Abstract
BACKGROUND
The purpose of this study was to determine the efficacy of a clinician referral and exercise program in improving exercise levels and quality of life for men with prostate cancer.
METHODS
This was a multicenter cluster randomized controlled trial in Melbourne, Australia comprising 15 clinicians: 8 clinicians were randomized to refer eligible participants (n = 54) to a 12-week exercise program comprising 2 supervised gym sessions and 1 home-based session per week, and 7 clinicians were randomized to follow usual care (n = 93). The primary outcome was self-reported physical activity; the secondary outcomes were quality of life, anxiety, and symptoms of depression.
RESULTS
A significant intervention effect was observed for vigorous-intensity exercise (effect size: Cohen's d, 0.46; 95% confidence interval [CI], 0.09-0.82; P = .010) but not for combined moderate and vigorous exercise levels (effect size: d, 0.08; 95% CI, −0.28 to 0.45; P = .48). Significant intervention effects were also observed for meeting exercise guidelines (≥150 min/wk; odds ratio, 3.9; 95% CI, 1.9-7.8; P = .002); positive intervention effects were observed in the intervention group for cognitive functioning (effect size: d, 0.34; 95% CI, −0.02 to 0.70; P = .06) and depression symptoms (effect size: d, −0.35; 95% CI, −0.71 to 0.02; P = .06). Eighty percent of participants reported that the clinician's referral influenced their decision to participate in the exercise program.
CONCLUSIONS
The clinician referral and 12-week exercise program significantly improved vigorous exercise levels and had a positive impact on mental health outcomes for men living with prostate cancer. Further research is needed to determine the sustainability of the exercise program and its generalizability to other cancer populations. Cancer 2015;121:2646–2654. © 2015 American Cancer Society.
Keywords: clinician referral, exercise program, physical activity, prostate cancer, quality of life
INTRODUCTION
Prostate cancer is the most commonly diagnosed male cancer in developed countries (with nonmelanoma skin cancer excluded)1 and accounts for approximately 30% of cancers diagnosed each year in Australian men.2
Across the disease trajectory, men living with prostate cancer have complex physical and psychological needs and subsequent long-term morbidity, with reduced functional capacity, decreased symptom control, and poorer quality-of-life outcomes.3–7
Exercise can have a positive impact on clinical outcomes in men with prostate cancer and has been shown to improve physiological and psychological outcomes by reducing treatment-related toxicities, preventing secondary comorbidities, enhancing functional capacity, and improving an individual's quality of life.8–13 However, many men with prostate cancer do not participate in regular exercise. Increasing age, comorbid conditions, cancer stage, side effects of treatment, and limited access to targeted programs and facilities have been identified as factors that influence participation in exercise for men living with the condition.8–12 Furthermore, men lack the confidence in their ability to be physically active and do not have knowledge about appropriate exercise levels.12,14,15
Addressing barriers to exercise participation requires a multipronged approach. One strategy is for clinicians to encourage exercise as part of patients' clinical care through a referral to an exercise program that is tailored to individual capabilities. Research has highlighted the importance of cancer clinician endorsement of physical activity recommendations to improving physical activity levels in men with prostate cancer,12 with cancer survivors increasingly looking for lifestyle advice, such as exercise, to improve their health outcomes.14 A recent study of men with prostate cancer suggested that encouragement from clinicians and referral to an exercise specialist were likely to give men more confidence to participate in physical activity.12 However, the effectiveness of this approach has not been tested because exercise programs do not form part of standard clinical care after a cancer diagnosis.16 Few clinicians view their role as health promotion advocates, with only 20% promoting lifestyle changes to their patients.17 A recent survey of clinicians who treated men with prostate cancer showed that more than half of clinicians (55%) reported that advising patients on physical activity was not part of their role, with “not enough time” and a “lack of knowledge/resources” being the most common barriers to promoting physical activity.18
The development of clinical partnerships and referral pathways with allied health personnel, such as exercise physiologists, could overcome the barriers experienced by clinicians. Such referrals would allow men with prostate cancer to receive an appropriate exercise dose to optimize therapeutic outcomes while receiving endorsement from their trusted treating clinicians.7
Study Aims and Hypotheses
The primary aim of this study was to assess the efficacy of a clinician-referred 12-week exercise program in comparison with usual care in increasing self-reported physical activity levels for men who had completed active treatment for prostate cancer. Secondary aims examined the effect of the exercise program on psychological well-being, quality of life, and objectively assessed physical activity. We hypothesized that participants in the intervention would be more physically active and have improved quality of life in comparison with participants in the control condition.
MATERIALS AND METHODS
Study Design
The study was a 2-armed, prospective, multicenter cluster randomized controlled trial evaluating the effects of a medical or nursing clinician referral to a 12-week exercise program (comprising 2 gym sessions and 1 home-based session per week and commencing 3 to 12 months after active treatment for prostate cancer) in comparison with usual care. The study was approved by the institutional research ethics committee. A detailed description of the study methods is available in the published protocol.19
Random Assignment
Fifteen clinicians (71%) who agreed to participate in the study were randomly allocated with an online random number generator to either the intervention (n = 8) or the control condition (n = 7). Clinicians remained in the 1 randomized condition for the entire recruitment period. Intervention clinicians were trained in the referral process, which comprised a standard script to follow and a referral slip19 to the supervised exercise program, which was handed to the participant during the consultation, as part of the standardized procedure.
Participant Recruitment
Recruitment occurred through urology and radiation oncology outpatient clinics across 3 major public health services and 4 private clinics located across Melbourne, Australia. Men were eligible to participate if they had completed active treatment for prostate cancer within the previous 3 to 12 months, were treated with curative intent, and could complete surveys in the English language. Men on hormone treatment were also eligible to take part in the study. Before a patient's consultation with a clinician at an outpatient clinic, eligible patients were informed about the study, provided with an information package, and followed up with respect to their interest in participating in the study. Those who were interested were asked to sign and return the consent form and baseline questionnaire before their participation in the study.
During the consultation, clinicians randomized to the intervention condition provided a standardized referral slip,19 which stated that the participant had been assigned to the exercise group and that the clinician recommended that they participate in the exercise program. Clinicians randomized into the control condition provided usual care regarding physical activity advice, which was typically minimal information. Clinician adherence to the study process was monitored each week.
The supervised exercise program was undertaken at each participant's local community gym and was supervised by exercise physiologists. The 12-week exercise program was based on exercise guidelines for cancer survivors developed by the American College of Sports Medicine20 and Exercise and Sport Science Australia21 and was guided by social cognitive theory.22 Postgraduate clinical exercise physiology students under the supervision of accredited exercise physiologists instructed participants during 2 supervised, 50-minute sessions per week at local gym facilities, and they advised participants on a weekly home-based session.
The primary outcome was the number of self-reported minutes of moderate-vigorous physical activity (MVPA) per week as measured with an adapted Godin-Shepherd Leisure Time Exercise Questionnaire, which was administered to assess participation in physical activity.23 Participants were asked to recall their average weekly frequency and duration of light-intensity activity (minimal effort, no perspiration), moderate-intensity activity (not exhausting, light perspiration), and vigorous-intensity activity (rapidly beating heart, sweating) in a typical week in the past month. We also calculated the percentage of participants meeting aerobic exercise guidelines of combined MVPA levels ≥ 150 min/wk and the number who reported zero MVPA levels.
The secondary outcomes included an objective assessment of physical activity as well as self-reported depressive symptoms, anxiety, and quality of life. All participants were asked to wear a hip-mounted accelerometer (GT1M; ActiGraph, Pensacola, FL) for 7 days at the baseline and immediately after the 12-week exercise program (equivalent time period for the control group) to obtain a quantitative measure of light-intensity activity and MVPA on an average day according to the Freedson adult cut points.24 Accelerometry data were processed with ActiLife software (version 6.7.1) and managed with a customized Excel macro. Minutes of daily light-intensity activity and MVPA were calculated by the summation of valid days (10 hours of wear time per day, with the removal of 60 minutes of consecutive zero counts), weighted by week and weekend days and divided by 7. Quality of life was measured with the European Organization for Research and Treatment of Cancer core quality-of-life questionnaire (QLQ-C30, version 3) and prostate tumor–specific module (QLQ-PR25).25 Prostate cancer–related anxiety was measured with the Memorial Anxiety Scale for Prostate Cancer (MAX-PC).26 Depressive symptoms were assessed with the 20-item Center for Epidemiological Studies Depression Inventory, with mean and total scores calculated.27
A satisfaction survey (intervention group) was conducted to assess the acceptability and feasibility of the clinician referral and exercise program. Participants were asked a series of questions about how much the clinician referral influenced their decision to participate in the 12-week program and their experience with the 12-week program, including whether they met their exercise goals, whether the gym location was convenient, and whether they intended to continue the exercise program after the study was completed.
Data Analysis
t tests and chi-square tests were used to compare baseline characteristics between the intervention and control groups. To assess the impact of the intervention on primary and secondary outcomes, time-by-treatment interactions were analyzed in a repeated measures split plot in time analysis of variance model for continuous outcomes. Post hoc contrasts were also conducted to determine follow-up by intervention impact. Repeated measures logistic regression was used for binary outcomes to evaluate time-by-treatment interactions. In both scenarios, model parameters were estimated with generalized estimating equations with an exchangeable working correlation matrix to take account of the repeated measures for each participant. Effect sizes (follow-up by intervention effect) were calculated with Cohen's d. Odds ratios were transformed into effect sizes with a logarithmic transformation.28 We performed an intention-to-treat analysis with a linear mixed model approach. Tests were 2-tailed with the statistical significance set at an α level of .05. The analyses were undertaken with STATA.
RESULTS
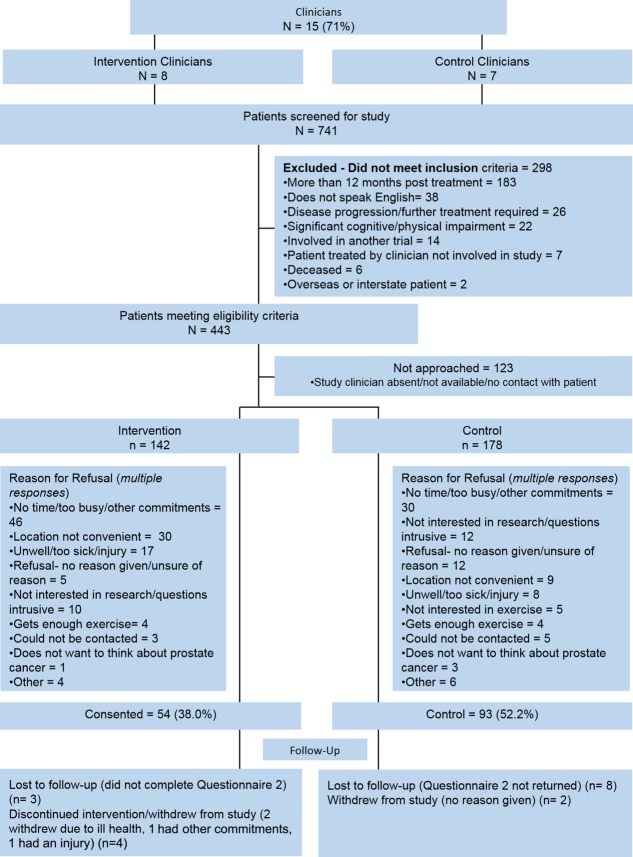
A consecutive series of men who completed active treatment for prostate cancer was screened from October 2011 to June 2013. In all, 741 patients were screened, and 443 (60%) met the eligibility criteria; 320 of these patients (72%) were approached, and 147 (46%) participated (Fig. 1). Participant compliance with questionnaire completion was very high; 95% completed each of the 2 time points, with minimal missing data for the primary and secondary outcomes (<1%). The overall attrition rate was 12% (control group, 11%; intervention group, 13%; P = .70).
Figure 1.
Consort diagram showing recruitment of patients into the ENGAGE study.
Participants ranged in age from 39 to 84 years (mean, 65.6 years; standard deviation, 8.5 years); the majority had stage I or II disease. At baseline, there were no significant differences in patient demographics or exercise levels. A statistically significant difference was observed, however, in stage of disease and treatment regimens between the 2 groups (Table 1).
TABLE 1.
Demographic and Clinical Characteristics of Participants in the ENGAGE Trial at Baseline by Condition
| Characteristics | Control | Intervention | P |
|---|---|---|---|
| Demographics | |||
| Age, mean (SD), years | 64.7 (8.7) | 66.9 (8.2) | .69 |
| Body mass index, mean (SD), kg/m2 | 28.0 (3.8) | 28.0 (3.5) | .97 |
| Marital status, No. (%) | .31 | ||
| Married/living with a partner | 69 (78.4) | 45 (83.3) | |
| Not married/living with a partner | 19 (21.6) | 9 (16.7) | |
| Education status, No. (%) | .85 | ||
| Primary | 6 (6.7) | 3 (5.7) | |
| Secondary | 31 (34.4) | 15 (28.3) | |
| Certificate/diploma | 30 (33.3) | 19 (35.9) | |
| University | 23 (25.6) | 16 (30.1) | |
| Private health service | 25 (26.9) | 14 (25.9) | .90 |
| MVPA combined, mean (SD), min/wk | 104.3 (18.4) | 167.7 (41.0) | .11 |
| VPA, mean (SD), min/wk | 25.7 (11.5) | 33.3 (11.7) | .66 |
| Moderate physical activity, mean (SD), min/wk | 78.6 (12.2) | 134.4 (34.6) | .08 |
| Exercising ≥150 min/wk, No. (%) | 15 (28.6) | 33 (35.8) | .36 |
| Inactive participants (0 min/wk of MVPA), No. (%) | 44 (48.4) | 22 (41.5) | .43 |
| Clinical data | |||
| Staging of disease, No. (%) | .01 | ||
| I | 28 (35) | 20 (48) | |
| II | 42 (52.5) | 11 (26) | |
| III | 10 (12.5) | 11 (26) | |
| Weeks since active treatment, mean (SD) | 25 (9.19) | 25 (11.39) | .89 |
| Treatment regimen, No. (%) | .03 | ||
| Surgery only | 46 (49.5) | 18 (33.3) | |
| Surgery and radiotherapy | 16 (17.2) | 15 (27.8) | |
| Surgery, radiotherapy, and ADT | 5 (5.4) | 3 (5.5) | |
| Radiotherapy only | 16 (17.2) | 5 (9.3) | |
| Radiotherapy and ADT | 8 (8.6) | 13 (24.1) | |
| Surgery and ADT | 2 (2.1) | 0 (0) |
Abbreviations: ADT, androgen-deprivation therapy; MVPA, moderate-vigorous physical activity; SD, standard deviation; VPA, vigorous physical activity.
For those who completed the supervised program, 85% of participants adhered to at least 18 of the 24 gym-based sessions (median, 88%; interquartile range, 17%). Among those who completed their home exercise diary (40 of 54 or 74%), an average of 81% (median, 88%; interquartile range, 17%) completed 9 to 12 of the prescribed home-based weekly sessions.
There were no significant intervention effects on MVPA (intervention impact, 33 minutes; effect size: d, 0.08; 95% confidence interval [CI], −0.28 to 0.45; P = .48; Table 2 and Fig. 2); however, significant intervention effects were observed for vigorous exercise (intervention impact: 45 minutes; effect size: d, 0.46; 95% CI, 0.09-0.82; P = .010). Significant intervention effects were observed for the percentage meeting aerobic exercise guidelines of combined MVPA levels ≥ 150 min/wk (odds ratio, 3.9; 95% CI, 1.9-7.8; P = .002; effect size: d, 0.75; 95% CI, 0.35-1.13; Table 3 and Fig. 2). At baseline, 48% of participants in the control condition (minimum, 0 mins; maximum, 1260 mins) and 41.5% in the intervention group (minimum, 0 mins; maximum, 1620 mins) reported zero MVPA (P = .43). At follow-up, 3 participants (6%) reported zero MVPA in the intervention group (minimum, 0 mins; maximum, 1500 mins), whereas 30 (32%) did in the control condition (minimum, 0 mins; maximum, 1560 mins; P = .0001; odds ratio, 4.8; 95% CI, 1.60-14.20).
TABLE 2.
Primary Outcome Measures (Self-Reported Physical Activity) for Intervention and Control Participants at Baseline and the 12-Week Follow-Up
| Physical Activity, Mean (SD), min/wk | Effect Size: Cohen's d (95% CI) | ||||||
|---|---|---|---|---|---|---|---|
| Control | Intervention | Effect Measure | |||||
| Baseline | Follow-Up | Baseline | Follow-Up | Follow-Up by Intervention P | Follow-Up by Intervention Effect (95% CI)a | ||
| Moderate-vigorous activity combined | 104 (176) | 153 (237) | 168 (298) | 252 (261.7) | .48 | 33 (−59 to 126) | 0.08 (−0.28 to 0.45) |
| Vigorous-intensity activity | 26 (110) | 42 (151) | 33 (85) | 94 (117) | .01 | 45 (11-79) | 0.46 (0.09-0.82) |
| Moderate-intensity activity | 79 (116) | 111 (169) | 134 (252) | 162 (254) | .90 | −6 (−94 to 82) | −0.03 (−0.40 to 0.33) |
Abbreviations: CI, confidence interval; SD, standard deviation.
Post hoc intervention by follow-up interaction contrast.
Figure 2.
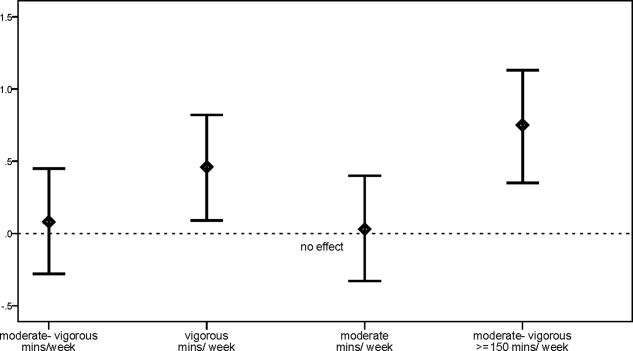
Effect sizes (95% confidence intervals) of physical activity levels for minutes of physical activity per week and for physical activity ≥ 150 min/wk.
TABLE 3.
Secondary Outcome Measures for Intervention and Control Participants at Baseline and the 12-Week Follow-Up
| Control | Intervention | Effect Measure | Effect Size: Cohen's d (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Baseline n = 91 | Follow-Up n = 83 | Baseline n = 53 | Follow-Up n = 46 | Follow-Up by Intervention P | Follow-Up by Intervention OR (95% CI)a | ||
| MVPA ≥ 150 min/wk, % | 28.6 | 43.4 | 35.8 | 69.6 | .002 | 3.9 (1.9-7.8) | 0.75 (0.35-1.13) |
| Control | Intervention | Effect Size: Cohen's d (95% CI) | |||||
| Baseline | Follow-Up | Baseline | Follow-Up | Follow-Up by Intervention P | Intervention Interaction Impact Effect (95% CI) | ||
| Accelerometer, mean (SD), min/day | n = 48 | n = 39 | n = 44 | n = 31 | |||
| MVPA | 38 (22) | 36 (23) | 40 (20.9) | 33 (16.5) | .31 | −4.5 (−13.1 to 4.2) | 0.17 (−0.33 to 0.67) |
| Vigorous intensity | 2 (12) | 0.6 (2) | 1 (4) | 1.5 (4) | .38 | −1.8 (−2.13 to 5.67) | −0.18 (−0.68 to 0.32) |
| Moderate intensity | 36 (19) | 36 (22) | 39 (20) | 31 (16) | .11 | 5.9 (−13.3 to 1.4) | 0.33 (−0.17 to 0.83) |
| Quality of life (EORTC-30), mean, SD | n = 91 | n = 83 | n = 53 | n = 47 | |||
| Physical | 91.9 (11.4) | 94.3 (10.2) | 91.6 (10.6) | 94.5 (9.2) | .83 | 0.3 (−2.3 to 2.9) | 0.02 (−0.3 to 0.4) |
| Cognitive | 85.2 (17.5) | 86.7 (16.8) | 83.3 (17.9) | 89.0 (16.8) | .06 | 4.0 (−0.2 to 8.2) | 0.34 (−0.02 to 0.7) |
| Emotional | 83.0 (21.3) | 88.4 (17.4) | 86.5 (17.8) | 88.7 (15.6) | .14 | −4.03 (−9.40 to 1.33) | −0.3 (−0.67 to 0.06) |
| Social | 85.0 (24.2) | 90.0 (19.3) | 84.3 (21.0) | 92.9 (13.3) | .45 | 3.1 (−4.9 to 11.2) | 0.08 (−0.3 to 0.4) |
| Role Functioning | 89.7 (22.9) | 93.0 (17.5) | 88.7 (19.3) | 94.7 (13.1) | .65 | 1.6 (−5.2 to 8.4) | 0.02 (−0.3 to 0.4) |
| Global | 77.5 (16.0) | 80.0 (15.9) | 75.9 (17.4) | 80.3 (14.7) | .37 | 2.2 (−2.6 to 6.9) | 0.2 (−0.2 to 0.5) |
| Anxiety (MAX-PC), mean (SD) | n = 91 | n = 82 | n = 53 | n = 47 | |||
| Total score | 12.7 (11.1) | 9.8 (9.6) | 9.1 (8.3) | 8.7 (8.8) | .02 | 2.48 (0.37-4.58) | 0.42 (0.06-0.79) |
| Prostate cancer anxiety (subscale 1) | 7.9 (8.6) | 5.9 (7.4) | 5.5 (6.3) | 3.9 (6.3) | .64 | 0.40 (−1.29 to 2.10) | 0.08 (−0.28 to 0.44) |
| PSA anxiety (subscale 2) | 0.6 (1.2) | 0.2 (0.7) | 0.1 (0.6) | 0.2 (1.2) | .03 | 0.43 (0.04-0.83) | 0.38 (−0.01 to 0.74) |
| Fear of recurrence anxiety (subscale 3) | 4.2 (3.3) | 3.8 (3.3) | 3.5 (3.1) | 4.5 (4.2) | .01 | 1.6 (0.27-2.93) | 0.45 (0.08-0.81) |
| Depression (CES-D), mean (SD) | n = 91 | n = 83 | n = 53 | n = 47 | |||
| Depression symptoms | 8.5 (7.0) | 7.6 (6.9) | 8.9 (9.3) | 6.5 (7.6) | .06 | −1.83 (−3.79 to 0.13) | −0.35 (−0.71 to 0.02) |
Abbreviations: CES-D, Center for Epidemiological Studies Depression Inventory; CI, confidence interval; EORTC-30, European Organization for Research and Treatment of Cancer 30; MAX-PC, Memorial Anxiety Scale for Prostate Cancer; MVPA, moderate-vigorous physical activity; OR, odds ratio; PSA, prostate-specific antigen; SD, standard deviation.
aPost hoc intervention by follow-up interaction contrast.
There were no significant differences between groups in objectively (accelerometer) assessed MVPA minutes on an average day (Table 3).
Borderline intervention effects were observed for the cognitive functioning subscale of the QLQ-C30 (effect size: d, 0.34, 95% CI, −0.02 to 0.70; P = .06) and Center for Epidemiological Studies Depression Inventory depression symptoms (effect size: d, −0.35; 95% CI, −0.71 to 0.02; P = .06). There was a significant decrease in total anxiety in the control group versus the intervention group (effect size: d, 0.42; 95% CI, 0.06-0.79; P = .02).
Forty-seven participants (87%) completed the intervention evaluation. Eighty percent of those participants reported that the clinician's referral influenced their decision to participate in the exercise program; 75% reported that they would recommend the exercise program to other people living with prostate cancer; 91.5% reported that the exercise program was rewarding; 94% reported that participating in the 12-week exercise program was extremely or quite useful; 88% reported that the program was extremely or quite beneficial to their health and well-being; 83% reported that they achieved their exercise goals; 79% reported that the location of the gym was convenient; and 45% reported an intention to continue with the physical activity program by joining a gym in the coming month.
DISCUSSION
This study involved a multipronged approach to improving exercise outcomes among men living with prostate cancer that included a standardized clinician referral to a supervised exercise program. Compared with men in the control condition, men in the intervention condition undertook more than twice as much vigorous exercise at follow-up and had almost 4 times the odds of meeting exercise guidelines (≥150 min/wk of MVPA) and nearly 5 times the odds of avoiding complete inactivity (0 MVPA minutes). However, the failure of our exercise intervention to significantly improve MVPA minutes may in part be due to the variation in the 2 groups both at baseline and at follow-up, which resulted in imprecise interval estimations. For example, 48% of those in the control condition and 41.5% in the intervention group did not participate in MVPA at the baseline, and this inflated the variance; at follow-up, 32% in the control group reported zero MVPA, whereas only 6% in the intervention group.
There were no significant intervention effects based on objectively measured physical activity. This may be due to the limited ability of the device to capture activities such as swimming, biking, and strength training29 or due to behavioral compensation in total daily physical activity (as reflected by the device's ability to capture all major bodily movement in comparison with the self-report measure, which is based mainly on structured activities) or the timing of wearing the accelerometer (ie, in the week immediately before and after the intervention).
The intervention did produce positive findings for depressive symptoms and quality-of-life outcomes (specifically improved cognitive function). The importance of exercise in improving quality-of-life outcomes has been supported by recently published Cochrane reviews and meta-analyses. However, because only 12% of the participants in the current study had clinical depression (≥16) at baseline, our ability to determine a significant impact of the program on depressive symptoms was limited. Cognitive dysfunction is a common outcome for men with prostate cancer and particularly for those on androgen-deprivation therapy. Regular exercise improves cardiovascular fitness, which has a positive association with cognitive performance in older adults and a protective effect against cognitive decline.30 There are several potential mechanisms explaining these associations: vascular contributions from exercise may delay or prevent the onset of cerebrovascular disease, exercise increases the expression of synaptic plasticity genes and results in increased brain neuroplasticity, and exercise increases resilience to brain aging and neurodegeneration through increases in brain volume.31 This study highlighted the potential importance of a targeted exercise program in improving cognitive functioning in men with prostate cancer.
Results from MAX-PC are somewhat difficult to interpret. The MAX-PC anxiety scale is focused on anxiety related to prostate-specific antigen scores, risk of recurrence, and generalized anxiety and may be more suitable for observing changes over longer periods of time. The follow-up of participants at 6 or 12 months, rather than 12 weeks, may provide a better understanding of the role of exercise and its impact on anxiety.
Nearly half of the participants in the intervention group indicated their intention to continue their program by joining their local community gym; the majority reported a benefit from the targeted program. Whether the program is sustained over time will determine the potential of the program to improve the medium- and long-term health outcomes for men living with the disease.32
Exercise adherence can be a challenge in cancer trials.33 However, this was not evident in the current study. In comparison with the control arm, there was a higher representation of patients who had surgery and completed adjuvant therapies, including radiotherapy and androgen-deprivation therapy, and participated in the intervention. The program was well tolerated by participants; only 13% completed fewer than 18 sessions, and this suggested that the exercise program was acceptable to participants. Attrition was substantially lower than that for other exercise trials, which reported attrition rates ranging from 0% to 44%.33 This reduces the risk of bias in the current study. The majority of the participants found the program rewarding and reported that it was beneficial to their health and well-being and that they achieved their exercise goals; this suggests that it was feasible. Supervised programs ensure that the exercise is targeted to the individual, and this can reduce the risk of injury and improve adherence to the program; the unsupervised home-based program potentially increases long-term adoption and maintenance of physical activity as part of one's daily routine.33,34
Importantly, among those who agreed to participate, 80% reported that the clinician's referral to take part in the 12-week program influenced their decision to participate. This has important clinical implications for how clinicians may assist and facilitate men's physical activity engagement in tailored exercise programs for improved physical activity and health outcomes.35
Our trial's strengths include recruitment of a sequential cohort of men with prostate cancer across public and private outpatient clinics, clinicians providing a standardized referral to men to a supervised exercise program, high adherence rates being achieved with the 2 gym sessions and 1 home-based session per week over the 12-week period, the use of previously validated measures, and 95% participant compliance with questionnaire completion.
Limitations of the study include the fact that the average MVPA levels at the baseline were higher than those found in the clinical trial from which we derived our initial power calculations,36 and this suggests that we may have recruited a more physically active sample of participants. Response bias is difficult to overcome in a study that relies on voluntary participation. Modest recruitment and fixed-term funding constraints resulted in a sample of 147 participants rather than the original target of 200.19 A post hoc power analysis was conducted and showed that there was 80% power to detect an interaction effect (time-by-treatment group) of 80 minutes.
Differential intervention effects between self-report and accelerometer data have been reported elsewhere in other cancer cohorts.37 However, a recent systematic review that compared self-reporting, including diaries, and direct measurement results found no clear trends in the overreporting or underreporting of physical activity by self-reporting versus direct methods.38 Researchers undertaking exercise studies should consider including self-report exercise behavior as well as objective measurements.32 We achieved a 46% participation rate, which was better than, or similar to, the rates in other randomized controlled trials involving prostate cancer survivors8,10,33; however, our sample was overrepresented by men with stage I and II disease. Future research should address different disease stages and exercise capacity in these vulnerable disease groups.
Our trial highlighted that clinicians are ideally suited to provide a teachable moment17 and refer men to exercise programs as part of their clinical care after active treatment for prostate cancer with an individually tailored supervised program to improve quality-of-life outcomes. Engagement with medical and allied health professionals in the establishment of a clinician referral pathway for exercise-based programs could reduce the barriers to exercise among prostate cancer survivors. Further study is required to determine the sustainability of the exercise program and its generalizability to other disease stage groups and cancer populations and investigate whether the supervised 12 week program is better suited for men who are more sedentary.10
FUNDING SUPPORT
This study was funded by the Australian Research Council (LP100200176) and the Prostate Cancer Foundation of Australia with in-kind support from YMCA Victoria, Eastern Health, Epworth Healthcare, North Eastern Metropolitan Integrated Cancer Service, and the Peter MacCallum Cancer Centre. Jo Salmon is supported by a principal research fellowship from the National Health and Medical Research Council (APP1026216). Kerry Courneya is supported by the Canada Research Chairs Program.
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Australian Institute of Health and Welfare. Cancer Incidence Projections: Australia, 2011 to 2020. Canberra, Australia: Australian Institute of Health and Welfare; 2012. Cancer series 66. [Google Scholar]
- 3.Smith DP, King MT, Egger S, et al. Quality of life three years after diagnosis of localised prostate cancer: population based cohort study. BMJ. 2009;339:b4817. doi: 10.1136/bmj.b4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drummond FJ, Kinnear H, O'Leary E, Donnelly, Gavin A, Sharp L. Long-term health-related quality of life of prostate cancer survivors varies by primary treatment. Results from the PiCTure (Prostate Cancer Treatment, Your Experience) study. J Cancer Surviv. doi: 10.1007/s11764-014-0419-6. In press. [DOI] [PubMed] [Google Scholar]
- 5.Davis KM, Kelly SP, Luta G, Tomko C, Miller AB, Taylor KL. The association of long-term treatment-related side effects with cancer-specific and general quality of life among prostate cancer survivors. Urology. 2014;84:300–306. doi: 10.1016/j.urology.2014.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elliott J, Fallows A, Staetsky L, et al. The health and well-being of cancer survivors in the UK: findings from a population-based survey. Br J Cancer. 2011;105:S11–S20. doi: 10.1038/bjc.2011.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Betof AS, Dewhirst MW, Jones LW. Effects and potential mechanisms of exercise training on cancer progression: a translational perspective. Brain Behav Immun. 2013;30(suppl):S75–S87. doi: 10.1016/j.bbi.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mishra SI, Scherer RW, Geigle PM, et al. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst Rev. 2012;8:CD007566. doi: 10.1002/14651858.CD007566.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baumann FT, Zopf EM, Bloch W. Clinical exercise interventions in prostate cancer patients–a systematic review of randomized controlled trials. Support Care Cancer. 2012;20:221–233. doi: 10.1007/s00520-011-1271-0. [DOI] [PubMed] [Google Scholar]
- 10.Bourke L, Homer KE, Thaha MA, et al. Interventions to improve exercise behaviour in sedentary people living with and beyond cancer: a systematic review. Br J Cancer. 2014;110:831–841. doi: 10.1038/bjc.2013.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gardner JR, Livingston PM, Fraser SF. Effects of exercise on treatment-related adverse effects for patients with prostate cancer receiving androgen-deprivation therapy: a systematic review. J Clin Oncol. 2014;32:335–346. doi: 10.1200/JCO.2013.49.5523. [DOI] [PubMed] [Google Scholar]
- 12.Craike MJ, Livingston PM, Botti M. An exploratory study of the factors that influence physical activity for prostate cancer survivors. Support Care Cancer. 2011;19:1019–1028. doi: 10.1007/s00520-010-0929-3. [DOI] [PubMed] [Google Scholar]
- 13.Antonelli J, Freedland SJ, Jones LW. Exercise therapy across the prostate cancer continuum. Prostate Cancer Prostatic Dis. 2009;12:110–115. doi: 10.1038/pcan.2009.4. [DOI] [PubMed] [Google Scholar]
- 14.Chipperfield K, Fletcher J, Millar J, et al. Factors associated with adherence to physical activity guidelines in patients with prostate cancer. Psychooncology. 2013;22:2478–2486. doi: 10.1002/pon.3310. [DOI] [PubMed] [Google Scholar]
- 15.Rogers LQ, Courneya KS, Gururaja RP, Markwell SJ, Imeokparia R. Lifestyle behaviors, obesity, and perceived health among men with and without a diagnosis of prostate cancer: a population-based, cross-sectional study. BMC Publ Health. 2008;8:23. doi: 10.1186/1471-2458-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lakoski SG, Eves ND, Douglas PS, Jones LW. Exercise rehabilitation in patients with cancer. Nat Rev Clin Oncol. 2012;9:288–296. doi: 10.1038/nrclinonc.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demark-Wahnefried W, Aziz NM, Rowland JH, Pinto BM. Riding the crest of the teachable moment: promoting long-term health after the diagnosis of cancer. J Clin Oncol. 2005;23:5814–5830. doi: 10.1200/JCO.2005.01.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spellman C, Craike M, Livingston PM. Knowledge, attitudes and practices (KAP) of clinicians in promoting physical activity to prostate cancer survivors. Health Educ J. 2014;73:566–575. [Google Scholar]
- 19.Livingston PM, Salmon J, Courneya KS, et al. Efficacy of a referral and physical activity program for survivors of prostate cancer [ENGAGE]: rationale and design for a cluster randomised controlled trial. BMC Cancer. 2011;11:237. doi: 10.1186/1471-2407-11-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2009;42:1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 21.Hayes SC, Spence RR, Galvao DA, Newton RU. Australian Association for Exercise and Sport Science position stand: optimising cancer outcomes through exercise. J Sci Med Sport. 2009;12:428–434. doi: 10.1016/j.jsams.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice-Hall; 1986. [Google Scholar]
- 23.Godin G, Shephard RJ. A simple method to assess exercise behaviour in the community. Can J Appl Sport Sci. 1985;10:141–146. [PubMed] [Google Scholar]
- 24.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30:777–781. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 25.Borghede G, Sullivan M. Measurement of quality of life in localized prostatic cancer patients treated with radiotherapy. Development of a prostate cancer-specific module supplementing the EORTC QLQ-C30. Qual Life Res. 1996;5:212–222. doi: 10.1007/BF00434743. [DOI] [PubMed] [Google Scholar]
- 26.Roth AJ, Nelson CJ, Rosenfeld B, et al. Assessing anxiety in men with prostate cancer: further data on the reliability and validity of the Memorial Anxiety Scale for Prostate Cancer (MAX-PC) Psychosomatics. 2006;47:340–347. doi: 10.1176/appi.psy.47.4.340. [DOI] [PubMed] [Google Scholar]
- 27.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 28.Chinn S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat Med. 2000;19:3127–3131. doi: 10.1002/1097-0258(20001130)19:22<3127::aid-sim784>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 29.Trost SG, McIver KL, Pate RR. Conducting accelerometer-based activity assessments in field-based research. Med Sci Sports Exerc. 2005;37(suppl):S531–S543. doi: 10.1249/01.mss.0000185657.86065.98. [DOI] [PubMed] [Google Scholar]
- 30.Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2008;3:CD005381. doi: 10.1002/14651858.CD005381.pub3. [DOI] [PubMed] [Google Scholar]
- 31.Ahlskog JE, Geda YE, Graff-Radford NR, Petersen RC. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc. 2011;86:876–884. doi: 10.4065/mcp.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newton RU, Galvao DA. Exercise in prevention and management of cancer. Curr Treat Options Oncol. 2008;9:135–146. doi: 10.1007/s11864-008-0065-1. [DOI] [PubMed] [Google Scholar]
- 33.Bourke L, Homer KE, Thaha MA, et al. Interventions for promoting habitual exercise in people living with and beyond cancer. Cochrane Database Syst Rev. 2013;9:CD010192. doi: 10.1002/14651858.CD010192.pub2. [DOI] [PubMed] [Google Scholar]
- 34.Cheifetz O, Park Dorsay J, Hladysh G, Macdermid J, Serediuk F, Woodhouse LJ. CanWell: meeting the psychosocial and exercise needs of cancer survivors by translating evidence into practice. Psychooncology. 2014;23:204–215. doi: 10.1002/pon.3389. [DOI] [PubMed] [Google Scholar]
- 35.Keogh JWL, Patel A, Macleod RD, Masters J. Perceived barriers and facilitators to physical activity in men with prostate cancer: possible influence of androgen deprivation therapy. Eur J Cancer Care (Engl) 2014;23:263–273. doi: 10.1111/ecc.12141. [DOI] [PubMed] [Google Scholar]
- 36.Demark-Wahnefried W, Clipp EC, Lipkus IM, et al. Main outcomes of the FRESH START trial: a sequentially tailored, diet and exercise mailed print intervention among breast and prostate cancer survivors. J Clin Oncol. 2007;25:2709–2718. doi: 10.1200/JCO.2007.10.7094. [DOI] [PubMed] [Google Scholar]
- 37.Boyle T, Lynch BM, Courneya KS, Vallance JK. Agreement between accelerometer-assessed and self-reported physical activity and sedentary time in colon cancer survivors. Support Care Cancer. 2015;23:1121–1126. doi: 10.1007/s00520-014-2453-3. [DOI] [PubMed] [Google Scholar]
- 38.Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56. doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]