Abstract
Decidual natural killer (dNK) cells are believed to be critical for maintaining maternal/fetal tolerance and regulating placental vascular remodeling based upon their abundance and unique phenotype during early pregnancy. However, the mechanism for how the dNK cells play such important roles in successful pregnancy remains undefined. Here, we identified a subtype of dNK cells characterized as having a CD3−CD56brightCD25+ phenotype. We found that CD56brightCD25+ NK cells preferentially localize to the maternal/fetal interface during early human pregnancy. CD25+ dNK cells account for approximately 75% of CD25-expressing decidual immune cells (DICs). However, less than 5% of CD25-positive peripheral blood mononuclear cells are CD25+ NK cells. Furthermore, CD25+ and CD25− dNK cells exhibit distinct phenotypes: CD25+ dNK cells display a more activated phenotype and greater cytokine-secreting capacity. Interestingly, coculture of peripheral NK (pNK) cells with primary trophoblasts upregulates the percentage of CD25-expressing pNK cells, resulting in increased expression of activation markers and cytokine production by pNK cells. In addition, we demonstrated that the CXCL12/CXCR4 axis is crucial for the recruitment of CD25+ dNK cells and contributes to the accumulation of CD3−CD56brightCD25+ dNK cells at the maternal/fetal interface. Thus, our data reveal that the crosstalk between trophoblasts and pNK cells leads to the accumulation of CD3−CD56brightCD25+ dNK cells, which exert a regulating effect at the maternal/fetal interface.
Keywords: CXCL12/CXCR4, CD3−CD56brightCD25+ NK cells, maternal/fetal interface, trophoblasts
Introduction
In a normal pregnancy, hemi-allogeneic cells from the fetus invade the maternal decidua but remain spared from attack by the maternal immune system through establishment of immune tolerance.1 Successful pregnancy requires the delicate and complicated crosstalk between the fetal-derived trophoblasts and the maternal-derived decidual cells. This meshwork of cells establishes a unique maternal–fetal immune environment via the production of regulatory factors, thus contributing to the maintenance of a normal pregnancy. However, the mechanism of this process is still not fully understood.2
Multiple mechanisms are thought to be responsible for promoting immune tolerance at the maternal/fetal interface. For example, TH2 cytokine bias,3 Fas ligand expression on fetal-derived trophoblasts4 and the inhibition of complement activation5 are critical for immune tolerance at the maternal/fetal interface. In addition, a delicate balance of inhibitory (PD-L1, Stat3 and TGF-β1) and stimulatory (CD80 and CD86) signals is observed during the establishment of immune privilege.6,7 Several unique immune cell subsets, including CD4+CD25+ regulatory T cells, also play vital roles in the maintenance of maternal–fetal tolerance.8 NK cells are a major component of innate immunity.9 Studies have shown that NK cells not only exert cell-mediated cytotoxicity against tumor cells or infected cells, but also regulate the function of other immune cells by secreting a variety of cytokines.10 Multiple activating and inhibitory receptors are expressed on the surface of NK cells. Currently, NK cell activity is thought to be controlled by a dynamic signaling balance between activating and inhibitory receptors, which are engaged upon interaction with their ligands, presented on the surface of specific target cells.11 In addition to having cytotoxic ability, NK cells act as a regulatory component in both innate and adaptive immune responses. In particular, NK cells may prime, influence and regulate the activities of adaptive immune responses through the crosstalk among NK cells, dendritic cells and T cells, cytokine secretion or cell-to-cell contact.12 These interactions imply that NK cells are crucial for immunity to infections and tumors.
One prominent feature during early human pregnancy is the striking abundance of decidual NK (dNK) cells. In contrast to NK cells in the peripheral blood, which account for approximately 10% of all peripheral lymphocytes, NK cells are the dominant cell type in the decidua during early human pregnancy.13 Interestingly, most dNK cells are CD56bright, whereas only a small fraction of peripheral NK cells are CD56bright. Thus, human dNK cells have been thought to play an important role in implantation and pregnancy, especially in early gestation. The precise functions of dNK cells in vivo remain unknown. At the maternal/fetal interface, dNK cells are in close contact with invading trophoblasts, which lack expression of classical HLA-A and -B antigens but selectively express HLA-C and the non-classical HLA-E, -G and CD1d molecules.14,15 This has led to the theory that trophoblasts interact with NK cells via their MHC antigens.16 In addition, a recent discovery has shown that dNK cells play a critical role in modulating trophoblast invasion and vascular remodeling.17 Because of their secretion of various cytokines, enzymes and other factors, dNK cells may play a role in the initiation of spiral arterial remodeling, as well as interacting with extravillous trophoblasts to aid in the completion of such processes.18 It was reported that the interaction of dNK and CD14+ cells lead to CD4+CD25+ regulatory T (Treg) cells induction and immunosuppression.19 Moreover, a recent study showed that CD56brightCD27+ NK cells promote immune tolerance and successful pregnancy through IFN-γ secretion, thereby inhibiting inflammatory TH17 cells.20
Similar to the TH1 and TH2 subsets of CD4+ T cells, NK cells are divided into NK1 and NK2 subpopulations based on their cytokine secretion profiles.21 The TH1 cytokine secreting NK1 subset, TH2 cytokine secreting NK2, TGF-β-secreting NK3 and IL-10-secreting NKr1 cells play major roles in immune regulation and may promote immune tolerance in transplantation and pregnancy.22 Regulatory NK cells, such as NK3 cells and NKr1, are the latest discoveries in the negative regulatory effects of NK cells on immune response.23 Unlike Treg cells, no specific surface marker for regulatory NK cells has been established. Studies using animal models suggest that, based on surface marker expression, DX5+CD3−CD25+Thy1.2brightc-kitdim NK cells may represent regulatory NK cells.24 Whether CD3−CD56brightCD25+ NK cells are present in human pregnancy decidua is still unknown.
In the present study, we identified a subtype of CD25-expressing dNK cells, which are preferentially recruited to the maternal/fetal interface in early human pregnancy. These CD56brightCD25+ NK cells possess an activated phenotype and are the main source of cytokines at the maternal/fetal interface. Furthermore, we investigated the role of trophoblasts in instructing peripheral NK cells to adopt the decidual phenotype of CD25+ NK cells based on the expression profile of cell surface molecules and intracellular cytokines. We also determined whether the CXCL12/CXCR4 axis was involved in the preferential recruitment of CD3−CD56brightCD25+ dNK cells to the maternal/fetal interface in early human pregnancy.
Materials and methods
Human villi and decidual tissue collection
First-trimester human villi and decidual tissues (gestational age 5–10 weeks) were obtained from clinically normal pregnancies, which were terminated for nonmedical reasons, at the Obstetrics and Gynecology Hospital of Fudan University. All the tissues were immediately collected into ice-cold Dulbecco's modified Eagle medium (DMEM) with high D-glucose or DMEM/F12 (Gibco, Grand Island, NY, USA), transported to the laboratory within 30 min after surgery, and washed in calcium- and magnesium-free Hanks balanced salt solution for trophoblasts or decidual immune cell (DIC) isolation. Each patient provided a signed, written consent form. All procedures involving study participants were approved by the Human Research Ethics Committee of the Obstetrics and Gynecology Hospital of Fudan University.
Isolation and purification of dNK cells
DICs were isolated by trypsin-DNase I digestion and discontinuous Percoll gradient centrifugation, as described in our previous study.25 DICs, which ranged in density between 1.062 and 1.077 g/ml, were collected and cultured in RPMI 1640 complete medium supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 mg/ml streptomycin in 5% CO2 at 37 °C. After primary culture overnight at 37 °C in 5% CO2, the non-adherent DICs were collected while adherent decidual stromal cells were discarded. Magnetic activated cell sorting human NK cell negative selection isolation kit (Miltenyi Biotec, Auburn, CA, USA) was used for NK cell enrichment from DICs according to the manufacturer's instructions.
Isolation and primary culture of human first-trimester trophoblasts
The first-trimester human villi were carefully separated from the decidua, minced into small fragments, and then treated by repeated trypsin digestions and Percoll gradient centrifugation as previously described.25 Briefly, the placental tissues obtained from 10–15 separate individuals were pooled and digested by 0.25% trypsin (Bio Basic Inco, Markham, Ontario, Canada) and 0.02% DNase type I at 37 °C with gentle agitation for 5 min. The liquid part of the digested suspension was discarded and the residual tissue was collected and subjected to four cycles of 10 min trypsin-DNase digestion. Trypsin digestion in each time was quenched with 10% FBS (Hyclone, Logan, UT, USA) and the liquid digest was harvested. The four digests were pooled, centrifuged at 1200 r.p.m. for 10 min, and the pellet was resuspended in 4 ml DMEM with high D-glucose. This suspension was carefully layered over a discontinuous Percoll gradient consisting of 70%–5% Percoll (v/v) in 5% increments of 2 ml each formed by diluting 90% Percoll with Hanks balanced salt solution. After centrifugation at 2000 r.p.m. for 20 min, the cells sedimenting at densities between 1.048 and 1.062 g/ml were collected and washed with DMEM-high glucose medium. These semi-purified cytotrophoblasts were maintained in DMEM-high glucose complete medium (2 mM glutamine, 25 mM HEPES, 100 UI/ml penicillin and 100 mg/ml streptomycin), supplemented with 15% heat-inactivated FBS (Hyclone) and incubated in 5% CO2 at 37 °C. The freshly isolated human trophoblasts were plated in plastic Petri dishes and incubated at 37 °C for 15 min to allow the contaminating macrophages to adhere to the plastic. The non-adherent cells (mainly trophoblasts) were transferred to 24-well plates pre-coated with matrigel at a concentration of 2×105 cells/ml, and cultured in DMEM-high glucose complete medium supplemented with 15% heat-inactivated FBS at 37 °C in 95% air and 5% CO2. This method designed by our laboratory allows for the recovery of a 95% pure population of trophoblasts. The characterization and purity of trophoblasts has been described in detail in our previous publication.25 After 72 h in culture, the trophoblast culture media (TCM) was recovered, passed through a 0.22-µm filter, and stored at −20 °C before use.
Isolation and purification of human peripheral NK cells (pNK)
Density centrifugation using Ficoll-Hypaque (Amersham Biosciences, Buckinghamshire, UK) was performed to isolate peripheral blood mononuclear cells (PBMCs) from the whole blood of women during early pregnancy. Whole blood (30 ml) containing acid citrate dextrose anticoagulant (Biological Specialty Corporation, Colmar, PA, USA) was diluted with an equal volume of phosphate-buffered saline and carefully layered over an equal volume of Ficoll density gradient medium. The tubes were centrifuged for 20 min at 2000 r.p.m. PBMCs were collected, washed 2–3 times in phosphate-buffered saline and centrifuged for 10 min at 1200 r.p.m. Magnetic activated cell sorting human NK cell negative selection isolation kit (Miltenyi Biotec, Auburn, CA, USA) were used for NK cells enrichment from PBMCs according to manufacturer's instructions.
Coculture of trophoblasts and pNK cells
The freshly isolated trophoblasts were seeded overnight at a density of 2×105 cells/ml per well in 24-well plates. The supernatants were aspirated completely and the cells were washed with 1× phosphate-buffered saline. The same number of magnetic activated cell sorting purified peripheral NK cells were added in each well and cocultured continuously for 48 h; the culture of purified peripheral NK cells alone was included as a control. The supernatants were collected and centrifuged at 1200 r.p.m. for 10 min, then transferred to FCM tubes for flow cytometric analysis. For intracellular cytokine analysis, brefeldin A (a Golgi inhibitor) (10 mg/ml) was used to block cytokine secretion into the media after the activation of cells using phorbol 12-myristate 13-acetate (50 ng/ml) and ionomycin (1 µg/ml) for 4 h at the end of the 48 h culture, and then the cells were harvested and analyzed by FCM for intracellular cytokine production.
Flow cytometry assay
NK cell purity, cell surface molecule expression and intracellular cytokine production were evaluated using flow cytometry. A minimum of 10 000 events was acquired using a Beckman-Coulter CyAN ADP flow cytometer and analyzed with FlowJo software (Tree Star, Ashland, OR, USA). FITC-conjugated anti-CD3 (Biolegend, San Diego, California, USA), PE-Cy7-conjugated anti-CD56 (Biolegend), PE-conjugated anti-CD25 and APC-conjugated anti-CD25 were used to identify NK cell subpopulations. Additional NK cell markers were used to assess NK cell phenotype: APC-conjugated anti-CD337 (NKp30), anti-CD336 (NKp44), anti-CD94 and anti-CXCR4; and PE-conjugated anti-CD335 (NKp46), anti-KIR2DL1 and anti-KIR3DL1. Intracellular cytokine analysis included APC-conjugated anti-IL-4, anti-IL-10 and anti-IFN-γ, and PE-conjugated anti-TGF-β and anti-IL-8 (Biolegend). Murine immunoglobulins of the same isotype were used as negative controls for nonspecific binding of mouse monoclonal antibodies to human antigens.
Chemotaxis assay
Chemotaxis was performed according to our previously published method.26 We used transwell plates (24-well, 6.5-mm diameter; Corning, New York, USA) containing polycarbonate filters of 5.0-µm pore size. The TCM of 800 µl with or without 1 µg/ml anti-CXCR4 (R&D Systems, Minneapolis, MN, USA), control medium, or control medium supplemented with recombinant human (rh) CXCL12 (R&D Systems), at concentrations from 1 to 100 ng/ml, were added to the bottom chamber of each well, and 200 µl of purified dNK suspensions containing 106 cells was added to the upper chamber. After incubation for 3 h at 37 °C in a standard tissue-culture incubator, all of the cells that migrated into each lower chamber were collected and labeled with fluorophore-conjugated Abs. The absolute number and percentage of migrated decidual NK subsets were analyzed by flow cytometry as previously described.26 All the assays were performed in triplicate for four independent experiments.
Statistical analysis
Statistically, one-way or two-way ANOVA was used for comparisons of cell markers and cytokine production. The post-hoc Dunnett's t-test was used to compare the significance between the control and various treatments. Error bars in the figures indicate standard errors. Statistical significance was set at P<0.05.
Results
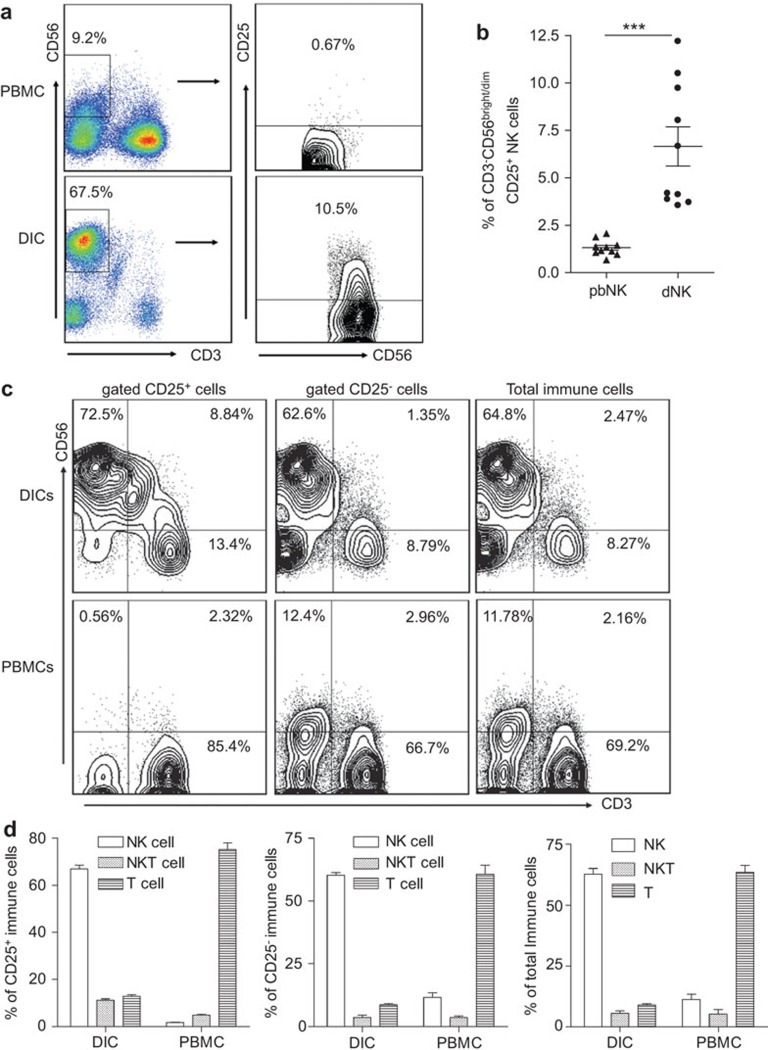
CD3−CD56brightCD25+ NK cells are preferentially recruited to the maternal/fetal interface in early human pregnancy
As shown in Figure 1a, peripheral blood NK (pNK) cells accounted for approximately 10% of the total lymphocytes. However, NK cells constituted 70% of decidual immune cells during a normal pregnancy. In addition, more than 90% of the NK cells in the decidua were CD3−CD56bright while most peripheral NK cells were CD3−CD56dim. Because CD25 is expressed on murine NK cells,24 we compared CD25 expression on human dNK cells with that on pNK cells. The results indicate that a much higher proportion of dNK cells (6.65%±3.25%) than pNK cells (1.31%±0.13%) were CD25+ (Figure 1b). We then investigated the distribution of CD25+ immune cells at the maternal/fetal interface and peripheral blood during early pregnancy, and found that CD25+ decidual immune cells consisted of 66.88%±5.40% CD3−CD56brightCD25+ NK cells, followed by 12.82%±2.09% CD3+CD56−CD25+ T cells and 11.16%±1.86% CD3+CD56+CD25+ NKT cells. In contrast, CD25+ peripheral blood leukocytes consisted of 71.17%±10.51% CD3+CD56−CD25+ T cells, followed by 5.86%±3.15% CD3+CD56+CD25+ NKT cells, and 1.78%±0.53% CD3−CD56+CD25+ NK cells (Figure 1c and d, left panels). We also analyzed the CD3 and CD56 expression after gating CD25− and the total immune cells to illustrate the exact cell phenotype and percentage. These data in Figure 1c and d (middle and right panels) showed that of CD25− decidual immune cells, approximately 65% were CD3−CD56bright NK cells, approximately 8.5% were CD3+CD56− T cells, and 2% were CD3+CD56+ NKT cells or other cell types. However, peripheral CD25− immune cells were more than 65% CD3+CD56− T cells, followed by approximately 12% CD3−CD56+ NK cells, and approximately 2.5% CD3+CD56+ NKT cells. It is interesting that the same pattern of cellular phenotype and frequency is found in the total immune cell population as in CD25− immune cells but not CD25+ immune cells.
Figure 1.
CD3−CD56brightCD25+ dNK cells are preferentially recruited to the maternal/fetal interface in early human pregnancy. (a, b) Comparison of the percentage of CD25+ NK cells in pNK and dNK cells in early human pregnancy. CD56+ NK cells were gated on prior to analysis of CD25 expression by FCM. The percentage of CD25+ NK cells was determined. (c, d) Comparison of the cell subsets constituting CD25+, CD25− or total immune cells from PBMC and DICs in early human pregnancy. Data represent the mean±standard error from ten independent experiments with ten deciduas. A representative image is shown. Data were analyzed using a paired t-test (b). ***P<0.001. DIC, decidual immune cell; dNK, decidual natural killer; PBMC, peripheral blood mononuclear cell; pNK, peripheral natural killer.
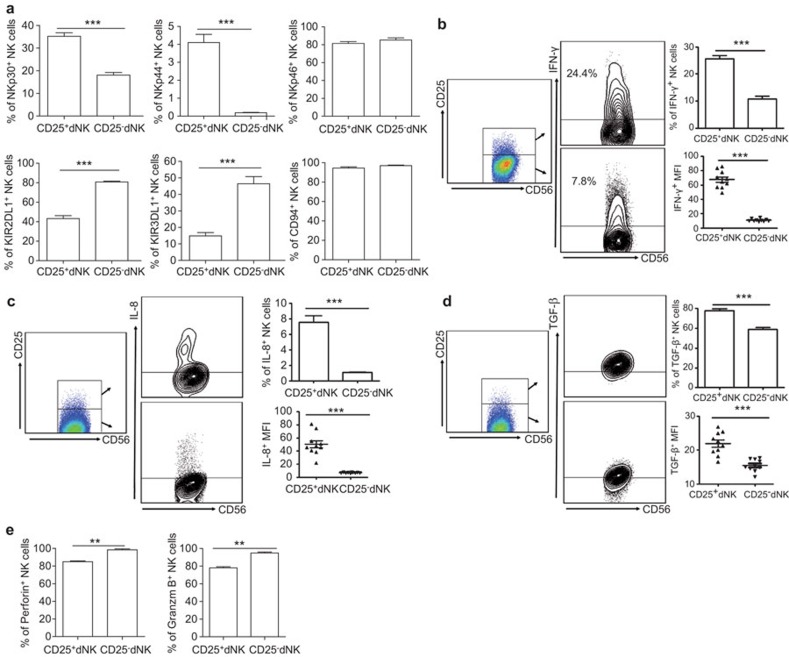
Decidual CD56brightCD25+ NK cells exhibit an activated phenotype and reduced cytotoxicity and are the main source of cytokines at the maternal-fetal interface
NK cells are believed to be phenotypically and functionally heterogeneous because of their differential expression of activating and inhibitory receptors. We compared the phenotypes of CD25+ and CD25− dNK cells and found that the CD25+ dNK subset expressed fewer inhibitory receptors, such as KIR2DL1 (42.23%±9.30% compared to 80.85%±2.62%) and KIR3DL1 (14.88%±6.32% compared to 46.40%±13.90%), and more activating receptors, such as NKp30 (35.20%±4.99% compared to 18.04%±3.68%) and NKp44 (4.10%±1.44% compared to 0.19%±0.07%). There was a slight decrease in NKp46 (81.45%±6.64% compared to 85.25%±7.67%) expression in the CD25+ NK cell population, although both CD25+ and CD25− subsets expressed high levels of this receptor. There was no significant difference in CD94 expression (94.56%±3.73% compared to 97.07%±1.57%) on either cell subset. These findings indicate that human CD25+ dNK cells display a more activated phenotype relative to that of CD25− dNK cells (Figure 2a). Given that our previous study suggested that dNK cells are activated cells but lack significant cytotoxic activity (data not shown), we hypothesized that their main function might be cytokine secretion. After in vitro stimulation with phorbol 12-myristate 13-acetate and ionomycin, we found that CD25+ dNK cells produced more IFN-γ (25.64%±3.88% compared to 10.72%±3.23%), TGF-β (77.1%±6.54% compared to 57.8%±6.87%) and IL-8 (7.54%±2.60% compared to 1.08%±0.22%) compared to CD25− dNK cells (Figure 2b–d). Approximately 80% of CD25+ dNK cells (77.1%±6.54%) produced TGF-β. making this an especially potent subset of TGF-β-producing leukocytes. On the other hand, we found that fewer CD25+ dNK cells produced IL-4 compared with CD25− dNK cells, and both CD25+ and CD25− dNK cells produced very little IL-10 though there was no significant difference between these subsets (data not shown). In addition, we measured the mean fluorescence intensity of intracellular cytokines. Accordantly, the mean fluorescence intensities for all cytokines examined in CD25+ dNK cells were higher than in CD25− dNK cells. Furthermore, fewer CD25+ dNK cells expressed perforin and Granzyme B, suggesting a reduced cytolytic capacity of CD25+ dNK cells relative to CD25− dNK cells (Figure 2e).
Figure 2.
Differences between CD25+ and CD25− dNK cells. (a) Flow cytometry analysis of activating and inhibitory receptors on CD3−CD56bright CD25+ and CD3−CD56brightCD25− dNK cells. (b–d) Flow cytometry analysis of intracellular cytokine percentage and MFI of IFN-γ, IL-8 and TGF-β on CD3−CD56brightCD25− and CD3−CD56brightCD25− dNK cells. (e) Flow cytometry analysis of perforin and Granzyme B expression in CD3−CD56bright CD25+ and CD3−CD56brightCD25− dNK cells. Data represent the mean±standard error from 10 independent experiments with different deciduas. *P<0.05, **P<0.01; ***P<0.001. dNK, decidual natural killer; MFI, mean fluorescence intensity.
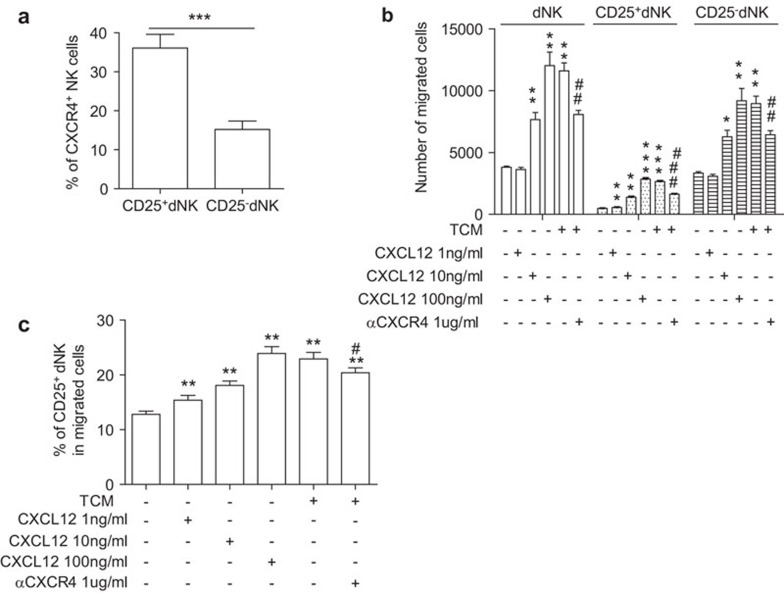
Trophoblasts recruit CXCR4+CD25+ dNK cells at the maternal/fetal interface via the CXCL12/CXCR4 axis
Our previous publications have demonstrated that the CXCL12/CXCR4 axis is crucial for recruiting decidual NK cells and creating a TH2 bias at the maternal/fetal interface by regulating the crosstalk between trophoblasts and decidual cells.25,27 We further investigated whether this axis was also involved in the preferential localization of CD3−CD56brightCD25+ NK cells at the maternal/fetal interface. First, we analyzed the frequency of CXCR4 expression in CD25+ and CD25− dNK cell subsets and found that a much higher proportion of CD3−CD56brightCD25+ dNK cells were CXCR4+ (36.08%±11.23%) compared to 15.22%±6.70% of CD3−CD56brightCD25− dNK cells (Figure 3a). We then measured the chemotactic activity of TCM and rhCXCL12 on purified dNK cells. The number of migrating dNK cells was analyzed by flow cytometry. Consistently, rhCXCL12 increased the number of migrating dNK cells in a concentration-dependent manner. Furthermore, TCM induced a 3.05-fold increase in the migration of purified dNK cells and a 5.56-fold increase in the migration of CD25+ dNK cells compared with the control. Treatment with anti-CXCR4 significantly inhibited the chemotactic activity of TCM in recruiting dNK cells (P<0.01) and CD25+ dNK cells (P<0.001) (Figure 3b). In addition, we assayed the percentage of CD25+ dNK that migrated in response to increasing concentrations of rhCXCL12 and TCM. Our results showed that rhCXCL12 at 1, 10 and 100 ng/ml induced a 1.20-, 1.41- and 1.87-fold increase in the migration of CD25+ dNK cells, respectively. Regarding the chemotactic activity of rhCXCL12, we also found that the TCM increased the migration frequency of CD25+ dNK cells 1.79-fold over the control wells, and its chemotactic activity was significantly inhibited by anti-CXCR4 (P<0.05) (Figure 3c). Furthermore, we measured the percentage of CD25+/CD25− dNK cells that migrated after treatment with CXCL12/TCM. These results showed that the ratio of CD25+/CD25− dNK cells that migrate increased with CXCL12/TCM treatment (Supplementary Figure 1), indicating that the CXCL12 axis preferentially induced migration of CD25+ over CD25− dNK cells.
Figure 3.
Trophoblasts attract CXCR4+CD25+ dNK cells to the maternal-fetal interface via secretion of CXCL12. (a) CD3−CD56brightCD25+ dNK cells exhibit higher CXCR4 expression at the maternal/fetal interface (n=10). (b) Analysis of the number of migrated dNK cells following treatment with rhCXCL12 in various concentrations or TCM. (c) Analysis of the percentage of CD25+ dNK that migrated toward various concentrations of rhCXCL12 and TCM (n=4). Data represent the mean±standard error from ten or four independent experiments with different deciduas. *P<0.05, **P<0.01, compared with control; #P<0.05, compared with TCM treatment. dNK, decidual natural killer; TCM, trophoblast culture media.
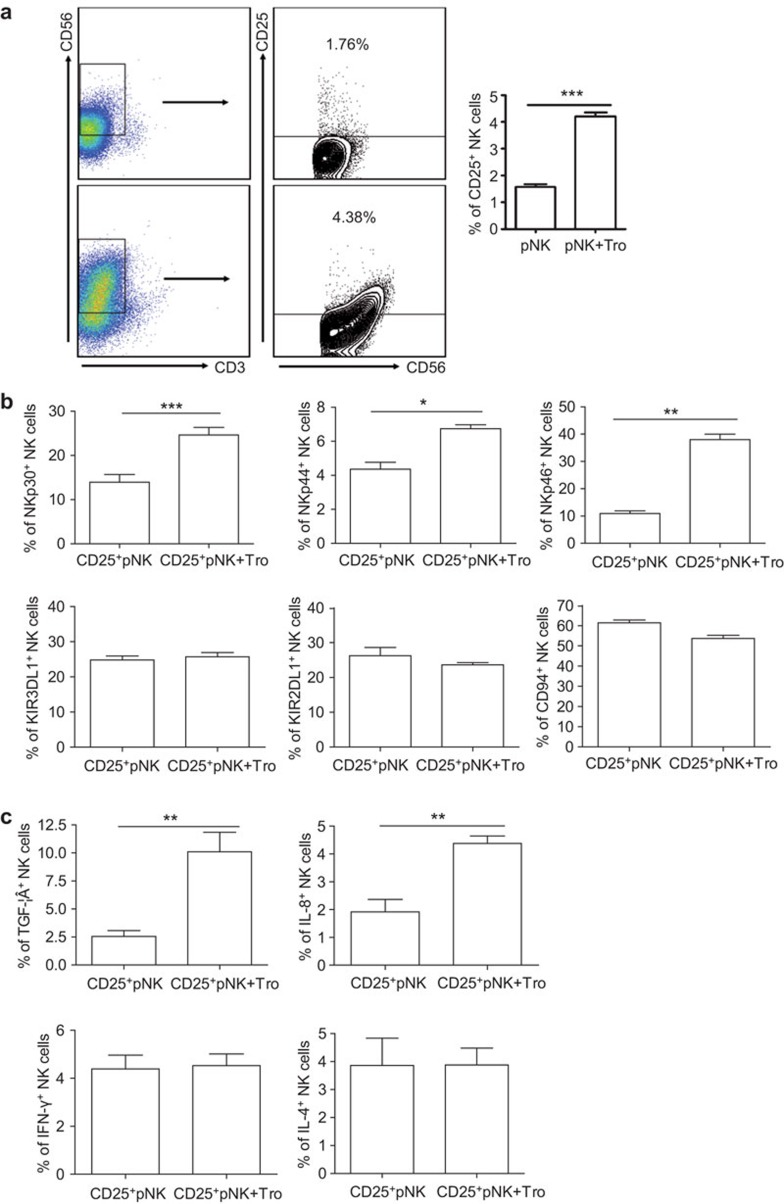
Trophoblasts instruct the phenotypical shift of CD25+ pNK cells to the CD25+ dNK phenotype
To investigate the interplay of trophoblasts and the accumulated NK cells, which were recruited from the periphery, at the maternal/fetal interface, we evaluated the change in NK cell phenotype and cytokine secretion using a trophoblast and pNK coculture model. As shown in Figure 4a, the coculture of trophoblasts with pNK cells increased the percentage of CD25+ NK cells (3.69%±0.55%) compared to NK cells alone (1.57%±0.23%). Analysis of activating and inhibitory receptor expression after gating on purified CD25+ pNK cells showed that following coculture, trophoblasts increased the expression of NKp30 (24.63%±2.97% compared to 13.97%±3.00% before coculture), NKp44 (6.74%±0.40% compared to 4.36%±0.69%) and NKp46 (38.00%±3.44% compared to 10.92%±1.67%). There was no significant shift in KIR2DL1 (P=0.290), KIR3DL1 (P=0.442) or CD94 expression (P=0.069) following coculture (Figure 4b). These findings indicate that trophoblasts induced activation of CD25+ pNK cells. In addition, intracellular cytokine production by pNK cells was determined by flow cytometry after coculture with primary human trophoblasts. The results shown in Figure 4c indicate that trophoblasts could upregulate TGF-β (10.11%±3.01% compared to 2.54%±0.90% before coculture) and IL-8 expression (4.38%±0.46% compared to 1.91%±0.78%) by NK cell. However, there was no significant effect on IFN-γ (P=0.726) or IL-4 (P=0.962) expression by pNK cells following coculture with trophoblasts.
Figure 4.
Trophoblasts instruct the phenotypic shift from a peripheral CD25+ NK (CD25+ pNK) toward a decidual CD25+ NK (CD25+ dNK)-like phenotype. (a) Trophoblasts increase the percentage of CD25-expressing NK cells (n=6). (b) Trophoblasts mediate the activation of CD3−CD56+CD25+ pNK cells. (n=3) (c) Trophoblasts increase the production TGF-β and IL-8 by pNK cells (n=3). Data represent the mean±standard error from six or three independent experiments with different deciduas. *P<0.05, **P<0.01; ***P<0.001. dNK, decidual natural killer; pNK, peripheral natural killer.
Discussion
In a normal pregnancy, it is intriguing that approximately 70% of the infiltrating CD45+ leukocytes in the decidua are CD56brightCD16− NK cells, with a small frequency of macrophages and T cells. dNK cells differ from peripheral blood NK cells in both phenotype and function, thus constituting a unique NK cell subset.28 However, the exact function of dNK cells remains poorly defined. A recent study showed that dNK but not pNK subtypes influence maternal spiral arterial remodeling and regulate trophoblast invasion, both in vitro and in vivo, through production of IL-8 and interferon-inducible protein-10.17
NK cells are phenotypically and functionally heterogeneous, and express various receptors known to recognize MHC class I molecules. Some of these receptors induce NK cell activation, while others inhibit their regulatory functions. A recent study suggested that different combinations of maternal KIR and fetal HLA-C genes influence the risk of developing preeclampsia.29 Overall, NK receptor–ligand interactions favoring dNK cell activation protect against preeclampsia via the secretion of large amounts of NK-cell derived growth factors and chemokines that support invading trophoblasts and decidual blood vessels.30 dNK cells are found in vivo in close contact with invasive trophoblasts, which are continuously exposed to the ligands for NK receptors. This is analogous to the chronic stimulation of activating receptors on dNK cells, which may induce NK cell tolerance to embryonic cells and enhance the ability of NK cells to produce growth factors. Increased production of IL-8 and interferon-inducible protein-10 was achieved by stimulating the NCR and NKG2D activating receptors on dNK cells, further emphasizing the developmental role of dNK cells as regulators at the maternal/fetal interface.31 It is reasonable to hypothesize that the unique functional properties of dNK cells might be attributed to the particular cytokine milieu in the decidua.32 In this study, we demonstrated that CD3−CD56brightCD25+ dNK cells preferentially localize to the maternal/fetal interface in early human pregnancy, and we also found that this cell subset displays a more activated phenotype than CD3−CD56brightCD25− dNK cells. Furthermore, coculture of pNK with trophoblasts increased CD25 expression and the frequency of TGF-β- and IL-8-producing pNK cells. Collectively, these data suggest that trophoblasts stimulate and educate pNK cells to have a memory-like phenotype.
Similar to the TH1 and TH2 subtypes of CD4+ T cells, NK cells are also divided into NK1 and NK2 subsets based on their distinct cytokine secretion profiles.21,22 The NK1 subpopulation predominantly secretes IFN-γ, but relatively no IL-4, IL-5 or IL-13. On the contrary, the NK2 subpopulation largely secretes IL-4, IL-5 and IL-13 but not IFN-γ. The TGF-β-secreting NK3 and IL-10-secreting NKr1 cells are thought to play important roles in immune regulation, promoting immune tolerance in transplantation and normal pregnancy.24,33 Recently, the negative regulatory functions of NK cells, such as NK3 and NKr1 cells, known as regulatory NK cells, were reported to be a part of normal and pathogenic immune responses.23 Here, we found a population of CD3−CD56brightCD25+ NK cells in the decidua. More than 75% of these dNK cells expressed TGF-β and approximately 25% produced IFN-γ, although very few expressed IL-4 (less than 3%) and almost none expressed IL-10 (data not shown). These data suggest that CD25+CD56bright dNK cells may represent a specific NK cell type in the decidua. However, they are more likely to be regulatory NK cells.
We found that IFN-γ, IL-8 and TGF-β were highly produced by CD25+ dNK cells compared with CD25− dNK cells from multiple donors. Interestingly, the cytotoxicity of CD25+ dNK cells was lower than that of CD25− dNK cells. TGF-β has been demonstrated to suppress NK cell cytotoxicity in vitro.34 In fact, we found that CD25+ dNK cells are an especially potent subset of TGF-β-producing cells. This might explain the abundance of TGF-β at the maternal/fetal interface during early pregnancy and contribute to the maintenance of the unique phenotype of dNK cells. In our research, we also found elevated levels of IFN-γ and IL-8 in CD25+ dNK cells, both of which are important in the initiation of spiral arterial remodeling through interactions with extravillous trophoblast.17 Our data suggested that the angiogeneic effects of dNK cells are mainly supplied by CD25+ rather than CD25− dNK cells. However, Treg cells also mediate maternal–fetal tolerance through IL-10 secretion,35 which is significantly reduced in the decidua of patients experiencing spontaneous abortion.36 Conversely, we found that very few CD25+ dNK cells produced IL-10, suggesting that CD25+ dNK cells promote immune tolerance mainly through secretion of TGF-β.
The origin of decidual NK cells remains the subject of some debate. Based on their close resemblance to the minor agranular CD56bright NK cells in the blood, some researchers believe that this NK cell population may migrate to the uterus, proliferate, and differentiate in the local hormone-rich environment. Indirect evidence suggests that CD56brightCD16− dNK cells originate from CD56brightCD16− pNK cells.37,38 In this study, our results showed that trophoblasts had no effect on the expression of cell surface molecules or intracellular cytokines by CD56bright pNK cells, also suggested the resemblance of peripheral CD56bright NK cells to dNK cells (Supplementary Figure 2). Our previous studies demonstrated that at the maternal/fetal interface, CXCL12 was mainly produced by trophoblasts and to a lesser extent by decidual stromal cells, while CXCR4 was intermediately expressed on dNK cells and highly expressed on peripheral NK cells (Ref. 27 and unpublished data). Studies indicate that CXCL12 expressed by invasive trophoblasts plays a role in the migration of NK cells to the decidua.25 In the present study, we confirmed that the CXCL12/CXCR4 axis is crucial for the accumulation of CD3−CD56bright dNK cells during early pregnancy. More interestingly, the CXCL12 axis preferentially induced migration of CD25+ over CD25− NK cells, indicating that CXCL12 secreted by trophoblasts preferentially recruits CD3−CD56brightCD25+ NK cells to the maternal-fetal interface.
As the most critical cell component in human placenta, embryo-derived trophoblasts exert an important role in shaping the immune milieu at the maternal/fetal interface.39,40 Both pro- and anti-inflammatory cytokines in trophoblasts were downregulated in spontaneously aborted pregnancies.41 Trophoblasts produce cytokines, such as TSLP, to instruct decidual dendritic cells and to induce a TH2 bias at the maternal/fetal interface.42 In the present study, we have shown that coculture of trophoblasts and purified peripheral NK cells can increase the surface expression of activating receptors and the secretion of TGF-β and IL-8 by CD3−CD56+CD25+ pNK cells. These data indicate that trophoblasts play an essential role in maintaining an activated phenotype on and cytokine production from CD25+ NK cells. Interestingly, there was no effect of trophoblasts on dNK cell phenotype or cytokine production (Supplementary Figure 3). One possible explanation for this is that dNK cells have already been instructed by the immune milieu at the maternal/fetal interface.
In summary, we have confirmed that CD3−CD56brightCD25+ dNK cells preferentially accumulate at the maternal/fetal interface in early human pregnancy. This population of CD25+ dNK displays an activated phenotype with a high frequency of cells producing IFN-γ, IL-8 and TGF-β. Trophoblasts play an important role in the recruitment of dNK cells, especially CD25+ dNK cells, to the maternal-fetal interface via CXCL12/CXCR4 interactions. Furthermore, trophoblasts mediate the shift in CD25+ pNK cells toward a more activated CD25+ dNK-like phenotype and cytokine profile, thereby shaping the immune milieu at the maternal/fetal interface during early gestation to maintain a normal pregnancy.
Acknowledgments
This work was supported by the Key Project of Shanghai Basic Research from Shanghai Municipal Science and Technology Commission (STCSM) (12JC1401600 to DJL), the Key Project of Shanghai Municipal Education Commission (MECSM) (14ZZ013 to MRD) and the Nature Science Foundation from National Nature Science Foundation of China (NSFC) (NSFC31270969 to DJL; NSFC81070537, NSFC31171437 and NSFC81370770 to MRD; NSFC31300751 to HLP; NSFC81370730 to QF).
Footnotes
Supplementary Information accompanies the paper on Cellular & Molecular Immunology's website (http://www.nature.com/cmi).
Supplementary Information
References
- 1Trowsdale J, Betz AG. Mother's little helpers: mechanisms of maternal–fetal tolerance. Nat Immunol 2006; 7: 241–246. [DOI] [PubMed] [Google Scholar]
- 2Munoz-Suano A, Hamilton AB, Betz AG. Gimme shelter: the immune system during pregnancy. Immunol Rev 2011; 241: 20–38. [DOI] [PubMed] [Google Scholar]
- 3Piccinni MP, Beloni L, Livi C, Maggi E, Scarselli G, Romagnani S. Defective production of both leukemia inhibitory factor and type 2 T-helper cytokines by decidual T cells in unexplained recurrent abortions. Nat Med 1998; 4: 1020–1024. [DOI] [PubMed] [Google Scholar]
- 4Makrigiannakis A, Zoumakis E, Kalantaridou S, Coutifaris C, Margioris AN, Coukos G et al. Corticotropin-releasing hormone promotes blastocyst implantation and early maternal tolerance. Nat Immunol 2001; 2: 1018–1024. [DOI] [PubMed] [Google Scholar]
- 5Xu C, Mao D, Holers VM, Palanca B, Cheng AM, Molina H. A critical role for murine complement regulator Crry in fetomaternal tolerance. Science 2000; 287: 498–501. [DOI] [PubMed] [Google Scholar]
- 6Ayatollahi M, Geramizadeh B, Samsami A. Transforming growth factor beta-1 influence on fetal allografts during pregnancy. Transplant Proc 2005; 37: 4603–4604. [DOI] [PubMed] [Google Scholar]
- 7Zhang J, Xiao X, Liu W, Demirci G, Li XC. Inhibitory receptors of the immune system: functions and therapeutic implications. Cell Mol Immunol 2009; 6: 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology 2004; 112: 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol 2008; 9: 503–510. [DOI] [PubMed] [Google Scholar]
- 10Raulet DH. Interplay of natural killer cells and their receptors with the adaptive immune response. Nat Immunol 2004; 5: 996–1002. [DOI] [PubMed] [Google Scholar]
- 11Lanier LL. NK cell recognition. Annu Rev Immunol 2005; 23: 225–274. [DOI] [PubMed] [Google Scholar]
- 12Andoniou CE, Coudert JD, Degli-Esposti MA. Killers and beyond: NK-cell-mediated control of immune responses. Eur J Immunol 2008; 38: 2938–2942. [DOI] [PubMed] [Google Scholar]
- 13Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol 2002; 2: 656–663. [DOI] [PubMed] [Google Scholar]
- 14Boyson JE, Rybalov B, Koopman LA, Exley M, Balk SP, Racke FK et al. CD1d and invariant NKT cells at the human maternal–fetal interface. Proc Natl Acad Sci USA 2002; 99: 13741–13746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15Kovats S, Main EK, Librach C, Stubblebine M, Fisher SJ, DeMars R. A class I antigen, HLA-G, expressed in human trophoblasts. Science 1990; 248: 220–223. [DOI] [PubMed] [Google Scholar]
- 16King A, Hiby SE, Gardner L, Joseph S, Bowen JM, Verma S et al. Recognition of trophoblast HLA class I molecules by decidual NK cell receptors—a review. Placenta 2000; 21: S81–S85. [DOI] [PubMed] [Google Scholar]
- 17Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S et al. Decidual NK cells regulate key developmental processes at the human fetal–maternal interface. Nat Med 2006; 12: 1065–1074. [DOI] [PubMed] [Google Scholar]
- 18Harris LK. Review: trophoblast–vascular cell interactions in early pregnancy: how to remodel a vessel. Placenta 2010; 31: S93–98. [DOI] [PubMed] [Google Scholar]
- 19Vacca P, Cantoni C, Vitale M, Prato C, Canegallo F, Fenoglio D et al. Crosstalk between decidual NK and CD14+ myelomonocytic cells results in induction of Tregs and immunosuppression. Proc Natl Acad Sci USA 2010; 107: 11918–11923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20Fu B, Li X, Sun R, Tong X, Ling B, Tian Z et al. Natural killer cells promote immune tolerance by regulating inflammatory TH17 cells at the human maternal–fetal interface. Proc Natl Acad Sci USA 2013; 110: E231–E240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21Peritt D, Robertson S, Gri G, Showe L, Aste-Amezaga M, Trinchieri G. Differentiation of human NK cells into NK1 and NK2 subsets. J Immunol 1998; 161: 5821–5824. [PubMed] [Google Scholar]
- 22Deniz G, Akdis M, Aktas E, Blaser K, Akdis CA. Human NK1 and NK2 subsets determined by purification of IFN-gamma-secreting and IFN-gamma-nonsecreting NK cells. Eur J Immunol 2002; 32: 879–884. [DOI] [PubMed] [Google Scholar]
- 23Zhang C, Zhang J, Tian Z. The regulatory effect of natural killer cells: do “NK-reg cells” exist? Cell Mol Immunol 2006; 3: 241–254. [PubMed] [Google Scholar]
- 24Saito S, Nakashima A, Myojo-Higuma S, Shiozaki A. The balance between cytotoxic NK cells and regulatory NK cells in human pregnancy. J Reprod Immunol 2008; 77: 14–22. [DOI] [PubMed] [Google Scholar]
- 25Wu X, Jin LP, Yuan MM, Zhu Y, Wang MY, Li DJ. Human first-trimester trophoblast cells recruit CD56brightCD16− NK cells into decidua by way of expressing and secreting of CXCL12/stromal cell-derived factor 1. J Immunol 2005; 175: 61–68. [DOI] [PubMed] [Google Scholar]
- 26Huang Y, Zhu XY, Du MR, Li DJ. Human trophoblasts recruited T lymphocytes and monocytes into decidua by secretion of chemokine CXCL16 and interaction with CXCR6 in the first-trimester pregnancy. J Immunol 2008; 180: 2367–2375. [DOI] [PubMed] [Google Scholar]
- 27Piao HL, Tao Y, Zhu R, Wang SC, Tang CL, Fu Q et al. The CXCL12/CXCR4 axis is involved in the maintenance of Th2 bias at the maternal/fetal interface in early human pregnancy. Cell Mol Immunol 2012; 9: 423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28Koopman LA, Kopcow HD, Rybalov B, Boyson JE, Orange JS, Schatz F et al. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med 2003; 198: 1201–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29Hiby SE, Walker JJ, O'Shaughnessy KM, Redman CW, Carrington M, Trowsdale J et al. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med 2004; 200: 957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Kalkunte SS, Mselle TF, Norris WE, Wira CR, Sentman CL, Sharma S. Vascular endothelial growth factor C facilitates immune tolerance and endovascular activity of human uterine NK cells at the maternal–fetal interface. J Immunol 2009; 182: 4085–4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31El Costa H, Casemayou A, Aguerre-Girr M, Rabot M, Berrebi A, Parant O et al. Critical and differential roles of NKp46- and NKp30-activating receptors expressed by uterine NK cells in early pregnancy. J Immunol 2008; 181: 3009–3017. [DOI] [PubMed] [Google Scholar]
- 32Verma S, Hiby SE, Loke YW, King A. Human decidual natural killer cells express the receptor for and respond to the cytokine interleukin 15. Biol Reprod 2000; 62: 959–968. [DOI] [PubMed] [Google Scholar]
- 33Zhou R, Wei H, Tian Z. NK3-like NK cells are involved in protective effect of polyinosinic–polycytidylic acid on type 1 diabetes in nonobese diabetic mice. J Immunol 2007; 178: 2141–2147. [DOI] [PubMed] [Google Scholar]
- 34Trotta R, Dal Col J, Yu J, Ciarlariello D, Thomas B, Zhang X et al. TGF-beta utilizes SMAD3 to inhibit CD16-mediated IFN-gamma production and antibody-dependent cellular cytotoxicity in human NK cells. J Immunol 2008; 181: 3784–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol 2004; 5: 266–271. [DOI] [PubMed] [Google Scholar]
- 36Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod 2004; 10: 347–353. [DOI] [PubMed] [Google Scholar]
- 37Hanna J, Wald O, Goldman-Wohl D, Prus D, Markel G, Gazit R et al. CXCL12 expression by invasive trophoblasts induces the specific migration of CD16− human natural killer cells. Blood 2003; 102: 1569–1577. [DOI] [PubMed] [Google Scholar]
- 38Carlino C, Stabile H, Morrone S, Bulla R, Soriani A, Agostinis C et al. Recruitment of circulating NK cells through decidual tissues: a possible mechanism controlling NK cell accumulation in the uterus during early pregnancy. Blood 2008; 111: 3108–3115. [DOI] [PubMed] [Google Scholar]
- 39Royle C, Lim S, Xu B, Tooher J, Ogle R, Hennessy A. Effect of hypoxia and exogenous IL-10 on the pro-inflammatory cytokine TNF-alpha and the anti-angiogenic molecule soluble Flt-1 in placental villous explants. Cytokine 2009; 47: 56–60. [DOI] [PubMed] [Google Scholar]
- 40Torricelli M, Voltolini C, Bloise E, Biliotti G, Giovannelli A, de Bonis M et al. Urocortin increases IL-4 and IL-10 secretion and reverses LPS-induced TNF-alpha release from human trophoblast primary cells. Am J Reprod Immunol 2009; 62: 224–231. [DOI] [PubMed] [Google Scholar]
- 41Scott VL, Shack LA, Eells JB, Ryan PL, Donaldson JR, Coats KS. Immunomodulator expression in trophoblasts from the feline immunodeficiency virus (FIV)-infected cat. Virol J 2011; 8: 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42Guo PF, Du MR, Wu HX, Lin Y, Jin LP, Li DJ. Thymic stromal lymphopoietin from trophoblasts induces dendritic cell-mediated regulatory TH2 bias in the decidua during early gestation in humans. Blood 2010; 116: 2061–2069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.