Abstract
Microvascular invasion (MVI) in hepatocellular carcinoma (HCC) is an independent predictor of poor outcomes subsequent to surgical resection or liver transplantation (LT); however, MVI currently cannot be adequately determined preoperatively. Radiogenomic venous invasion (RVI) is a contrast-enhanced computed tomography (CECT) biomarker of MVI derived from a 91-gene HCC “venous invasion” gene expression signature. Preoperative CECTs of 157 HCC patients who underwent surgical resection (N = 72) or LT (N = 85) between 2000 and 2009 at three institutions were evaluated for the presence or absence of RVI. RVI was assessed for its ability to predict MVI and outcomes. Interobserver agreement for scoring RVI was substantial among five radiologists (κ = 0.705; P < 0.001). The diagnostic accuracy, sensitivity, and specificity of RVI in predicting MVI was 89%, 76%, and 94%, respectively. Positive RVI score was associated with lower overall survival (OS) than negative RVI score in the overall cohort (P < 0.001; 48 vs. >147 months), American Joint Committee on Cancer tumor-node-metastasis stage II (P < 0.001; 34 vs. >147 months), and in LT patients within Milan criteria (P < 0.001; 69 vs. >147 months). Positive RVI score also portended lower recurrence-free survival at 3 years versus negative RVI score (P = 0.001; 27% vs. 62%). Conclusion: RVI is a noninvasive radiogenomic biomarker that accurately predicts histological MVI in HCC surgical candidates. Its presence on preoperative CECT is associated with early disease recurrence and poor OS and may be useful for identifying patients less likely to derive a durable benefit from surgical treatment. (Hepatology 2015;62:792–800)
Hepatocellular carcinoma (HCC) is the sixth-most common cancer worldwide and the third-leading cause of cancer-related deaths.1 For patients with early-stage HCC, surgical resection and liver transplantation (LT) are potentially curative.2 However, recurrence subsequent to surgical treatment is common, with 5-year rates reaching 70% after surgical resection and 35% post-LT.3–6 Given the scarcity of organs available for LT and the morbidity risks associated with both procedures, there remains a need to better select patients who will gain enduring benefit from surgical therapies. A major obstacle to improving patient selection is the absence of diagnostic tools capable of identifying biologically aggressive disease and predicting postsurgical recurrence.
Microvascular invasion (MVI) is a powerful validated, independent predictor of early recurrence and poor overall survival (OS) after surgical treatment of HCC.7–9 Currently, the diagnosis of MVI can only reliably be made by histology of explanted tissue when its clinical utility is marginal. A noninvasive test capable of accurately identifying MVI preoperatively would be of great benefit in better stratifying HCC patients for surgical management.
Recently, we reported that global HCC gene expression patterns could be reconstructed using conventional contrast-enhanced computed tomography (CECT) imaging.10 This approach represents an emerging field, known as radiogenomics, where tumor imaging features are mapped to corresponding gene expression profiles.11 Using this method, we prospectively defined a CECT imaging biomarker, termed radiogenomic venous invasion (RVI), for histological MVI. In contrast to conventional imaging features previously proposed for noninvasive diagnosis of MVI, which have been identified through imaging histopathology correlation studies,12,13 RVI was instead derived by association to a previously characterized HCC-specific “venous invasion” gene signature.14 RVI was validated by demonstrating a strong relationship with histological MVI and prediction of MVI in an independent cohort.10 Although confirmatory, this validation study was limited to a small population of surgically resected patients with brief clinical follow-up; thus, the true impact of this biomarker remains unknown.
This multicenter study was performed to assess the diagnostic accuracy and prognostic significance of the RVI biomarker in a large, multi-institutional population of surgical candidates with HCC. Our primary aim was to determine the diagnostic accuracy of RVI in predicting histology-confirmed MVI. The ability of RVI to predict OS and recurrence-free survival (RFS) were assessed as secondary endpoints. Last, clinical applicability of RVI was examined in the context of standard-of-care staging paradigms.
Patients and Methods
Study Design
This study was a prospective evaluation of an imaging biomarker, RVI, conducted on a retrospective cohort with cross-sectional and longitudinal components. RVI was derived using a radiogenomic approach outlined in Fig. 1A.10 Patients had histology-confirmed HCC treated by surgical resection or LT between 2000 and 2009 and underwent CECT within 12 months before surgery. Patients were excluded if they received locoregional therapy (i.e., ablation or transarterial chemoembolization) before the time of imaging owing to the potential confounding effects of post-treatment changes in radiogenomic evaluation.
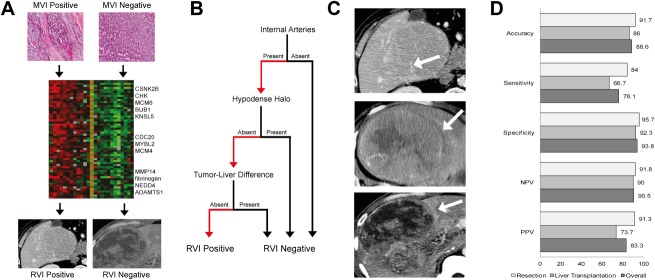
Fig. 1.
RVI biomarker. (A) Gene expression analysis of HCC tumors with histological MVI yielded a 91-gene “venous invasion” gene signature.14 Association mapping of CECT traits with this gene signature enabled derivation of the RVI radiogenomic biomarker for MVI.10 (B) RVI score is defined by a three-trait decision tree in patients with HCC. (C) Examples of the three CECT traits that compose the RVI biomarker: Top, internal arteries; middle, hypodense halo; bottom, tumor-liver difference. (D) Diagnostic performance of RVI in surgical resection, LT, and overall cohorts. Abbreviations: NPV, negative predictive value; PPV, positive predictive value.
Patients were obtained from three comprehensive cancer centers at the University of California Los Angeles (UCLA), Stanford University, and University of California San Diego (UCSD). Institutional review board approval for this study was granted for each site. Data on MVI, nuclear grade (Edmondson-Steiner system), and cirrhosis were obtained from pathology reports. Laboratory values, including alpha-fetoprotein (AFP), bilirubin, albumin, and prothrombin time, as well as clinical outcome, were obtained from medical records and the Social Security Death Index. Dates of preoperative computed tomography (CT) imaging, surgery, and locoregional therapy were also recorded. Patients were classified by the American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) staging criteria based on CT imaging and the medical history taken at the time of CT.15 LT patients were also classified by the Milan and University of California San Francisco (UCSF) staging criteria.16,17
CT Imaging
CECT scans were performed using either bi- or triphasic imaging protocols. Image acquisition parameters are detailed in the Supporting Appendix.
RVI Determination
The presence or absence of RVI on preoperative CECT was defined according to the algorithm shown in Fig. 1B. RVI consists of three separate imaging features: “Internal arteries” is the persistence of discrete arterial enhancement within the tumor in the venous phase of imaging; “hypodense halo” is a rim of hypoattenuation partially or completely circumscribing the tumor; and “tumor-liver difference” is a focal or circumferential sharp transition in attenuation between the tumor and the adjacent liver parenchyma in the absence of a hypodense halo (Fig. 1B,C; Supporting Appendix).10
RVI score was independently determined by five radiologists (C.B.S., M.D.K., D.S.W., M.G.C., and R.L.K.), blinded to the clinical and pathological data, with 13, 11, 3, 1, and 18 years of abdominal oncological imaging experience, respectively. Initial training was based on the set of cases from which the RVI biomarker was originally defined.10 Radiologists could use arterial phase images for lesion localization; however, all feature scoring was based exclusively on portal venous phase images.10 In patients with multiple tumors, RVI was assessed on the largest lesion (index tumor). Discrepancies in RVI scoring were resolved by consensus review. Diameter of the index tumor and the total number of lesions were recorded.
Statistical Analysis
Summary statistics of baseline characteristics were reported. Kruskal-Wallis’ tests were used to test differences among the three institutions in continuous and ordinal variables. Reader agreements of RVI scoring were quantified using kappa statistics. Receiver operating characteristic analyses were used to test diagnostic performance of RVI against histology of the resected tumors, which is the gold standard for the diagnosis of MVI. Diagnostic accuracy, sensitivity, and specificity of RVI were assessed in the surgical resection, LT, and overall cohorts. In the subset of patients who underwent preoperative biopsy, the diagnostic accuracy of assessing MVI from needle-core biopsy samples was separately compared to RVI. Additionally, Mann-Whitney's U tests were used to test differences in baseline characteristics based on RVI score.
Uni- and multivariate regression analyses using Cox's proportional hazard models were used to evaluate predictors of OS and RFS. OS was defined as the time from surgery to the date of death or last follow-up. RFS was defined as the time from surgery to the date of recurrence based on imaging, last follow-up, or death. Covariates included demographic characteristics and variables that were significant in univariate analysis. Kaplan-Meier's plot with log-rank tests were used to compare OS and RFS based on RVI or MVI status.
RVI was evaluated in subgroups defined by tumor size (≤3 or >3 cm), AFP values (≤20 or >20 ng/mL), AJCC-TNM stage, and Milan status.18,19 Within each subgroup, univariate Cox's proportional hazard model or Kaplan-Meier's plots with log-rank tests were used to compare OS between RVI-positive and -negative patients. In addition, interactions between RVI and tumor size or RVI and AFP were evaluated under Cox's model. No adjustments were used in multiple comparisons in exploratory analyses. Additionally, OS for RVI-positive and -negative patients were compared for LT patients who underwent bridging locoregional therapy after CT imaging. All statistical analyses were performed with STATA (v12.0; StataCorp LP, College Station, TX) and SPSS software (v22.0; IBM Corp, Armonk, NY)).
Results
Patient Characteristics
Of 278 patients, 157 met inclusion criteria (85 UCLA, 50 Stanford, and 22 UCSD). Seventy-two patients (46%) underwent surgical resection, whereas 85 (54%) underwent LT. MVI was diagnosed in explanted tissue of 45 patients (29%). Sites were similar in distribution of age, sex, index tumor size, and AJCC stage. The proportion of patients with MVI was greater at UCLA (38%) than UCSD (24%) and Stanford (18%; P = 0.05). Median times to follow-up for OS and RFS were 50 (interquartile range [IQR]: 21-68) and 42 months (IQR, 12-66), respectively. Additional patient characteristics are detailed in Table 1 and the Supporting Appendix.
Table 1.
Baseline Patient Demographic and Clinical Characteristics
| Total (N = 157) | |
|---|---|
| Median age, years (IQR) | 56 (50-64) |
| Sex (%) | |
| Male | 117(74.5) |
| Female | 40 (25.5) |
| Surgery (%) | |
| Resection* | 72 (45.9) |
| Liver Transplantation* | 85 (54.1) |
| Etiology of Liver Disease (%) | |
| HCV* | 48 (30 6) |
| HBV | 38 (24.2) |
| Alcohol | 8 (5.1) |
| Multiple | 29 (18.4) |
| Unknown | 34 (21.7) |
| Median AFP, ng/mL (IQR) | 17.4 (5-136.3) |
| Locoregional Therapy (%) | |
| Bridging* | 35 (22.3) |
| Downstaging | 6 (3.8) |
| Imaging (IQR) | |
| Median time from imaging to surgery, months | 2.3 (0.75-5.25) |
| Median tumor size, cm | 2.8 (1.8-4.5) |
| ≤ 3 cm (%) | 82 (52.2) |
| > 3 cm (%) | 75 (47.8) |
| Number of Lesions (%) | |
| Single | 120 (76.4) |
| 2 to 3 | 37 (23.5) |
| Pathology (%) | |
| Grade 1* | 33 (21) |
| Grade 2 | 40 (25.5) |
| Grade 3 | 47 (29.9) |
| Grade 4 | 13(8.3) |
| Unknown | 24 (15.3) |
| MVI† | 45 (28.7) |
| Cirrhosis | 107 (68.2) |
| Staging | |
| Median Follow-Up OS, months (IQR) | 49.6 (20.6-68.4) |
| Median Follow-Up RFS, months (IQR) | 41.9 (12.3-66.3) |
| AJCC-TNM (%) | |
| Stage 1 | 92 (58.6) |
| Stage 2 | 56 (35.6) |
| Stage 3a | 9 (5.7) |
| Child-Pugh Score (%) | |
| A | 81(51.6) |
| B | 57 (36.3) |
| C | 10(6.4) |
| Milan (%) | 78 (91.8) |
| UCSF(%) | 85(100) |
*P < 0.05 between institutions (Kruskal Wallis test for continuous scale and Chi-squared test with ties for categorical scores).
Diagnostic Accuracy of RVI in Predicting MVI
Agreement among the five radiologists for RVI scoring was substantial (κ = 0.705; 95% confidence interval [CI]: 0.635-0.775). Forty-one patients scored positive for RVI on their preoperative CECTs, whereas 116 scored RVI negative. Diagnostic assessments of RVI in predicting histological MVI status in the surgical resection, LT, and overall cohorts were 91.7%, 86.0%, and 88.6% in accuracy; 84.0%, 66.7%, and 76.1% in sensitivity; and 95.7%, 92.3%, and 93.8% in specificity, respectively (Fig. 1D). Differences in diagnostic accuracy were not statistically significant among institutions (P = 0.104) and in tumors greater or less than 3 cm (P = 0.262).
In the subset of 22 patients who underwent preoperative needle-core biopsy, identification of MVI through biopsy had a diagnostic accuracy of 63.6%, sensitivity of 12.5%, and specificity of 92.9%, whereas positive RVI had a diagnostic accuracy of 95.4%, sensitivity of 87.5%, and specificity of 100%.
Characteristics of RVI-Positive Patients
Comparison of patients based on RVI status revealed that RVI-positive patients had larger tumors (P = 0.024; 3.5 vs. 2.6 cm), higher AFP values (P = 0.002; 89.8 vs. 14.7 ng/mL), higher nuclear grade (P = 0.008; 3 vs. 2), and were less likely to have cirrhosis (P = 0.042; 57.9% vs. 75.2%). Additionally, patients with RVI had less time between imaging to surgery than patients without RVI (P = 0.001; 2.4 vs. 3.4 months). There were no differences in median age, sex, type of surgery, etiology of liver disease, number of lesions, AJCC-TNM stage, or Child-Pugh score between the two groups (Supporting Table 1).
Prognostic Factors for OS and RFS
In univariate analysis of OS, higher AFP level (hazard ratio [HR]: 1.25; P = 0.029; 95% CI: 1.02-1.54), larger index tumor size (HR, 1.11; P < 0.001; 95% CI: 1.05-1.19), higher nuclear grade (HR, 1.49; P = 0.004; 95% CI: 1.14-1.96), positive MVI (HR, 3.08; P < 0.001; 95% CI: 1.38-4.29), and positive RVI score (HR, 2.93; P < 0.001; 95% CI: 1.65-5.20) were significant factors of poor prognosis. Age (HR, 1.0; P = 0.905; 95% CI: 0.98-1.03), male gender (HR, 1.73; P = 0.157; 95% CI: 0.81-3.69), chronic hepatitis B virus (HBV) infection (HR, 0.93; P = 0.816; 95% CI: 0.52-1.68), chronic hepatitis C virus (HCV) infection (HR, 1.04; P = 0.893; 95% CI: 0.59-1.82), and multiple lesions (HR, 1.24; P = 0.567; 95% CI: 0.74-1.73) were not significant prognostic factors. On multivariate analysis of OS, larger index tumor size (HR, 1.14; P < 0.001; 95% CI: 1.07-1.22), and positive RVI score (HR, 2.74; P = 0.001; 95% CI: 1.47-5.09) were significant prognostic factors of poor survival. In multivariate analysis, MVI and RVI were evaluated separately owing to colinearity between MVI and RVI (Table 2).
Table 2.
Uni- and Multivariate Analyses of Predictors of OS
| Univariate |
Multivariate (MVI) |
Multivariate (RVI) |
||||||
|---|---|---|---|---|---|---|---|---|
| No. of Pts | Median Overall Survival (months) | P | HR (95% Cl) | P | HR (95% Cl) | P | HR (95% Cl) | |
| Age† | 157 | 89 | 0.905 | 1.0 (0.98-1.03) | 0.512 | 0.99 (0.96-1.02) | 0.513 | 0.990 (0.96-1.02) |
| Sex | ||||||||
| Male | 117 | 95 | 0.157 | 1.73 (0.81-3.69) | 0.535 | 1.36 (0.51-3.60) | 0.567 | 1.33 (0.50-3.52) |
| Female | 40 | |||||||
| HBV | ||||||||
| Yes | 55 | 147 | 0.816 | 0.93 (0.52-1.68) | ||||
| No | 102 | 105 | ||||||
| HCV | ||||||||
| Yes | 77 | 95 | 0.893 | 1.04(0.59-1.82) | ||||
| No | 80 | 147* | ||||||
| AFP† | 144 | 85 | 0.029 | 1.25(1.02-1.54) | 0.259 | 1.14(0.91-1.43) | 0.168 | 1.18(0.93-1.50) |
| Locoregional therapy | ||||||||
| Bridging | 35 | 105 | 0.338 | 0.69 (0.32-1.47) | ||||
| Downstaging | 6 | 147* | 0.873 | 1.13(0.26-4.81) | ||||
| Tumor size† | 157 | 85 | <0.001 | 1.11 (1.05-1.19) | <0.001 | 1.14(1.07-1.23) | <0.001 | 1.14(1.07-1.22) |
| No. of Lesions† | ||||||||
| Single | 120 | 105 | ||||||
| Two to three | 37 | 85 | 0.567 | 1.24 (0.74-1.73) | 0.109 | 1.53 (0.91-2.57) | 0.145 | 1.47 (0.87-2.46) |
| Nuclear grade† | 133 | 66 | 0.004 | 1.49(1.14-1.96) | 0.098 | 1.29 (0.95-1.74) | 0.043 | 1.37 (1.01-1.85) |
| MVI | ||||||||
| Yes | 45 | 63 | <0.001 | 3.08(1.38-4.29) | 0.079 | 1.81 (0.93-3.53) | ||
| No | 112 | 147* | ||||||
| RVI | ||||||||
| Yes | 41 | 48 | <0.001 | 2.93(1.65-5.20) | 0.001 | 2.74 (1.47-5.09) | ||
| No | 116 | 147* | ||||||
*Median overal survival was greater than maximum follow-up time.
†For a continous variable, median survival is listed for the subjects who had greater than the average of variable.
In multivariate analysis of 3-year RFS, larger index tumor size (HR, 1.18; P < 0.001; 95% CI: 1.09-1.28) and positive RVI score (HR, 2.74; P = 0.007; 95% CI: 1.31-5.73) were significant prognostic factors of recurrence (Supporting Table 2).
Comparison of MVI and RVI in OS and RFS
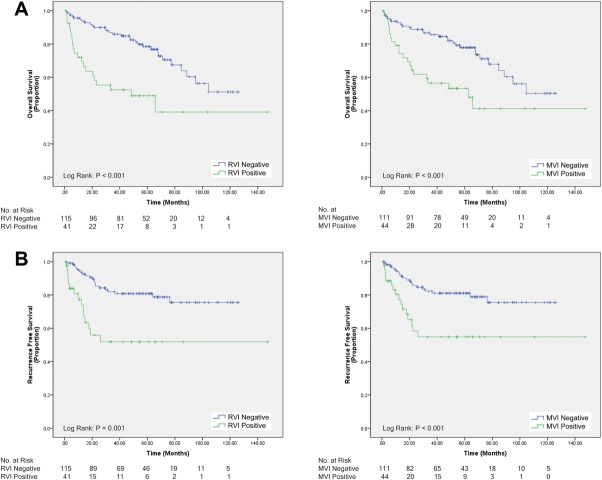
Median OS for patients with positive RVI score was less than patients with negative RVI score (P < 0.001; 48 vs. >147 months). Analogously, patients with histological MVI had lower median OS compared to those without (P < 0.001; 63 vs. >147 months; Fig. 2A). Patients with negative RVI score were more likely to be recurrence free at 3 years than those with positive RVI score (P = 0.001; 62% vs. 27%). Similarly, patients with histological MVI were more likely to be recurrence free after 3 years than those without (P < 0.001; 61% vs. 33%; Fig. 2B).
Fig. 2.
(A) OS of 157 HCC patients who underwent surgical resection or LT: left, RVI status; right, MVI status. (B) Three-year RFS of 157 HCC patients who underwent surgical resection or LT: left, RVI status; right, MVI status.
OS of RVI and MVI in Subgroup Analyses
The prognostic significance of RVI was evaluated within subgroups defined by established predictors of prognosis in HCC.20,21 In index tumor size, positive RVI score was associated with a poor prognosis in patients with tumors 3 cm or smaller in diameter (HR, 3.87; P = 0.001; 95% CI: 1.71-8.72) and in those with tumors greater than 3 cm (HR, 2.39; P = 0.035; 95% CI: 1.06-5.38). Interaction between RVI and tumor size was not significant (P = 0.49). In AFP values, positive RVI score was associated with a poor prognosis in patients with values 20 ng/mL and less (HR, 3.15; P = 0.02; 95% CI: 1.19-8.12) as well as greater than 20 ng/mL (HR, 3.71; P = 0.001; 95% CI: 1.76-8.05; Supporting Table 3). Interaction between RVI and AFP value was not significant (P = 0.69).
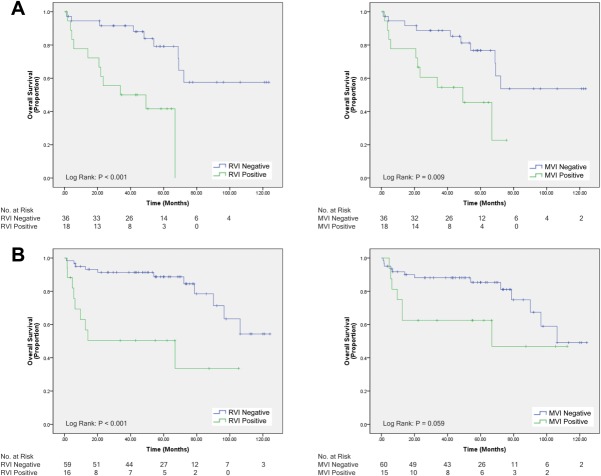
In patients classified as AJCC stage I, positive RVI score had statistically different, but clinically comparable, median OS compared to negative RVI score (P = 0.01; 105 vs. 106 months). In parallel, median OS for patients with histological MVI was also equivalent to those without (P = 0.048; 105 vs. 106 months; Supporting Fig. 1). However, in AJCC stage II, positive RVI score was associated with worse median OS, compared to negative RVI score (P < 0.001; 34 vs. >147 months), and histological MVI was associated with lower median OS than those without (P = 0.009; 49 vs. >147 months; Fig. 3A). For LT patients who were within Milan criteria, positive RVI score was associated with lower median OS, compared to negative RVI score (P < 0.001; 69 vs. >147 months). In the same group, patients with histological MVI had lower median OS than those without that approached statistical significance (P = 0.059; 67 vs. 106 months; Fig. 3B). Last, in 35 patients who underwent locoregional bridging therapy in the interval between CT imaging and LT to maintain transplant eligibility,22 positive RVI score was again associated with lower median OS, compared to negative RVI score (P < 0.001; 34 vs. 106 months; Supporting Fig. 2).
Fig. 3.
(A) OS of 56 HCC patients classified as AJCC stage 2 who underwent surgical resection or LT: left, RVI status; right, MVI status. (B) OS of 78 HCC patients within Milan criteria who underwent LT: left, RVI status; right, MVI status.
Discussion
Surgical treatment of HCC suffers from high rates of disease recurrence.3–5 One explanation is that current management paradigms do not account for histological MVI, a known predictor of disease recurrence and poor clinical outcome after surgical treatment.7,8,20 In this multicenter study, we evaluated a novel imaging biomarker derived from a gene expression signature of vascular invasion in HCC.10 We found that RVI was a strong predictor of MVI, with a diagnostic accuracy of 89%, sensitivity of 76%, and specificity of 94% in the overall cohort. Diagnostic performance was comparatively high in both surgical resection and LT subgroups. Furthermore, diagnostic performance did not significantly vary among institutions or with tumor size. These data show that RVI is a robust predictor of MVI and may have broad clinical applicability.
RVI also predicts clinical outcomes in a manner similar to MVI. We found that both positive RVI score and positive MVI were associated with poor OS and increased recurrence at 3 years. Importantly, RVI remained prognostic when the study population was classified by standard-of-care clinical staging systems, such as the AJCC-TNM and Milan criteria.15,16 We found that patients with a positive RVI score were associated with worse OS than negative RVI score in AJCC stage II and Milan patients. This was also the case in LT patients who underwent preoperative locoregional therapy to maintain transplant eligibility; those with positive RVI score had lower median OS, compared to those with negative RVI score. Our results suggest that many patients with a positive RVI score who undergo surgical resection or LT take on the risks associated with these procedures with diminished likelihood of a favorable clinical outcome.
Interestingly, in multivariate proportional hazard models, we found that RVI was an independent prognostic factor of OS and 3-year RFS whereas MVI was not. Additionally, Kaplan-Meier's analysis of LT patients within the Milan criteria showed that RVI, but not MVI, was a statistically significant factor in survival. A possible reason for this discrepancy is that RVI was derived from a gene expression profile for MVI, not histological MVI itself, and may capture a more fundamental phenotype of aggressive disease.
Numerous studies have shown that tumor size and serum AFP are predictors of poor prognosis in HCC.20,23 This was also true in our cohort, where higher AFP level was associated with poor OS whereas larger tumor size was associated with both poor OS and increased disease recurrence. Interestingly, in subgroup analyses, we found that RVI provided additional prognostic information in patients with favorable characteristics, such as tumors 3 cm or smaller and AFP values less than 20 ng/mL. We found that RVI had a lower HR in tumors greater than 3 cm, compared to those less than 3 cm, suggesting that the biomarker has less discriminating power in large tumors, which are known to be associated with poor prognosis.20 Furthermore, there was no evidence of statistical interaction between RVI and tumor size or between RVI and AFP, indicating that the magnitude of the associations between the subgroups was not different. Finding that RVI provides prognostic information in patients with small lesions and in those with clinically insignificant AFP values suggests a possible role for RVI as an early detector of aggressive disease.
Detection of MVI using preoperative biopsy has proven unreliable owing to intratumoral heterogeneity causing sampling error.24 Our results confirm the inadequacy of biopsy, where we observed a sensitivity of just over 12% in detecting MVI. Similarly, nuclear grade, which is a known independent predictor of prognosis, also relies on tissue procurement and, when assessed on preoperative biopsy specimens, is equally susceptible to sampling error.24 The same is true of more recent proteomic and genomic markers of MVI, which require invasive tissue acquisition.25,26 Previous attempts to noninvasively predict MVI have utilized demographic criteria, serum proteins, and imaging features.12,21,27 However, these approaches make use of retrospectively determined factors that are likely to vary with local population characteristics or institutional acquisition parameters. In contrast, RVI was derived from a gene expression profile associated with a number of different biological processes, including angiogenesis, cellular proliferation, and matrix invasion.10,14 Therefore, RVI utilizes a biology-driven strategy for predicting MVI through noninvasive interrogation of the molecular basis of venous invasion.
Given that RVI scoring was performed up to 12 months before pathological evaluation, it is possible that MVI may have developed during this interval. This raises the question of whether RVI predicts future development of MVI or current MVI. Given that RVI was derived from a vascular invasion gene profile, it is likely that both current MVI and “pre-MVI” tumors manifest RVI traits. Although conceptually interesting, this distinction does not affect the high concordance of RVI with MVI in surgically removed tumors or undermine its utility as a predictor of postsurgical outcome.
The limitations of this study include its retrospective cohort and potential variations in CECT acquisition parameters. It is possible that additional accuracy could be yielded with standardization of CT protocols and thinner slice reconstruction. However, our data do not necessarily support this given that no significant differences in diagnostic accuracy were observed for tumors less than 3 cm in diameter, compared to those greater than 3 cm. Additionally, we achieved a κ of 0.705, suggesting substantial agreement among radiologists with a wide range of experience. Furthermore, varied imaging protocols reflect actual clinical practice patterns, underscoring the immediate clinical applicability of this radiogenomic biomarker. Another potential limitation is the use of CT, rather than magnetic resonance imaging. Whereas CT is standard for HCC imaging, MRI provides greater tissue and microstructure evaluation, particularly with the application of diffusion-weighted imaging and hepatobiliary contrast agents, and may provide additional information.28
In this study, we analyzed a CECT biomarker for histological MVI derived from HCC-specific vascular invasion gene expression patterns. RVI predicts MVI with a high degree of accuracy and risk stratifies OS and RFS in a similar manner to MVI. Noninvasive preoperative evaluation of RVI score may alter indications for surgical treatment of HCC and thus potentially reduce rates of disease recurrence in appropriately selected patients.
Glossary
- AFP
alpha-fetoprotein
- AJCC
American Joint Committee on Cancer
- CECT
contrast-enhanced computed tomography
- CI
confidence interval
- CT
computed tomography
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HR
hazard ratio
- IQR
interquartile range
- LT
liver transplantation
- MVI
microvascular invasion
- OS
overall survival
- RVI
radiogenomic venous invasion
- RFS
recurrence-free survival
- TNM
tumor-node-metastasis
- UCLA
University of California Los Angeles
- UCSD
University of California San Diego
- UCSF
University of California San Francisco
Supporting Information
Additional Supporting Information may be found at http://onlinelibrary.wiley.com/doi/10.1002/hep.27877/suppinfo.
Supplemental Figure 1- Overall survival of 92 HCC patients classified as AJCC stage 1 who underwent either surgical resection or LT: left, RVI status; right, MVI status.
Supplemental Figure 2- Overall survival of 35 HCC patients within Milan criteria who underwent bridging locoregional therapy prior to LT: left, RVI status; right, MVI status.
Supplemental Table 1- Differences in characteristics based on RVI status
Supplemental Table 2- Univariate and multivariate analyses of predictors of three year recurrence free survival.
Supplemental Table 3- Univariate analysis of overall survival by positive RVI status in a subdivided cohort based on tumor size and AFP level.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 3.Fong Y, Sun RL, Jarnagin W, Blumgart LH. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg. 1999;229:790–799. doi: 10.1097/00000658-199906000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zimmerman MA, Ghobrial RM, Tong MJ, Hiatt JR, Cameron AM, Hong J, Busuttil RW. Recurrence of hepatocellular carcinoma following liver transplantation: a review of preoperative and postoperative prognostic indicators. Arch Surg. 2008;143:182–188. doi: 10.1001/archsurg.2007.39. [DOI] [PubMed] [Google Scholar]
- 5.Nakashima Y, Nakashima O, Tanaka M, Okuda K, Nakashima M, Kojiro M. Portal vein invasion and intrahepatic micrometastasis in small hepatocellular carcinoma by gross type. Hep Res. 2003;26:142–147. doi: 10.1016/s1386-6346(03)00007-x. [DOI] [PubMed] [Google Scholar]
- 6.Grazi GL, Ercolani G, Pierangeli F, Del Gaudio M, Cescon M, Cavallari A, Mazziotti A. Improved results of liver resection for hepatocellular carcinoma on cirrhosis give the procedure added value. Ann Surg. 2001;234:71–78. doi: 10.1097/00000658-200107000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim KC, Chow PK, Allen JC, Chia GS, Lim M, Cheow PC, et al. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg. 2011;254:108–113. doi: 10.1097/SLA.0b013e31821ad884. [DOI] [PubMed] [Google Scholar]
- 8.Tsai TJ, Chau GY, Lui WY, Tsay SH, King KL, Loong CC, et al. Clinical significance of microscopic tumor venous invasion in patients with resectable hepatocellular carcinoma. Surgery. 2000;127:603–608. doi: 10.1067/msy.2000.105498. [DOI] [PubMed] [Google Scholar]
- 9.Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11–e22. doi: 10.1016/S1470-2045(11)70175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Segal E, Sirlin CB, Ooi C, Adler AS, Gollub J, Chen X, et al. Decoding global gene expression programs in liver cancer by noninvasive imaging. Nat Biotechnol. 2007;25:675–680. doi: 10.1038/nbt1306. [DOI] [PubMed] [Google Scholar]
- 11.Kuo MD, Yamamoto S. Next generation radiologic-pathologic correlation in oncology: Rad-Path 2.0. Am J Roentgenol. 2011;197:990–997. doi: 10.2214/AJR.11.7163. [DOI] [PubMed] [Google Scholar]
- 12.Chandarana H, Robinson E, Hajdu CH, Drozhinin L, Babb JS, Taouli B. Microvascular invasion in hepatocellular carcinoma: is it predictable with pretransplant MRI? Am J Roentgenol. 2011;196:1083–1089. doi: 10.2214/AJR.10.4720. [DOI] [PubMed] [Google Scholar]
- 13.Chou CT, Chen RC, Lee CW, Ko CJ, Wu HK, Chen YL. Prediction of microvascular invasion of hepatocellular carcinoma by pre-operative CT imaging. Br J Radiol. 2012;85:778–783. doi: 10.1259/bjr/65897774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X, Cheung ST, So S, Fan ST, Barry C, Higgins J, et al. Gene expression patterns in human liver cancers. Mol Biol Cell. 2002;13:1929–1939. doi: 10.1091/mbc.02-02-0023.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henderson JM, Sherman M, Tavill A, Abecassis M, Chejfec G, Gramlich T. AHPBA/AJCC consensus conference on staging of hepatocellular carcinoma: consensus statement. HPB (Oxford) 2003;5:243–250. doi: 10.1080/13651820310015833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 17.Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 18.Pons F, Varela M, Llovet JM. Staging systems in hepatocellular carcinoma. HPB (Oxford) 2005;7:35–41. doi: 10.1080/13651820410024058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riaz A, Ryu RK, Kulik LM, Mulcahy MF, Lewandowski RJ, Minocha J, et al. Alpha-fetoprotein response after locoregional therapy for hepatocellular carcinoma: oncologic marker of radiologic response, progression, and survival. J Clin Oncol. 2009;27:5734–5742. doi: 10.1200/JCO.2009.23.1282. [DOI] [PubMed] [Google Scholar]
- 20.Duffy JP, Vardanian A, Benjamin E, Watson M, Farmer DG, Ghobrial RM, et al. Liver transplantation criteria for hepatocellular carcinoma should be expanded: a 22-year experience with 467 patients at UCLA. Ann Surg. 2007;246:502–509. doi: 10.1097/SLA.0b013e318148c704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shim JH, Han S, Lee YJ, Lee SG, Kim KM, Lim YS, et al. Half-life of serum alpha-fetoprotein: an early prognostic index of recurrence and survival after hepatic resection for hepatocellular carcinoma. Ann Surg. 2013;257:708–717. doi: 10.1097/SLA.0b013e318273be70. [DOI] [PubMed] [Google Scholar]
- 22.Yao FY, Hirose R, LaBerge JM, Davern TJ, Bass NM, Kerlan RK, et al. A prospective study on downstaging of hepatocellular carcinoma prior to liver transplantation. Liver Transpl. 2005;11:1505–1514. doi: 10.1002/lt.20526. [DOI] [PubMed] [Google Scholar]
- 23.Arrieta O, Cacho B, Morales-Espinosa D, Ruelas-Villavicencio A, Flores-Estrada D, Hernández-Pedro N. The progressive elevation of alpha fetoprotein for the diagnosis of hepatocellular carcinoma in patients with liver cirrhosis. BMC Cancer. 2007;7:28. doi: 10.1186/1471-2407-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pawlik TM, Gleisner AL, Anders RA, Assumpcao L, Maley W, Choti MA. Preoperative assessment of hepatocellular carcinoma tumor grade using needle biopsy: implications for transplant eligibility. Ann Surg. 2007;245:435–442. doi: 10.1097/01.sla.0000250420.73854.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poté N, Alexandrov T, Le Faouder J, Laouirem S, Léger T, Mebarki M, et al. Imaging mass spectrometry reveals modified forms of histone H4 as new biomarkers of microvascular invasion in hepatocellular carcinomas. Hepatology. 2013;58:983–994. doi: 10.1002/hep.26433. [DOI] [PubMed] [Google Scholar]
- 26.Nault JC, De Reyniès A, Villanueva A, Calderaro J, Rebouissou S, Couchy G, et al. A hepatocellular carcinoma 5-gene score associated with survival of patients after liver resection. Gastroenterology. 2013;145:176–187. doi: 10.1053/j.gastro.2013.03.051. [DOI] [PubMed] [Google Scholar]
- 27.Kim BK, Han KH, Park YN, Park MS, Kim KS, Choi JS, et al. Prediction of microvascular invasion before curative resection of hepatocellular carcinoma. J Surg Oncol. 2008;97:246–252. doi: 10.1002/jso.20953. [DOI] [PubMed] [Google Scholar]
- 28.Kitao A, Matsui O, Yoneda N, Kozaka K, Kobayashi S, Koda W, et al. Hypervascular hepatocellular carcinoma: correlation between biologic features and signal intensity on gadoxetic acid-enhanced MR images. Radiology. 2012;265:780–789. doi: 10.1148/radiol.12120226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1- Overall survival of 92 HCC patients classified as AJCC stage 1 who underwent either surgical resection or LT: left, RVI status; right, MVI status.
Supplemental Figure 2- Overall survival of 35 HCC patients within Milan criteria who underwent bridging locoregional therapy prior to LT: left, RVI status; right, MVI status.
Supplemental Table 1- Differences in characteristics based on RVI status
Supplemental Table 2- Univariate and multivariate analyses of predictors of three year recurrence free survival.
Supplemental Table 3- Univariate analysis of overall survival by positive RVI status in a subdivided cohort based on tumor size and AFP level.