, naive and memory B cells from HC subjects. In contrast, IL-6, IL-17, IL-21, and IL-33 did not activate B cells in vitro. Moreover, all stimulator molecules, except IL-6 and IL-33, upregulated CXCR3 expression (Figure 4A). We observed that the plasma IFN-α and sCD40L levels were significantly increased in IA patients compared with HC subjects and IT patients. Importantly, the plasma levels of sCD40L in IC and RP patients were significantly decreased compared with IA patients. However, the levels of plasma IFN-α in IC and RP subjects were not downregulated (Figure 4B). These results indicated that the increased B-cell activation in IA patients likely reflected elevated plasma IFN-α and sCD40L levels, which could be partially reversed in IC patients but completely restored to normal levels in RP patients.
Figure 4.
IFN-α and sCD40L preferentially activate B cells in vitro. (A) The expression levels of CD71, CD69, and CXCR3 on cultured B-cell subsets are shown after in vitro stimulation with IL-6, IL-17, IFN-α, sCD40L, IL-21, or IL-33 for 1 day. B cells from HC subjects (n = 6) and IA patients (n = 6) were purified with CD19 MicroBeads and subsequently cultured with various cytokines. The data are presented as the means ± standard deviation. *P < 0.05, **P < 0.01. (B) Plasma IFN-α and sCD40L levels in HC, IT, IA, ENH, and RP subjects were determined using ELISA. Each dot represents one individual. P-values less than 0.05 are shown.
HBsAg-specific IgG-secreting B cells are reduced in patients with chronic HBV infection but restored in RP subjects
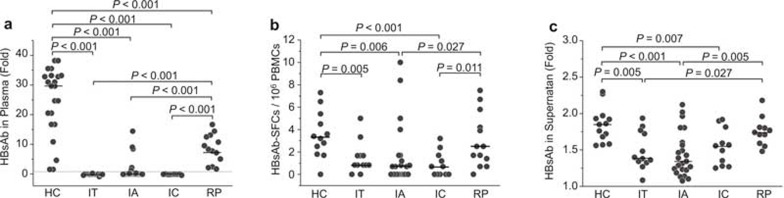
The HBsAb released from HBsAg-specific B cells protects the host from re-infection and is highly important for the clearance of HBV virions or antigens in vivo. Therefore, we monitored the plasma HBsAb levels in the five subject groups. As shown in Figure 5A, only 12.5% of IA patients (4/32) and none of the IT or IC patients had detectable plasma HBsAb, while 100% of the HC and RP subjects were HBsAb positive. However, the titer of serum HBsAb in RP subjects was significantly lower than in healthy individuals (24.7 ± 11.6 vs. 8.6 ± 4.9) (Figure 5A). We also observed that the frequencies of HBsAg-specific IgG-secreting B cells and the levels of HBsAb in the culture supernatant were significantly lower in IT, IA, and IC patients than in HC subjects, but these effects were partially recovered in RP subjects (Figure 5B and C). These results indicated that HBsAg-specific B cells might be deficient in CHB patients, which could lead to a decrease in plasma anti-HBsAg neutralizing antibodies; however, impaired HBV-specific B cells are partially restored in RP subjects with HBsAg seroconversion.
Figure 5.
HBsAg-specific IgG-producing B cells are reduced in HBV-infected patients but restored in RP subjects. (A) HBsAb levels in serum were determined using ELISA. The data are shown as fold-changes over the negative control. The dashed horizontal line indicates the cut-off value calculated as 2.1 times the negative control. (B) HBsAg-specific IgG-producing B cells were significantly reduced in HBV-infected patients but restored in RP subjects with HBsAg seroconversion. (C) HBsAb levels in the culture supernatant were determined using ELISA. The data are shown as fold-changes over the negative control. SFC, spot-forming cells. Each dot represents one individual. P-values less than 0.05 are shown.
DISCUSSION
Although several limited studies have characterized peripheral B cells in patients with chronic HBV infection,14,15,16,17,18,19 the precise characteristics of B cells, particularly HBsAg-specific B cells, during the natural history of chronic HBV infection and the association of these cells with disease outcome remain unclear. In the present study, phenotypic and functional analyses of B cells were comprehensively performed using a substantial number of IT, IA, IC patients, and RP subjects with HBsAg seroconversion. We observed a dichotomy in the hyperactivation of total B cells and the impairment of HBsAg-specific B cells in CHB patients, and these effects were reversed in subjects with HBsAg seroconversion.
The present study provided some important insights into the B-cell activation observed in the immunopathogenesis of chronic HBV infection. However, the downregulation of CXCR3 on B cells in CHB patients is different from the observations reported in a recent study,16 which might partially be attributed to the different patient cohorts examined. In addition, the increased activation of B cells, as indicated by the CD71 and CD69 expression on cells from CHB patients, was also translated into hyper-functionality, as evidenced by the elevated plasma IgG and IgM levels and the increased secretion of IgG and IgM upon stimulation in vitro. Although chronic microbial infections are frequently associated with persistent polyclonal B-cell activation, potentially leading to highly dysfunctional immune disorders, such as chronic HIV infection,21,22 the underlying mechanisms of general B-cell hyperactivation remain unknown. The results of the present study supported the notion that IFN-α and sCD40L might preferentially activate B cells, as indicated by increased CD71, CD69, and CXCR3 expression on both naive and memory B cells in vitro, whereas other cytokines, such as IL-6 and IL-21, which are upregulated in CHB patients,23,24 did not activate B cells. We employed sCD40L, a surrogate of T cell help, and IFN-α, an innate immune-derived signal-engaging toll-like receptor (TLR)-9, expressed predominantly by B cells and plasmacytoid dendritic cells. Thus, the elevated TLR9-mediated signals25 and T cell likely contribute to the functional hyperactivation of B cells in CHB patients, as previously observed in HIV and HCV infections, which induce chronic liver inflammation.21,26 Future studies should address whether and how continuous B-cell activation in CHB patients might lead to B-cell exhaustion, as observed in HIV infection.22,27 However, human peripheral B cells are also activated by sCD40L in vitro as surrogated antigen-presenting cells, which represent a potential approach for anti-HBV immunotherapy through the induction of HBsAg-specific B cells.28
Similar to previous reports,14,15,18,19 the impaired generation of HBsAg-specific B cells and plasma anti-HBsAg in CHB patients was observed in the present study. Thus, the undetectable anti-HBsAg in CHB patients is likely an important factor leading to HBV persistence. However, several CHB patients showed detectable levels of anti-HBsAg- and HBsAg-specific B cells in the present study, indicating that the B cells from CHB patients retain the potential to produce HBsAb. This finding is consistent with the clinical observation that a small proportion of CHB patients have a low HBsAb titer. Indeed, studies using sensitive immunoassays for the detection of antibodies in the presence of excess plasma antigens have revealed that anti-HBsAg is produced in a majority of CHB patients, but these antibodies are typically complexed with HBsAg present at high concentrations in the plasma of these patients.14,29,30
The results of the present study supported the notion that the antigen-presenting capacity of B cells was also potentially impaired in CHB patients, evidenced by significantly decreased CD80 expression. Because the ligation of CD28 on CD4 T cells by CD80/CD86 on B cells represents a dominant co-stimulatory pathway that plays a critical role in CD4 T cell activation and proliferation, the downregulation of CD80 on B cells in CHB patients likely reflects the insufficient induction of CD4 T cell activation and proliferation. Indeed, in chronic HBV infection, not only are HBV-specific CD4 and CD8 T cell responses significantly diminished,31,32 but a generalized T cell hypo-responsiveness has also been demonstrated.33 This T-cell hyporesponsiveness might be a consequence of functionally impaired dendritic cells.34 Thus, as critical antigen-presenting cells, B cells might also be involved in T-cell impairment in chronic HBV infection.
More importantly, the general activation of B cells and the functional impairment of HBsAg-specific B cells observed in the present study could be partially reversed in IC patients but almost completely reversed in responders with HBsAg seroconversion, compared with IA patients. This finding also supports the hypothesis that HBsAg might play a key role in the inhibition of HBV-specific responses, thus leading to the failure of HBV clearance in CHB patients. However, not all RP subjects displayed a recovery of plasma HBsAb and HBsAg-specific B cells. Together with recent studies quantifying HBsAg in the natural history of chronic HBV infection,35,36 the results of the present study suggest preliminary associations between HBsAg levels and HBsAg-specific B-cell responses in CHB patients. Future longitudinal studies should comprehensively dissect the dynamics of B cell immune responses to further validate the influences of HBsAg levels on host T- and B-cell immune responses.
Taken together, these findings provide new insights into B-cell functions during different phases of chronic HBV infection and suggest that the promotion of HBsAg-specific B cells would be a favorable new therapeutic strategy for this disease.
POTENTIAL CONFLICTS OF INTEREST
None reported.
Acknowledgments
This work was supported by grants from the National Grand Program on Key Infectious Disease (2013ZX10002001-001-003, 2012ZX10002-007-002), the National Natural Science Foundation of China (81373137), the National Key Basic Research Program of China (2012CB519005), and the National Science Fund for Outstanding Young Scholars (81222024).
References
- 1European Association for the Study of the Liver. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol 2012; 57: 167–185. [DOI] [PubMed] [Google Scholar]
- 2Wang FS, Zhang Z. Host immunity influences disease progression and antiviral efficacy in humans infected with hepatitis B virus. Expert Rev Gastroenterol Hepatol 2009; 3: 499–512. [DOI] [PubMed] [Google Scholar]
- 3Schurich A, Khanna P, Lopes AR et al. Role of the coinhibitory receptor cytotoxic T lymphocyte antigen-4 on apoptosis-prone CD8 T cells in persistent hepatitis B virus infection. Hepatology 2011; 53: 1494–1503. [DOI] [PubMed] [Google Scholar]
- 4Bertoletti A, Ferrari C. Innate and adaptive immune responses in chronic hepatitis B virus infections: towards restoration of immune control of viral infection. Gut 2011; 61: 1754–1764. doi:10.1136/gutjnl-2011-301073. [DOI] [PubMed] [Google Scholar]
- 5Zhang Z, Zhang JY, Wang LF, Wang FS. Immunopathogenesis and prognostic immune markers of chronic hepatitis B virus infection. J Gastroenterol Hepatology 2012; 27: 223–230. [DOI] [PubMed] [Google Scholar]
- 6Lo CM, Fung JT, Lau GK et al. Development of antibody to hepatitis B surface antigen after liver transplantation for chronic hepatitis B. Hepatology 2003; 37: 36–43. [DOI] [PubMed] [Google Scholar]
- 7Luo Y, Lo CM, Cheung CK, Lau GK, Fan ST, Wong J. Identification of hepatitis B virus-specific lymphocytes in human liver grafts from HBV-immune donors. Liver Transpl 2007; 13: 71–79. [DOI] [PubMed] [Google Scholar]
- 8Dahmen U, Li J, Dirsch O et al. Adoptive transfer of donor-derived immunity by liver transplantation: a potential avenue to prevent hepatitis B virus reinfection. J Viral Hepat 2003; 10: 31–36. [DOI] [PubMed] [Google Scholar]
- 9Schumann A, Lindemann M, Valentin-Gamazo C et al. Adoptive immune transfer of hepatitis B virus specific immunity from immunized living liver donors to liver recipients. Transplantation 2009; 87: 103–111. [DOI] [PubMed] [Google Scholar]
- 10Dervite I, Hober D, Morel P. Acute hepatitis B in a patient with antibodies to hepatitis B surface antigen who was receiving rituximab. N Engl J Med 2001; 344: 68–69. [DOI] [PubMed] [Google Scholar]
- 11Westhoff TH, Jochimsen F, Schmittel A et al. Fatal hepatitis B virus reactivation by an escape mutant following rituximab therapy. Blood 2003; 102: 1930. [DOI] [PubMed] [Google Scholar]
- 12Niscola P, Del Principe MI, Maurillo L et al. Fulminant B hepatitis in a surface antigen-negative patient with B-cell chronic lymphocytic leukaemia after rituximab therapy. Leukemia 2005; 19: 1840–1841. [DOI] [PubMed] [Google Scholar]
- 13Yeo W, Chan TC, Leung NW et al. Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol 2009; 27: 605–611. [DOI] [PubMed] [Google Scholar]
- 14Maruyama T, McLachlan A, Iino S, Koike K, Kurokawa K, Milich DR. The serology of chronic hepatitis B infection revisited. J Clin Invest 1993; 91: 2586–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15Bocher WO, Herzog-Hauff S, Herr W et al. Regulation of the neutralizing anti-hepatitis B surface (HBs) antibody response in vitro in HBs vaccine recipients and patients with acute or chronic hepatitis B virus (HBV) infection. Clin Exp Immunol 1996; 105: 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16Oliviero B, Cerino A, Varchetta S et al. Enhanced B-cell differentiation and reduced proliferative capacity in chronic hepatitis C and chronic hepatitis B virus infections. J Hepatol 2011; 55: 53–60. [DOI] [PubMed] [Google Scholar]
- 17Dusheiko GM, Hoofnagle JH, Cooksley WG, James SP, Jones EA. Synthesis of antibodies to hepatitis B virus by cultured lymphocytes from chronic hepatitis B surface antigen carriers. J Clin Invest 1983; 71: 1104–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Barnaba V, Valesini G, Levrero M et al. Immunoregulation of the in vitro anti-HBs antibody synthesis in chronic HBsAg carriers and in recently boosted anti-hepatitis B vaccine recipients. Clin Exp Immunol 1985; 60: 259–266. [PMC free article] [PubMed] [Google Scholar]
- 19Barnaba V, Levrero M, Ruberti G et al. In vitro anti-HBs antibody synthesis from anti-hepatitis B vaccine recipients. Clin Exp Immunol 1987; 70: 283–288. [PMC free article] [PubMed] [Google Scholar]
- 20Zhang Z, Xu X, Lu J et al. B and T lymphocyte attenuator down-regulation by HIV-1 depends on type I interferon and contributes to T-cell hyperactivation. J Infect Dis 2011; 203: 1668–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21Moir S, Fauci AS. Pathogenic mechanisms of B-lymphocyte dysfunction in HIV disease. J Allergy Clin Immunol 2008; 122: 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22Moir S, Fauci AS. B cells in HIV infection and disease. Nat Rev Immunol 2009; 9: 235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23Chu CM, Sheen IS, Yeh CT, Hsieh SY, Tsai SL, Liaw YF. Serum levels of interferon-alpha and -gamma in acute and chronic hepatitis B virus infection. Dig Dis Sci 1995; 40: 2107–2112. [DOI] [PubMed] [Google Scholar]
- 24Gora-Gebka M, Liberek A, Szydlowska-Lysiak W, Bako W, Korzon M. Serum interleukin 6 and interleukin 12 levels in children with chronic hepatitis HBV treated with interferon alpha. Ann Hepatol 2003; 2: 92–97. [PubMed] [Google Scholar]
- 25Zhang Z, Zou ZS, Fu JL et al. Severe dendritic cell perturbation is actively involved in the pathogenesis of acute-on-chronic hepatitis B liver failure. J Hepatol 2008; 49: 396–406. [DOI] [PubMed] [Google Scholar]
- 26Dustin LB, Rice CM. Flying under the radar: the immunobiology of hepatitis C. Annu Rev Immunol 2007; 25: 71–99. [DOI] [PubMed] [Google Scholar]
- 27Moir S, Ho J, Malaspina A et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med 2008; 205: 1797–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28Wu C, Liu Y, Zhao Q et al. Soluble CD40 ligand-activated human peripheral B cells as surrogated antigen presenting cells: a preliminary approach for anti-HBV immunotherapy. Virol J 2010; 7: 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol 1995; 13: 29–60. [DOI] [PubMed] [Google Scholar]
- 30Maruyama T, Iino S, Koike K, Yasuda K, Milich DR. Serology of acute exacerbation in chronic hepatitis B virus infection. Gastroenterology 1993; 105: 1141–1151. [DOI] [PubMed] [Google Scholar]
- 31Chang JJ, Wightman F, Bartholomeusz A et al. Reduced hepatitis B virus (HBV)-specific CD4+ T-cell responses in human immunodeficiency virus type 1-HBV-coinfected individuals receiving HBV-active antiretroviral therapy. J Virol 2005; 79: 3038–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32Maini MK, Boni C, Lee CK et al. The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J Exp Med 2000; 191: 1269–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33Boni C, Penna A, Ogg GS et al. Lamivudine treatment can overcome cytotoxic T-cell hyporesponsiveness in chronic hepatitis B: new perspectives for immune therapy. Hepatology 2001; 33: 963–971. [DOI] [PubMed] [Google Scholar]
- 34Chen L, Zhang Z, Chen W et al. B7-H1 up-regulation on myeloid dendritic cells significantly suppresses T cell immune function in patients with chronic hepatitis B. J Immunol 2007; 178: 6634–6641. [DOI] [PubMed] [Google Scholar]
- 35Jaroszewicz J, Calle SB, Wursthorn K et al. Hepatitis B surface antigen (HBsAg) levels in the natural history of hepatitis B virus (HBV)-infection: a European perspective. J Hepatol 2010; 52: 514–522. [DOI] [PubMed] [Google Scholar]
- 36Nguyen T, Thompson AJ, Bowden S et al. Hepatitis B surface antigen levels during the natural history of chronic hepatitis B: a perspective on Asia. J Hepatol 2010; 52: 508–513. [DOI] [PubMed] [Google Scholar]