Cardiac transplantation is currently the preferred choice of treatment for end-stage cardiac disease. Despite great advances have been made in the prevention and treatment of acute transplant rejection, accelerated cardiac allograft vasculopathy (CAV) still limits the long-term success of heart transplantation. CAV is a rapidly progressive form of atherosclerosis described by vascular remodeling of the graft coronary arteries that occurs uniquely in transplant recipients. In the early stages, CAV is characterized by intimal proliferation, while luminal stenosis of epicardial branches, occlusion of smaller arteries and myocardial infarction occur in the later stages.1 These events are the results of cumulative endothelial injuries induced by nonspecific insults, such as ischemia–reperfusion injury, as well as by alloimmune responses. Thus, innate and adaptive immune responses are involved in the pathogenesis of this disease, as supported by the evidence that syngeneic transplants and transplants into immune deficient mice (such as RAG 1−/− mice) fail to develop CAV.2,3
Neutrophils are commonly associated with ischemia reperfusion injury.4,5,6 They are recruited to transplants through inflammatory chemokines secreted by the graft vasculature, such as tumor-necrosis factor (TNF) and interleukin-1 (IL-1), expressed during reperfusion.6 King et al. investigated the role of neutrophils in CAV development in a mouse model. In this study, aortic transplants were performed in the presence of therapeutic levels of cyclosporine A and subsequently aortas were harvested at different times after transplantation. The authors reported that the peaks of neutrophil flux into the media occurred at 1 day and 1 week post-transplant and correlated with medial smooth muscle cell (SMC) destruction. This loss was significantly reduced by neutrophil depletion.6 In the same view, So et al.7 investigated the role of neutrophils in the loss of SMC at 1 day and the failure to recover by 2 weeks in the presence of prolonged (60-min) cold ischemia in neutrophil loss-of-function recipient B6 mice (NOX2−/−). They first observed that the loss of SMC was lower in grafts transplanted into NOX2−/− as compared to wild-type B6 recipients. Moreover, grafts transplanted into NOX2−/− recipients exhibited significant SMC repopulation at 2 weeks after transplant, despite exposure to 60-min cold ischemia.
To evaluate the potential role of immunosuppression in the prevention of CAV in heart transplant recipients, Vitiello et al.8 evaluated the effect of different routinely used immunosuppressive drugs (ID) cyclosporine A (CsA), tacrolimus (TAC), mycophenolic acid (MPA), sirolimus (SIR) or everolimus (EVE) on neutrophil response.8
First, they assessed the capacity of selected pro-inflammatory agonists N-Formyl-Met-Leu-Phe, bacterial lipopolysaccharide (LPS), TNF-α or control vehicle (phosphate-buffered saline) to induce the release of cytokines by human neutrophils. They observed that treatment with these agonists induced the release of vascular endothelial growth factor (VEGF), IL-1 receptor antagonist (IL-1RA) and IL-8 by neutrophils, and the most potent effect was evoked by LPS. Most pre-treatments with ID, alone or in combination significantly decreased IL-8 release. Highest concentration of SIR or TAC had no effect or even increased IL-8, while the greatest inhibition of basal IL-8 release was observed with EVE (−90%) alone or in combination with other ID. Moreover, pre-treatment with SIR and EVE alone or in combination with CsA or TAC significantly decreased the release of VEGF by neutrophils under all proinflammatory stimulations, while no effects were observed with CsA. Notably, the combination of SIR and MPA was associated with a complete or partial loss of SIR capacity to prevent VEGF release, whereas EVE in combination to MPA maintained EVE activity.
Interestingly, EVE was the only ID capable of promoting the release of the anti-inflammatory cytokine, IL-1RA. Thus the authors showed that pre-treatment with MPA or SIR, at all concentrations had a tendency to reduce IL-1RA release induced by N-Formyl-Met-Leu-Phe, LPS and TNF-α, whereas pre-treatment with CsA or TAC had no or minor inhibitory effect on IL-1RA release. On the other hand, LPS- or TNF-induced IL-1RA release was increased twofold by EVE, even in combination with CSA, TAC or MPA.
Vitiello et al. further assessed the effects of different ID used alone or in combination on neutrophil adhesion onto human umbilical vein endothelial cells or human extracellular matrix. The resultant data showed that when stimulated with TNF-α, the pre-treatment with CsA or MPA provided a non-significant increase of neutrophils adhesion onto human umbilical vein endothelial cells, whereas pre-treatments with TAC, SIR or EVE decreased neutrophils adhesion to human umbilical vein endothelial cells by about 74%. This effect was largely dependent on the ability of these compounds to alter β2-integrin/CD18 activation.
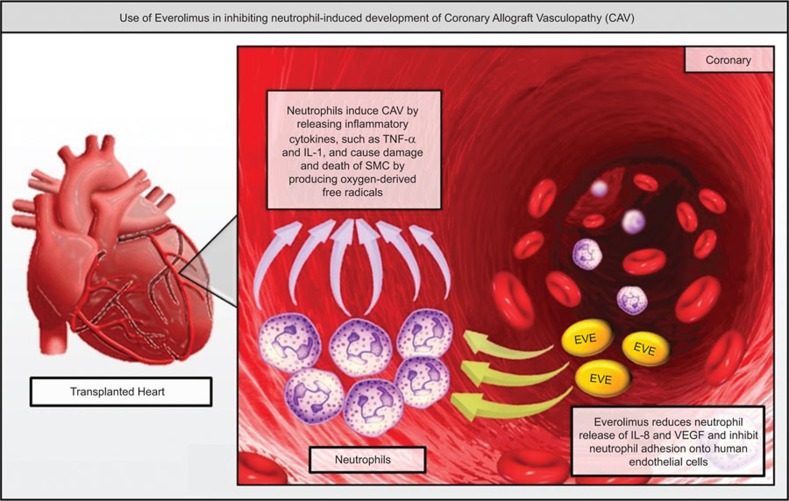
In summary, the Vitiello study shows the mechanisms by which different ID affect neutrophil activity. Based on these findings, EVE represents the most effective ID in preventing neutrophil adhesion and cytokine release, which are essential for the development and progression of CAV (Figure 1). The results of this study may lead to the development of innovative prophylactic strategies based on the use of EVE, alone or in combination with other ID. In future studies, it will be interesting to add to these results data on the individual patient's pharmacokinetics and immune system pre-treatment status. This integrated approach may represent a major step forward in order to develop individualized patient-tailored strategies and to improve the long-term prognosis of heart transplant patients.
Figure 1.
Use of everolimus in inhibiting neutrophil-induced development of CAV. Neutrophils can induce CAV by releasing inflammatory cytokines, such as TNF-α and IL-1, and cause damage and death of SMC by producing oxygen-derived free radicals. EVE is able to reduce the release of release of IL-8 and VEGF by neutrophils and to increase the production of anti-inflammatory cytokine IL-1RA. Moreover, EVE can inhibit neutrophil adhesion onto human endothelial cells and human extracellular matrix by altering β2-integrin/CD18 activation. CAV, coronary allograft vasculopathy; EVE, everolimus; IL-1RA, interleukin-1 receptor antagonist; IL-8, interleukin-8; SMC, smooth muscle cell; TNF-α, tumor-necrosis factor-α VEGF, vascular endothelial growth factor.
References
- 1Weis M. Cardiac allograft vasculopathy: prevention and treatment options. Transplant Proc 2002; 34: 1847–1849. [DOI] [PubMed] [Google Scholar]
- 2Wanders A, Akyurek ML, Waltenberger J, Ren ZP, Stafberg C, Funa K et al. Ischemia-induced transplant arteriosclerosis in the rat. Arterioscler Thromb Vasc Biol 1995; 15: 145–155. [PubMed] [Google Scholar]
- 3Tanaka M, Mokhtari G, Terry R, Gunawan F, Baslsam L, Hoyt G et al. Prolonged cold ischemia in rat cardiac allografts promotes ischemia–reperfusion injury and the development of graft coronary artery disease in a linear fashion. J Heart Lung Transplant 2005; 24: 1906–1914. [DOI] [PubMed] [Google Scholar]
- 4El-Sawy T, Fahmy NM, Fairchild RL. Chemokines: directing leukocyte infiltration into allografts. Curr Opin Immunol 2002; 14: 562–568. [DOI] [PubMed] [Google Scholar]
- 5Jaeschke H, Smith CW. Mechanisms of neutrophil-induced parenchymal cell injury. J Leuk Biol 1997; 61: 647–653. [DOI] [PubMed] [Google Scholar]
- 6King CL, Devitt JJ, Lee TD, Hancock Friesen CL. Neutrophil mediated smooth muscle cell loss precedes allograft vasculopathy. J Cardiothorac Surg 2010; 5: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7So M, Lee TD, Hancock Friesen CL. Neutrophils are responsible for impaired medial smooth muscle cell recovery and exaggerated allograft vasculopathy in aortic allografts exposed to prolonged cold ischemia. J Heart Lung Transplant 2013; 32: 360–367. [DOI] [PubMed] [Google Scholar]
- 8Vitiello D, Neagoe PE, Sirois MG, White M. Effect of everolimus on the immunomodulation of human neutrophils inflammatory response and activation. Cell Mol Immunol 2014; in press. [DOI] [PMC free article] [PubMed]